Design, Synthesis, Antitumor, and Antiplasmodial Evaluation of New 7-Chloroquinoline–Benzimidazole Hybrids
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
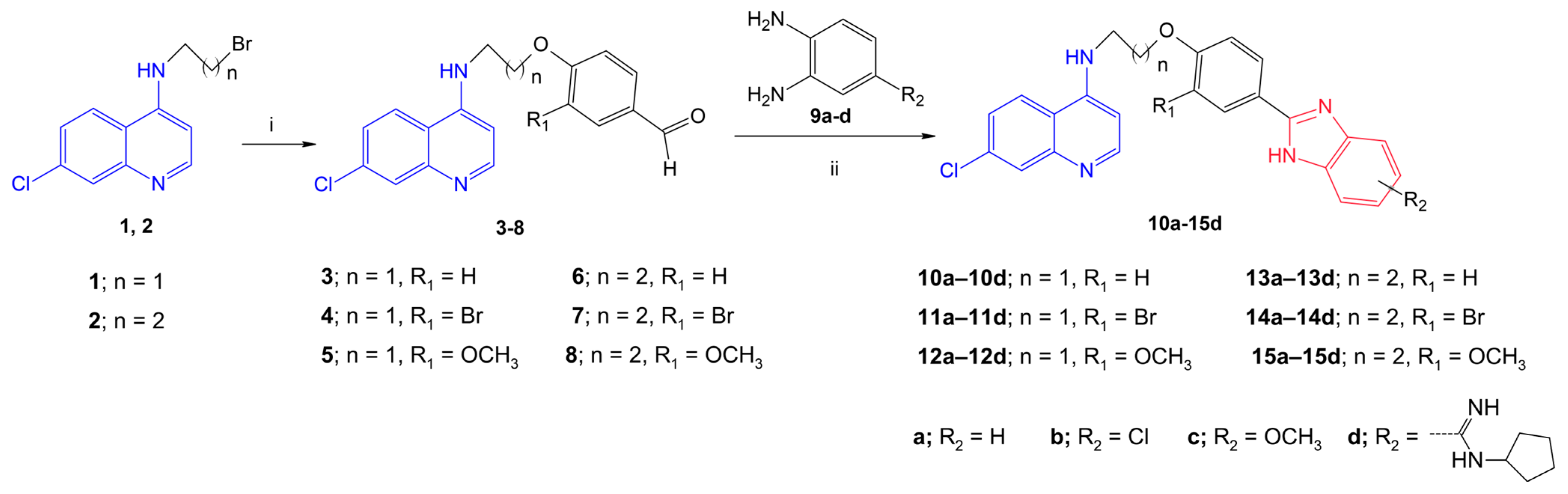
Design and Synthesis of New 7-Chloro-4-aminoquinoline–benzimidazoles
2.2. Biological Activity
2.2.1. Evaluation of Antiproliferative Activity of the Novel Compounds on Human Cells In Vitro
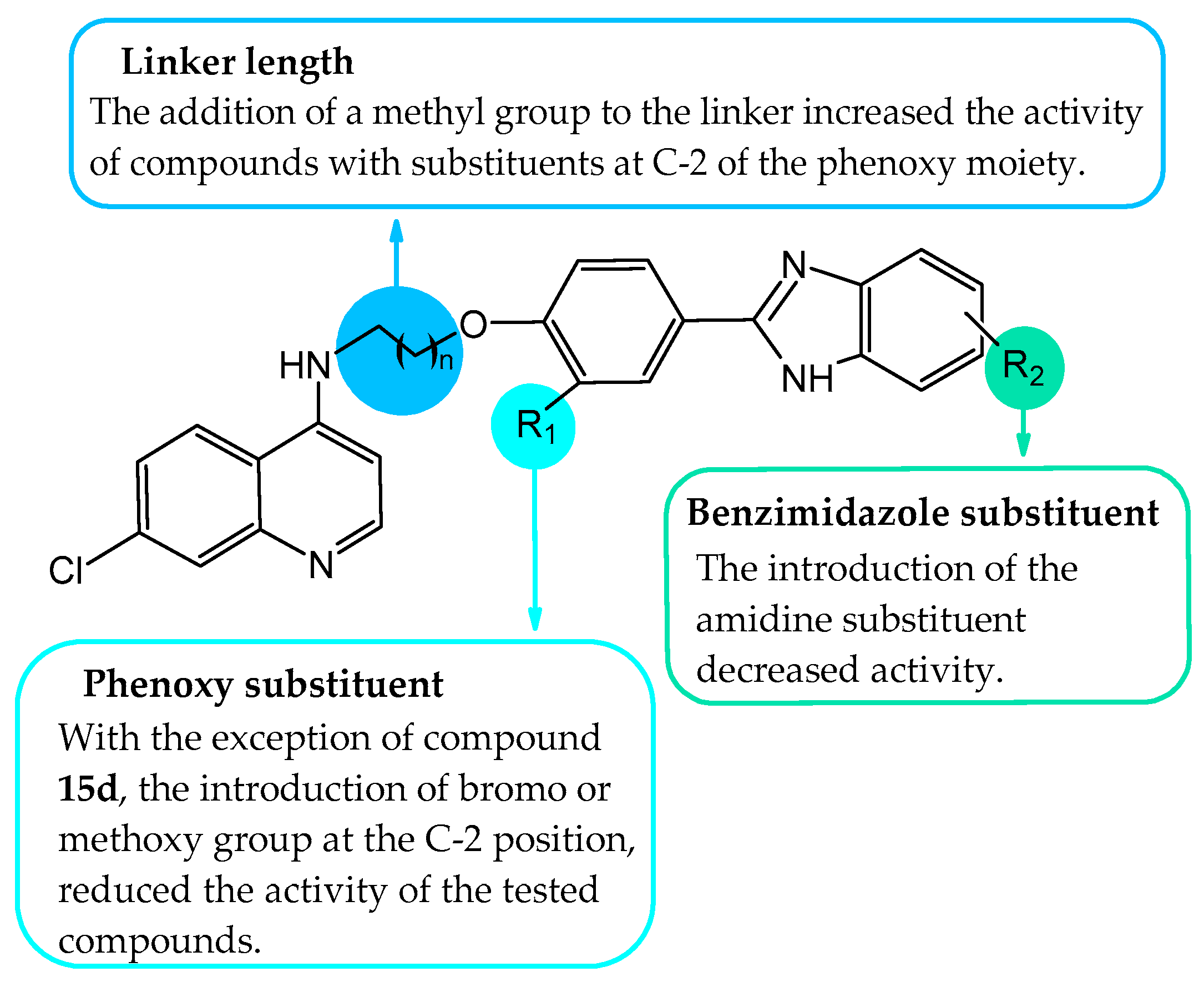
2.2.2. Evaluation of Antiplasmodial Activity via In Vitro and SAR Analysis
2.3. QSAR Models for Antiplasmodial Activities
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure for the Synthesis of Compounds 4 and 6–8
- 3-bromo-4-{2-[(7-chloroquinolin-4-yl)amino]ethoxy}benzaldehyde (4)
- 4-{3-[(7-chloroquinolin-4-yl)amino]propoxy}benzaldehyde (6)
- 3-bromo-4-{3-[(7-chloroquinolin-4-yl)amino]propoxy}benzaldehyde (7)
- 4-{3-[(7-chloroquinolin-4-yl)amino]propoxy}-3-methoxybenzaldehyde (8)
3.1.2. General procedure for the synthesis of compounds 10a–15d
- N-{2-[4-(1H-benzo[d]imidazole-2-yl)phenoxy]ethyl)}-7-chloroquinolin-4-amine (10a)
- 7-chloro-N-{2-[4-(5(6)-chloro-1H-benzo[d]imidazole-2-yl)phenoxy]ethyl}quinolin-4-amine (10b)
- 7-chloro-N-{2-[4-(5(6)-methoxy-1H-benzo[d]imidazole-2-yl)phenoxy]ethyl}quinolin-4-amine (10c)
- N-{2-[4-(1H-benzo[d]imidazole-2-yl)-2-bromophenoxy]ethyl}-7-chloroquinolin-4-amine (11a)
- N-{[2-bromo-4-(5(6)-chloro-1H-benzo[d]imidazole-2-yl)phenoxy]ethyl}-7-chloroquinolin-4-amine (11b)
- N-{[2-bromo-4-(5(6)-methoxy-1H-benzo[d]imidazole-2-yl)phenoxy]ethyl}-7-chloroquinolin-4-amine (11c)
- 2-{3-bromo-4-[2-(7-chloroquinolin-4-ylamino)ethoxy]phenyl}-N-cyclopentyl-1H-benzo[d]imidazole-5(6)-carboximidamide trihydrochloride (11d)
- N-{[4-(1H-benzo[d]imidazole-2-yl)-2-methoxyphenoxy]ethyl}-7-chloroquinolin-4-amine (12a)
- 7-chloro-N-{[4-(5(6)-chloro-benzo[d]imidazole-2-yl)-2-methoxyphenoxy]ethyl}quinolin-4-amine (12b)
- 7-chloro-N-{[4-(5(6)-methoxy-benzo[d]imidazole-2-yl)-2-methoxyphenoxy]ethyl}quinolin-4-amine (12c)
- 2-{3-methoxy-4-[2-(7-chloroquinolin-4-ylamino)ethoxy]phenyl}-N-cyclopentyl-1H-benzo[d]imidazole-5(6)-carboximidamide trihydrochloride (12d)
- N-{2-[4-(1H-benzo[d]imidazole-2-yl)phenoxy]propyl)}-7-chloroquinolin-4-amine (13a)
- 7-chloro-N-{2-[4-(5(6)-chloro-1H-benzo[d]imidazole-2-yl)phenoxy]propyl}quinolin-4-amine (13b)
- 7-chloro-N-{2-[4-(5(6)-methoxy-1H-benzo[d]midazole-2-yl)phenoxy]propyl}quinolin-4-amine (13c)
- 2-{4-[2-(7-chloroquinolin-4-ylamino)propoxy]phenyl}-N-cyclopentyl-1H-benzo[d]imidazole-5(6)-carboximidamide trihydrochloride (13d)
- N-{2-[4-(1H-benzo[d]imidazole-2-yl)-2-bromophenoxy]propyl}-7-chloroquinolin-4-amine (14a)
- N-{[2-bromo-4-(5(6)-chloro-1H-benzo[d]imidazole-2-yl)phenoxy]propyl}-7-chloroquinolin-4-amine (14b)
