Comparative Exergy and Environmental Assessment of the Residual Biomass Gasification Routes for Hydrogen and Ammonia Production
Abstract
1. Introduction
- Fluid dynamics (updraft, downdraft),
- Modes of heat transfer to the gasification process (auto thermal or directly heated gasifiers and allothermal or indirectly heated gasifiers),
- Gasification agents (air, oxygen, or steam blown), and
- Pressure (atmospheric or pressurized).
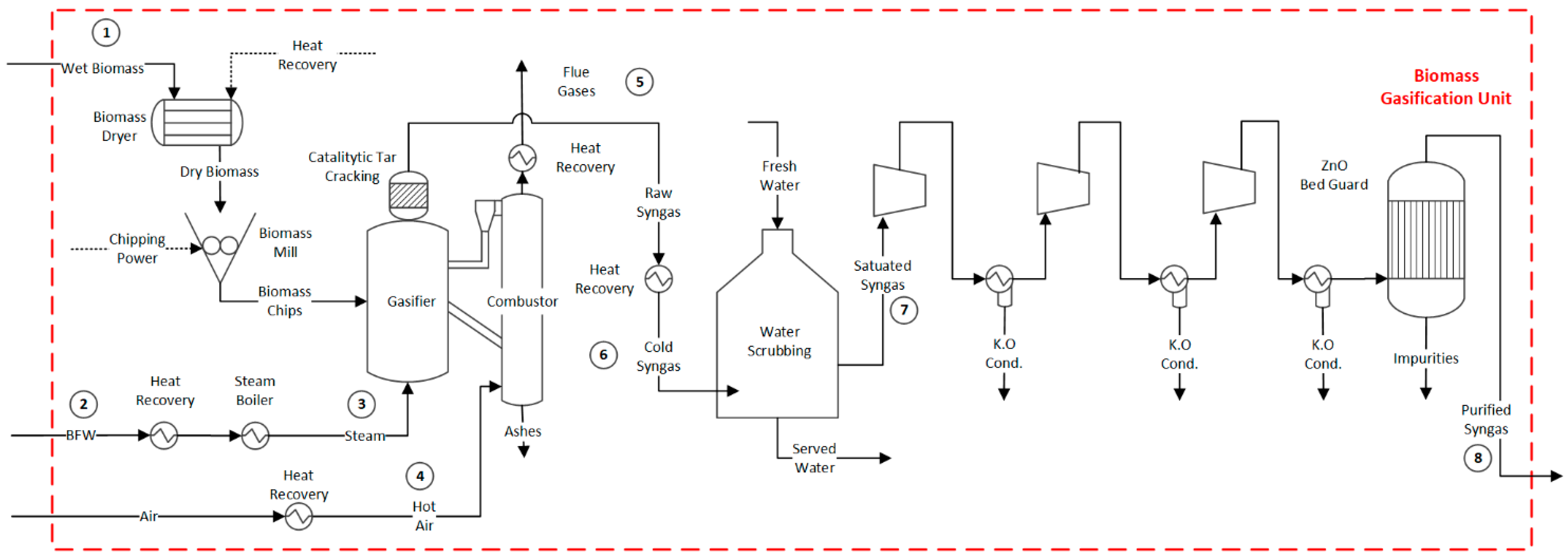
2. Process Description
2.1. Biomass Drying and Chipping Process
2.2. Gasification Process
2.3. Syngas Conditioning Process
2.4. Carbon Dioxide Capture and Methanation Processes
2.5. Pressure Swing Adsorption and Hydrogen Compression in Hydrogen Production Route
2.6. Ammonia Synthesis Loop
2.7. Integrated Flowsheets of the Ammonia and Hydrogen Production Routes Using Residual Biomass
3. Materials and Methods
3.1. Process Modeling
3.2. CO2 Emissions
3.3. Exergy Efficiency
3.4. Definition of the Optimization Problem
4. Results and Discussion
4.1. Energy Integration Analysis and Power Generation Potential
4.2. General CO2 Emissions Balance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Latin symbols | |
| M | moisture content (%) |
| VM | volatile matter content (%) |
| FC | fixed carbon content (%) |
| C | carbon (%) |
| H | hydrogen (%) |
| N | nitrogen (%) |
| S | sulphur (%) |
| Cl | chlorine (%) |
| O | oxygen (%) |
| b | specific chemical exergy (kJ/kg) |
| h | enthalpy (kJ/kg) |
| B | chemical exergy flow rate (kW) |
| W | electrical power (kW) |
| R | cascaded heat transfer rate (kW) |
| Q | heat exchanged (kJ) |
| y | molar fraction (-) |
| T | Temperature (°C, K) |
| N | number of intervals (-) |
| Superscript | |
| CH | chemical exergy |
| Subscripts | |
| db | dry basis |
| r | interval of temperature |
| Greek symbols | |
| ratio of specific chemical exergy | |
| exergy efficiency | |
| Moisture (%) | |
| Abbreviations | |
| DEPG | dimethyl ethers of polyethylene glycols |
| PSA | pressure swing adsorption |
| GBE | general balance of emissions (tCO2/tproduct) |
| LHV | lower heating value (kJ/kg) |
| MER | minimum energy requirement (kW) |
References
- Thraen, D.; Shaubach, K.; Global Wood Pellet Industry and Trade Study 2017. IEA Bioenergia 2017. Available online: https://www.ieabioenergy.com/blog/publications/two-page-summary-global-wood-pellet-industry-and-trade-study-2017/ (accessed on 5 September 2022).
- EPE. Brazilian Energy Balance Year 2016. 2016. Available online: https://www.epe.gov.br/en/publications/publications/brazilian-energy-balance/brazilian-energy-balance-2016 (accessed on 2 March 2023).
