Optimization of Flavonoid Extraction from Salix babylonica L. Buds, and the Antioxidant and Antibacterial Activities of the Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instruments and Reagents
2.2. Samples and Processing
2.3. Experimental Design
2.3.1. Single-Factor Experiments
2.3.2. Optimization of Extraction Conditions by Box–Behnken Design (BBD)
2.3.3. Determination of TFW Content
2.4. Component Analysis and Detection Condition
2.5. Antioxidant Capacity Assay
2.6. Antibacterial Capacity Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effects of Ethanol Volume Fraction on the Yield of TFW
3.2. Effects of Ultrasonic Extraction Time on the Yield of TFW
3.3. Effects of Solvent-to-Material Ratio on the Yield of TFW
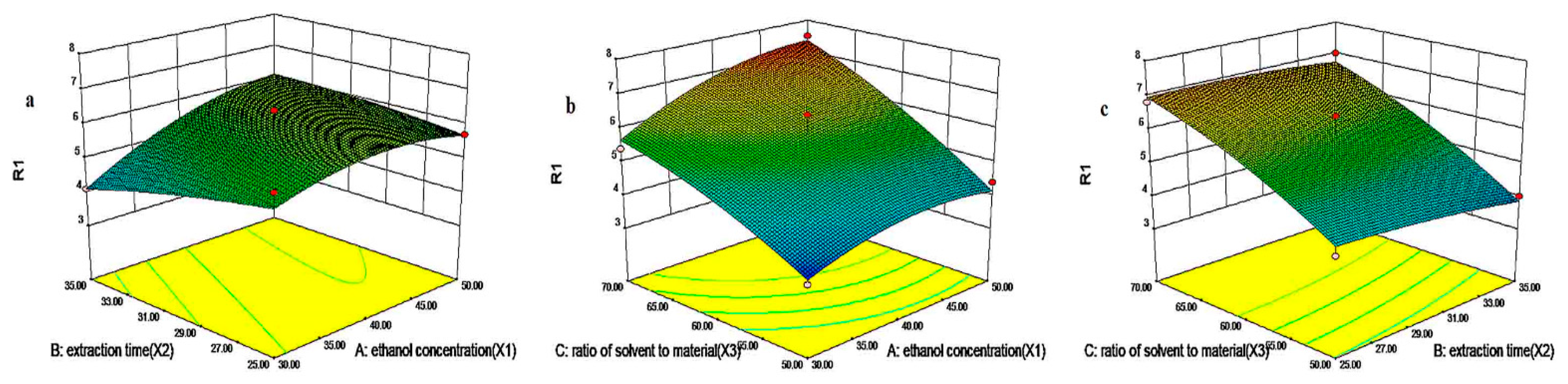
3.4. Optimization of Extraction Conditions by BBD
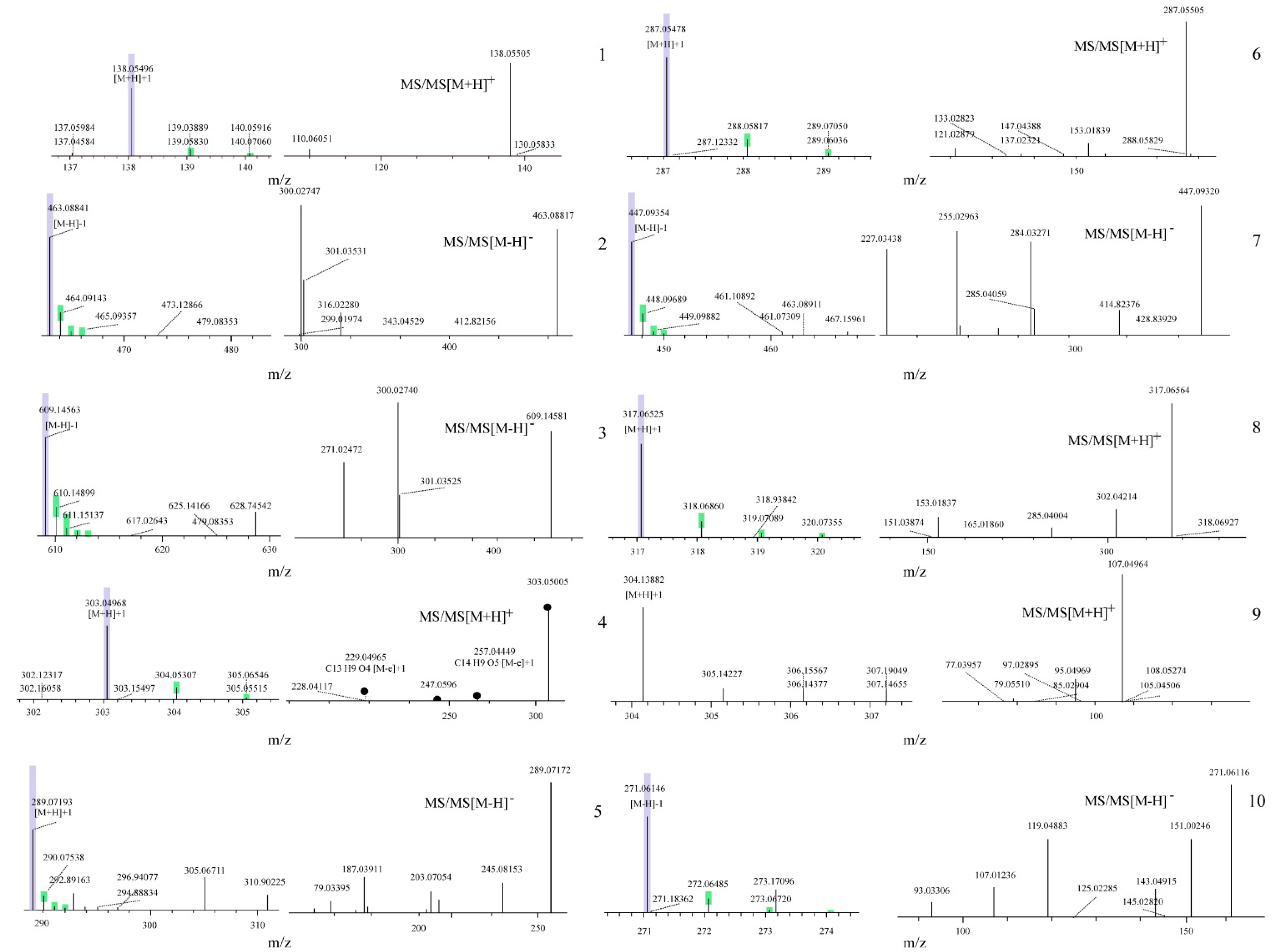
3.5. Chemical Component Analysis of TFW Extractives
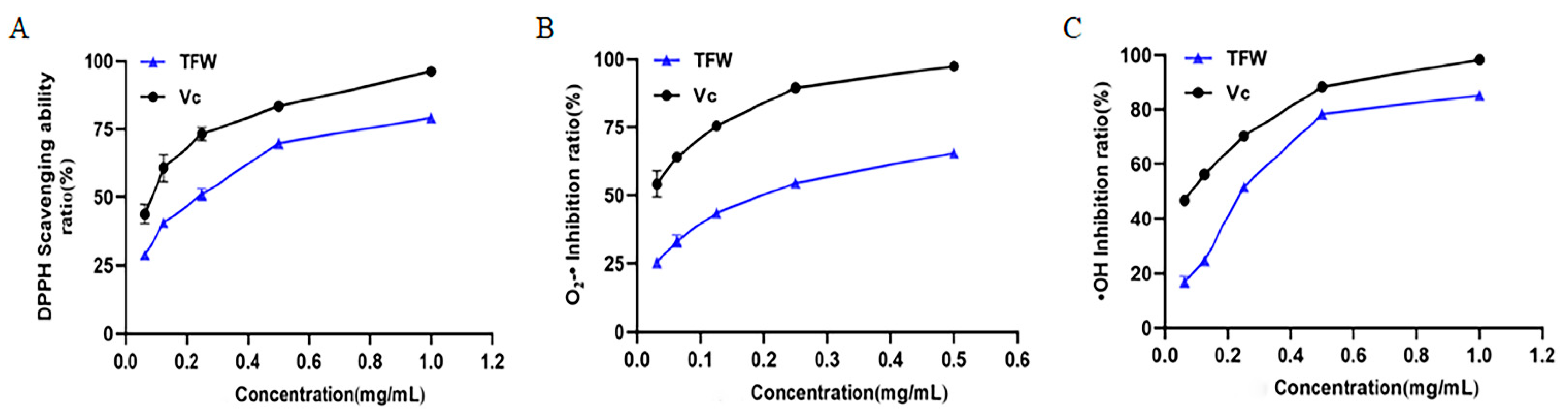
3.6. Antioxidant Capacity
3.7. Antibacterial Capacity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhang, H.; Wang, X.; He, D.; Zou, D.; Zhao, R.; Wang, H.; Li, S.; Xu, Y.; Abudureheman, B. Optimization of Flavonoid Extraction from Xanthoceras sorbifolia Bunge Flowers, and the Antioxidant and Antibacterial Capacity of the Extract. Molecules 2021, 27, 113. [Google Scholar] [CrossRef]
- Khalfallah, A.; Berrehal, D.; Bensouici, C.; Kabouche, A.; Semra, Z.; Voutquenne-Nazabadioko, L.; Alabdul Magid, A.; Kabouche, Z. Flavonoids, cytotoxic, antioxidant and antibacterial activities of Evax pygmaea. Pharm. Biol. 2017, 55, 2292–2296. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Chen, M.; Liu, T.; Zhang, P.; Sheng, J. Rhizoma drynariae total flavonoids inhibit the inflammatory re-sponse and matrix degeneration via MAPK pathway in a rat degenerative cervical intervertebral disc model. Biomed. Pharmacother. 2021, 138, 111466. [Google Scholar] [CrossRef] [PubMed]
- Catalkaya, G.; Venema, K.; Lucini, L.; Rocchetti, G.; Delmas, D.; Daglia, M.; Capanoglu, E. Interaction of dietary polyphenols and gut microbiota: Microbial metabolism of polyphenols, influence on the gut microbiota, and implications on host health. Food Front. 2020, 1, 109–133. [Google Scholar] [CrossRef]
