New Hydrazinothiazole Derivatives of Usnic Acid as Potent Tdp1 Inhibitors
Abstract
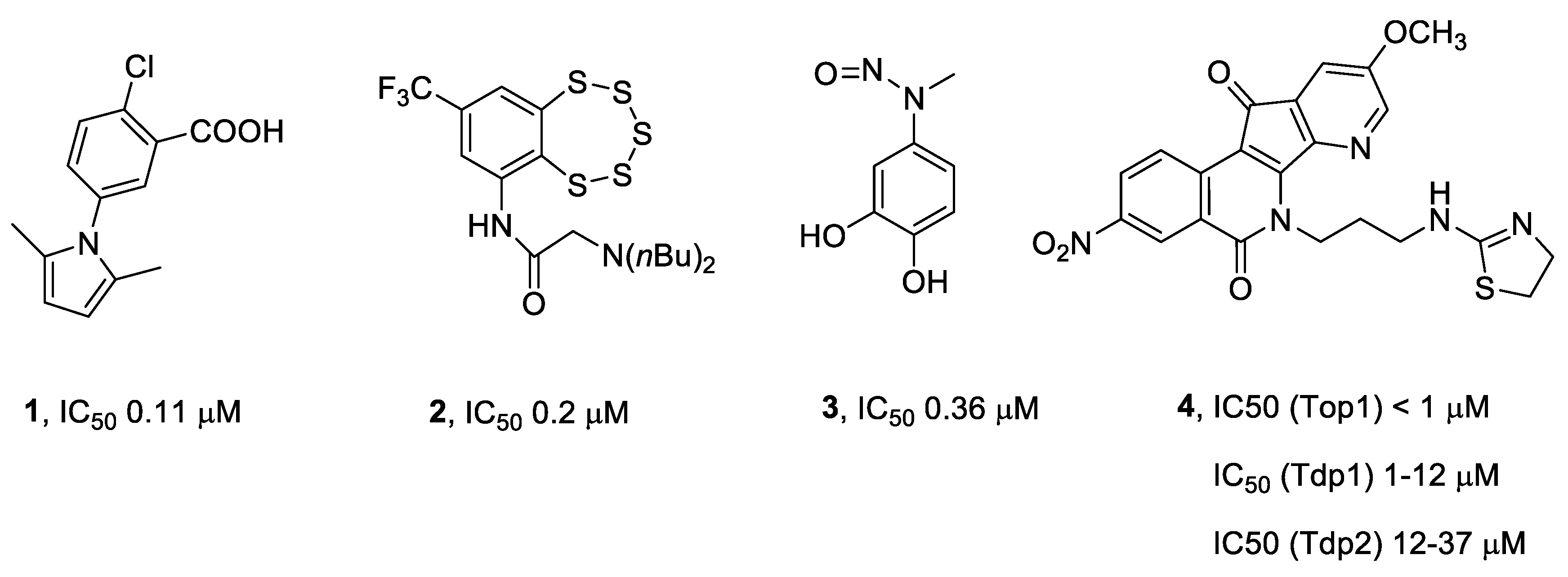
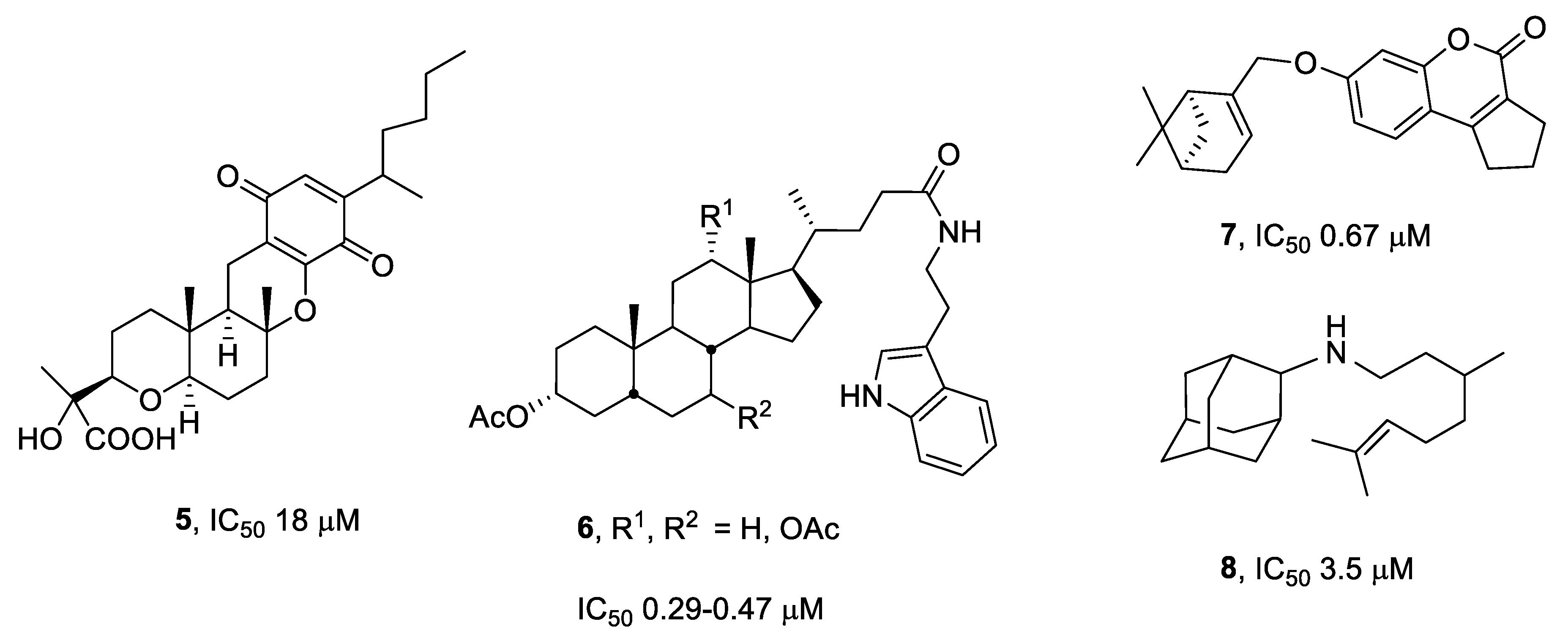
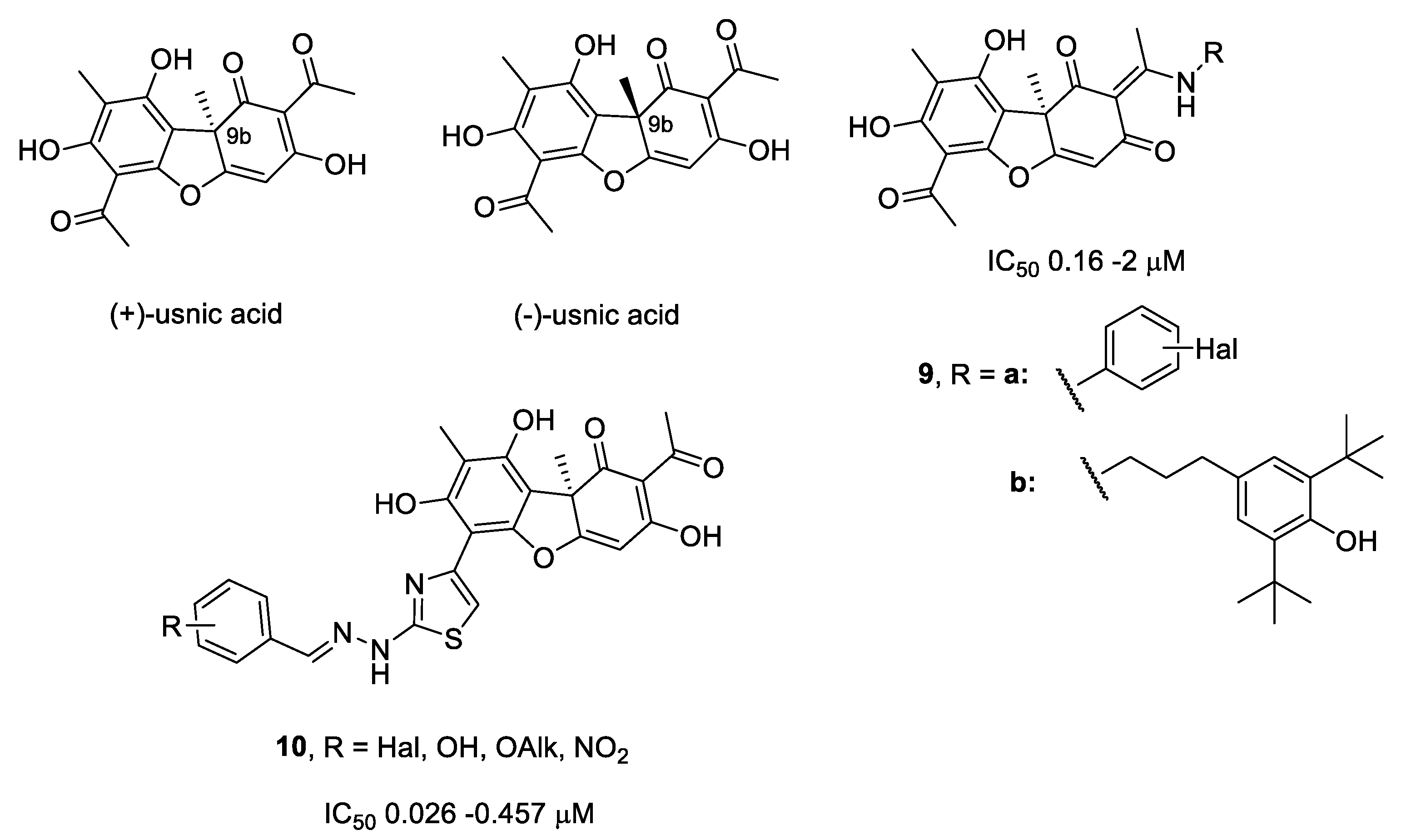
:1. Introduction
2. Results and Discussion
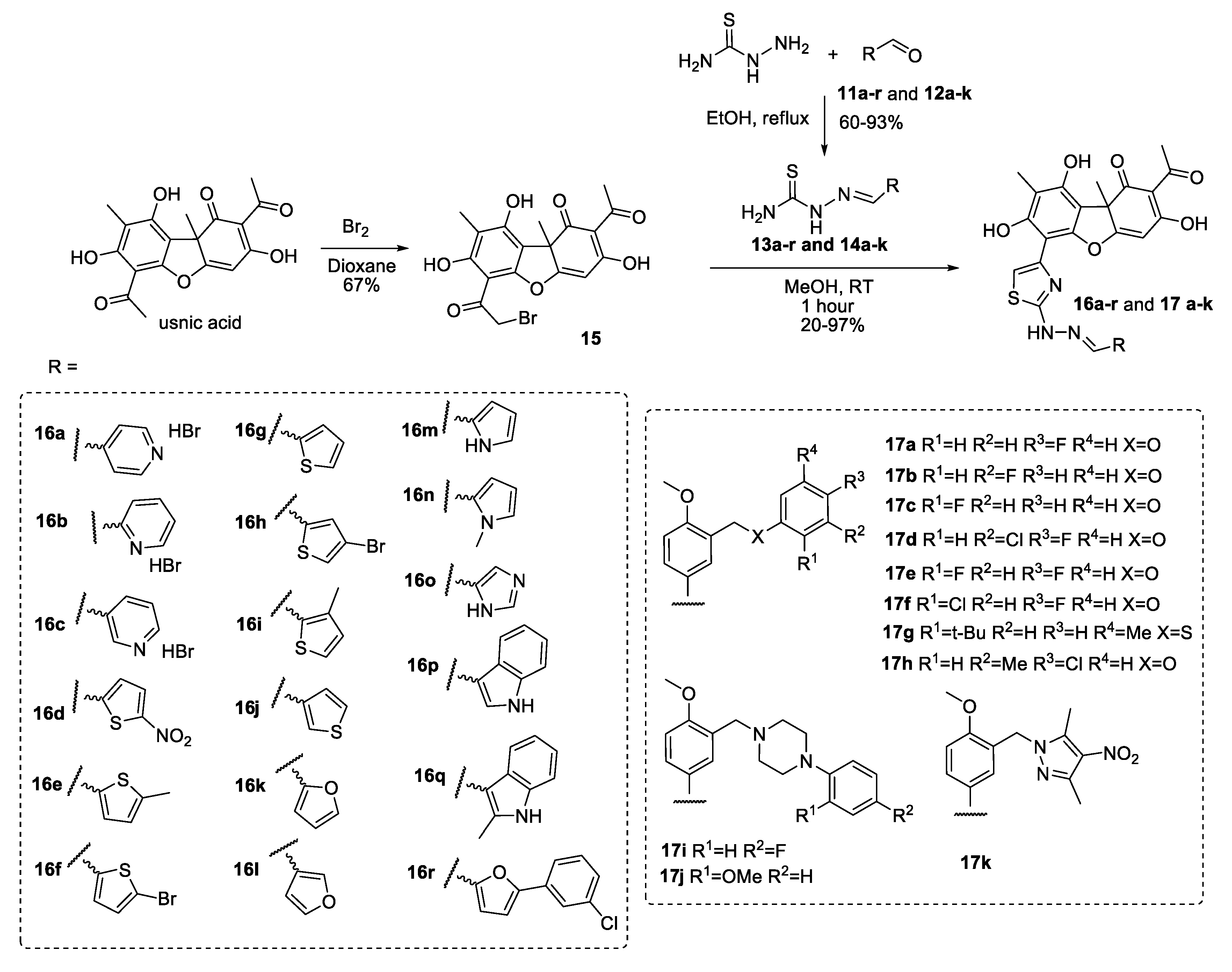
2.1. Chemistry
2.2. Biology
2.2.1. Structure–Activity Relationship Analysis
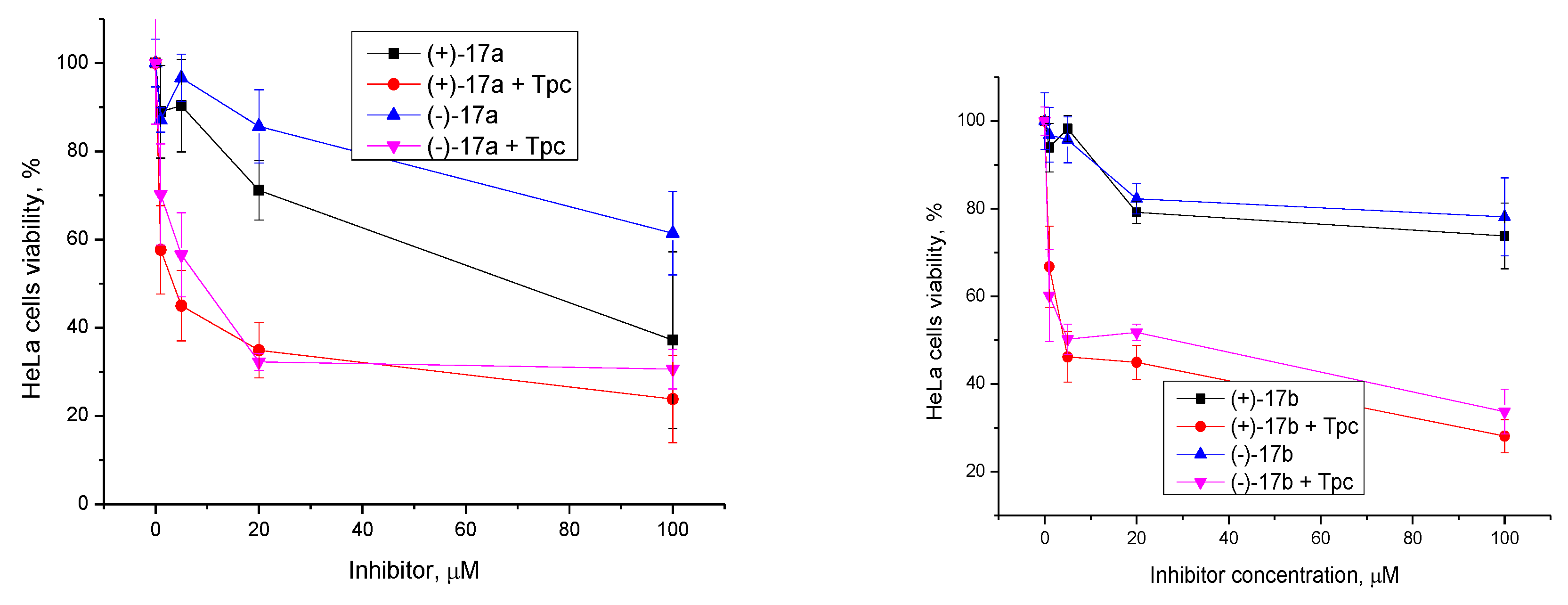
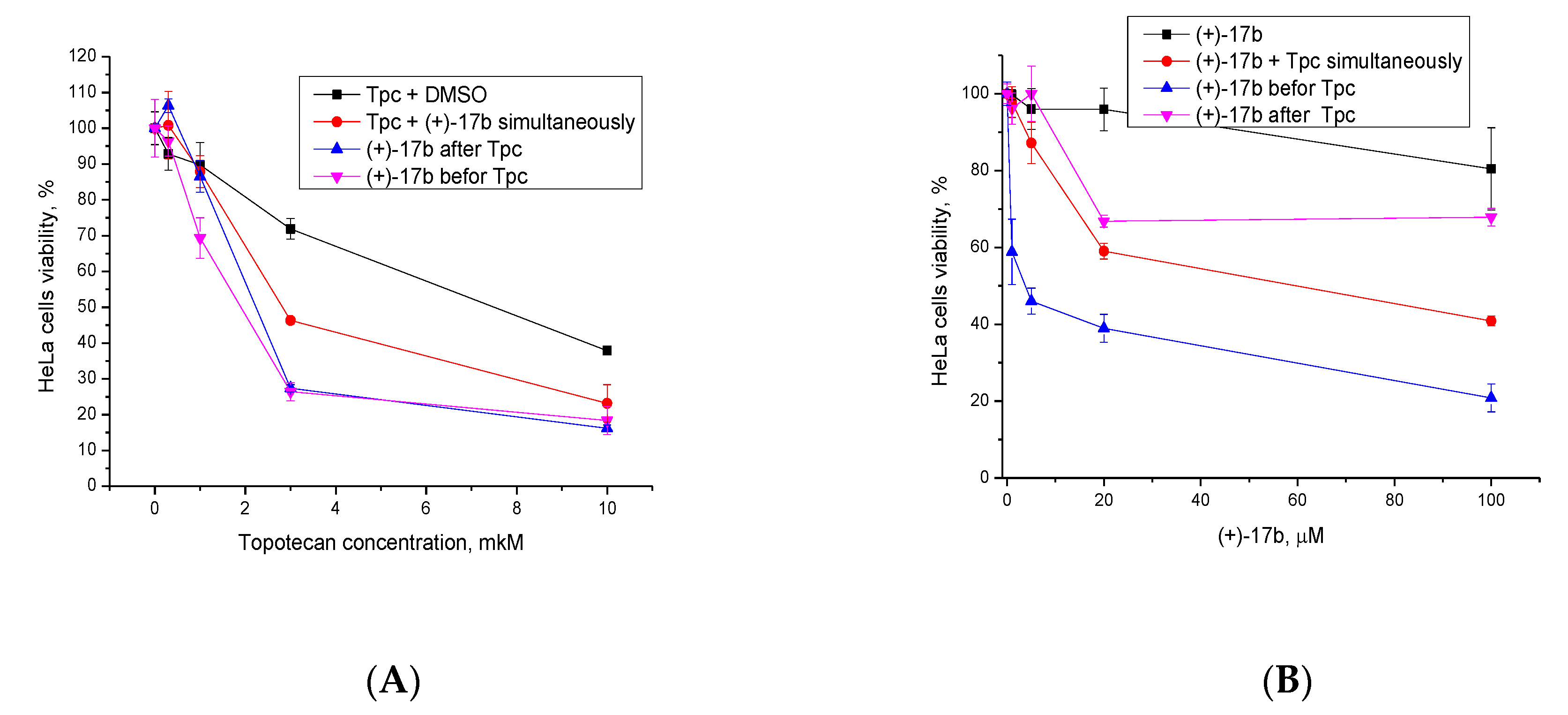
2.2.2. Synergistic Effect
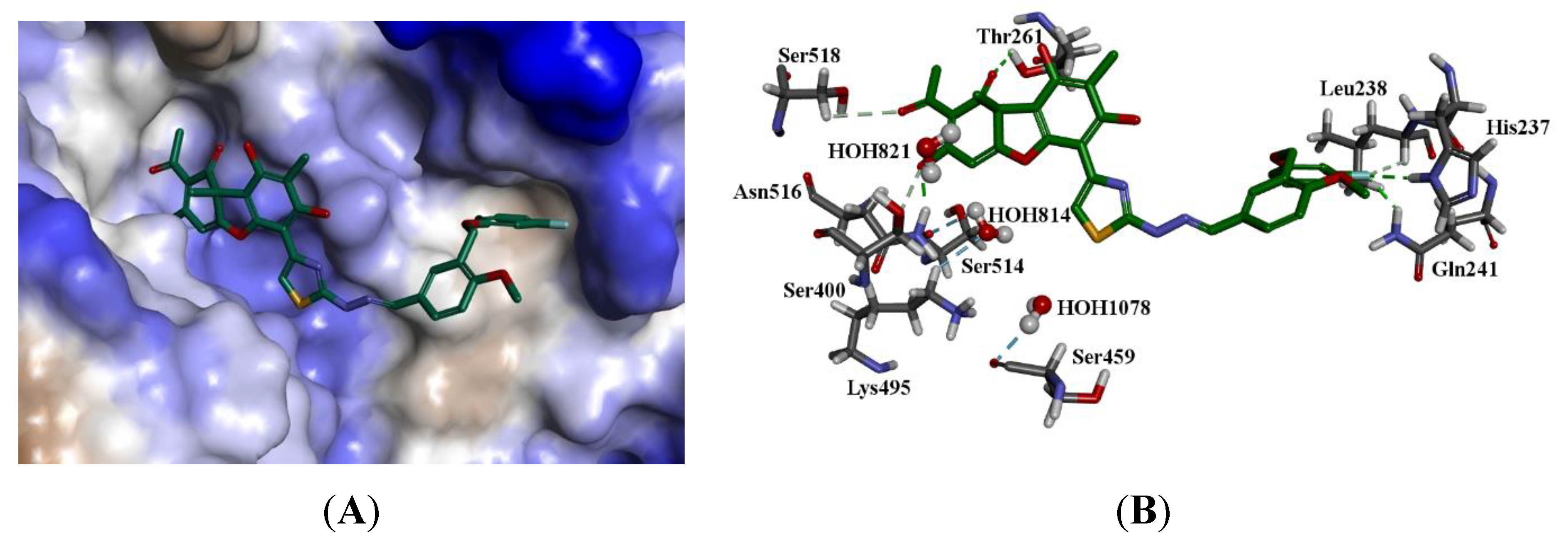
2.3. Molecular Modeling
2.4. Chemical Space
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure for the Synthesis of Compounds 13a–r and 14a–k
3.1.2. General Procedure for the Synthesis of Compounds 16a–r and 17a–k
3.2. Real-Time Detection of Tdp1 Activity
3.3. Binding Assay
3.4. Cytotoxicity Assay
3.5. Modeling and Screening
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hosoya, N.; Miyagawa, K. Targeting DNA damage response in cancer therapy. Cancer Sci. 2014, 105, 370–388. [Google Scholar] [CrossRef] [Green Version]
- Mangoni, A.A.; Eynde, J.J.V.; Jampilek, J.; Hadjipavlou-Litina, D.; Liu, H.; Reynisson, J.; Sousa, M.E.; Gomes, P.A.C.; Prokai-Tatrai, K.; Tuccinardi, T.; et al. Breakthroughs in Medicinal Chemistry: New Targets and Mechanisms, New Drugs, New Hopes–5. Molecules 2019, 24, 2415. [Google Scholar] [CrossRef] [PubMed]
- Interthal, H.; Pouliot, J.J.; Champoux, J.J. The tyrosyl-DNA phosphodiesterase Tdp1 is a member of the phospholipase D superfamily. Proc. Natl. Acad. Sci. USA 2001, 98, 12009–12014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.C. Mechanism of DNA Binding and Cleavage. Nat. Rev. Mol. Cell. Biol. 2002, 3, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Laev, S.S.; Salakhutdinov, N.F.; Lavrik, O.I. Tyrosyl-DNA phosphodiesterase inhibitors: Progress and potential. Bioorg. Med. Chem. 2016, 24, 5017–5027. [Google Scholar] [CrossRef] [PubMed]
- Kawale, A.S.; Povirk, L.F. Tyrosyl-DNA phosphodiesterases: Rescuing the genome from the risks of relaxation. Nucleic Acids Res. 2018, 46, 520–537. [Google Scholar] [CrossRef]
- Arabshahi, H.J.; van Rensburg, M.; Pilkington, L.I.; Jeon, C.Y.; Song, M.; Gridel, L.-M.; Leung, E.; Barker, D.; Vuica-Ross, M.; Volcho, K.P.; et al. A synthesis, in silico, in vitro and in vivo study of thieno[2,3-b]pyridine anticancer analogues. Med. Chem. Commun. 2015, 6, 1987–1997. [Google Scholar] [CrossRef]
- Dean, R.A.; Fam, H.K.; An, J.; Choi, K.; Shimizu, Y.; Jones, S.J.; Boerkoel, C.F.; Interthal, H.; Pfeifer, T.A. Identification of a putative Tdp1 inhibitor (CD00509) by in vitro and cell-based assays. J. Biomol. Screen. 2014, 10, 1372–1382. [Google Scholar] [CrossRef]
- Zakharenko, A.; Khomenko, T.; Zhukova, S.; Koval, O.; Zakharova, O.; Anarbaev, R.; Lebedeva, N.; Korchagina, D.; Komarova, N.; Vasiliev, V.; et al. Synthesis and biological evaluation of novel tyrosyl-DNA phosphodiesterase 1 inhibitors with a benzopentathiepine moiety. Bioorg. Med. Chem. 2015, 23, 2044–2052. [Google Scholar] [CrossRef]
- Marchand, C.; Lea, W.A.; Jadhav, A.; Dexheimer, T.S.; Austin, C.P.; Inglese, J.; Pommier, Y.; Simeonov, A. Identification of phosphotyrosine mimetic inhibitors of human tyrosyl-DNA phosphodiesterase I by a novel AlphaScreen high-throughput assay. Mol. Cancer Ther. 2009, 8, 240–248. [Google Scholar] [CrossRef]
- Wang, P.; Elsayed, M.S.A.; Plescia, C.B.; Ravji, A.; Redon, C.E.; Kiselev, E.; Marchand, C.; Zeleznik, O.; Agama, K.; Pommier, Y.; et al. Synthesis and Biological Evaluation of the First Triple Inhibitors of Human Topoisomerase 1, Tyrosyl-DNA Phosphodiesterase 1 (Tdp1), and Tyrosyl-DNA Phosphodiesterase 2 (Tdp2). J. Med. Chem. 2017, 60, 3275–3288. [Google Scholar] [CrossRef] [PubMed]