- Marcio, R.; Santos, D.; De Alencar Nääs, I.; Neto, M.M.; Vendrametto, O. An overview on the brazilian orange juice production chain 1 uma visão da produção brasileira de suco de laranja. Rev. Bras. Frutic. Jaboticabal SP 2013, 35, 218–255. [Google Scholar]
- Nakashima, R.; Flórez-Orrego, D.; de Oliveira Junior, S. Integrated anaerobic digestion and gasification processes for upgrade of ethanol biorefinery residues. J. Power Technol. 2019, 99, 104–114. [Google Scholar]
- Pellegrini, L.F.; Deoliveirajr, S. Exergy analysis of sugarcane bagasse gasification. Energy 2007, 32, 314–327. [Google Scholar] [CrossRef]
- González, D.; Colón, J.; Gabriel, D.; Sánchez, A. The effect of the composting time on the gaseous emissions and the compost stability in a full-scale sewage sludge composting plant. Sci. Total Environ. 2019, 654, 311–323. [Google Scholar] [CrossRef]
- Swati, A.; Hait, S. Fate and bioavailability of heavy metals during vermicomposting of various organic wastes—A review. Process Saf. Environ. Prot. 2017, 109, 30–45. [Google Scholar] [CrossRef]
- Biagini, E.; Barontini, F.; Tognotti, L. Gasification of agricultural residues in a demonstrative plant: Corn cobs. Bioresour. Technol. 2014, 173, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Vera, D.; Jurado, F.; Margaritis, N.K.; Grammelis, P. Experimental and economic study of a gasification plant fuelled with olive industry wastes. Energy Sustain. Dev. 2014, 23, 247–257. [Google Scholar] [CrossRef]
- Biagini, E.; Barontini, F.; Tognotti, L. Gasification of agricultural residues in a demonstrative plant: Vine pruning and rice husks. Bioresour. Technol. 2015, 194, 36–42. [Google Scholar] [CrossRef]
- Guo, X.M.; Trably, E.; Latrille, E.; Carrère, H.; Steyer, J.-P. Hydrogen production from agricultural waste by dark fermentation: A review. Int. J. Hydrog. Energy 2010, 35, 10660–10673. [Google Scholar] [CrossRef]
- Ghani, W.A.W.A.K.; Moghadam, R.A.; Salleh, M.A.M.; Alias, A.B. Air Gasification of Agricultural Waste in a Fluidized Bed Gasifier: Hydrogen Production Performance. Energies 2009, 2, 258–268. [Google Scholar] [CrossRef]
- Durán-Sarmiento, M.A.; Del Portillo-Valdés, L.A.; Rueda-Ordonez, Y.J.; Florez-Rivera, J.S.; Rincón-Quintero, A.D. Study on the gasification process of the biomass obtained from agricultural waste with the purpose of estimating the energy potential in the Santander region and its surroundings. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1253, 012001. [Google Scholar] [CrossRef]
- Widjaya, E.R.; Chen, G.; Bowtell, L.; Hills, C. Gasification of non-woody biomass: A literature review. Renew. Sustain. Energy Rev. 2018, 89, 184–193. [Google Scholar] [CrossRef]
- Göransson, K.; Söderlind, U.; He, J.; Zhang, W. Review of syngas production via biomass DFBGs. Renew. Sustain. Energy Rev. 2011, 15, 482–492. [Google Scholar] [CrossRef]