- Raza, A.; Xu, X.; Xia, L.; Xia, C.; Tang, J.; Ouyang, Z. Quercetin-Iron Complex: Synthesis, Characterization, Antioxidant, DNA Binding, DNA Cleavage, and Antibacterial Activity Studies. J. Fluoresc. 2016, 26, 2023–2031. [Google Scholar] [CrossRef]
- Harasstani, O.A.; Tham, C.L.; Israf, D.A. Kaempferol and Chrysin Synergies to Improve Septic Mice Survival. Molecules 2017, 22, 92. [Google Scholar] [CrossRef]
- Shilpi, S.; Dubey, V.; Meena, A.; Siddiqui, L.; Maurya, A.K.; Luqman, S. Rutin restricts hydrogen peroxide-induced alterations by up- regulating the redox-system: An in vitro, in vivo and in silico study. Eur. J. Pharmacol. 2018, 835, 115–125. [Google Scholar] [CrossRef]
- Wahab, A.G.; Sallam, A.; Elgaml, A.; Lahloub, M.F.; Afif, M.S. Antioxidant and antimicrobial activities of Salix babylonica extracts. World. Pharm. Sci. 2018, 6, 1–6. [Google Scholar]
- Shakibaei, M.; Allaway, D.; Nebrich, S.; Mobasheri, A. Botanical Extracts from Rosehip (Rosa canina), Willow Bark (Salix alba), and Nettle Leaf (Urtica dioica) Suppress IL-1β-Induced NF-κB Activation in Canine Articular Chondrocytes. Evid. Based Complement. Altern. Med. 2012, 2012, 1–16. [Google Scholar] [CrossRef]
- Rivero-Perez, N.; Hernández-Alvarado, J.L.; Valladares-Carranza, B.; Delgadillo-Ruiz, L.; Ojeda-Ramírez, D.; Sosa-Gutiérrez, C.G.; Morales-Ubaldo, A.L.; Vega-Sanchez, V.; Zaragoza-Bastida, A. Salix babylonica L. as a Natural Anticoccidial Alternative in Growing Rabbits. Evid. Based Complement. Altern. Med. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- González-Alamilla, E.N.; Gonzalez-Cortazar, M.; Valladares-Carranza, B.; Rivas-Jacobo, M.A.; Herrera-Corredor, C.A.; Ojeda-Ramírez, D.; Zaragoza-Bastida, A.; Rivero-Perez, N. Chemical Constituents of Salix babylonica L. and Their Antibacterial Activity Against Gram-Positive and Gram-Negative Animal Bacteria. Molecules 2019, 24, 2992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salem, A.Z.M.; Salem, M.Z.; Gonzalez-Ronquillo, M.; Camacho, L.M.; Cipriano, M. Major chemical constituents of Leucaena leucocephala and Salix babylonica leaf extracts. J. Trop. Agric 2011, 49, 95–98. [Google Scholar]
