Identification of Degradation Products and Components in Shellfish Purple by Ultrahigh Performance Liquid Chromatography Coupled with Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Stock Solution Preparation for Direct Infusion
2.3. UHPLC-MS/MS Analysis
2.4. Chromatographic Conditions
2.5. MS/MS Conditions
2.6. Sample Preparation
2.7. Aging Process
3. Results
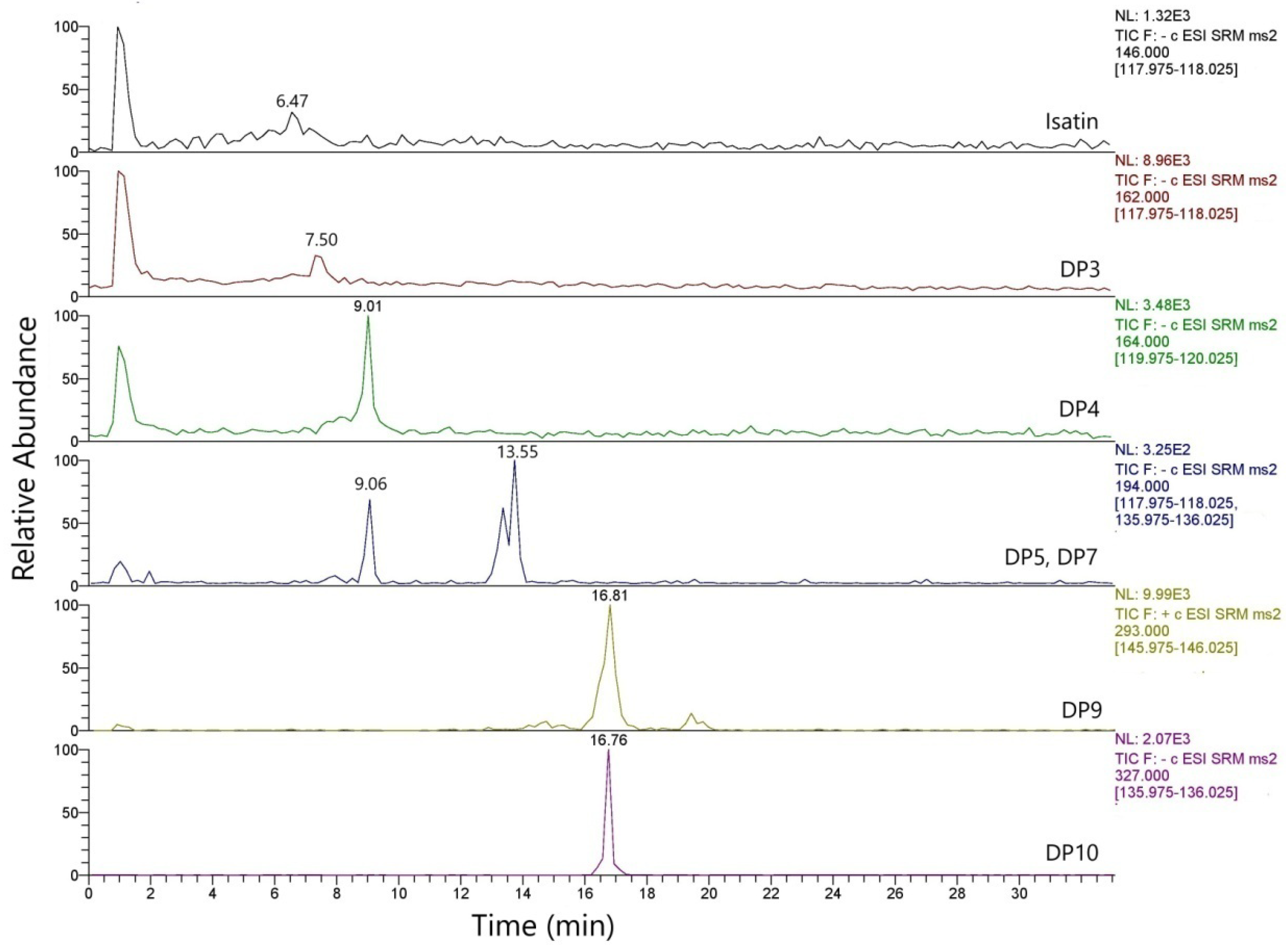
3.1. Degradation Products of Indigotin (IND) in Standard Solutions
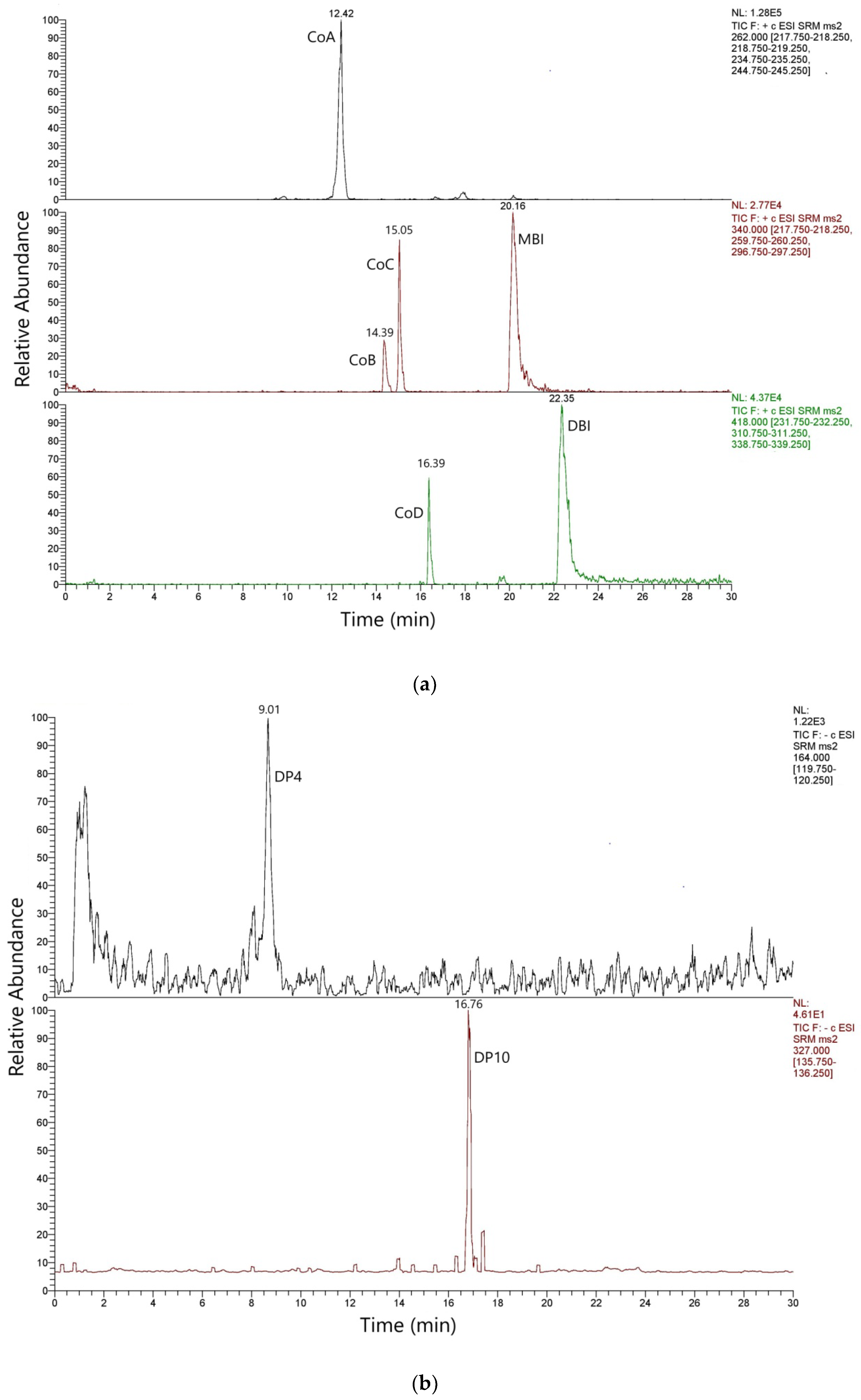
3.2. Coloring Components and Degradation Products in Hexaplex trunculus L. Pigment
3.3. Degradation Products in Dyed Textiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cardon, D. Natural Dyes: Sources, Traditions, Technology and Science; Archetype: London, UK, 2007. [Google Scholar]
- Clark, R.J.H.; Cooksey, C.J.; Daniels, M.A.M.; Withnall, R. Indigo, woad, and Tyrian Purple: Important vat dyes from antiquity to the present. Endeavour 1993, 17, 191–199. [Google Scholar] [CrossRef]
- Koren, Z.C. New Chemical Insights into the Ancient Molluskan Purple Dyeing Process; Chapter 3; ACS Symposium Series; American Chemmical Society: Washigton, DC, USA, 2013. [Google Scholar]
- Maravelaki-Kalaitzaki, P.; Kallitrakas-Kontos, N. Pigment and terracotta of Hellenistic figurines in Crete. Anal. Chim. Acta 2003, 497, 209–225. [Google Scholar] [CrossRef]
- Ribechini, E.; Pérez-Arantegui, J.; Pavan, A.; Degano, I.; Zanaboni, M.; Colombini, M.P. First evidence of purple pigment production and dyeing in southern Arabia (Sumhuram, Sultanate of Oman) revealed by mass spectrometric and chromatographic techniques. J. Cult. Herit. 2016, 19, 486–491. [Google Scholar] [CrossRef]
- Koren, Z.C. Archaeo-chemical analysis of Royal Purple on a Darius I stone jar. Microchim. Acta 2008, 162, 381–392. [Google Scholar] [CrossRef]
- March, R.E.; Papanastasiou, M.; McMahon, A.W.; Allen, N.S. An investigation of paint from a mural in the church of Sainte Madeleine, Manas, France. J. Mass Spectrom. 2011, 46, 816–820. [Google Scholar] [CrossRef]
- Breniquet, C.; Desrosiers, S.; Nowik, W.; Rast-Eicher, A. Les textiles découverts dans les tombes de l’agedu Bronze moyen a Chagar Bazar (Syrie). In Chagar Bazar (Syrie) VIII. Les Tombes Ordinaires d’ L’¯ageduBronzeAncien et Moyen des Chantiers D-F-H-I (1999–2011); Tunca, O., Baghdo, A., Eds.; Peeters Publishers: Lueven-Liege, Belgium, 2018; pp. 11–32. [Google Scholar]