- Perez, D.L.; Brown, A.; Mudhoo, A.; Timko, M.; Rostagno, M.; Forster-Carneiro, T. Applications of subcritical and supercritical water conditions for extraction, hydrolysis, gasification, and carbonization of biomass: A critical review. Biofuel Res. J. 2017, 4, 611–626. [Google Scholar] [CrossRef]
- Koppatz, S.; Pfeifer, C.; Rauch, R.; Hofbauer, H.; Marquard-Moellenstedt, T.; Specht, M. H2 rich product gas by steam gasification of biomass with in situ CO2 absorption in a dual fluidized bed system of 8 MW fuel input. Fuel Process Technol. 2009, 90, 914–921. [Google Scholar] [CrossRef]
- Leckner, B. Developments in fluidized bed conversion of solid fuels. Therm. Sci. 2016, 20, 135. [Google Scholar] [CrossRef]
- Kraussler, M.; Binder, M.; Hofbauer, H. Behavior of GCMS tar components in a water gas shift unit operated with tar-rich product gas from an industrial scale dual fluidized bed biomass steam gasification plant. Biomass Convers. Biorefinery 2016, 7, 69–79. [Google Scholar] [CrossRef]
- Thunman, H.; Seemann, M.; Vilches, T.B.; Maric, J.; Pallares, D.; Ström, H.; Berndes, G.; Knutsson, P.; Larsson, A.; Breitholtz, C.; et al. Advanced biofuel production via gasification—Lessons learned from 200 man-years of research activity with Chalmers’ research gasifier and the GoBiGas demonstration plant. Energy Sci. Eng. 2018, 6, 6–34. [Google Scholar] [CrossRef]
- Flórez-Orrego, D.; Maréchal, F.; Junior, S.D.O. Comparative exergy and economic assessment of fossil and biomass-based routes for ammonia production. Energy Convers. Manag. 2019, 194, 22–36. [Google Scholar] [CrossRef]
- Budzianowski, W.M. Low-carbon power generation cycles: The feasibility of CO2 capture and opportunities for integration. J. Power Technol. 2011, 91, 6–13. [Google Scholar]
- Palacios-Bereche, R.; Mosqueira-Salazar, K.J.; Modesto, M.; Ensinas, A.V.; Nebra, S.A.; Serra, L.M.; Lozano, M.-A. Exergetic analysis of the integrated first- and second-generation ethanol production from sugarcane. Energy 2013, 62, 46–61. [Google Scholar] [CrossRef]
- Ribeiro Domingos, M.; Florez-Orrego, D.; Maréchal, F. Comparison of regression techniques for generating surrogate models to predict the thermodynamic behavior of biomass gasification systems. In Proceedings of the 33rd European Symposium on Computer Aided Process Engineering, Athens, Greece, 18–21 June 2023. [Google Scholar]
- Allesina, G.; Pedrazzi, S.; Guidetti, L.; Tartarini, P. Modeling of coupling gasification and anaerobic digestion processes for maize bioenergy conversion. Biomass Bioenergy 2015, 81, 444–451. [Google Scholar] [CrossRef]