- N-{[2-bromo-4-(5(6)-methoxy-1H-benzo[d]imidazole-2-yl)phenoxy]propyl}-7-chloroquinolin-4-amine (14c)
- 2-{3-bromo-4-[2-(7-chloroquinolin-4-ylamino)propoxy]phenyl}-N-cyclopentyl-1H-benzo[d]imidazole-5(6)-carboximidamide trihydrochloride (14d)
- N-{[4-(1H-benzo[d]imidazole-2-yl)-2-methoxyphenoxy]propyl}-7-chloroquinolin-4-amine (15a)
- 7-chloro-N-{[4-(5(6)-chloro-benzo[d]imidazole-2-yl)-2-methoxyphenoxy]propyl}quinolin-4-amine (15b)
- 7-chloro-N-{[4-(5(6)-methoxy-benzo[d]imidazole-2-yl)-2-methoxyphenoxy]propyl}quinolin-4-amine (15c)
- 2-{3-methoxy-4-[2-(7-chloroquinolin-4-ylamino)propoxy]phenyl}-N-cyclopentyl-1H-benzo[d]imidazole-5(6)-carboximidamide trihydrochloride (15d)
3.2. Biological Activity
3.2.1. Evaluation of the Antiproliferative Activity of the Novel Compounds on Human Cells
Cell Lines and Cell Culturing
Proliferation Assay
3.2.2. Evaluation of Activity against Erythrocytic Stages of P. falciparum
3.3. QSAR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Snow, R.W.; Guerra, C.A.; Noor, A.M.; Myint, H.Y.; Hay, S.I. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 2005, 434, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.J.; Lucas, T.C.; Nguyen, M.; Nandi, A.K.; Bisanzio, D.; Battle, K.E.; Cameron, E.; Twohig, K.A.; Pfeffer, D.A.; Rozier, J.A.; et al. Mapping the global prevalence, incidence, and mortality of Plasmodium falciparum, 2000–17: A spatial and temporal modelling study. Lancet 2019, 394, 322–331. [Google Scholar] [CrossRef]
- World Health Organization. World Malaria Report. December 2022. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023 (accessed on 10 April 2024).
- Ippolito, M.M.; Moser, K.A.; Kabuya, J.-B.B.; Cunningham, C.; Juliano, J.J. Antimalarial Drug Resistance and Implications for the WHO Global Technical Strategy. Curr. Epidemiol. Rep. 2021, 8, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Nocht; Werner, H. Beobachtungen über relative Chininresistenz bei Malaria aus Brasilien. Dtsch. Med. Wochenschrift. 1910, 36, 1557–1560. [Google Scholar] [CrossRef][Green Version]
- Moore, D.V.; Lanier, J.E. Observations on two Plasmodium falciparum infections with an abnormal response to chloroquine. Am. J. Trop. Med. Hyg. 1961, 10, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, E.F.; Webster, H.K.; Pavanand, K.; Thosingha, L. Type II mefloquine resistance in thailand. Lancet 1982, 320, 1335. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.-N.; Ma, N.; Meng, Y.; Zhang, X.; Wong, Y.-K.; Xu, C.; Liao, F.; Jiang, T.; Tu, Y.; Wang., J. Study towards improving artemisinin-based combination therapies. Nat. Prod. Rep. 2021, 38, 1229–1412. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Su, X.-z. Discovery, mechanisms of action and combination therapy of artemisinin. Expert Rev. Anti Infect. Ther. 2009, 7, 999–1013. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, F.A.; Liang, X.; Cui, L. Plasmodium falciparum resistance to ACTs: Emergence, mechanisms, and outlook. Int. J. Parasitol. Drugs Drug Resist. 2021, 16, 102–118. [Google Scholar] [CrossRef]