- Abdel-Kareem, O.; Alawi, M.A.; Mubarak, M.S. Identification of Dyestuffs in a Rare Coptic Garment Using High Performance Liquid Chromatography with Photodiode Array Detection (HPLC-PDA). JTATM 2010, 6, 1–7. [Google Scholar]
- James, M.A.; Reifarth, N.; Mukherjee, A.J.; Crump, M.P.; Gates, P.J.; Sandor, P.; Robertson, F.; Pfälzner, P.; Evershed, R.P. High prestige royal purple dyed textiles from the Bronze Age royal tomb at Qatna, Syria. Antiquity 2009, 83, 1109–1118. [Google Scholar] [CrossRef]
- Hartl, A.; van Bommel, M.R.; Joosten, I.; Hofmann-de Keijzer, R.; Grömer, K.; Rösel-Mautendorfer, H.; Reschreiter, H. Reproducing colourful woven bands from the Iron Age salt mine of Hallstatt in Austria: An interdisciplinary approach to acquire knowledge of prehistoric dyeing technology. J. Archaeol. Sci. Rep. 2015, 2, 569–595. [Google Scholar] [CrossRef]
- Koh, A.A.; Betancourt, P.P.; Pareja, M.N.; Brogan, T.M.; Apostolakou, V. Organic residue analysis of pottery from the dye workshop at Alatsomouri-Pefka, Crete. J. Archaeol. Sci. Rep. 2016, 7, 536–538. [Google Scholar] [CrossRef]
- Benkendorff, K.; Rudd, D.; Nongmaithem, B.D.; Liu, L.; Young, F.; Edwards, V.; Avila, C.; Abbott, C.A. Are the Traditional Medical Uses of Muricidae Molluscs Substantiated by Their Pharmacological Properties and Bioactive Compounds? Mar. Drugs 2015, 13, 5237–5275. [Google Scholar] [CrossRef]
- Katsifas, C.; Ignatiadou, D.; Zacharopoulou, A.; Kantiranis, N.; Karapanagiotis, I.; Zachariadis, G. Non-Destructive X-ray Spectrometric and Chromatographic Analysis of Metal Containers and Their Contents, from Ancient Macedonia. Separations 2018, 5, 32. [Google Scholar] [CrossRef]
- Vermeulen, M.; Tamburini, D.; McGeachy, A.C.; Meyers, R.D.; Walton, M.S. Multiscale characterization of shellfish purple and other organic colorants in 20th-century traditional enredos from Oaxaca, Mexico. Dyes Pigments 2022, 206, 110663. [Google Scholar] [CrossRef]
- Michel, R.H.; Lazar, J.; McGovern, P.E. Indigoid dyes in Peruvian and Coptic textiles of the University 632 Museum of Archaeology and Anthropology. Archeomaterials 1992, 6, 69–83. [Google Scholar]
- Chavez, E.A.; Michel-Morfin, J.E. Strategies for sustainable dye harvest of the purple conch Plicopurpura pansa (Gould, 1853) from west central Mexico. Am. Malacol. Bull. 2006, 21, 51–57. [Google Scholar]
