Topic Editors


The Tumor Microenvironment, Immuno-Oncology, and Immune Checkpoint: Implications for Current and Emergent Immunotherapies, 2nd Edition
Topic Information
Dear Colleagues,
Immunotherapy uses the body’s natural defenses (immune system) to fight disease. For example, our immune system can help to destroy cancer cells. Immuno-oncology is a type of immunotherapy that is directed against cancer.
There are two main parts of the immune system:
(1) Innate immunity (also known as built-in immune protection) has mechanisms that are prepared to act immediately, such as the skin, inner lining of the gut and lungs, stomach acid, intestinal bacteria, and leukocytes (neutrophils) that can find and kill bacteria.
In innate immunity, pathogens are recognized by receptors encoded in the germline. The receptors, known as pattern recognition receptors (PPRs), have a broad specificity for pathogen-associated molecular patterns (PAMPs).
Innate immune cells are granulocytes (neutrophils, eosinophils, basophils, mast cells), monocytes, macrophages, dendritic cells, and innate lymphoid cells (cytotoxic Natural Killer (NK) cells, and several noncytotoxic cells (IL1, 2, etc.)).
(2) Acquired immunity is immune protection that is “learned.”
After being exposed to certain diseases and a type of bacteria, fungus, or virus for the first time, the immune system develops memory and responds quicker and better the second time.
Lymphocytes are involved in the acquired immune response.
B cells/lymphocytes make antibodies that lock onto the surface of bacteria or viruses. B lymphocytes are part of the memory of the immune system.
There are different kinds of T lymphocytes: helper T cells (Th) and killer/cytotoxic T cells (Tc). Th cells stimulate B cells to make antibodies and help Tc cells to develop. Tc cells kill the body’s own cells infected by viruses or bacteria.
In adaptive immunity, pathogens are recognized by receptors that are generated randomly. These receptors recognize epitopes (polypeptides). The receptors are B-cell (BCR) and T-cell (TCR) receptors. The response is slow (3–5 days) and has memory.
The tumor microenvironment (TME) is comprised of the tumor, the extracellular matrix (ECM), and variable infiltration by nontransformed cells, including vascular vessels, fibroblasts, and immune system infiltrates. The TME plays a major role in tumor initiation, progression, infiltration and metastasis, and resistance to treatment. The tumoral immune microenvironment comprises variable percentages of CD8+Tc, naïve CD4+T cells, FOXP3+ regulatory T lymphocytes (Tregs), B cells, neutrophils, macrophages, NK cells, and dendritic cells. Macrophages, known as tumor-associated macrophages (TAMs), are a key component of the microenvironment. These M2-like TAMs are associated with tumor migration and invasion, metastasis, growth, angiogenesis, tissue remodeling, and inhibiting the host immune response (Treg induction, inactivation of T cells, etc.). Relevant markers associated with TAMs are PD-L1/L2, MMPs, M-CSF, CSF1R, IL-10, TGFB, prostaglandins, IDO1/2, etc.