- Tian, C.; Zhang, P.; Yang, C.; Gao, X.; Wang, H.; Guo, Y.; Liu, M. Extraction Process, Component Analysis, and In Vitro Antioxidant, Antibacterial, and Anti-Inflammatory Activities of Total Flavonoid Extracts from Abutilon theophrasti Medic. Leaves. Mediat. Inflamm. 2018, 2018, 1–17. [Google Scholar] [CrossRef]
- Qiao, Z.; Han, L.; Liu, X.; Dai, H.; Liu, C.; Yan, M.; Li, W.; Han, W.; Li, X.; Huang, S.; et al. Extraction, Radical Scavenging Activities, and Chemical Composition Identification of Flavonoids from Sunflower (Helianthus annuus L.) Receptacles. Molecules 2021, 26, 403. [Google Scholar] [CrossRef] [PubMed]
- Llorent-Martínez, E.J.; Zengin, G.; Sinan, K.I.; Polat, R.; Canlı, D.; Picot-Allain, M.C.N.; Mahomoodally, M.F. Impact of different extraction solvents and techniques on the biological activities of Cirsium yildizianum (Asteraceae: Cynareae). Ind. Crop. Prod 2020, 144, 112033. [Google Scholar] [CrossRef]
- Wei, Q.; Yue, W.; Lyu, K.B.; Yang, W.T.; Zhu, W.T. Study on extracting technologyby orthogonal design and antibacterial activity of procyanidinsfrom chestnut shells. Food Ferment. Ind. 2016, 42, 214–219. [Google Scholar]
- Ghasemzadeh, A.; Baghdadi, A.; Jaafar, H.; Swamy, K.M.; Wahab, M.E.P. Optimization of flavonoid extraction from red and brown rice bran and evaluation of the antioxidant properties. Molecules 2018, 23, 1863. [Google Scholar] [CrossRef]
- Krongrawa, W.; Limmatvapirat, S.; Saibua, S.; Limmatvapirat, C. Optimization of Ultrasound-Assisted Extraction of Yields and Total Methoxyflavone Contents from Kaempferia parviflora Rhizomes. Molecules 2022, 27, 4162. [Google Scholar] [CrossRef]
- Czyrski, A.; Sznura, J. The application of Box-Behnken-Design in the optimization of HPLC separation of fluoroquinolones. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Song, H.; Moon, E.; Ha, J.H. Application of response surface methodology based on a box-behnken design to determine optimal parameters to produce brined cabbage used in Kimchi. Foods 2021, 10, 1935. [Google Scholar] [CrossRef]