- Rilov, G. Multi-species collapses at the warm edge of a warming sea. Sci. Rep. 2016, 6, 36897. [Google Scholar] [CrossRef] [PubMed]
- Koren, Z.C. High-Performance Liquid Chromatographic analysis of an ancient tyrian purple dyeing vat from Israel. Isr. J. Chem. 1995, 35, 117–124. [Google Scholar] [CrossRef]
- Koren, Z.C. HPLC-PDA analysis of brominated indirubinoid, indigoid, and isatinoid dyes. In Indirubin, the Red Shade of Indigo; Meijer, L., Guyard, N., Skaltsounis, A.L., Eisenbrand, G., Eds.; Life in Progress Editions; Station Biologique: Roscoff, France, 2006; Chapter 5; pp. 45–53. [Google Scholar]
- Nowik, W.; Marcinowska, R.; Kusyk, K.; Cardon, D.; Trojanowicz, M. High performance liquid chromatography of slightly soluble brominated indigoids from Tyrian Purple. J. Chromatogr. A 2011, 1218, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Surowiec, I.; Nowik, W.; Moritz, T. Mass spectrometric identification of new minor indigoids in shellfish purple dye from Hexaplex trunculus. Dyes Pigments 2012, 94, 363–369. [Google Scholar] [CrossRef]
- Grosjean, D.; Whitmore, P.M.; Cass, G.R.; Druzlk, J.R. Ozone fanding of natural organic colorants: Me chanisms and products of the reaction of ozone with indigos, Environ. Sci. Technol. 1988, 22, 292–298. [Google Scholar] [CrossRef]
- Vasileiadou, A.; Karapanagiotis, I.; Zotou, A. UV-induced degradation of wool and silk dyed with shellfish purple. Dyes Pigments 2019, 168, 317–326. [Google Scholar] [CrossRef]
- Molino, R.J.E.J.; Junio, H.A. Profiling the Philippine Blue: Liquid chromatography/mass spectrometry-based metabolomics study on Philippine Indigofera. Rapid Commun. Spectrom. 2021, 35, 9037. [Google Scholar] [CrossRef]
- Novotna, P.; Boon, J.J.; van der Horst, J.; Paakova, V. Photodegradation of indigo in dichloromethane solution. Color. Technol. 2003, 119, 121–217. [Google Scholar] [CrossRef]
- Sousa, M.M.; Miguel, C.; Rodrigues, I.; Parola, A.J.; Pina, F.; de Melo, J.S.S.; Melo, M.J. A photochemical study on the blue dye indigo: From solution to ancient Andean textiles. Photochem. Photobiol. Sci. 2008, 7, 1353–1359. [Google Scholar] [CrossRef]
- Poulin, J. Identification of indigo and its degradation products on a silk textile fragment using gas chromatography-mass spectrometry. J. Can. Assoc. Conserv. 2007, 32, 48–56. [Google Scholar]