- Conrad, M.D.; Asua, V.; Garg, S.; Giesbrecht, D.; Niaré, K.; Smith, S.; Namuganga, J.F.; Katairo, T.; Legac, J.; Crudale, R.M.; et al. Evolution of Partial Resistance to Artemisinins in Malaria Parasites in Uganda. N. Engl. J. Med. 2023, 389, 722–732. [Google Scholar] [CrossRef]
- Dhorda, M.; Chanaki, A.; Dondorp, A.M. Artemisinin and multidrug-resistant Plasmodium falciparum—A threat for malaria control and elimination. Curr. Opin. Infect. Dis. 2021, 34, 432–439. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Pomeroy, A.E.; Schmidt, E.V.; Sorger, P.K.; Palmer, A.C. Drug independence and the curability of cancer by combination chemotherapy. Trends Cancer 2022, 8, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Gilad, Y.; Gellerman, G.; Lonard, D.M.; O’Malley, B.W. Drug Combination in Cancer Treatment—From Cocktails to Conjugated Combinations. Cancers 2021, 13, 669. [Google Scholar] [CrossRef] [PubMed]
- Nussinov, R.; Tsai, C.J.; Jang, H. Anticancer Drug Resistance: An Update and Perspective. Drug Resist. Updat. 2021, 59, 100796. [Google Scholar] [CrossRef]
- Emran, T.B.; Shahriar, A.; Mahmud, A.R.; Rahman, T.; Abir, M.H.; Siddiquee, M.F.; Ahmed, H.; Rahman, N.; Nainu, F.; Wahyudin, E.; et al. Multidrug Resistance in Cancer: Understanding Molecular Mechanisms, Immunoprevention and Therapeutic Approaches. Front. Oncol. 2022, 12, 891652. [Google Scholar] [CrossRef] [PubMed]
- Krstulović, L.; Stolić, I.; Jukić, M.; Opačak-Bernardi, T.; Starčević, K.; Bajić, M.; Glavaš-Obrovac, L. New quinoline-arylamidine hybrids: Synthesis, DNA/RNA binding and antitumor activity. Eur. J. Med. Chem. 2017, 137, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Krstulović, L.; Leventić, M.; Rastija, V.; Starčević, K.; Jirouš, M.; Janić, I.; Karnaš, M.; Lasić, K.; Bajić, M.; Glavaš-Obrovac, L. Novel 7-Chloro-4-aminoquinoline-benzimidazole Hybrids as Inhibitors of Cancer Cells Growth: Synthesis, Antiproliferative Activity, In Silico ADME Predictions, and Docking. Molecules 2023, 28, 540. [Google Scholar] [CrossRef]
- Krstulović, L.; Mišković Špoljarić, K.; Rastija, V.; Filipović, N.; Bajić, M.; Glavaš-Obrovac, L. Novel 1,2,3-Triazole-Containing Quinoline–Benzimidazole Hybrids: Synthesis, Antiproliferative Activity, In Silico ADME Predictions, and Docking. Molecules 2023, 28, 6950. [Google Scholar] [CrossRef]