- Ribeiro Domingos, M.E.G.; Flórez-Orrego, D.; Teles Dos Santos, M.; Oliveira, S., Jr.; Marechal, F. Incremental financial analysis of black liquor upgraded gasification in integrated kraft pulp and ammonia production plants under uncertainty of feedstock costs and carbon taxes. In Proceedings of the 32nd European Symposium on Computer Aided Process Engineering, Toulouse, France, 12–15 June 2022. [Google Scholar]
- Gassner, M.; Maréchal, F. Increasing Efficiency of Fuel Ethanol Production from Lignocellulosic Biomass by Process Integration. Energy Fuels 2013, 27, 2107–2115. [Google Scholar] [CrossRef]
- Telini, R.; Florez-Orrego, D.; Oliveira, S., Jr. Techno-economic and environmental assessment of ammonia production from residual bagasse gasification: A decarbonization pathway for nitrogen fertilizers. Front. Energy Res. 2022, 10, 881263. [Google Scholar] [CrossRef]
- Tock, L.; Maréchal, F. Co-production of hydrogen and electricity from lignocellulosic biomass: Process design and thermo-economic optimization. Energy 2012, 45, 339–349. [Google Scholar] [CrossRef]
- Vargas, G.G.; Oliveira, S. Exergy Assessment of Electricity Generation via Biomass Gasification by Neural Network Algorithm. In Proceedings of the 36th International Conference on Efficiency, Cost, Optimization, Simulation and Environmental Impact of Energy Systems, Las Palmas, Spain, 25–30 June 2023. [Google Scholar]
- Ribeiro Domingos, M.E.G.; Flórez-Orrego, D.; Teles Dos Santos, M.; Oliveira Junior, S.; Maréchal, F. Multi-time integration approach for combined pulp and ammonia production and seasonal CO2 management. Comput. Chem. Eng. 2023, 176, 108305. [Google Scholar] [CrossRef]
- Vargas, G.G.; Florez-Orrego, D.; Oliveira, S. Comparative exergy assessment of residual biomass gasification routes for hydrogen and ammonia production. In Proceedings of the 35th International Conference on Efficiency, Cost, Optimization, Simulation and Environmental Impact of Energy Systems, Copenhagen, Denmark, 3–7 July 2022. [Google Scholar]
- SABESP. State Basic Sanitation Company from Sao Paulo. Available online: www.sabesp.gov.br (accessed on 2 March 2023). (In Portugese)
- Sues Caula, A. Are European Bioenergy Targets Achievable?: An Evaluation Based on Thermoeconomic and Environmental Indicators; Technische Universiteit Eindhoven: Eindhoven, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification and Pyrolysis: Pratical Design and Theory; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Bergman, P.C.A.; Boersman, A.R.; Kiel, J.H.A.; Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Torrefaction for Entrained-Flow Gasification of Biomass Revisions A B Made by. Available online: www.ecn.nl/biomass (accessed on 11 August 2021).