- Witkoś, K.; Lech, K.; Jarosz, M. Identification of degradation products of indigoids by tandem mass spectrometry. J. Mass Spectrom. 2015, 50, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Karapanagiotis, I.; Mantzouris, D.; Cooksey, C.; Mubarak, M.; Tsiamyrtzis, P. An improved HPLC method coupled to PCA for the identification of Tyrian purple in archaeological and historical samples. Microchem. J. 2013, 110, 70–80. [Google Scholar] [CrossRef]
- Vasileiadou, A.; Karapanagiotis, I.; Zotou, A. Determination of Tyrian purple by high performance liquid chromatography with diode array detection. J. Chromatogr. A 2016, 1448, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E. Characterization of historical organic dyestuffs by liquid chromatography-mass spectrometry. Anal. Bioanal. Chem. 2008, 391, 33–57. [Google Scholar] [CrossRef]
- Lech, K.; Fornal, E. A Mass Spectrometry-Based Approach for characterization of red, blue, and purple natural dyes. Molecules 2020, 25, 3223. [Google Scholar] [CrossRef]
- Serrano, A.; van Bommel, M.; Hallett, J. Evaluation between ultrahigh pressure liquid chromatography and high-performance liquid chromatography analytical methods for characterizing natural dyestuffs. J. Chromatogr. A 2013, 1318, 102–111. [Google Scholar] [CrossRef]
- Szostek, B.; Orska-Gawrys, J.; Surowiec, I.; Trojanowicz, M. Investigation of natural dyes occurring in historical Coptic textiles by high-performance liquid chromatography with UV/Vis and mass spectrometric detection. J. Chromatogr. A 2003, 1012, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Puchalska, M.; Polec-Pawlak, K.; Zadrozna, I.; Hryszko, H.; Jarosz, M. Identification of indigoid dyes in natural organic pigments used in historical art objects by high-performance liquid chromatography coupled to electrospay ionization mass spectrometry. J. Mass Spectrom. 2004, 39, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Degano, I.; Biesaga, M.; Colombini, M.P.; Trojanowicz, M. Historical and archaeological textiles: An insight on degradation of wool and silk yarns. J. Chromatogr. A 2011, 1218, 5837–5847. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, A.; Bonaduce, I.; Colombini, M.P.; Ribechini, E. Characterization of natural indigo and shellfish purple by mass spectrometric techniques. Rapid Commun. Mass Spectrom. 