- Gonzalez, S.; Dumitrascuta, M.; Eiselt, E.; Louis, S.; Kunze, L.; Blasiol, A.; Vivancos, M.; Previti, S.; Dewolf, E.; Martin, C.; et al. Optimized Opioid-Neurotensin Multitarget Peptides: From Design to Structure–Activity Relationship Studies. J. Med. Chem. 2020, 63, 12929–12941. [Google Scholar] [CrossRef]
- Tiglani, D.; Salahuddin; Mazumder, A.; Yar, M.S.; Kumar, R.; Ahsan, M.J. Benzimidazole-Quinoline Hybrid Scaffold as Promising Pharmacological Agents: A Review. Polycycl. Aromat. Comp. 2022, 42, 5044–5066. [Google Scholar] [CrossRef]
- Marinho, J.A.; Guimaras, D.S.M.; Glanzmann, N.; Almeida Pimentel, G.; da Costa Nunes, I.K.; Pereira, H.M.G.; Navarro, M.; de Pilla Varotti, F.; da Silva, A.D.; Abramo, C. In vitro and in vivo antiplasmodial activity of novel quinoline derivative compounds by molecular hybridization. Eur. J. Med. Chem. 2021, 215, 113271. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Kumar, A.; Singh, H.; Sonawane, P.; Paliwal, H.; Thareja, S.; Pathak, P.; Grishina, M.; Jaremko, M.; Emwas, A.H.; et al. Concept of Hybrid Drugs and Recent Advancements in Anticancer Hybrids. Pharmaceuticals 2022, 15, 1071. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.-S.; Xu, Z.; Chang, L.; Li., C.; Yan, X.-F.; Gao, C.; Ding, C.; Zhao, F.; Shi, F.; Xiang, W. Hybrid molecules with potential in vitro antiplasmodial and in vivo antimalarial activity against drug-resistant Plasmodium falciparum. Med. Res. Rev. 2020, 40, 931–971. [Google Scholar] [CrossRef] [PubMed]
- Basavarajaiah, S.M. The Versatile Quinoline and Its Derivatives as anti-Cancer Agents: An Overview. Polycycl. Aromat. Comp. 2022, 43, 4333–4345. [Google Scholar] [CrossRef]
- Saxena, A.; Majee, S.; Ray, D.; Saha, B. Inhibition of cancer cells by Quinoline-Based compounds: A review with mechanistic insights. Bioorg. Med. Chem. 2024, 103, 117681. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, N.; Shepherd, S.; Mohammed, K.; Lee, K.A.; Allen, M.; Johnston, S.; Kipps, E.; McGrath, S.; Noble, J.; Parton, M.; et al. Neratinib in advanced HER2-positive breast cancer: Experience from the royal Marsden hospital. Breast Cancer Res. Treat. 2022, 195, 333–340. [Google Scholar] [CrossRef]
- Al-Salama, Z.T.; Syed, Y.Y.; Scott, L.J. Lenvatinib: A Review in Hepatocellular Carcinoma. Drugs 2019, 79, 665–674. [Google Scholar] [CrossRef]
- Gambacorti-Passerini, C.; Le Coutre, P.; Piazza, R. The role of bosutinib in the treatment of chronic myeloid leukemia. Future Oncol. 2019, 16, 4395–4408. [Google Scholar] [CrossRef]
- Maroto, P.; Porta, C.; Capdevila, J.; Apolo, A.B.; Viteri, S.; Rodriguez-Antona, C.; Martin, L.; Castellano, D. Cabozantinib for the treatment of solid tumors: A systematic review. Ther. Adv. Med. Oncol. 2022, 14, 17588359221107112. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.K.; Saadeldin, M.K.; Salem, A.H.; Ibrahim, S.A.; Shouman, S.; Abdel-Naim, A.B.; Orecchia, R. A Critical Review of Chloroquine and Hydroxychloroquine as PotentialAdjuvant Agents for Treating People with Cancer. Future Pharmacol. 2022, 2, 431–443. [Google Scholar] [CrossRef]