- ASPENTECH. Aspen Plus V8.8; Aspen Technology Inc.: Bedford, MA, USA, 2015. [Google Scholar]
- Ribeiro Domingos, M.E.G.; Orrego, D.F.; dos Santos, M.T.; Velásquez, H.I.; Junior, S.D.O. Exergy and environmental analysis of black liquor upgrading gasification in an integrated kraft pulp and ammonia production plant. Int. J. Exergy 2021, 35, 35. [Google Scholar] [CrossRef]
- Kinchin, C.M.; Bain, R.L. Hydrogen Production from Biomass via Indirect Gasification: The Impact of NREL Process Development Unit Gasifier Correlations; National Renewable Energy Laboratory: Golden, CO, USA, 2009. [CrossRef]
- Puig-Gamero, M.; Argudo-Santamaria, J.; Valverde, J.; Sánchez, P.; Sanchez-Silva, L. Three integrated process simulation using aspen plus®: Pine gasification, syngas cleaning and methanol synthesis. Energy Convers. Manag. 2018, 177, 416–427. [Google Scholar] [CrossRef]
- Marcantonio, V.; De Falco, M.; Capocelli, M.; Bocci, E.; Colantoni, A.; Villarini, M. Process analysis of hydrogen production from biomass gasification in fluidized bed reactor with different separation systems. Int. J. Hydrog. Energy 2019, 44, 10350–10360. [Google Scholar] [CrossRef]
- Spath, P.; Aden, A.; Eggeman, T.; Ringer, M.; Wallace, B.; Jechura, J. Biomass to Hydrogen Production Detailed Design and Economics Utilizing the Battelle Columbus Laboratory Indirectly-Heated Gasifier; National Renewable Energy Laboratory: New York, NY, USA, 2005. [CrossRef]
- Spath, P.L.; Dayton, D.C. Preliminary Screening—Technical and Economic Assessment of Synthesis Gas to Fuels and Chemicals with Emphasis on the Potential for Biomass-Derived Syngas. 2003. Available online: http://www.osti.gov/bridge (accessed on 3 May 2022).
- Gail, E.; Gos, S.; Kulzer, R.; Lorösch, J.; Rubo, A. Ullmann’s Encyclopedia of Industrial Chemistry, 5th ed.; Wiley: Hoboken, NJ, USA, 2012; pp. 673–710. [Google Scholar]
- Flórez-Orrego, D.; Oliveira Junior, S. On the allocation of the exergy costs and CO2 emission cost for an integrated syngas and ammonia production plant. In Proceedings of the ECOS 2015—28th International Conference on Efficiency Cost, Optimization Simulation and Environmental Impact Energy Systtems, Pau, France, 29 June–3 July 2015. [Google Scholar]
- Flórez-Orrego, D.; Oliveira Junior, S. On the efficiency, exergy costs and CO2 emission cost allocation for an integrated syngas and ammonia production plant. Energy 2016, 117, 341–360. [Google Scholar] [CrossRef]
- Adams, T.A.; Salkuyeh, Y.K.; Nease, J. Chapter 6—Processes and simulations for solvent-based CO2 capture and syngas cleanup. In Reactor and Process Design in Sustainable Energy Technology; Shi, F., Ed.; Elsevier: Amsterdam, The Netherland, 2014; pp. 163–231. [Google Scholar]
- Kuo, P.-C.; Illathukandy, B.; Wu, W.; Chang, J.-S. Energy, exergy, and environmental analyses of renewable hydrogen production through plasma gasification of microalgal biomass. Energy 2021, 223, 120025. [Google Scholar] [CrossRef]