- Takagi, M.; Ueda, J.Y.; Hwang, J.H.; Hashimoto, J.; Izumikawa, M.; Murakami, H.; Sekido, Y.; Shin-ya, K. Tyrosyl-DNA phosphodiesterase 1 inhibitor from an anamorphic fungus. J. Nat. Prod. 2012, 75, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Salomatina, O.V.; Popadyuk, I.I.; Zakhcarenko, A.L.; Zakharova, O.D.; Fadeev, D.S.; Komarova, N.I.; Reynisson, J.; Arabshahi, H.I.; Chand, R.; Volcho, K.P.; et al. Novel Semisynthetic Derivatives of Bile Acids as Effective Tyrosyl-DNA Phosphodiesterase 1 Inhibitors. Molecules 2018, 23, 679. [Google Scholar] [CrossRef] [PubMed]
- Khomenko, T.; Zakharenko, A.; Odarchenko, T.; Arabshahi, H.J.; Sannikova, V.; Zakharova, O.; Korchagina, D.; Reynisson, J.; Volcho, K.; Salakhutdinov, N.; et al. New inhibitors of tyrosyl-DNA phosphodiesterase I (Tdp 1). Bioorg. Med. Chem. 2016, 24, 5573–5581. [Google Scholar] [CrossRef] [PubMed]
- Ponomarev, K.Y.; Suslov, E.V.; Zakharenko, A.L.; Zakharova, O.D.; Rogachev, A.D.; Korchagina, D.V.; Zafar, A.; Reynisson, J.; Nefedov, A.A.; Volcho, K.P.; et al. Aminoadamantanes containing monoterpene-derived fragments as potent tyrosyl-DNA phosphodiesterase 1 inhibitors. Bioorg. Chem. 2018, 76, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Mozhaitsev, E.S.; Zakharenko, A.L.; Suslov, E.V.; Korchagina, D.V.; Zakharova, O.D.; Vasil’eva, I.A.; Chepanova, A.A.; Black, E.; Patel, J.; Chand, R.; et al. Novel Inhibitors of DNA Repair Enzyme TDP1 Combining Monoterpenoid and Adamantane Fragments. Anti-Cancer Agents Med. Chem. 2019, 19, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Chepanova, A.A.; Mozhaitsev, E.S.; Munkuev, A.A.; Suslov, E.V.; Korchagina, D.V.; Zakharova, O.D.; Zakharenko, A.L.; Patel, J.; Ayine-Tora, D.M.; Reynisson, J.; et al. The development of Tyrosyl-DNA phosphodiesterase 1 inhibitors. Combination of monoterpene and adamantine moieties via amide or thioamides bridges. Appl. Sci. 2019, 9, 2767. [Google Scholar] [CrossRef]
- Zakharenko, A.; Luzina, O.; Koval, O.; Nilov, D.; Gushchina, I.; Dyrkheeva, N.; Švedas, V.; Salakhutdinov, N.; Lavrik, O. Tyrosyl-DNA phosphodiesterase 1 inhibitors: Usnic acid enamines enhance the cytotoxic effect of camptothecin. J. Nat. Prod. 2016, 79, 2961–2967. [Google Scholar] [CrossRef]
- Zakharenko, A.L.; Luzina, O.A.; Sokolov, D.N.; Kaledin, V.I.; Nikolin, V.P.; Popova, N.A.; Patel, J.; Zakharova, O.D.; Chepanova, A.A.; Zafar, A.; et al. Novel tyrosyl-DNA phosphodiesterase 1 inhibitors enhance the therapeutic impact of topoteCan on in vivo tumor models. Eur. J. Med. Chem. 2019, 161, 581–593. [Google Scholar] [CrossRef]
- Koldysheva, E.V.; Men’shchikova, A.P.; Lushnikova, E.L.; Popova, N.A.; Kaledin, V.I.; Nikolin, V.P.; Zakharenko, A.L.; Luzina, O.A.; Salakhutdinov, N.F.; Lavrik, O.I. Antimetastatic Activity of Combined Topotecan and Tyrosyl-DNA Phosphodiesterase-1 Inhibitor on Modeled Lewis Lung Carcinoma. Bull. Exp. Biol. Med. 2019, 166, 661–666. [Google Scholar] [CrossRef]
- Zakharenko, A.L.; Luzina, O.A.; Sokolov, D.N.; Zakharova, O.D.; Rakhmanova, M.E.; Chepanova, A.A.; Dyrkheeva, N.S.; Lavrik, O.I.; Salakhutdinov, N.F. Usnic Acid Derivatives Are Effective Inhibitors of Tyrosyl-DNA Phosphodiesterase 1. Russ. J. Bioorg. Chem. 2017, 43, 84–90. [Google Scholar] [CrossRef]
- Luzina, O.A.; Salakhutdinov, N.F. Biological activity of usnic acid and its derivatives: Part 1. Activity against unicellular organisms. Russ. J. Bioorg. Chem. 2016, 42, 115–132. [Google Scholar] [CrossRef]
- Luzina, O.A.; Salakhutdinov, N.F. Biological activity of usnic acid and its derivatives: Part 2. effects on higher organisms. Molecular and physicochemical aspects. Russ. J. Bioorg. Chem. 2016, 42, 249–268. [Google Scholar] [CrossRef]