- Ebenezer, O.; Jordaan, M.A.; Carena, G.; Bono, T.; Shapi, M.; Tuszynski, J.A. An Overview of the Biological Evaluation of Selected Nitrogen-Containing Heterocycle Medicinal Chemistry Compounds. Int. J. Mol. Sci. 2022, 23, 8117. [Google Scholar] [CrossRef] [PubMed]
- Tahlan, S.; Kumar, S.; Narasimhan, B. Pharmacological significance of heterocyclic 1H-benzimidazole scaffolds: A review. BMC Chem. 2019, 13, 101. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Benzimidazole and its derivatives as cancer therapeutics: The potential role from traditional to precision medicine. Acta. Pharm. Sin. B. 2023, 13, 478–497. [Google Scholar] [CrossRef] [PubMed]
- Tran, B.; Cohen, M.S. The discovery and development of binimetinib for the treatment of melanoma. Expert Opin. Drug Discov. 2020, 15, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Lalic, H.; Aurer, I.; Batinic, D.; Visnjic, D.; Smoljo, T.; Babic, A. Bendamustine: A review of pharmacology, clinical use and immunological effects. Oncol. Rep. 2022, 47, 114. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.H.; Alva, A.S.; Larson, T.; Szpakowski, S.; Purkaystha, D.; Amin, A.; Karpiak, L.; Piha-Paul, S.A. A mutation-specific, single-arm, phase 2 study of dovitinib in patients with advanced malignancies. Oncotarget 2020, 11, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Leshabane, M.; Dziwornu, G.A.; Coertzen, D.; Reader, J.; Moyo, P.; van der Watt, M.; Chisanga, K.; Nsanzubuhoro, C.; Ferger, R.; Erlank, E.; et al. Benzimidazole Derivatives Are Potent against Multiple Life Cycle Stages of Plasmodium falciparum Malaria Parasites. ACS Infect. Dis. 2021, 9, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- Escala, N.; Pineda, L.M.; Ng, M.G.; Coronado, L.M.; Spadafora, C.; Del Olmo, E. Antiplasmodial activity, structure-activity relationship and studies on the action of novel benzimidazole derivatives. Sci Rep. 2023, 6, 285. [Google Scholar] [CrossRef]
- Hranjec, M.; Starčević, K.; Zamola, B.; Mutak, S.; Đerek, M.; Karminski-Zamola, G. New amidino-benzimidazolyl derivatives of tylosin and desmycosin. J. Antibiot. 2002, 55, 308–314. [Google Scholar] [CrossRef][Green Version]
- Fairley, T.T.; Tidwell, R.R.; Donkor, I.; Naiman, N.A.; Ohemeng, K.A.; Lombardy, R.J.; Bentley, J.A.; Cory, M. Structure, DNA Minor Groove Binding, and Base Pair Specificity of Alkyland Aryl-Linked Bis(amidinobenzimidazoles) and Bis(amidinoindoles). J. Med. Chem. 1993, 36, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Abou-Elkhair, R.A.I.; Hassan, A.E.A.; Boykin, D.W.; Wilson, W.D. Lithium Hexamethyldisilazane Transformation of Transiently Protected 4-Aza/Benzimidazole Nitriles to Amidines and their Dimethyl Sulfoxide Mediated Imidazole Ring Formation. Org. Lett. 2016, 18, 4714–4717. [Google Scholar] [CrossRef] [PubMed]