- Pires, A.P.B.; Filho, V.F.D.S.; Alves, J.L.F.; Marangoni, C.; Bolzan, A.; Machado, R.A.F. Application of a new pilot-scale distillation system for monoethylene glycol recovery using an energy saving falling film distillation column. Chem. Eng. Res. Des. 2019, 153, 263–275. [Google Scholar] [CrossRef]
- ASPENTECH. Aspen Physical Property System—Physical Property Methods; Aspen Technology, Inc.: Cambridge, MA, USA, 2011. [Google Scholar]
- Field, R.P.; Brasington, R. Baseline Flowsheet Model for IGCC with Carbon Capture. Ind. Eng. Chem. Res. 2011, 50, 11306–11312. [Google Scholar] [CrossRef]
- Puig-Arnavat, M.; Bruno, J.C.; Coronas, A. Modified Thermodynamic Equilibrium Model for Biomass Gasification: A Study of the Influence of Operating Conditions. Energy Fuels 2012, 26, 1385–1394. [Google Scholar] [CrossRef]
- Szargut, J.; Morris, D.R.; Steward, F.R. Exergy Analysis of Thermal, Chemical, and Metallurgical Processes; Hemisphere: New York, NY, USA, 1987. [Google Scholar]
- Channiwala, S.; Parikh, P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Larson, D.C.; Weinberger, C.B.; Lawley, A.; Thomas, D.H.; Moore, T.W. Fundamentals of Engineering Energy. J. Eng. Educ. 1994, 83, 325–330. [Google Scholar] [CrossRef]
- Flórez-Orrego, D.; Silva, J.A.; de Oliveira, S., Jr. Renewable and non-renewable exergy cost and specific CO2 emission of electricity generation: The Brazilian case. Energy Convers. Manag. 2014, 85, 619–629. [Google Scholar] [CrossRef]
- Flórez-Orrego, D.; da Silva, J.A.M.; Velásquez, H.; de Oliveira, S. Renewable and non-renewable exergy costs and CO2 emissions in the production of fuels for Brazilian transportation sector. Energy 2015, 88, 18–36. [Google Scholar] [CrossRef]
- Chen, Y.C.; Kuo, J. Potential of greenhouse gas emissions from sewage sludge management: A case study of Taiwan. J. Clean. Prod. 2016, 129, 196–201. [Google Scholar] [CrossRef]
- Yoo, M.-J.; Lessard, L.; Kermani, M.; Maréchal, F. OsmoseLua—An Integrated Approach to Energy Systems Integration with LCIA and GIS. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Linnhoff, B.; Hindmarsh, E. The pinch design method for heat exchanger networks. Chem. Eng. Sci. 1983, 38, 745–763. [Google Scholar] [CrossRef]
- de Souza, A.C.C.; Luz-Silveira, J.; Sosa, M.I. Physical-Chemical and Thermodynamic Analyses of Ethanol Steam Reforming for Hydrogen Production. J. Fuel Cell Sci. Technol. 2006, 3, 346–350. [Google Scholar] [CrossRef]
- da Silva, A.L.; de Fraga Malfatti, C.; Müller, I.L. Thermodynamic analysis of ethanol steam reforming using Gibbs energy minimization method: A detailed study of the conditions of carbon deposition. Int. J. Hydrog. Energy 2009, 34, 4321–4330. [Google Scholar] [CrossRef]