- Weber, L.; Hammoud, M.D.; Jankuhn, S.; Lipowicz, B.; Vissiennon, C. Bioactive Plant Compounds in Coffee Charcoal (Coffeae carbo) Extract Inhibit Cytokine Release from Activated Human THP-1 Macrophages. Molecules 2019, 24, 4263. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Xu, L.; Hu, H.; Yang, Y.; Zhang, X.; Peng, Y.; Xiao, P. DPPH Radical Scavenging and Postprandial Hyperglycemia Inhibition Activities and Flavonoid Composition Analysis of Hawk Tea by UPLC-DAD and UPLC-Q/TOF MSE. Molecules 2017, 22, 1622. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhu, M.; Wang, K.; Yang, E.; Su, J.; Wang, Q.; Cheng, N.; Xue, X.; Wu, L.; Cao, W. Identification and quantitation of bioactive components from honeycomb (Nidus Vespae). Food Chem. 2019, 314, 126052. [Google Scholar] [CrossRef] [PubMed]
- Abreu, I.N.; Ahnlund, M.; Moritz, T.; Albrectsen, B.R. UHPLC-ESI/TOFMS determination of salicylate-like phenolic gycosides in Populus tremula leaves. J. Chem. Ecol. 2011, 37, 857–870. [Google Scholar] [CrossRef]
- Rodríguez-Valdovinos, K.Y.; Salgado-Garciglia, R.; Vázquez-Sánchez, M.; Álvarez-Bernal, D.; Oregel-Zamudio, E.; Ceja-Torres, L.F.; Medina-Medrano, J.R. Quantitative Analysis of Rutin by HPTLC and In Vitro Antioxidant and Antibacterial Activities of Phenolic-Rich Extracts from Verbesina sphaerocephala. Plants 2021, 10, 475. [Google Scholar] [CrossRef]
- Veiko, A.G.; Lapshina, E.A.; Zavodnik, I.B. Comparative analysis of molecular properties and reactions with oxidants for quercetin, catechin, and naringenin. Mol. Cell Biochem. 2021, 476, 4287–4299. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.Y.; Liao, P.R.; Zhao, M.Z.; Gong, C.; Dang, Y.; Qu, Y.; Qiu, L.S. Optimization of Ultrasonic Flavonoid Extraction from Saussurea involucrate, and the Ability of Flavonoids to Block Melanin Deposition in Human Melanocytes. Molecules 2020, 25, 313. [Google Scholar] [CrossRef]
- Yin, X.S.; Zhang, X.Q.; Li, D.Q. Simultaneous optimization of ultrasound-assisted extraction of antioxidants and tyrosinase inhibitory activities of Semen Oroxyli flavonoids using response surface methodology. J. Food Meas. Charact. 2020, 14, 694–707. [Google Scholar] [CrossRef]