- Luzina, O.A.; Salakhutdinov, N.F. Usnic acid and its derivatives for pharmaceutical use: A patent review (2000–2017). Expert Opin. Ther. Pat. 2018, 28, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Galanty, A.; Pasko, P.; Podolak, I. Enantioselective activity of usnic acid: A comprehensive review and future perspectives. Phytocem Rev 2019, 18, 527–548. [Google Scholar] [CrossRef]
- Fonseca, N.C.; Faria da Cruz, L.; da Silva Villela, F.; Aparecida, G.P.; de Siqueira-Neto, J.L.; Kellar, D.; Suzuki, B.M.; Ray, D.; de Souza, T.B.; Alves, R.J.; et al. Synthesis of a Sugar-Based Thiosemicarbazone Series and Structure-Activity Relationship versus the Parasite Cysteine Proteases Rhodesain, Cruzain, and Schistosoma mansoni Cathepsin B1. Antimicrob. Agents Chemother 2015, 59, 2666–2677. [Google Scholar] [CrossRef] [Green Version]
- Polovinka, M.P.; Salakhutdinov, N.F.; Panchenko, M.Y. Method for Preparing Usninic Acid. Patent RU2317076, 20 February 2008. [Google Scholar]
- Luzina, O.A.; Sokolov, D.N.; Shernyukov, A.V.; Salakhutdinov, N.F. Synthesis of aurones based on usninic acid. Chem. Nat. Compd. 2012, 48, 385–391. [Google Scholar] [CrossRef]
- Li-Zhulanov, N.S.; Zakharenko, A.L.; Chepanova, A.A.; Patel, J.; Zafar, A.; Volcho, K.P.; Salakhutdinov, N.F.; Reynisson, J.; Leung, I.K.H.; Lavrik, O.I. A novel class of tyrosyl-DNA phosphodiesterase 1 inhibitors that contain the octahydro-2H-chromen-4-ol scaffold. Molecules 2018, 23, 2468. [Google Scholar] [CrossRef]
- Martino, E.; Della Volpe, S.; Terribile, E.; Benetti, E.; Sakaj, M.; Centamore, A.; Sala, A.; Collina, S. The long story of camptothecin: From traditional medicine to drugs. Bioorg. Med. Chem. Lett. 2017, 27, 701–707. [Google Scholar] [CrossRef]
- Pommier, Y.; Leo, E.; Zhang, H.L.; Marchand, C. DNA Topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010, 17, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Perego, P.; Cossa, G.; Tinelli, S.; Corna, E.; Carenini, N.; Gatti, L.; De Cesare, M.; Ciusani, E.; Zunino, F.; Luison, E.; et al. Role of tyrosyl-DNA phosphodiesterase 1 and inter-players in regulation of tumor cell sensitivity to topoisomerase I inhibition. Biochem. Pharmacol. 2012, 83, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.F. Designing Drugs to Avoid Toxicity. Prog. Med. Chem. 2011, 50, 1–47. [Google Scholar] [PubMed]
- Lountos, G.T.; Zhao, X.Z.; Kiselev, E.; Tropea, J.E.; Needle, D.; Pommier, Y.; Burke, T.R.; Waugh, D.S. Identification of a ligand binding hot spot and structural motifs replicating aspects of tyrosyl-DNA phosphodiesterase I (TDP1) phosphoryl recognition by crystallographic fragment cocktail screening. Nucleic Acids Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.R.; Interthal, H.; Champoux, J.J.; Hol, W.G.J. Insights into Substrate Binding and Catalytic Mechanism of Human Tyrosyl-DNA Phosphodiesterase (Tdp1) from Vanadate and Tungstate-inhibited Structures. J. Mol. Biol. 2002, 324, 917. [Google Scholar] [CrossRef]
- Zhu, F.; Logan, G.; Reynisson, J. Wine Compounds as a Source for HTS Screening Collections. A Feasibility Study. Mol. Inf. 2012, 31, 847. [Google Scholar] [CrossRef]
- Eurtivong, C.; Reynisson, J. The Development of a Weighted Index to Optimise Compound Libraries for High Throughput Screening. Mol. Inform. 2018, 38, 1800068. [Google Scholar] [CrossRef]
- Obushak, N.D.; Lesyuk, A.I.; Gorak, Y.I.; Matiichuk, V.S. Mechanism of Meerwein Arylation of Furan Derivatives. Russ. J. Org. Chem. 2009, 45, 1375–1381. [Google Scholar] [CrossRef]