2004, 18, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Koh, H.L. Determination of indican, isatin, indirubin and indigotin in Isatis indigotica by liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Sanz, E.; Arteaga, A.; Garcia, M.A.; Camara, C.; Dietz, C. Chromatographic analysis of indigo from Maya Blue by LC-DAD-QTOF. J. Archaeol. Sci. 2012, 39, 3516–3523. [Google Scholar] [CrossRef]
- Virgiliou, C.; Sampsonidis, I.; Gika, H.G.; Raikos, N.; Theodoridis, G.A. Development and Validation of a HILIC-MS/MS multitargeted method for metabolomics applications. Electrophoresis 2015, 36, 2215–2225. [Google Scholar] [CrossRef]
- Giles, C.H. The fading of colouring matters. J. Appl. Chem. 1965, 15, 541–550. [Google Scholar] [CrossRef]
- Crews, P.C. The fading rates of some natural dyes. Stud. Conserv. 1987, 32, 65–72. [Google Scholar] [CrossRef]
- Cristea, D.; Vilarem, G. Improving light fastness of natural dyes on cotton yarn. Dyes Pigments 2006, 70, 238–245. [Google Scholar] [CrossRef]
- Ford, B.L. Monitoring colour change in textiles on display. Stud. Conserv. 1992, 37, 1–11. [Google Scholar] [CrossRef]
- Daniels, V. The light-fastness of textiles dyed with 6,6´-dibromoindigotin (Tyrian purple). J. Photochem. Photobiol. A 2006, 184, 73–77. [Google Scholar] [CrossRef]
| Time (min) | Flow (mL min−1) | A (%) | B (%) |
|---|---|---|---|
| 0 | 0.3 | 95 | 5 |
| 3 | 0.3 | 95 | 5 |
| 13 | 0.3 | 70 | 30 |
| 19 | 0.3 | 40 | 60 |
| 22 | 0.3 | 40 | 60 |
| 37 | 0.3 | 5 | 95 |
| 37.01 | 0.3 | 95 | 5 |
| 38 | 0.3 | 95 | 5 |
| Compounds | Chemical Formula | Rt (min) | Ion Mode | Precursor Ion (m/z) | Fragment Ion (m/z) and CE (V) |
|---|---|---|---|---|---|
| IS | C8H5NO2 | 6.47 | − | 146 | 118.03 (14) 42.45 (21) 90.10 (23) |
| DP3 | C8H5NO3 | 7.52 | − | 162 | 118 |
| DP4 | C8H13NO3 | 9.01 | − | 164 | 120 |
| DP5 | C9H15NO4 | 9.06 | − | 194 | 136 |
| DP6 | C9H13NO3 | 9.51 | − | 178 | 134 |
| DP7 | C9H13NO4 | 13.55 | − | 194 | 118 |
| DP9 | C16H10N2O4 | 16.81 | + | 293 | 146 |
| DP10 | C15H12N2O6 | 16.76 | − | 327 | 135 |
| Co A | C16H11ON3 | 12.42 | + | 262 | 219235 245 |
| Co B | C16H10ON3Br | 14.39 | + | 340 | 218 260 297 |
| Co C | C16H10ON3Br | 15.05 | + | 341 | 219 260 297 |
| Co D | C16H9ON3Br2 | 16.39 | + | 418 | 232 311 339 |
| IND | C16H10O2N2 | 17.9 | + | 262.8 | 218.81 (25) 234.51 (25) 189.75 (37) |