- Li, B.; Li, Y.; Hu, Q. Antioxidant activity of flavonoids from tartary buckwheat bran. Toxicol. Environ. Chem. 2015, 98, 1–16. [Google Scholar] [CrossRef]
- Piątczak, E.; Dybowska, M.; Płuciennik, E.; Kośla, K.; Kolniak-Ostek, J.; Kalinowska-Lis, U. Identification and accumulation of phenolic compounds in the leaves and bark of Salix alba (L.) and their biological potential. Biomolecules 2020, 10, 1391. [Google Scholar] [CrossRef]
- Kim, M.H. Antioxidant Activity and Anti-inflammatory Effects of Salix Koreensis Andersson Branches Extracts. J. Korean Soc. Food Cult. 2016, 6, 28.1–28.6. [Google Scholar] [CrossRef]
- Izu, A.; Solomon, F.; Nzenze, S.A.; Mudau, A.; Zell, E.; O’Brien, L.K.; Whitney, G.C.; Verani, J.; Groome, M.; Madhi, A.S. Pneumococcal conjugate vaccines and hospitalization of children for pneumonia: A time-series analysis, South Africa, 2006–2014. Bull. World Health Organ. 2017, 95, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Van der Poll, T.; Opal, S.M. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet 2009, 374, 1543–1556. [Google Scholar] [CrossRef]
- Machado, I.; Garrido, V.; Hernandez, I.L.; Botero, J.; Bastida, N.; San-Roman, B.; Grilló, M.J.; Hernandez, J.F. Rapid and specific detection of Salmonella infections using chemically modified nucleic acid probes. Anal. Chim. Acta 2018, 1054, 157–166. [Google Scholar] [CrossRef]
- Ramadhani, S.; Santoni, A.; Mai, E. Antibacterial Activity and Structure Elucidation of Salicin from Stem Bark of Salix tetrasperma ROXB. Indones. J. Fundam. Appl. Chem. 2019, 4, 47–52. [Google Scholar] [CrossRef]
- González-Alamilla, E.; Rivas-Jacobo, M.; Sosa-Gutiérrez, C.; Delgadillo-Ruiz, L.; Valladares-Carranza, B.; Rosenfeld-Miranada, C.; Zaragoza-Bastida, A.; Rivero-Pérez, N. Efecto antibacteriano del extracto metanolico de Salix babylonica sobre bacterias de importancia en salud pública. Abanico Vet. 2020, 10, 1–11. [Google Scholar]

| Levels | Ethanol Concentration (X1)/(%) | Extraction Time (X2)/(min) | Ratio of Solvent to Material (X3)/(mL/g) |
|---|---|---|---|
| –1 | 30 | 25 | 50 |
| 0 | 40 | 30 | 60 |
| 1 | 50 | 35 | 70 |
| No. | Independent Variable Levels | Response | ||
|---|---|---|---|---|