The immune checkpoint refers to the mechanisms the immune system uses to avoid collateral damage during the physiological immune response and maintain self-tolerance. Ligand–receptor interactions are mediated by co-stimulatory molecules such as CD80, CD86, GITRL, and ICOSLG expressed by antigen-presenting cells (APCs), and CD28, GITR, and ICOS expressed by T cells. Conversely, coinhibitory signaling suppresses T cell activation using PD-1, CTLA-4, and LAG3 expressed by T cells.
In the tumor microenvironment, cancer cells take advantage of the coinhibitory pathway, expressing markers such as PD-L1 (Figure 1). In addition, tumor-associated macrophages (TAMs) can also express PD-L1 and CSF1R (Figure 2).
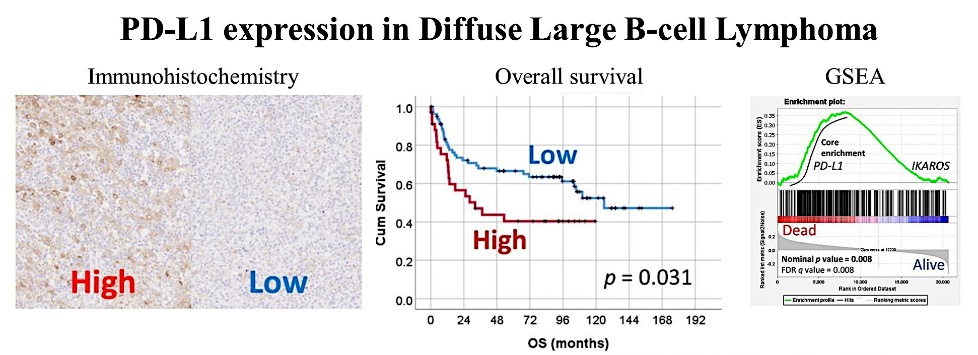
Figure 1. PD-L1 expression in diffuse large B-cell lymphoma (DLBCL) and the correlation with the poor prognosis of patients.
AI 2021, 2(1), 106-134; https://doi.org/10.3390/ai2010008
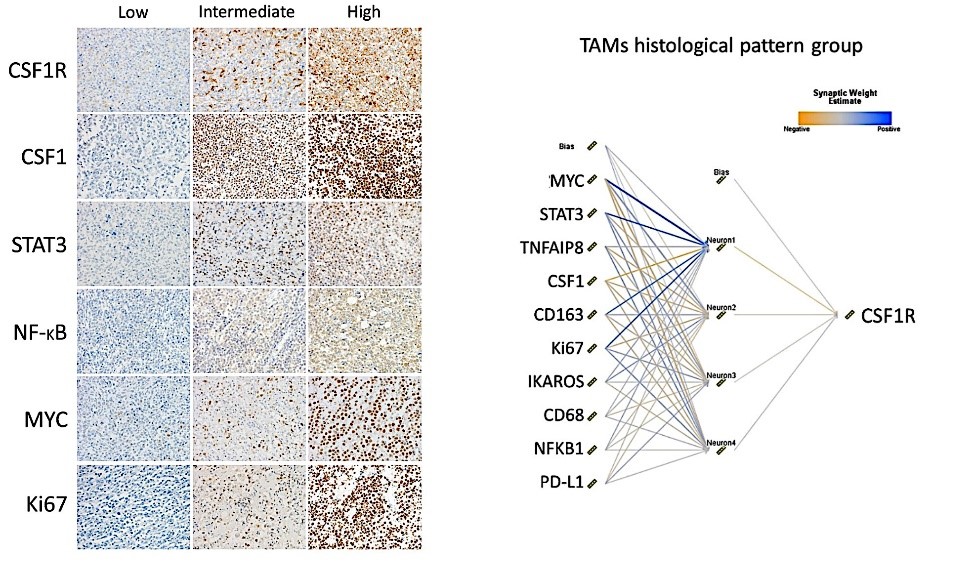
Figure 2. CSF1R expression by tumor-associated macrophages (TAMs) in diffuse large B-cell lymphoma (DLBCL) and the correlation with other markers using a neural network.
Hemato 2021, 2(2), 182-206; https://doi.org/10.3390/hemato2020011
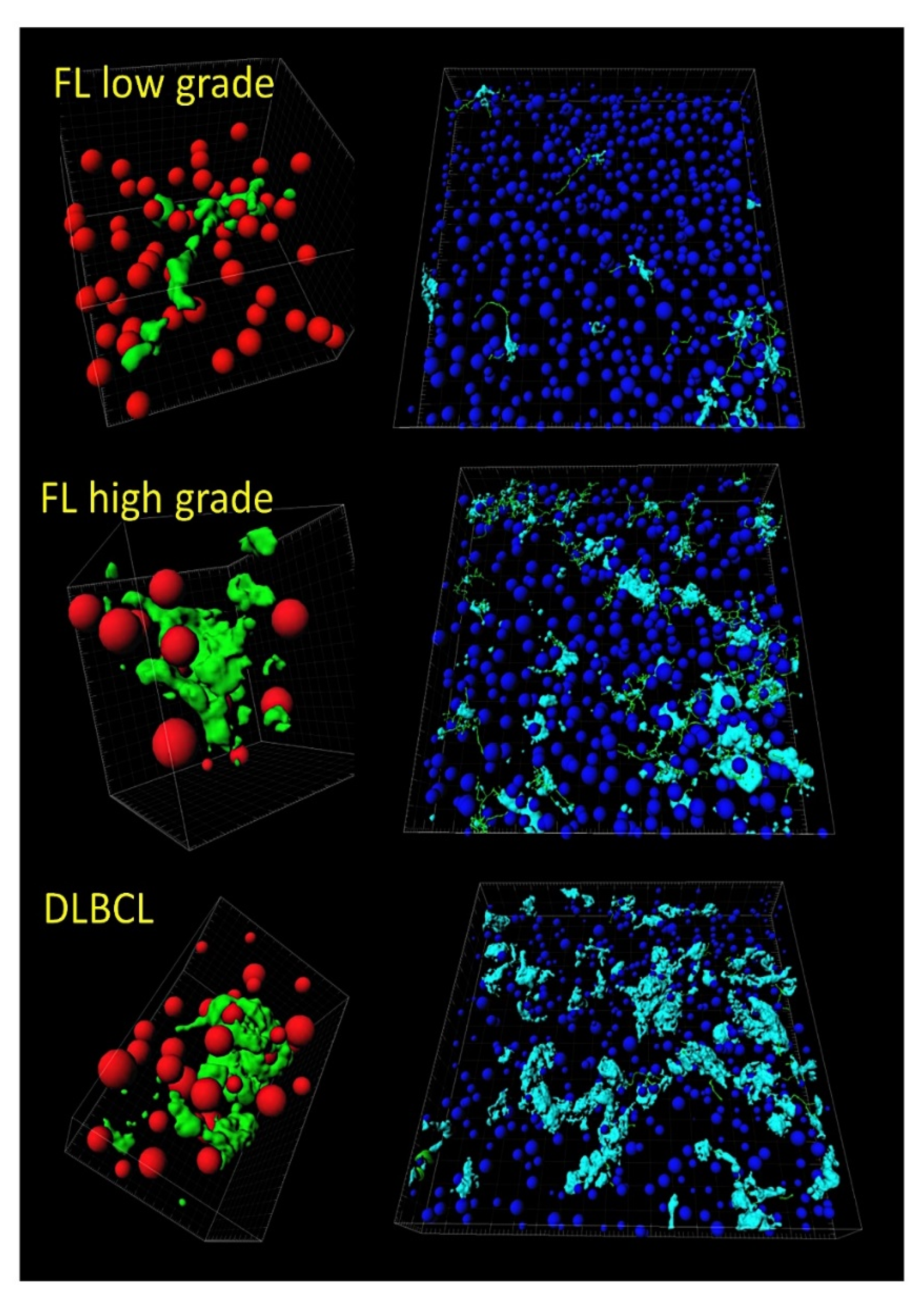
Figure 3. Tridimensional analysis of tumor-associated macrophages (TAMs) in follicular lymphoma and transformation to diffuse large B-cell lymphoma (DLBCL). Both the quantity and the shape of TAMs increase in disease progression and transformation to high-grade B-cell lymphoma.
Cancers 2022, 14(21), 5318; https://doi.org/10.3390/cancers14215318
Other factors are immunostimulatory signals for turning up cold tumors, which are not restricted to neoantigens derived from mutations. For instance, these include stress signals (DAMPs), the DNA damage response, and metabolic changes in the TME. Finally, the key role of immunosenescence and/or epitranscriptomic changes in immune cells that challenge tumor immunity must also be considered.
Some kinds of cancer are susceptible to treatment with immunotherapy because cancer cells are different from normal cells, so the immune system recognizes them and kills these abnormal cells. There are different types of immunotherapies:
- Monoclonal antibodies (MABs), which recognize and attach certain proteins on cell surfaces;
- Checkpoint inhibitors (immune checkpoint blockade (ICB) therapies);
- Cytokines, which help boost the immune system;
- Vaccines, which help recognize and attack cancer;
- CAR T-cell therapy (also known as adoptive cell transfer).
Since standard chemotherapeutic agents and radiation, as well as targeted therapies, elicit/instigate an antitumor immune response, the combination of therapeutic modalities is the best treatment approach. Nevertheless, the current ICB therapies have a limitation in that they acquire resistance mechanisms.
This Topic accepts manuscripts that deal with all aspects of the immune response in hematological neoplasia and cancer, from basic to clinical research and integrative analyses (including mice models and artificial intelligence analyses).
Dr. Joaquim Carreras
Dr. Luis J. Castro-Vega
Topic Editors
Keywords
- hematological neoplasia
- cancer
- immune microenvironment
- immune checkpoint
- immuno-oncology
- artificial intelligence
- tumor-associated macrophages
Participating Journals
| Journal Name | Impact Factor | CiteScore | Launched Year | First Decision (median) | APC | |
|---|---|---|---|---|---|---|