- Khachatryan, D.S.; Razinov, A.L.; Kolotaev, A.V.; Beluś, S.K.; Matevosyan, K.R. Alkylation of -NH, -OH, and -SH acids in the presence of potassium carbonate 1. Functionalization of chloromethyl group of alkoxysubstituted aromatic aldehydes. Russ. Chem. Bull. 2015, 64, 395–404. [Google Scholar] [CrossRef]
- Zakharenko, A.L.; Luzina, O.A.; Kaledin, V.I.; Nikolin, V.P.; Popova, N.A.; Patel, J.; Zakharova, O.D.; Sokolov, D.N.; Chepanova, A.A.; Zafar, A.; et al. Novel Tyrosyl-DNA Phosphodiesterase 1 Inhibitors Enhance the Therapeutic Impact of TopoteCan on In Vivo Tumor Models. Eur. J. Med. Chem. 2019, 161, 581–593. [Google Scholar] [CrossRef]
- Cardoso, M.V.; de Siqueira, L.R.; da Silva, E.B.; Costa, L.B.; Hernandes, M.Z.; Rabello, M.M. 2-Pyridyl thiazoles as novel anti-T. cruzi agents: Structural design, synthesis and Pharmacological evaluation. Eur. J. Med. Chem. 2014, 86, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, A.C.; Aamer, S.; Fayaz, A.; Muhammad, R.; Zaman, A.; Farukh, J.; Tanzeela, A.F. Synthesis, computational studies and enzyme inhibitory kinetics of substituted methyl[2-(4-dimethylamino-benzylidene)-hydrazono)-4-oxo-thiazolidin-5-ylidene]acetates as mushroom tyrosinase inhibitors. Bioorg. Med. Chem. 2017, 25, 5929–5938. [Google Scholar]
- Xu, J.; Liu, J.; Zhu, X.; Yu, Y.; Cao, S. Novel inhibitors of tyrosinase produced by the 4-substitution of TCT. Food Chem. 2017, 221, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Dong, H.; Yu, Y.; Cao, S. Inhibitory effect of synthetic aromatic heterocycle thiosemicarbazone derivatives on mushroom tyrosinase: Insights from fluorescence, 1H NMR titration and molecular docking studies. Food Chem. 2016, 190, 709–716. [Google Scholar] [CrossRef]
- Cohen, M.; Ladd, J.R. Dimethylvinylethoxysilane and Methylvinyldiethoxysilane. Am. Chem. Soc. 1953, 75, 988. [Google Scholar] [CrossRef]
- Alomar, K.; Landreau, A.; Kempf, M.; Khan, M.A.; Allain, M.; Bouet, G. Synthesis, crystal structure, characterization of zinc(II), cadmium(II) complexes with 3-thiophene aldehyde thiosemicarbazone (3TTSCH). Biological activities of 3TTSCH and its complexes. J. Inorg. Biochem. 2010, 104, 397–404. [Google Scholar] [CrossRef] [Green Version]
- Abram, U.; Ortner, K.; Gust, R.; Sommer, K. Gold complexes with thiosemicarbazones: Reactions of bi- and tridentate thiosemicarbazones with dichloro[2-(dimethylaminomethyl)phenyl-C1,N]gold(III), [Au(damp-C1,N)Cl2]. J. Chem. Soc. Dalton Trans. 2000, 735–744. [Google Scholar] [CrossRef]
- Reis, D.C.; Recio Despaigne, A.A.; Da Silva, J.G.; Silva, N.F.; Vilela, C.F.; Mendes, I.C.; Takahashi, J.A.; Beraldo, H. Structural Studies and Investigation on the Activity of Imidazole-Derived Thiosemicarbazones and Hydrazones against Crop-Related Fungi. Molecules 2013, 18, 12645–12662. [Google Scholar] [CrossRef] [Green Version]
- Yi, W.; Dubois, C.; Yahiaoui, S.; Haudecoeur, R.; Belle, C.; Song, H.; Hardré, R.; Réglier, M.; Boumendjel, A. Refinement of arylthiosemicarbazone pharmacophore in inhibition of mushroom tyrosinase. Eur. J. Med. Chem. 2011, 45, 4330–4335. [Google Scholar] [CrossRef]
- Lebedeva, N.A.; Rechkunova, N.I.; Lavrik, O.I. AP-site cleavage activity of tyrosyl-DNA phosphodiesterase 1. FEBS Lett. 2011, 585, 683–686. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T.J. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.; Henrick, K.; Nakamura, H. Announcing the worldwide protein data bank. Nat. Struct. Mol. Biol. 2003, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Scigress: Version FJ 2.6 (EU 3.1.7), Fujitsu Limited 2008–2016. Available online: https://www.fqs.pl/en/chemistry/products/scigress (accessed on 20 September 2019).