| INR | C16H10O2N2 | 18.8 | + | 262.8 | 218.79 (24) 189.83 (36) 234.63 (22) |
| MBI | C16H9O2N2Br | 20.16 | + | 342.3 | 261.62 (28) 204.76 (49) 340,1 (14) |
| 6′MBIR | C16H9O2N2Br | 20.8 | + | 341 | 296.32 (25) 204.93 (46) 340,1 (10) |
| 6MBIR | C16H9O2N2Br | 21.08 | + | 341 | 261.56 (19) 233.72 (27) 204.76 (51) |
| DBI | C16H8O2N2Br2 | 22.35 | + | 419 | 184.84 (22) 340.83 (6) 63.14 (36) |
| DBIR | C16H8O2N2Br2 | 24.08 | + | 419 | 282.6 (29) 255.1 (35) 189.2 (38) |
| Compounds | IND Solution | Shellfish Purple Pigment | Silk Dyed with Shellfish Purple | ||||
|---|---|---|---|---|---|---|---|
| FRESH | AGED 1 | FRESH | AGED 1 | FRESH | AGED 2 | AGED 1 | |
| IS | + | + | + | ||||
| DP3 | + | + | + | ||||
| DP4 | + | + | + | + | + | + | |
| DP5 | + | ||||||
| DP6 | + | + | |||||
| DP7 | + | ||||||
| DP9 | + | + | |||||
| DP10 | + | + | + | + | + | ||
| Co A | ++ | ++ | ++ | ++ | |||
| Co B | ++ | ++ | ++ | ++ | |||
| Co C | ++ | ++ | ++ | ++ | |||
| Co D | ++ | ++ | ++ | ++ | |||
| IND | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| INR | ++ | ++ | ++ | ++ | ++ | ||
| MBI | +++ | +++ | +++ | +++ | +++ | ||
| 6′MBIR | + | + | |||||
| 6MBIR | + | + | |||||
| DBI | +++ | +++ | +++ | +++ | +++ | ||
| DBIR | ++ | + | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasileiadou, A.; Sampsonidis, I.; Theodoridis, G.; Zotou, A.; Karapanagiotis, I.; Kalogiannis, S. Identification of Degradation Products and Components in Shellfish Purple by Ultrahigh Performance Liquid Chromatography Coupled with Tandem Mass Spectrometry. Heritage 2024, 7, 1935-1946. https://doi.org/10.3390/heritage7040092
Vasileiadou A, Sampsonidis I, Theodoridis G, Zotou A, Karapanagiotis I, Kalogiannis S. Identification of Degradation Products and Components in Shellfish Purple by Ultrahigh Performance Liquid Chromatography Coupled with Tandem Mass Spectrometry. Heritage. 2024; 7(4):1935-1946. https://doi.org/10.3390/heritage7040092
Chicago/Turabian StyleVasileiadou, Athina, Ioannis Sampsonidis, Georgios Theodoridis, Anastasia Zotou, Ioannis Karapanagiotis, and Stavros Kalogiannis. 2024. "Identification of Degradation Products and Components in Shellfish Purple by Ultrahigh Performance Liquid Chromatography Coupled with Tandem Mass Spectrometry" Heritage 7, no. 4: 1935-1946. https://doi.org/10.3390/heritage7040092









