1. Introduction
Inorganic pigments are widely used as coloring materials for ceramics, glasses, plastics, and glazes because they have good thermal stabilities and resistances to climatic conditions. Among various colors, a greenish color makes us feel reposed, healed and relaxed; therefore, it has been used for a variety of applications. A characteristic of green is that it brings peaceful images of woods and forests. In addition, green signifies safety on traffic signs and public guideboards, and such feelings are widely accepted by people [
1]. Thus, green is also used for information boards in evacuation centers and an emergency exits, etc. Moreover, because green is an intermediate color on the hue circle, it is used in various situations for harmonization with other colors.
Currently, chromium oxide (Cr
2O
3), chromium green, cobalt green (CoO·nZnO), and so on are used as commercially available inorganic green pigments. For Cr
2O
3, studies have been conducted to improve its optical reflectance properties by mixing it with TiO
2, Al
2O
3, and V
2O
5 [
2], or by substituting other metal ions at the Cr
3+ site, as in Cr
2−xAl
xO
3 [
3]. Sangeetha et al. found that Cr
2O
3 added with rare earth elements improved the greenness of the reflectance without decreasing the greenness [
4]. Cr
3+ is not a harmful ion but Cr
2O
3 has been synthesized by reducing the Na
2Cr
2O
7 or pyrolysis of CrO
3, which contains Cr
6+ [
5]. Chromium green is composed of iron blue (Fe
4[Fe(CN)
6]
3) and chrome yellow (PbCrO
4) and is used in paints because of its high hiding power. However, chrome yellow contains Pb and Cr
6+. Cr
6+ is identified as a carcinogen by the International Agency for Research on Cancer (IARC), and so is well known as a highly toxic element. At the same time, Pb is identified as possible carcinogenesis. On the other hand, the safety of CoO in cobalt green has also been questioned due to the concern about adverse effects on the human body. The use of these pigments has been restricted because they contain harmful elements (e.g., Cd, Pb, and Cr) and CoO, which has deleterious effect on the human body and the environment. Therefore, there is a strong desire to develop environmentally friendly inorganic green pigments to replace harmful inorganic pigments.
Based on this background, in order to develop novel environmentally friendly inorganic green pigments, we took note of pentavalent manganese (Mn
5+) ions as a coloring source. Mn ions are not only harmless but also inexpensive and exhibit various valences to be utilized in multiple applications. Mn
5+ absorbs light with a wavelength of 400 nm or less based on ligand-to-metal charge transfer (LMCT) transition and d-d transition (
3A
2 →
3T
1 (
3P)) [
6,
7]. Visible light around 650 nm is also absorbed by d-d transition attributed to
3A
2 →
3T
1 (
3F), and therefore, green coloration can be expected by using Mn
5+. Although Mn
5+ ions tend to be generally unstable, they can be stable in the following situation: Mn ions are substituted in tetrahedral sites as well as a host compound, which is comprised of larger ionic radius elements such as alkali earth metal (Sr and Ba) and smaller ionic radius elements such as P and Al [
6]. Actually, various Mn
5+-containing compounds have been reported [
7,
8,
9,
10,
11,
12] such as turquoise color pigments based on apatite-type Ba
5Mn
3−xMxO
12Cl (M = V, P) [
13] and Ba
3(MO
4)
2:Mn
5+ (M = V, P) phosphors [
14].
We focused on LaSr
2AlO
5 as a host material because it contains only low toxicity elements. LaSr
2AlO
5 adopts a tetragonal structure (space group:
I4/
mcm) [
15]. This complex oxide is stacked alternately with an 8-coordinated La
3+/Sr
2+ layer, a 10-coordinatd Sr
2+ layer, and a 4-coordinated Al
3+ layer through O
2-. A number of papers have also reported the use of LaSr
2AlO
5 as a base material for photocatalysts and phosphors [
15,
16,
17,
18,
19,
20]. There are many compounds in which La
3+ is replaced by other elements, but few in which the Al
3+ is replaced.
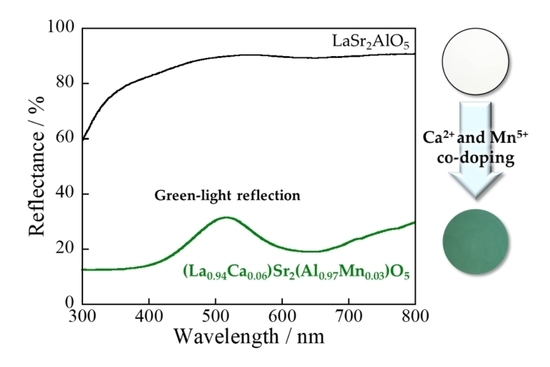
In this study, therefore, La1.03Sr1.97Al0.97M0.03O5 (M = Fe, Co, Ni, and Cu) samples, whose Al3+ site was substituted by various transition metal elements, had their color properties characterized. Among them, the Co-doped sample exhibited a vivid yellow, but not green. Thus, La1−2xCa2xSr2Al1−xMnxO5 (x = 0 and 0.03) samples were synthesized to obtain a new environmentally friendly green pigment. The Al3+ site in the LaSr2AlO5 host was partially substituted by Mn5+ ions. At the same time, the La3+ site was replaced by Ca2+ for charge compensation. The color properties of the samples were evaluated and compared with commercially available inorganic green pigments.