Biomedicines
|
3.9 | 6.8 | 2013 | 17 Days | CHF 2600 | Submit |
Cancers
|
4.4 | 8.8 | 2009 | 20.3 Days | CHF 2900 | Submit |
Cells
|
5.2 | 10.5 | 2012 | 16 Days | CHF 2700 | Submit |

Journal of Clinical Medicine
|
2.9 | 5.2 | 2012 | 17.7 Days | CHF 2600 | Submit |

Pharmaceutics
|
5.5 | 10.0 | 2009 | 14.9 Days | CHF 2900 | Submit |

Reports
|
0.6 | - | 2018 | 21.7 Days | CHF 1400 | Submit |

Allergies
|
- | - | 2021 | 34.8 Days | CHF 1000 | Submit |

Preprints.org is a multidisciplinary platform offering a preprint service designed to facilitate the early sharing of your research. It supports and empowers your research journey from the very beginning.
MDPI Topics is collaborating with Preprints.org and has established a direct connection between MDPI journals and the platform. Authors are encouraged to take advantage of this opportunity by posting their preprints at Preprints.org prior to publication:
- Share your research immediately: disseminate your ideas prior to publication and establish priority for your work.
- Safeguard your intellectual contribution: Protect your ideas with a time-stamped preprint that serves as proof of your research timeline.
- Boost visibility and impact: Increase the reach and influence of your research by making it accessible to a global audience.
- Gain early feedback: Receive valuable input and insights from peers before submitting to a journal.
- Ensure broad indexing: Web of Science (Preprint Citation Index), Google Scholar, Crossref, SHARE, PrePubMed, Scilit and Europe PMC.