- Allinger, N.L. Conformational analysis. 130. MM2. A hydrocarbon force field utilizing V1 and V2 torsional terms. J. Am. Chem. Soc. 1977, 99, 8127. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727. [Google Scholar] [CrossRef]
- Eldridge, M.D.; Murray, C.W.; Auton, T.R.; Paolini, G.V.; Mee, R.P. Empirical scoring functions: I. The development of a fast empirical scoring function to estimate the binding affinity of ligands in receptor complexes. J. Comput. Aided Mol. Des. 1997, 11, 425. [Google Scholar] [CrossRef]
- Verdonk, M.L.; Cole, J.C.; Hartshorn, M.J.; Murray, C.W.; Taylor, R.D. Improved protein–ligand docking using GOLD. Proteins 2003, 52, 609. [Google Scholar] [CrossRef]
- Korb, O.; Stutzle, T.; Exner, T.E. Empirical scoring functions for advanced protein− ligand docking with PLANTS. J. Chem. Inf. Model 2009, 49, 84. [Google Scholar] [CrossRef]
- Mooij, W.; Verdonk, M.L. General and targeted statistical potentials for protein–ligand interactions. Proteins 2005, 61, 272. [Google Scholar] [CrossRef]
- QikProp Version 3.2: Schrödinger, New York QikProp Version 3.2 2009. Available online: https://www.schrodinger.com/qikprop (accessed on 20 September 2019).
- Ioakimidis, L.; Thoukydidis, L.; Mirza, A.; Naeem, S.; Reynisson, J. Benchmarking the reliability of QikProp. Correlation between experimental and predicted values. QSAR Comb. Sci. 2008, 27, 445. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 16a–r and 17a–k are available from the authors. |


| Code | Compound, R | (+)-enantiomer | (−)-enantiomer | ||
|---|---|---|---|---|---|
| IC50/nM | KD/µM | IC50/nM | KD/µM | ||
| 16a |  | 160 ± 16 | 139 ± 32 | 139 ± 38 | n.d. |
| 16b |  | 492 ± 88 | 77 ± 24 | 1480 ± 265 | n.d. |
| 16c | 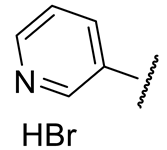 | 353 ± 17 | n.d.* | 154 ± 18 | 262 ± 25 |
| 16d |  | 70 ± 4 | 597 ± 98 | 142 ± 4 | n.d. |
| 16e | 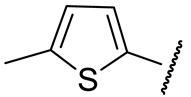 | 91 ± 7 | n.d. | 54 ± 25 | n.d. |
| 16f | 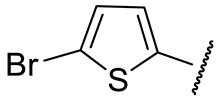 | 88 ± 3 | 95 ± 11 | 43 ± 1 | 148 ± 9 |
| 16g |  | 52 ± 18 | n.d. | 169 ± 6 | 281 ± 25 |
| 16h | 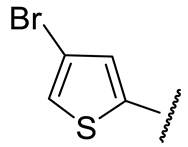 | 70 ± 18 | n.d. | 56 ± 4 | 355 ± 50 |
| 16i | 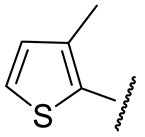 | 72 ± 3 | n.d. | 121 ± 25 | n.d. |
| 16j | 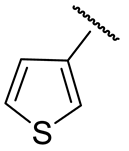 | 107 ± 31 | 65 ± 7 | 40 ± 3 | 771 ± 46 |
| 16k |  | 120 ± 15 | 67 ± 9 | 46 ± 24 | 250 ± 24 |
| 16l |  | 77 ± 6 | n.d. | 178 ± 4 | 233 ± 9 |
| 16m |  | 188 ± 3 | n.d. | 18 ± 1 | 354 ± 40 |
| 16n | 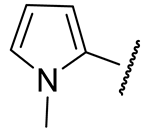 | 151 ± 14 | 719 ± 17 | 29 ± 9 | n.d. |
| 16o | 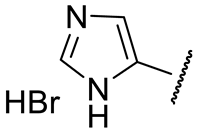 | 1690 ± 890 | 38 ± 2 | 570 ± 109 | 51 ± 4 |
| 16p | 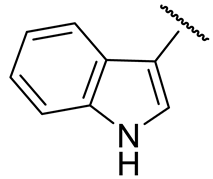 | 138 ± 3 | 268 ± 11 | 57 ± 1 | n.d. |
| 16q | 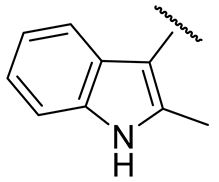 | 21 ± 6 | n.d. | 81 ± 20 | 58±4 |
| 16r |  | 55 ± 8 | 113 ± 52 | 53 ± 3 | n.d. |
| Code | R | (+)-enantiomer | (−)-enantiomer | ||
|---|---|---|---|---|---|
| IC50/nM | KD/µM | IC50/nM | KD/µM | ||
| 17a | 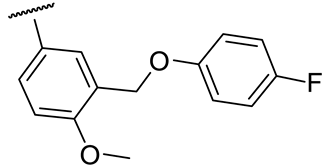 | 26 ± 8 | 212 ± 25 | 54 ± 3 | n.d.* |
| 17b | 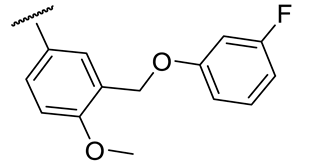 | 26 ± 4 | 131 ± 15 | 78 ± 3 | n.d. |
| 17c |  | 41 ± 7 | 204 ± 23 | 37 ± 10 | 74 ± 4 |
| 17d |  | 77 ± 3 | 130 ± 29 | 18 ± 1 | 76 ± 5 |
| 17e | 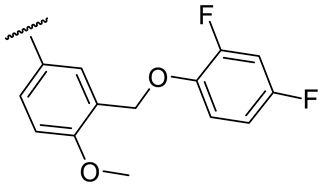 | 64 ± 6 | 213 ± 4 | 30 ± 11 | 130 ± 8 |
| 17f |  | 60 ± 1 | 127 ± 14 | 21 ± 5 | 536 ± 90 |
| 17g | 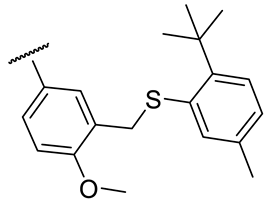 | 122 ± 25 | 223 ± 42 | 94 ± 20 | n.d. |
| 17h | 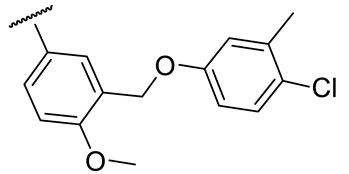 | 74 ± 1 | 188 ± 22 | 48 ± 2 | n.d. |
| 17i |  | 80 ± 7 | 161 ± 22 | 59 ± 5 | n.d. |
| 17j |  | 69 ± 14 | 69 ± 14 | 71 ± 15 | n.d. |
| 17k | 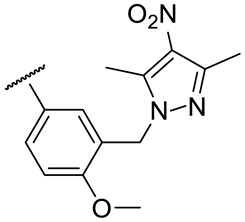 | 54 ± 8 | 156 ± 8 | 46 ± 5 | 142 ± 11 |
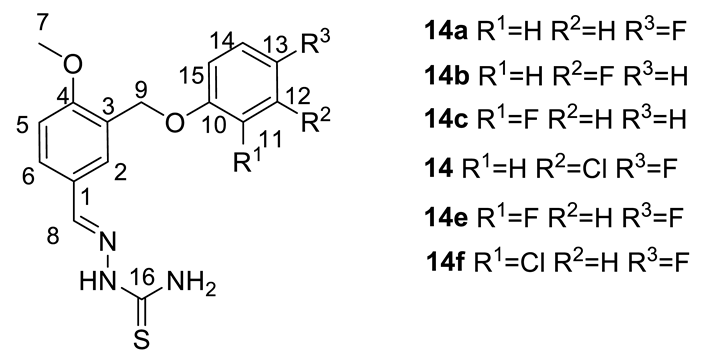
| № | 14a | 14b | 14c | 14d | 14e | 14f |
|---|---|---|---|---|---|---|
| H-2 | s 8.00 | s 8.01 | s 8.01 | s 8.00 | s 8.01 | s 8.01 |
| H-5 | m 7.00–7.15 | m 7.08–7.30 | d 7.09 (J = 8.7 Hz) | d 7.07 (J = 8.6 Hz) | d 7.01 (J = 8.6 Hz) | d 7.01 (J = 8.6 Hz) |
| H-6 | m 7.72 | m 7.75 | m 7.77 | m 7.72 | m 7.75 | m 7.77 |
| H-7 | s 3.86 | s 3.86 | s 3.85 | s 3.85 | s 3.86 | s 3.86 |
| H-8 | s 8.12 | s 8.13 | s 8.16 | s 8.14 | s 8.13 | s 8.15 |
| H-9 | s 5.00 | s 5.09 | s 5.09 | s 5.02 | s 5.04 | s 5.09 |
| H-11 | m 7.00–7.15 | m 7.08–7.30 | t 7.35 (J = 9.1 Hz) | |||
| H-12 | m 7.00–7.15 | m 7.21 | m 6.77 | m 7.42 | ||
| H-13 | m 7.08–7.30 | m 7.28 | ||||
| H-14 | m 7.00–7.15 | m 7.08–7.30 | t 7.11 (J = 7.7 Hz) | m 7.27 | m 6.77 | m 7.28 |
| H-15 | m 7.00–7.15 | m 6.95 | m 6.95 | m 7.02 | m 6.77 | m 7.17 |
| NH | s 11.32 | s 11.33 | s 11.35 | s 11.34 | s 11.33 | s 11.33 |
| NH2 | m 7.90 | m 7.89 | m 7.90 | m 7.92 | m 7.93 | m 7.87 |
| № | 14a | 14b | 14c | 14d | 14e | 14f |
|---|---|---|---|---|---|---|
| C-1 | 125.39 | 124.99 | 124.57 | 124.94 | 124.53 | 124.93 |
| C-2 | 129.11 | 129.54 | 129.12 | 129.27 | 129.57 | 129.20 |
| C-3 | 126.95 | 126.97 | 126.56 | 126.99 | 127.01 | 126.98 |