| Parameter | Sugar Cane Bagasse [43] | Sewage Sludge [44] | Orange Bagasse [45] |
|---|---|---|---|
| Proximate analysis | |||
| Fixed Carbon | 50.00 | 18.40 | 9.23 |
| Volatile Material | 14.32 | 7.60 | 73.20 |
| Moisture | 83.54 | 64.90 | 20.60 |
| Ash | 2.14 | 27.50 | 6.20 |
| Ultimate Analysis (%) db | |||
| Carbon | 46.70 | 33.90 | 46.40 |
| Hydrogen | 6.02 | 6.30 | 5.54 |
| Oxygen | 44.95 | 25.50 | 40.15 |
| Nitrogen | 0.17 | 5.88 | 1.70 |
| Sulphur | 0.02 | 0.67 | 0.00 |
| Chlorine | 0.00 | 0.21 | 0.00 |
| Reaction | (kJ/mol) | Name | No. |
|---|---|---|---|
| C + O2 CO2 | −394 | Complete combustion | (R. 1) |
| C + CO2 2CO | +173 | Boudouard reaction | (R. 2) |
| C + H2O CO + H2 | +131 | Char steam gasification | (R. 3) |
| C + 2 H2 CH4 | −75 | Char gasification | (R. 4) |
| CO + ½ O2 CO2 | −283 | Carbon oxidation | (R. 5) |
| H2 + ½ O2 H2O | −242 | Hydrogen oxidation | (R. 6) |
| CH4 + 2 O2 CO2 + 2H2O | −283 | Methane oxidation | (R. 7) |
| CO + H2O CO2 + H2 | −41 | Water-gas shift reaction | (R. 8) |
| 6CO + 9H2 6H2O + C6H6 | −1583 | Tar formation | (R. 9) |
| Reaction | (kJ/mol) | Name | |
|---|---|---|---|
| CH4 + H2O CO + 3H2 | +206 | Steam reform | (R. 10) |
| CO + H2O CO2 + H2 | −41 | Water gas shift reaction | (R. 11) |
| Biomass | LHV (MJ/kg) | bCH (MJ/kg) |
|---|---|---|
| Sugar cane bagasse | 17.39 | 19.50 |
| Sewage sludge | 19.25 | 16.13 |
| Orange bagasse | 25.24 | 20.26 |
| Sugar Cane Bagasse | Sewage Sludge | Orange Bagasse | |
|---|---|---|---|
| Gasification (%) | 68.1 | 69.8 | 73.6 |
| Chipping (%) | 2.0 | 2.1 | 2.2 |
| Dryer (%) | 3.5 | 2.6 | 1.6 |
| Scrubber (%) | 3.6 | 3.9 | 2.7 |
| ATR (%) | 2.3 | 2.5 | 2.1 |
| Shift reactors (%) | 0.9 | 0.9 | 0.8 |
| Physical absorption (%) | 3.4 | 2.9 | 3.3 |
| Methanator (%) | 0.2 | 0.2 | 0.3 |
| Compression (%) | 4.3 | 3.3 | 2.4 |
| Ammonia reactors (%) | 4.0 | 4.0 | 3.1 |
| Others (%) | 7.7 | 7.8 | 7.9 |
| Sugar Cane Bagasse | Sewage Sludge | Orange Bagasse | |
|---|---|---|---|
| Gasification (%) | 56.7 | 57.8 | 63 |
| Chipping (%) | 4.6 | 4.6 | 4.3 |
| Dryer (%) | 2.6 | 1.9 | 1.2 |
| Scrubber (%) | 2.7 | 2.9 | 2.1 |
| Compression (%) | 2.4 | 2.7 | 1.9 |
| Shift reactors (%) | 0.3 | 0.3 | 0.2 |
| Physical absorption (%) | 7.0 | 5.9 | 6.0 |
| PSA combustor (%) | 19.9 | 19.8 | 17.6 |
| Others (%) | 3.8 | 4.1 | 3.7 |
| Chemical Plant | Power Generated |
|---|---|
| Sugarcane bagasse to hydrogen | 6208 kW |
| Sugarcane bagasse to ammonia | 7259 kW |
| Sewage sludge to hydrogen | 11,835 kW |
| Sewage sludge to ammonia | 13,147 kW |
| Orange bagasse to hydrogen | 13,735 kW |
| Orange bagasse to ammonia | 15,171 kW |
| Process Parameter | Sugarcane Bagasse | Sewage Sludge | Orange Bagasse |
|---|---|---|---|
| Biomass Consumption (tbiomass/tH2) | 27.39 | 20.54 | 15.86 |
| Syngas produced in the gasifier (MJ/kgH2) | 188.82 | 187.96 | 237.24 |
| Hydrogen Produced (tH2/day) | 23.32 | 31.13 | 40.32 |