- Held, J.; Gebru, T.; Kalesse, M.; Jansen, R.; Gerth, K.; Müller, R.; Mordmüller, B. Antimalarial activity of the myxobacterial macrolide chlorotonil A. Antimicrob. Agents Chemother. 2023, 58, 6378–6384. [Google Scholar] [CrossRef] [PubMed]
- Noedl, H.; Bronnert, J.; Yingyuen, K.; Attlmayr, B.; Kollaritsch, H.; Fukuda, M. Simple histidine-rich protein 2 double-site sandwich enzyme-linked immunosorbent assay for use in malaria drug sensitivity testing. Antimicrob. Agents Chemother. 2005, 49, 3575–3577. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.P.; Paul, S.; Mitra, I.; Roy, K. On two novel parameters for validation of predictive QSAR models. Molecules 2009, 14, 1660–1701. [Google Scholar] [CrossRef] [PubMed]
- Kiralj, R.; Ferreira, M.M.C. Basic Validation Procedures for Regression Models in QSAR and QSPR Studies: Theory and Application. J. Braz. Chem. 2009, 20, 770–787. [Google Scholar] [CrossRef]
- Chirico, N.; Gramatica, P. Real External Predictivity of QSAR Models: How To Evaluate It? Comparison of Different Validation Criteria and Proposal of Using the Concordance Correlation Coefficient. J. Chem. Inf. Model. 2011, 51, 2320–2335. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Kar, S.; Ambure, P. On a simple approach for determining applicability domain of QSAR models. Chemom. Intell. Lab. Syst. 2015, 145, 22–29. [Google Scholar] [CrossRef]
- Todeschini, R.; Consonni, V. Molecular Descriptors for Chemoinformatics: Volume I: Alphabetical Listing/Volume II: Appendices, References; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Mswahili, M.E.; Martin, G.L.; Woo, J.; Choi, G.J.; Jeong, Y.-S. Antimalarial Drug Predictions Using Molecular Descriptors and Machine Learning against Plasmodium falciparum. Biomolecules 2021, 11, 1750. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility andpermeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Sanchez, M.; Menunier, B. Hybrid Molecules QA where Q is an aminoquinoline and A is an antibiotic residue, their Synthesis and their uses as antibacterial agents, 2006. WO 2006/02474.
- Boyd, M.R.; Paull, K.D. Some practical considerations and applications of the national cancer institute in vitro anticancer drug discovery screen. Drug Dev. Res. 1995, 34, 91–109. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing Version 4.1.3; R Foundation for Statistical Computing: Vienna, Austria, 2011; Available online: https://www.R-project.org/ (accessed on 14 April 2024).