| Ethanol Concentration (X1)/(%) | Extraction Time (X2)/(min) | Ratio of Solvent to Material (X3)/(mL/g) | ||
| 1 | 30 | 25 | 60 | 5.8 |
| 2 | 50 | 35 | 60 | 5.7 |
| 3 | 40 | 30 | 60 | 5.8 |
| 4 | 50 | 25 | 60 | 5.7 |
| 5 | 40 | 25 | 70 | 6.8 |
| 6 | 40 | 35 | 70 | 7.0 |
| 7 | 30 | 30 | 50 | 3.4 |
| 8 | 30 | 35 | 60 | 4.1 |
| 9 | 50 | 30 | 50 | 4.4 |
| 10 | 40 | 30 | 60 | 6.4 |
| 11 | 40 | 25 | 50 | 4.2 |
| 12 | 30 | 30 | 70 | 5.4 |
| 13 | 40 | 30 | 60 | 5.5 |
| 14 | 50 | 30 | 70 | 7.5 |
| 15 | 40 | 30 | 60 | 5.7 |
| 16 | 40 | 35 | 50 | 4 |
| 17 | 40 | 30 | 60 | 5.8 |
| Time (min) | A (%) | B (%) |
|---|---|---|
| 0 | 98 | 2 |
| 1 | 98 | 2 |
| 5 | 80 | 20 |
| 10 | 50 | 50 |
| 15 | 20 | 80 |
| 20 | 5 | 95 |
| 25 | 5 | 95 |
| 26 | 98 | 2 |
| 30 | 98 | 2 |
| Variables | Sum of Squares | DF | Mean Square | F Value | p-Value |
|---|---|---|---|---|---|
| Mode | 19.5 | 9 | 2.17 | 13.02 | 0.0014 |
| X1 | 2.65 | 1 | 2.65 | 15.90 | 0.0053 |
| X2 | 0.36 | 1 | 0.36 | 2.17 | 0.1841 |
| X3 | 14.31 | 1 | 14.31 | 86.03 | <0.0001 |
| X1*X2 | 0.72 | 1 | 0.72 | 4.34 | 0.0756 |
| X1*X3 | 0.30 | 1 | 0.30 | 1.82 | 0.2195 |
| X2*X3 | 0.040 | 1 | 0.040 | 0.24 | 0.6389 |
| X12 | 0.74 | 1 | 0.74 | 4.46 | 0.0725 |
| X22 | 0.038 | 1 | 0.038 | 0.23 | 0.6473 |
| X32 | 0.25 | 1 | 0.25 | 1.52 | 0.2575 |
| Residual | 1.16 | 7 | 0.17 | ||
| Lack of Fit | 0.71 | 3 | 0.24 | 2.10 | 0.2428 |
| Pure Error | 0.45 | 4 | 0.11 | ||
| Cor total | 20.66 | 16 | |||
| R2 | 0.9436 | ||||
| RAdj2 | 0.8712 | ||||
| RPred2 | 0.4142 | ||||
| Adeq Precision | 12.227 | ||||
| C.V.% | 7.44 |
| No. | Rt (min) | [M–H]– | MS/MS [M–H]– | [M+H]– | MS/MS [M+H]– | Calculated Mass | Formula | Proposed Molecule | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1c | 2.61 | _ | _ | 138.1 | 110.1, 139.1 | 137.0 | C7H7NO2 | trigonelline | [21] |
| 2a | 5.22 | 463.1 | 299.0, 301.0, 300.0 | _ | _ | 464.1 | C21H20O12 | isoquercitrin | [22] |
| 3a | 5.66 | 609.1 | 300.0, 301.0, 271.0 | _ | _ | 610.2 | C27H30O16 | rutin | [23] |
| 4a | 5.67 | _ | _ | 303.0 | 257.0, 229.1, 247.1 | 302.0 | C15H10O7 | quercetin | [22] |
| 5a | 5.79 | 289.1 | 203.1, 245.1_ | _ | _ | 290.1 | C15H14O6 | catechin | [22] |