| Heating requirement 1 (GJ/tH2) | 0.00 | 0.00 | 0.00 |
| Cooling requirement 1 (GJ/tH2) | 47.60 | 75.66 | 83.64 |
| Captured CO2 (tCO2/tbiomass) | 0.503 | 0.534 | 0.901 |
| Fossil CO2 emitted—indirect 2 (kgCO2/kgH2) | 1.919 | 3.896 | 1.629 |
| Indirect emitted CO2—EE (%) | 0.081 | 0.078 | 0.072 |
| Indirect emitted CO2—Biomass (%) | 0.919 | 0.922 | 0.928 |
| Total fossil CO2 emitted (kgCO2/kgH2) | 1.919 | 3.896 | 1.629 |
| Biogenic CO2 emissions avoided 3 (kgCO2/kg H2) | 13.682 | 10.869 | 14.166 |
| Biogenic CO2 emitted—direct (kgCO2/kg H2) | 6.527 | 6.747 | 6.584 |
| Total atmospheric emissions (kgCO2/kg H2) | 8.447 | 10.643 | 8.213 |
| General balance of CO2 emissions 4 (kgCO2/kg H2) | −5.235 | −0.226 | −5.953 |
| Process Parameter | Sugarcane Bagasse | Sewage Sludge | Orange Bagasse |
|---|---|---|---|
| Biomass Consumption (tbiomass/tNH3) | 3.93 | 3.05 | 2.26 |
| Syngas produced in the gasifier (MJ/kgNH3) | 28.02 | 27.93 | 34.99 |
| Ammonia produced (tNH3/day) | 157.16 | 209.47 | 273.43 |
| Heating requirement 1 (GJ/tNH3) | 0.00 | 0.00 | 0.00 |
| Cooling requirement 1 (GJ/tNH3) | 6.72 | 11.28 | 11.36 |
| Captured CO2 (tCO2/tbiomass) | 0.603 | 0.668 | 1.096 |
| Fossil CO2 emitted—indirect 2 (kgCO2/kgNH3) | 0.272 | 0.572 | 0.230 |
| Indirect emitted CO2—EE (%) | 0.101 | 0.093 | 0.091 |
| Indirect emitted CO2—Biomass (%) | 0.899 | 0.907 | 0.909 |
| Total fossil CO2 emitted (kgCO2/kgNH3) | 0.272 | 0.572 | 0.230 |
| Biogenic CO2 emissions avoided 3 (kgCO2/kgNH3) | 2.351 | 2.022 | 2.450 |
| Biogenic CO2 emitted—direct (kgCO2/kgNH3) | 0.936 | 1.003 | 0.606 |
| Total atmospheric emissions (kgCO2/kgNH3) | 1.208 | 1.574 | 0.835 |
| General balance of CO2 emissions 4 (kgCO2/kgNH3) | −1.142 | −0.448 | −1.615 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas, G.G.; Flórez-Orrego, D.A.; de Oliveira Junior, S. Comparative Exergy and Environmental Assessment of the Residual Biomass Gasification Routes for Hydrogen and Ammonia Production. Entropy 2023, 25, 1098. https://doi.org/10.3390/e25071098
Vargas GG, Flórez-Orrego DA, de Oliveira Junior S. Comparative Exergy and Environmental Assessment of the Residual Biomass Gasification Routes for Hydrogen and Ammonia Production. Entropy. 2023; 25(7):1098. https://doi.org/10.3390/e25071098
Chicago/Turabian StyleVargas, Gabriel Gomes, Daniel Alexander Flórez-Orrego, and Silvio de Oliveira Junior. 2023. "Comparative Exergy and Environmental Assessment of the Residual Biomass Gasification Routes for Hydrogen and Ammonia Production" Entropy 25, no. 7: 1098. https://doi.org/10.3390/e25071098
APA StyleVargas, G. G., Flórez-Orrego, D. A., & de Oliveira Junior, S. (2023). Comparative Exergy and Environmental Assessment of the Residual Biomass Gasification Routes for Hydrogen and Ammonia Production. Entropy, 25(7), 1098. https://doi.org/10.3390/e25071098