| C-4 | 159.03 | 159.18 | 158.78 | 159.07 | 159.17 | 159.10 |
| C-5 | 111.60 | 111.74 | 111.32 | 111.65 | 111.68 | 111.78 |
| C-6 | 129.85 | 129.99 | 129.57 | 130.08 | 130.30 | 129.83 |
| C-7 | 56.23 | 56.27 | 55.86 | 56.25 | 56.26 | 56.29 |
| C-8 | 142.52 | 142.41 | 141.99 | 142.42 | 142.33 | 142.44 |
| C-9 | 65.62 | 66.17 | 65.75 | 66.03 | 66.12 | 66.78 |
| C-10 | 155.33 | d 146.83 (J = 10 Hz) | d 146.42 (J = 11 Hz) | d 155.62 (J = 2.4 Hz) | t 161.17 (J = 14 Hz) | d 151.14 (J = 2.4 Hz) |
| C-11 | d 116.37 (J = 4 Hz) | 115.69 | d 150.60 (J = 248 Hz) | d 115.53 (J = 7 Hz) | d 162.40 (J = 244 Hz) | d 122.50 (J = 10 Hz) |
| C-12 | d 116.19 (J = 11 Hz) | d151 (J = 244 Hz) | d 115.96 (J = 18 Hz) | d 120.08 (J = 17 Hz) | t 96.65 (J = 27 Hz) | d 117.51 (J = 28 Hz) |
| C-13 | d 155.83 (J = 240 Hz) | d 116.38 (J = 18 Hz) | d 121.21 (J = 7 Hz) | d 151.12 (J = 247 Hz) | d 162.25 (J = 244 Hz) | d 155.08 (J = 247 Hz) |
| C-14 | d 116.19 (J = 11 Hz) | d 125.30 (J = 3 Hz) | d 124.88 (J = 3 Hz) | d 117.56 (J = 22 Hz) | m 99.15 | d 115.01 (J = 21 Hz) |
| C-15 | d 116.37 (J = 4 Hz) | d 121.63 (J = 7 Hz) | 115.27 | 116.63 | m 99.43 | d 115.54 (J = 9.4 Hz) |
| C-16 | 178.04 | 178.06 | 177.64 | 178.06 | 178.07 | 178.09 |
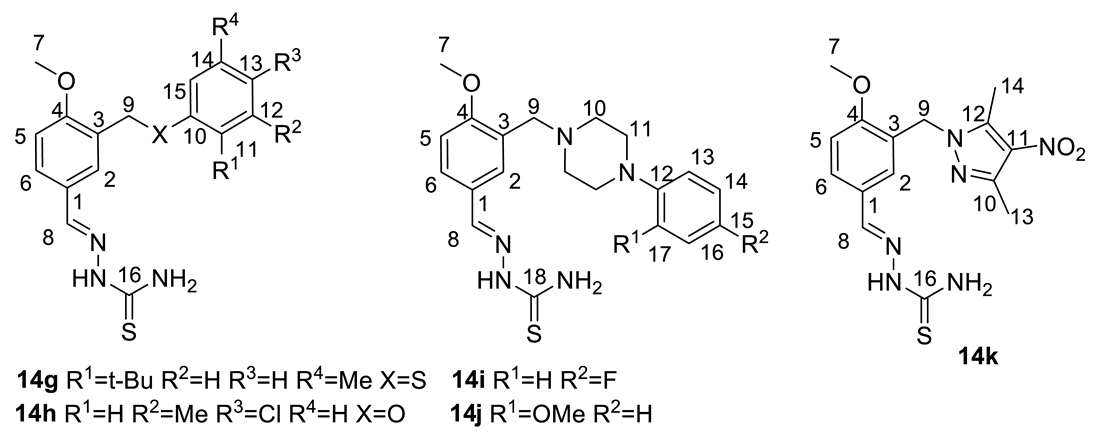
| № | 14g | 14h | 14i | 14j | 14k |
|---|---|---|---|---|---|
| H-2 | s 7.91 | s 7.99 | s 8.00 | s 8.00 | s 7.89 |
| H-5 | d 7.00 (J = 9.3 Hz) | m 7.04 | m 7.02 | d 7.05 (J = 8.5 Hz) | d 7.03 (J = 8.5 Hz) |
| H-6 | m 7.62 | m 7.72 | m 7.69 | bs 7.75 | m 7.63 |
| H-7 | s 3.80 | s 3.85 | s 3.83 | s 3.83 | s 3.81 |
| H-8 | s 8.14 | s 8.14 | s 8.11 | s 8.15 | s 7.97 |
| H-9 | s 4.09 | s 5.00 | s 3.55 | s 3.68 | s 5.21 |
| H-10 | bs 2.57 | bs 2.69 | |||
| H-11 | s 1.19 (t-Bu) | bs 3.07 | bs 2.93 | ||
| H-12 | s 7.09 | s 2.28 (Me) | |||
| H-13 | s 7.18 | m 7.06 | m 6.90 | m 6.87–6.94 | 2.34 |
| H-14 | s 2.21 (Me) | m 7.28 | m 7.02 | m 6.87–6.94 | 2.67 |
| H-15 | s 7.09 | m 6.86 | m 6.87–6.94 | ||
| H-16 | m 7.02 | m 6.87–6.94 | |||
| H-17 | m 6.90 | s 3.74 (OMe) | |||
| NH | s 11.32 | s 11.33 | s 11.29 | s 11.34 | s 11.33 |
| NH2 | s 7.62 and s 7.80 | s 7.89 and s 7.93 | m 7.69 and s 7.85 | bs 7.72 and bs 7.90 | s 7.75 and s 7.78 |

| № | 14g | 14h | 14i | 14j | 14k |
|---|---|---|---|---|---|
| C-1 | 125.87 | 124.88 | 127.95 | 126.36 | 123.74 |
| C-2 | 129.13 | 129.36 | 126.19 | 128.64 | 129.05 |
| C-3 | 126.31 | 126.52 | 127.95 | 126.36 | 126.59 |
| C-4 | 158.47 | 158.57 | 159.03 | 159.28 | 158.26 |
| C-5 | 111.11 | 111.19 | 111.16 | 111.38 | 111.30 |
| C-6 | 129.59 | 129.50 | 129.64 | 130.46 | 129.37 |
| C-7 | 55.77 | 55.80 | 55.67 | 55.89 | 55.83 |
| C-8 | 142.02 | 142.12 | 142.24 | 142.42 | 141.87 |
| C-9 | 31.52 | 64.84 | 48.73 | 49.30 | 48.10 |
| C-10 | 134.24 | 157.29 | 55.06 | 55.05 | 145.04 |
| C-11 | 148.8 (C, Ar) 30.88 (Me t-Bu) 34.14 (C, t-Bu) | 117.36 | 52.36 | 52.55 | 130.02 |
| C-12 | 128.25 | 128.60 (C, Ar) 19.79 (Me) | 147.75 | 152.02 | 141.38 |
| C-13 | 125.88 | 136.52 | 117.02 (J = 7 Hz) | 111.92 | 11.39 |
| C-14 | 134.58 (C, Ar) 19.32 (Me) | 124.70 | 115.06 (J = 22 Hz) | 118.13 | 13.90 |
| C-15 | 123.05 | 113.82 | d 155.00 (J = 232 Hz) | 122.77 | |
| C-16 | 177.58 | 177.66 | 115.06 (J = 22 Hz) | 120.92 | 177.67 |
| C-17 | 117.02 (J = 7 Hz) | 140.08 | |||
| C-18 | 177.60 | 177.64 |
| № | 16a | 16b | 16c | 16d | 16e |
|---|---|---|---|---|---|
| Structure | 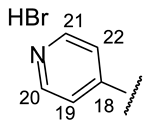 | 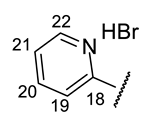 | 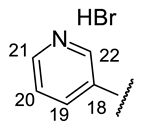 | 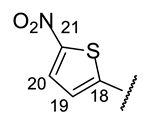 | 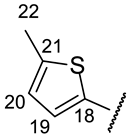 |
| H-14 | s 7.39 | s 7.39 | s 7.38 | s 7.27 | s 7.23 |
| H-17 | s 8.16 | s 8.15 | s 8.22 | s 8.08 | s 8.16 |
| H-19 | d 8.10 (J = 5.5 Hz) | d 8.07 (J = 8.0 Hz) | s 9.13 | d 7.34 (J = 4.5 Hz) | d 7.19 (J = 4.9 Hz) |
| H-20 | d 8.84 (J = 5.5 Hz) | t 7.66 (J = 5.9 Hz) | d 8.85 (J = 5.1 Hz) | d 7.98 (J = 4.5 Hz) | m 6.79 |
| H-21 | d 8.84 (J = 5.5 Hz) | t 8.21 (J = 5.1 Hz) | m 8.21 | ||
| H-22 | d 8.10 (J = 5.5 Hz) | d 8.71 (J = 5.1 Hz) | d 8.70 (J = 5.1 Hz) | s 2.46 | |
| NH | bs 12.37 | bs 12.45 | --- | s 12.78 | --- |
| OH-3 | --- | bs 18.82 | bs 18.76 | bs 18.78 | bs 18.82 |
| OH-7 | s 13.28 | s 13.05 | s 12.96 | s 12.49 | s 12.27 |
| OH-9 | --- | bs 10.30 | bs 10.30 | s 10.23 | s 10.27 |
| № | 16a | 16b | 16c | 16d | 16e |
|---|---|---|---|---|---|
| Structure | 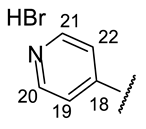 | 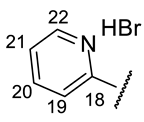 |  | 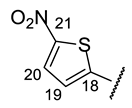 |  |
| C-17 | 137.15 | 146.33 | 137.05 | 135.56 | 138.62 |
| C-18 | 148.81 | 150.12 | 133.07 | 146.60 | 136.51 |
| C-19 | 122.41 | 124.82 | 141.66 | 130.58 | 130.36 |
| C-20 | 143.16 | 138.72 | 143.08 | 128.13 | 126.38 |
| C-21 | 143.16 | 121.21 | 126.76 | 149.84 | 142.34 |
| C-22 | 122.41 | 140.70 | 139.95 | 15.35 |
| № | 16f | 16g | 16h | 16i | 16j |
|---|---|---|---|---|---|
| Structure | 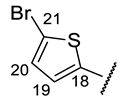 |  | 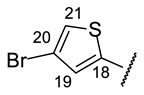 | 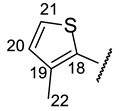 | 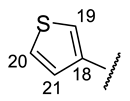 |
| H-14 | s 7.11 | s 7.08 | s 7.04 | s 7.09 | s 7.12 |