| 6a | 5.91 | _ | _ | 287.1 | 121.0, 153.0 | 286.0 | C15H10O6 | kaempferol | [23] |
| 7a | 6.35 | 447.1 | 284.0, 255.0, 227.0 | _ | _ | 448.1 | C21H20O11 | astragalin | [22] |
| 8a | 6.73 | _ | _ | 317.1 | 302.0, 153.0, 285.0 | 316.1 | C16H12O7 | isorhamnetin | [23] |
| 9b | 7.25 | _ | _ | 304.1 | 107.1 | 286.1 | C13H18O7 | salicin | [24] |
| 10a | 7.58 | 271.1 | 93.0, 107.0, 119.0, 151.0 | _ | _ | 272.1 | C15H12O5 | naringenin | [23] |
| Indicators | Antioxidants | R2 of Linear Fit | IC50 (mg/mL) |
|---|---|---|---|
| DPPH | TFW | 0.9941 | 0.20 |
| VC | 0.9921 | 0.08 | |
| O2–· | TFW | 0.9911 | 0.18 |
| VC | 0.9931 | 0.03 | |
| ·OH | TFW | 0.9951 | 0.24 |
| VC | 0.9982 | 0.08 |
| Bacterial Strain | Doxycycline (µg/mL) | Levofloxacin (µg/mL) | TFW (mg/mL) |
|---|---|---|---|
| Escherichia coli (ATCC25922) | 0.5 | 0.0625 | 5 |
| Streptococcus pneumoniae (ATCC49619) | 0.0625 | 2 | 5 |
| Staphylococcus aureus (ATCC29213) | 0.25 | 0.25 | 2.5 |
| Salmonella enterica (ATCC 51812) | 8 | 4 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Song, Y.; Wang, H.; Fu, Y.; Zhang, Y.; Pavlovna, K.I. Optimization of Flavonoid Extraction from Salix babylonica L. Buds, and the Antioxidant and Antibacterial Activities of the Extract. Molecules 2022, 27, 5695. https://doi.org/10.3390/molecules27175695
Zhang P, Song Y, Wang H, Fu Y, Zhang Y, Pavlovna KI. Optimization of Flavonoid Extraction from Salix babylonica L. Buds, and the Antioxidant and Antibacterial Activities of the Extract. Molecules. 2022; 27(17):5695. https://doi.org/10.3390/molecules27175695
Chicago/Turabian StyleZhang, Peng, Yuwen Song, Hongling Wang, Yujie Fu, Yingying Zhang, and Korotkova Irina Pavlovna. 2022. "Optimization of Flavonoid Extraction from Salix babylonica L. Buds, and the Antioxidant and Antibacterial Activities of the Extract" Molecules 27, no. 17: 5695. https://doi.org/10.3390/molecules27175695
APA StyleZhang, P., Song, Y., Wang, H., Fu, Y., Zhang, Y., & Pavlovna, K. I. (2022). Optimization of Flavonoid Extraction from Salix babylonica L. Buds, and the Antioxidant and Antibacterial Activities of the Extract. Molecules, 27(17), 5695. https://doi.org/10.3390/molecules27175695