- Hocquet, A.; Langgård, M. An evaluation of the MM+ force field. J Mol Model 1998, 4, 94–112. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods I. Method. J. Comput. Chem. 1989, 10, 209–220. [Google Scholar] [CrossRef]
- Gramatica, P.; Sangion, A. A Historical Excursus on the Statistical Validation Parameters for QSAR Models: A Clarification Concerning Metrics and Terminology. J. Chem. Inf. Model. 2016, 56, 1127–1131. [Google Scholar] [CrossRef] [PubMed]

| IC50 a (µM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Comp. | n | R1 | R2 | MRC-5 | HeLa | CaCo-2 | THP-1 | Hut78 | HL-60 |
| 10a | 1 | H | H | 3.1 ± 0.3 | 3.4 ± 0.5 | 6.1 ± 4.9 | 2.4 ± 0.2 | 2.8 ± 0.5 | 1.3 ± 0.9 |
| 10b | 1 | H | Cl | 4.0 ± 1.1 | 2.7 ± 0.2 | 1.9 ± 0.2 | 3.4 ± 0.8 | 1.6 ± 1.2 | 2.0 ± 0.4 |
| 10c | 1 | H | OCH3 | 4.9 ± 1.7 | 2.6 ± 0.4 | 1.7 ± 0.1 | 3.8 ± 0.4 | 2.3 ± 0.8 | 1.8 ± 0.5 |
| 10d | 1 | H | Am | 99.2 ± 8.3 | >100 b | >100 b | >100 | 92± 6.1 | >100 |
| 11a | 1 | Br | H | 2.2 ± 0.8 | 2.6 ± 0.2 | 2.1 ± 0.6 | 4.9 ±7.3 | 2.4 ± 0.6 | 0.3 ± 0.3 |
| 11b | 1 | Br | Cl | 3.0 ± 0.9 | 5.4 ± 4.6 | 13.3 ± 2.5 | 4.4 ±5.1 | 5.8 ± 1.9 | 1.3 ± 0.9 |
| 11c | 1 | Br | OCH3 | 5.3 ± 2.4 | 2.5 ± 0.4 | 1.5 ± 0.3 | 3.5 ±7.3 | 3.0 ± 1.1 | 2.2 ± 0.4 |
| 11d | 1 | Br | Am | >100 | >100 | >100 | 33.9 ± 3.6 | 17.3 ± 9.2 | 44.3 ±1.2 |
| 12a | 1 | OCH3 | H | 3.8 ± 0.4 | 2.2 ± 0.1 | 2.0 ± 0.3 | 3.0 ± 0.6 | 4.2 ± 2.1 | 2.0 ± 0.3 |
| 12b | 1 | OCH3 | Cl | 3.0 ± 1.6 | 5.6 ± 1.3 | 4.7 ± 4.0 | 6.1 ± 6.9 | 3.7 ± 5.2 | 3.3 ± 1.1 |
| 12c | 1 | OCH3 | OCH3 | 1.1 ± 0.8 | 2.7 ± 0.2 | 2.1 ± 0.3 | 3.1 ± 0.8 | 2.4 ± 1.1 | 0.2 ± 0.0 |
| 12d | 1 | OCH3 | Am | >100 | >100 | >100 | 100 | 16.1 ± 4.7 | 52.5 ± 5.1 |
| 13a | 2 | H | H | 0.3 ± 0.2 | 3.0 ± 2.3 | 1.1 ± 0.4 | 3.0 ± 0.8 | 1.6 ± 0.8 | 0.4 ± 0.2 |
| 13b | 2 | H | Cl | 0.4 ± 0.1 | 0.6 ± 0.1 | 1.6 ± 0.4 | 2.1 ± 0.7 | 0.3 ± 0.2 | 0.2 ± 0.1 |
| 13c | 2 | H | OCH3 | 2.7 ± 0.5 | 0.2 ± 0.2 | 1.0 ± 0.5 | 2.6 ± 0.0 | 1.1 ± 0.5 | 0.3 ± 0.2 |
| 13d | 2 | H | Am | 83.0 ± 5.4 | >100 | >100 | 14.4 ± 3.1 | 15.2 ± 3.0 | 18.2 ± 6.7 |
| 14a | 2 | Br | H | 0.6 ± 0.6 | 1.2 ± 0.7 | 5.0 ± 7.3 | 5.1 ± 1.7 | 1.7 ± 0.4 | 0.7 ± 0.5 |
| 14b | 2 | Br | Cl | 2.0 ± 1.8 | 1.5 ± 1.0 | 1.5 ± 1.1 | 6.8 ± 3.5 | 0.9 ± 0.6 | 0.4 ± 0.1 |
| 14c | 2 | Br | OCH3 | 0.4 ± 0.1 | 0.8 ± 0.7 | 1.6 ± 0.9 | 5.2 ± 1.8 | 0.6 ± 0.1 | 0.3 ± 0.1 |
| 14d | 2 | Br | Am | >100 | >100 | >100 | 100 | 19.7 ± 4.0 | 24.4 ± 4.4 |
| 15a | 2 | OCH3 | H | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.8 ± 0.1 | 2.2 ± 0.5 | 1.5 ± 0.3 | 0.4 ± 0.1 |
| 15b | 2 | OCH3 | Cl | 0.3 ± 0.0 | 0.3 ± 0.1 | 1.1 ± 0.6 | 2.5 ± 0.3 | 0.7 ± 0.4 | 0.2 ± 0.1 |
| 15c | 2 | OCH3 | OCH3 | 0.7 ± 0.6 | 1.2 ± 0.6 | 1.5 ± 0.3 | 2.2 ± 0.3 | 0.7 ± 0.6 | 0.2 ± 0.0 |