2. Materials and Methods
2.1. Synthesis
The LaSr2(Al0.97Fe0.03)O5, La1.03Sr1.97(Al0.97M0.03)O5 (M: Ni and Cu), and La1+xSr2−x(Al1−xCox)O5 (x = 0, 0.03, 0.05 and 0.10) samples were synthesized using a citrate sol–gel method. La(NO3)3·6H2O (FUJIFILM Wako Pure Chemical Industries Ltd., Osaka, Japan, 99.9%), Sr(NO3)2 (FUJIFILM Wako Pure Chemical Industries Ltd., 98.0%), Al(NO3)3·9H2O (FUJIFILM Wako Pure Chemical Industries Ltd., 99.9%), Fe(NO3)3·9H2O (FUJIFILM Wako Pure Chemical Industries Ltd., 99.9%), Co(NO3)2·6H2O (FUJIFILM Wako Pure Chemical Industries Ltd., 99.5%), Cu(NO3)2·3H2O (FUJIFILM Wako Pure Chemical Industries Ltd., 99.9%), and Ni(NO3)2·6H2O (Kishida Chemical Co. Ltd., Osaka, Japan, 98.0%) were weighed so as to obtain the objective compositions and were dissolved in deionized water to adjust the La, Sr, and (Al + M) concentrations to 0.2 mol L−1. After the solution was stirred homogeneously, citric acid (FUJIFILM Wako Pure Chemical Industries Ltd., 98.0%) was added as a chelating agent to complex the cations into the solution in the mole ratio 2:1 with regard to the total cations (La, Sr, Al, and M). The mixed solution was stirred at 80 °C until a gel was obtained. Then, the gel was heated in an oven at 120 °C for 24 h and completely dried at 350 °C on a mantle heater. The dried gel was calcined in an alumina crucible at 500 °C for 8 h in air. After the calcination, the sample was heated again in an alumina boat at 1400 °C for 9 h in air. Before characterization, the sample was ground in an agate mortar with agate pestle.
(La1−2xCa2x)Sr2(Al1−xMnx)O5 (x = 0 and 0.03) samples were prepared by the same procedure described above. The starting materials, La(NO3)3·6H2O, Ca(NO3)2·4H2O (FUJIFILM Wako Pure Chemical Industries Ltd., 98.5%), Sr(NO3)2, Al(NO3)3·9H2O, and (CH3COO)2Mn·4H2O (FUJIFILM Wako Pure Chemical Industries Ltd., 99.9%), were weighed stoichiometrically.
2.2. Characterization
X-ray powder diffraction (XRD) was conducted using an X-ray diffractometer (Rigaku Corporation, Tokyo, Japan Ultima IV) to identify the crystal phase and structure. The XRD patterns were taken with Cu-Kα radiation operating with a tube voltage of 40 kV and a tube current of 40 mA. The data were collected by scanning over the 2
θ range of 20–80°. The sampling width was 0.02° and the scan speed was 6° min
−1. The lattice volumes were calculated from the XRD peak angles refined by α-Al
2O
3 as a standard and using the CellCalc Ver. 2.20 software. The Rietveld refinement of the resulting XRD patterns was performed by the RIETAN-FP software (version 3.12) package to determine the precise crystal structure [
21]. From the Rietveld refinement, the following final R-factors were obtained: Rwp (R-weighed pattern), Rp (R-pattern), Re (R-expected), S (goodness-of-fit indicator), and RF (R-structure factor). The optical reflectance spectra of the as-prepared samples were recorded on an ultraviolet-visible (UV-Vis) spectrometer (JASCO Corporation, Tokyo, Japan, V-770 with an integrating sphere attachment) using a standard white plate as a reference. The step width was 1 nm and the scan rate was 1000 nm min
−1. The color properties of the powder samples were evaluated in terms of the Commission Internationale de l’Éclairage (CIE)
L*
a*
b*
Ch° system using a colorimeter (Konica-Minolta, INC., Tokyo, Japan, CR-400). A standard C illuminant was used for the colorimetric measurements. The
L* parameter indicates the brightness or darkness in a neutral grayscale. The
a* values represent the red–green axes, and the
b* value yellow–blue axes. The chroma parameter (
C) means the color saturation and is calculated with the formula,
C = [(
a*)
2 + (
b*)
2]
1/2. The hue angle (
h°) ranges from 0 to 360° and is calculated with the formula
h° = tan
−1(
b*/
a*). The standard deviations of all values for the
L*
a*
b*
Ch° color coordinate data were less than 0.1.