| H-17 | s 7.58 | s 7.81 | bs 8.12 | s 7.73 | s 7.78 |
| H-19 | d 6.77 (J = 3.8 Hz) | d 7.08 (J = 3.4 Hz) | s 7.04 | s 7.43 | |
| H-20 | d 6.86 (J = 3.8 Hz) | m 6.94 | d 6.72 (J = 5.3 Hz) | d 7.46 (J = 4.9 Hz) | |
| H-21 | d 7.27 (J = 5.0 Hz) | s 7.19 | d 7.14 (J = 4.9 Hz) | m 7.31 | |
| H-22 | s 2.23 | ||||
| NH | bs 9.06 | bs 9.48 | --- | bs 9.11 | bs 8.99 |
| OH-3 | s 18.79 | s 18.78 | s 18.79 | s 18.78 | s 18.79 |
| OH-7 | --- | --- | --- | --- | --- |
| OH-9 | s 10.29 | s 10.27 | s 10.40 | s 10.26 | s 10.28 |
| № | 16f | 16g | 16h | 16i | 16j |
|---|---|---|---|---|---|
| Structure | 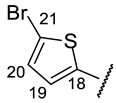 |  | 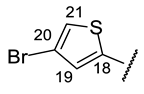 | 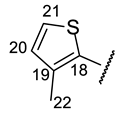 | 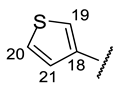 |
| C-17 | 135.97 | 142.67 | 138.68 | 136.47 | 137.90 |
| C-18 | 139.39 | 138.12 | 138.72 | 131.71 | 136.51 |
| C-19 | 128.64 | 137.70 | 131.84 | 138.95 | 124.77 |
| C-20 | 129.92 | 127.87 | 110.30 | 126.73 | 126.09 |
| C-21 | 115.29 | 129.21 | 125.35 | 130.60 | 126.59 |
| C-22 | 13.95 |
| № | 16k | 16l | 16m | 16n | 16o |
|---|---|---|---|---|---|
| Structure | 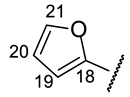 | 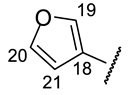 |  | 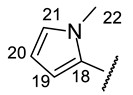 | 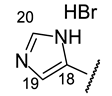 |
| H-14 | s 7.15 | s 7.11 | s 7.24 | s 7.09 | s 7.37 |
| H-17 | s 7.60 | s 7.63 | s 7.94 | s 7.68 | s 8.09 |
| H-19 | d 6.65 (J = 2.5 Hz) | s 7.59 | s 6.46 | m 6.39 | s 8.00 |
| H-20 | m 6.45 | s 7.39 | s 6.14 | m 6.11 | s 9.17 |
| H-21 | d 7.49 (J = 2.2 Hz) | m 6.75 | s 6.92 | s 6.72 | |
| H-22 | s 3.91 | ||||
| NH | s 8.88 | bs 8.93 | --- | bs 8.68 | --- |
| NH (HetAr) | s 11.30 | s 12.60 | |||
| OH-3 | s 18.77 | s 18.79 | bs 18.77 | s 18.79 | --- |
| OH-7 | bs 12.40 | --- | s 12.05 | --- | s 12.71 |
| OH-9 | s 10.26 | s 10.95 | s 10.28 | s 10.28 | s 10.31 |
| № | 16k | 16l | 16m | 16n | 16o |
|---|---|---|---|---|---|
| Structure | 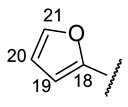 |  | 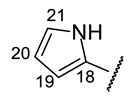 | 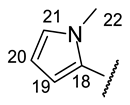 | 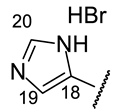 |
| C-17 | 132.37 | 135.09 | 136.59 | 136.37 | 130.90 |
| C-18 | 148.62 | 121.97 | 126.80 | 126.39 | 128.58 |
| C-19 | 112.28 | 143.42 | 109.33 | 115.74 | 119.55 |
| C-20 | 111.72 | 144.00 | 112.13 | 108.03 | 132.36 |
| C-21 | 144.26 | 107.19 | 122.14 | 128.01 | |
| C-22 | 37.09 |
| № | 16p | 16q | 16r |
|---|---|---|---|
| Structure |  |  | 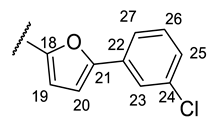 |
| H-14 | s 7.24 | s 7.22 | s 7.12 |
| H-17 | s 8.30 | s 8.33 | s 7.68 |
| H-19 | m 8.21 | d 6.69 (J = 3.3 Hz, AB-system) | |
| H-20 | d 6.66 (J = 3.3 Hz, AB-system) | ||
| H-21 | s 7.45 | m 7.34 | |
| H-22 | m 7.21 | m 7.13 | |
| H-23 | m 7.21 | m 7.13 | s 7.62 |
| H-24 | d 7.83 (J = 2.7 Hz) | m 8.10 | |
| H-25 | d 7.52 (J = 7.5 Hz) | ||
| H-26 | s 2.51 | m 7.21–7.30 | |
| H-27 | m 7.21–7.30 | ||
| NH | s 12.08 | s 12.00 | --- |
| NH (HetAr) | s 11.59 | s 11.51 | |
| OH-3 | bs 18.82 | bs 18.82 | s 18.76 |
| OH-7 | s 13.03 | bs 13.05 | --- |
| OH-9 | s 10.30 | s 10.31 | s 10.26 |
| № | 16n | 16o | 16r |
|---|---|---|---|
| Structure | 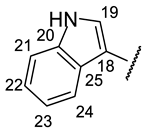 | 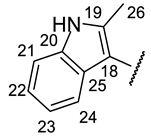 | 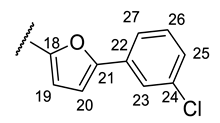 |
| C-17 | 140.39 | 140.29 | 132.95 |
| C-18 | 111.35 | 107.42 | 148.80 |
| C-19 | 130.13 | 135.76 | 108.17 |
| C-20 | 137.11 | 139.52 | 114.29 |
| C-21 | 111.95 | 110.99 | 153.82 |
| C-22 | 121.59 | 120.63 | 131.35 |
| C-23 | 120.59 | 120.46 | 123.92 |
| C-24 | 122.64 | 121.83 | 134.78 |
| C-25 | 124.02 | 124.02 | 127.90 |
| C-26 | 11.53 | 129.91 | |
| C-27 | 122.03 |
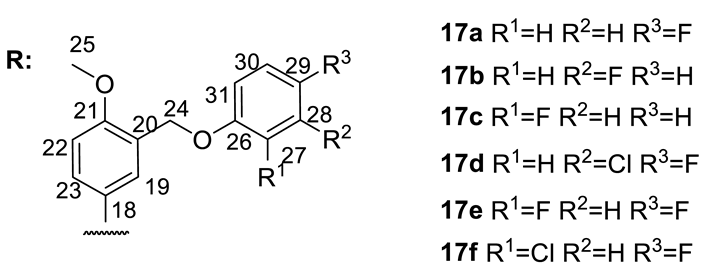
| № | 17a | 17b | 17c | 17d | 17e | 17f |
|---|---|---|---|---|---|---|
| H-14 | s 7.09 | s 7.02 | m 7.05 | s 7.06 | s 7.09 | s 7.07 |
| H-17 | m 7.45–7.65 | s 7.81 | s 7.68 | s 7.64 | s 7.60 | s 7.85 |
| H-19 | m 7.45–7.65 | s 7.71 | s 7.62 | s 7.61 | s 7.56 | s 7.71 |
| H-22 | m 6.82–6.97 | d 6.77 (J = 8.6 Hz) | d 6.78 (J = 7.0 Hz) | m 6.82 | d 6.51 (J = 7.9 Hz) | m 6.90 |
| H-23 | m 7.45–7.65 | d 7.44 (J = 8.6 Hz) | d 7.44 (J = 7.0 Hz) | d 7.48 (J = 7.3 Hz) | d 7.48 (J = 8.3 Hz) | d 7.41 (J = 8.1 Hz) |
| H-24 | s 5.00 | s 5.07 | s 5.09 | s 4.97 | s 5.00 | s 5.04 |
| H-25 | s 3.83 | s 3.79 | s 3.79 | s 3.82 | s 3.84 | s 3.83 |
| H-27 | m 6.82–6.97 | m 6.90–7.10 | s 6.91 | m 7.01 | d 8.47 (J = 8.5 Hz) | |
| H-28 | m 6.82–6.97 | m 6.90–7.10 | m 7.05 | d 6.82 (J = 7.9 Hz) | m 6.90 | |
| H-29 | m 6.90–7.10 | m 7.05 | ||||
| H-30 | m 6.82–6.97 | m 7.05 | m 7.01 | t 6.41 (J = 8.4 Hz) | m 7.12 | |
| H-31 | m 6.82–6.97 | m 6.90–7.10 | m 6.82 | |||
| NH | bs 8.92 | --- | bs 9.45 | bs 9.45 | bs 8.93 | bs 9.60 |
| OH-3 | s 18.78 | s 18.77 | s 18.76 | s 18.77 | s 18.78 | s 18.75 |
| OH-7 | --- | --- | --- | --- | bs 12.21 | --- |
| OH-9 | s 10.24 | s 10.29 | s 10.23 | s 10.24 | s 10.23 | s 10.22 |
| № | 17a | 17b | 17c | 17d | 17e | 17f |
|---|---|---|---|---|---|---|
| C-17 | 142.43 | 143.82 | 143.09 | 142.48 | 142.30 | 142.35 |
| C-18 | 125.64 | 125.43 | 125.30 | 125.01 | 124.84 | 125.12 |
| C-19 | 127.14 | 127.14 | 127.13 | 127.07 | 127.11 | 127.14 |
| C-20 | 126.07 | 125.93 | 126.00 | 126.04 | 126.20 | 126.28 |
| C-21 | 158.06 | 158.19 | 158.08 | 158.03 | 158.10 | 158.05 |
| C-22 | 110.26 | 110.21 | 110.14 | 110.25 | 110.41 | 110.34 |
| C-23 | 127.69 | 128.96 | 127.93 | 127.96 | 128.06 | 128.65 |
| C-24 | 65.26 | 65.91 | 65.88 | 65.32 | 65.16 | 65.22 |
| C-25 | 55.47 | 55.46 | 55.36 | 55.46 | 55.53 | 55.78 |
| C-26 | d 154.74 (J = 2.2 Hz) | d 146.61 (J = 10 Hz) | d 146.61 (J = 11 Hz) | d 154.81 (J = 2.4 Hz) | t 160.57 (J = 13.4 Hz) | d 150.14 (J = 2.4 Hz) |
| C-27 | d 115.56 (J = 12 Hz) | 115.50 | d 151.42 (J = 247 Hz) | 116.44 | d 162.68 (J = 244 Hz) | d 121.50 (J = 10 Hz) |
| C-28 | d 115.83 (J = 3.2 Hz) | d 151.84 (J = 249 Hz) | d 116 (J = 16 Hz) | 120.83 (J = 19 Hz) | t 96.47 (J = 29 Hz) | d 118.51 (J = 28 Hz) |
| C-29 | d 155.68 (J = 239 Hz) | d 116.00 (J = 19 Hz) | d 121.24 (J = 7 Hz) | d 151.54 (J = 247 Hz) | d 162.55 (J = 244 Hz) | d 156.08 (J = 247 Hz) |
| C-30 | d 115.83 (J = 3.2 Hz) | d 124.08 (J = 3.5 Hz) | d 124.10 (J = 3.4 Hz) | d 116.51 (J = 22 Hz) | m 98.50 | d 114.06 (J = 21 Hz) |
| C-31 | d 115.56 (J = 12 Hz) | d 121.25 (J = 8 Hz) | 115.41 | d 114.30 (J = 6.7 Hz) | m 98.50 | d 114.67 (J = 9.4 Hz) |

| № | 17g | 17h | 17i | 17j | 17k |
|---|---|---|---|---|---|
| H-14 | s 7.08 | s 7.07 | s 7.00 | s 6.98 | s 7.23 |
| H-17 | s 7.49 | s 7.69 | bs 7.98 | s 8.13 | s 7.26 |
| H-19 | s 7.32 | s 7.64 | bs 7.98 | s 8.05 | s 7.67 |
| H-22 | d 6.76 (J = 8.5 Hz) | m 6.76 | d 6.56 (J = 8.0 Hz) | d 6.46 (J = 8.1 Hz) | d 7.08 (J = 8.4 Hz) |
| H-23 | m 7.40 | d 7.51 (J = 8.3 Hz) | m 7.34 | m 6.84-7.03 | d 7.50 (J = 8.4 Hz) |
| H-24 | s 4.04 | s 4.99 | s 4.11 | s 4.19 | s 5.27 |
| H-25 | s 3.77 | s 3.84 | s 3.61 | bs 3.51 | s 3.85 |
| H-26 | s 3.29 | bs 3.51 | |||
| H-27 | s 1.22 (t-Bu) | d 6.88 (J = 2.4 Hz) | s 3.48 | m 3.85 | |
| H-28 | m 7.23 | s 2.34 (Me) | |||
| H-29 | m 7.08 | m 6.83–6.94 | m 6.84–7.03 | s 2.40 | |
| H-30 | s 2.33 (Me) | d 7.21 (J = 8.6 Hz) | m 6.83–6.94 | m 3.85 (OMe) | s 2.59 |
| H-31 | m 7.32 | d 6.83 (J = 8.5 Hz) | m 6.84–7.03 | ||
| H-32 | m 6.83–6.94 | m 6.84–7.03 | |||
| H-33 | m 6.83–6.94 | m 6.84–7.03 | |||
| NH | bs 9.09 | bs 9.56 | bs 11.22 | bs 11.22 | bs 12.28 |
| OH-3 | s 18.79 | s 18.79 | bs 18.7 | bs 18.76 | bs 18.80 |
| OH-7 | --- | --- | bs 12.64 | bs 12.63 | bs 12.77 |
| OH-9 | s 10.26 | s 10.27 | s 10.24 | s 10.23 | s 10.30 |
| № | 17g | 17h | 17i | 17j | 17k |
|---|---|---|---|---|---|
| C-17 | 142.74 | 143.61 | 141.77 | 141.03 | 142.40 |
| C-18 | 125.70 | 125.47 | 127.27 | 127.72 | 123.52 |
| C-19 | 127.77 | 127.19 | 129.01 | 129.77 | 126.09 |
| C-20 | 126.47 | 125.90 | 127.27 | 127.72 | 126.99 |
| C-21 | 158.61 | 158.08 | 158.70 | 158.36 | 157.16 |
| C-22 | 110.38 | 110.21 | 110.52 | 110.89 | 110.26 |
| C-23 | 128.79 | 129.46 | 131.17 | 131.96 | 128.05 |
| C-24 | 32.56 | 64.74 | 48.25 | 47.48 | 47.97 |
| C-25 | 55.50 | 55.45 | 55.35 | 55.26 | 55.41 |
| C-26 | 134.44 | 157.12 | 54.52 | 54.64 | 140.77 |
| C-27 | 149.06 (CAr) 31.09 (t-Bu) | 113.28 | 51.98 | 52.05 | 145.97 |
| C-28 | 129.57 | 127.92 20.25 (Me) | 146.65 | 151.77 | 130.90 |
| C-29 | 128.79 | 136.97 | d 115.45 (J = 22 Hz) | 139.11 (CAr) 54.96 (O-Me) | 11.32 |
| C-30 | 135.72 (CAr) 19.73 (Me) | 126.01 | d 118.53 (J = 7.43 Hz) | 110.89 | 14.07 |
| C-31 | 123.63 | 117.22 | d 156.02 (J = 240 Hz) | 118.75 | |
| C-32 | d 118.53 (J = 7.43 Hz) | 124.02 | |||
| C-33 | d 115.45 (J = 22 Hz) | 121.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filimonov, A.S.; Chepanova, A.A.; Luzina, O.A.; Zakharenko, A.L.; Zakharova, O.D.; Ilina, E.S.; Dyrkheeva, N.S.; Kuprushkin, M.S.; Kolotaev, A.V.; Khachatryan, D.S.; et al. New Hydrazinothiazole Derivatives of Usnic Acid as Potent Tdp1 Inhibitors. Molecules 2019, 24, 3711. https://doi.org/10.3390/molecules24203711
Filimonov AS, Chepanova AA, Luzina OA, Zakharenko AL, Zakharova OD, Ilina ES, Dyrkheeva NS, Kuprushkin MS, Kolotaev AV, Khachatryan DS, et al. New Hydrazinothiazole Derivatives of Usnic Acid as Potent Tdp1 Inhibitors. Molecules. 2019; 24(20):3711. https://doi.org/10.3390/molecules24203711
Chicago/Turabian StyleFilimonov, Aleksander S., Arina A. Chepanova, Olga A. Luzina, Alexandra L. Zakharenko, Olga D. Zakharova, Ekaterina S. Ilina, Nadezhda S. Dyrkheeva, Maxim S. Kuprushkin, Anton V. Kolotaev, Derenik S. Khachatryan, and et al. 2019. "New Hydrazinothiazole Derivatives of Usnic Acid as Potent Tdp1 Inhibitors" Molecules 24, no. 20: 3711. https://doi.org/10.3390/molecules24203711
APA StyleFilimonov, A. S., Chepanova, A. A., Luzina, O. A., Zakharenko, A. L., Zakharova, O. D., Ilina, E. S., Dyrkheeva, N. S., Kuprushkin, M. S., Kolotaev, A. V., Khachatryan, D. S., Patel, J., Leung, I. K. H., Chand, R., Ayine-Tora, D. M., Reynisson, J., Volcho, K. P., Salakhutdinov, N. F., & Lavrik, O. I. (2019). New Hydrazinothiazole Derivatives of Usnic Acid as Potent Tdp1 Inhibitors. Molecules, 24(20), 3711. https://doi.org/10.3390/molecules24203711