| 15d | 2 | OCH3 | Am | 95.2 ± 9.7 | >100 | >100 | 29.6 ± 0.3 | 12.8 ± 5.5 | 19.5 ± 1.1 |
| 5-FU | 54.1 c | 8.2 c | 5.9 c | >100 c | >100 c | 76.4 c | |||
| IC50 a (nM) | |||||
|---|---|---|---|---|---|
| Comp. | n | R1 | R2 | Pf3D7 | PfDd2 |
| 10a | 1 | H | H | 2.7 ± 0.2 | 3.3 ± 0.1 |
| 10b | 1 | H | Cl | 3.5 ± 1.5 | 4.9 ± 0.8 |
| 10c | 1 | H | OCH3 | 6.8 ± 1.8 | 10.1 ± 5.0 |
| 10d | 1 | H | Am | 58.5 ± 5.9 | 36.9 ± 17.7 |
| 11a | 1 | Br | H | 36.9 ± 1.5 | 8.7 ± 3.4 |
| 11b | 1 | Br | Cl | 87.3 ± 3.3 | 88.3 ± 3.1 |
| 11c | 1 | Br | OCH3 | 19.7 ± 4.2 | 28.0 ± 0.6 |
| 11d | 1 | Br | Am | 321.4 ± 32.8 | 984.5 ± 520.8 |
| 12a | 1 | OCH3 | H | 14.5 ± 4.0 | 91.2 ± 9.5 |
| 12b | 1 | OCH3 | Cl | 11.1 ± 0.3 | 21.5 ± 2.5 |
| 12c | 1 | OCH3 | OCH3 | 9.7 ± 0.2 | 15.4 ± 2.0 |
| 12d | 1 | OCH3 | Am | 190.5 ± 11.5 | 120.0 ± 7.5 |
| 13a | 2 | H | H | 2.6 ± 0.7 | 4.8 ± 0.3 |
| 13b | 2 | H | Cl | 2.4 ± 1.4 | 3.9 ± 1.1 |
| 13c | 2 | H | OCH3 | 12.8 ± 0.5 | 11.9 ± 1.6 |
| 13d | 2 | H | Am | 57.4 ± 1.9 | 198.3 ± 0.1 |
| 14a | 2 | Br | H | 9.3 ± 0.9 | 13.0 ± 1.9 |
| 14b | 2 | Br | Cl | 5.0 ± 1.4 | 14.2 ± 1.2 |
| 14c | 2 | Br | OCH3 | 11.4 ± 4.0 | 11.8 ± 3.2 |
| 14d | 2 | Br | Am | 158.6 ± 22.6 | 450.8 ± 40.3 |
| 15a | 2 | OCH3 | H | 4.4 ± 0.3 | 11.2 ± 0.5 |
| 15b | 2 | OCH3 | Cl | 4.7 ± 1.5 | 5.2 ± 0.5 |
| 15c | 2 | OCH3 | OCH3 | 6.9 ± 1.7 | 16.5 ± 5.7 |
| 15d | 2 | OCH3 | Am | 28.7 ± 9.4. | 22.9 ± 4.3 |
| CQ | 11.1 ± 0.5 | 360.0 ± 27.15 | |||
| Statistical Parameters | Model 1 | Model 2 | Validation Criteria Thresholds |
|---|---|---|---|
| Ntr | 20 | 20 | |
| Next | 5 | 5 | |
| R2 | 0.886 | 0.859 | ≥0.6 |
| R2adj | 0.865 | 0.832 | ≥0.6 |
| s | 0.219 | 0.288 | as low as possible |
| F | 41.589 | 32.525 | significant at p < 0.05 |
| Kxx | 0.053 | 0.217 | as low as possible |
| ΔK | 0.328 | 0.102 | ≥0.05 |
| RMSEtr | 0.196 | 0.257 | close to zero |
| MAEtr | 0.159 | 0.207 | close to zero |
| CCCtr | 0.940 | 0.924 | ≥0.80 |
| Q2LOO | 0.840 | 0.799 | >0.5 |
| RMSEcv | 0.232 | 0.307 | RMSEtr < RMSEcv |
| MAEcv | 0.192 | 0.256 | close to zero |
| CCCcv | 0.915 | 0.889 | ≥ 0.80 |
| R2Yscr | 0.160 | 0.158 | < 0.2 |
| Q2Yscr | −0.341 | −0.353 | R2yscr > Q2yscr |
| RMSEext | 0.201 | 0.266 | close to zero |
| MAEext | 0.147 | 0.250 | close to zero |
| R2ext | 0.937 | 0.878 | ≥ 0.6 |
| CCCext | 0.934 | 0.890 | ≥ 0.80 |
| Q2F1 | 0.908 | 0.808 | > 0.6 |
| Q2F2 | 0.893 | 0.797 | > 0.6 |
| Q2F3 | 0.880 | 0.850 | > 0.6 |
| r2m average | 0.685 | 0.827 | ≥ 0.5 |
| r2m difference | 0.111 | 0.030 | as low as possible |
| Applicability domain | |||
| N compounds outlier | - | 1 (12a) | |
| N compounds out of app.dom. | 1 (14d) | - | |
| h* | 0.600 | 0.600 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krstulović, L.; Rastija, V.; Pessanha de Carvalho, L.; Held, J.; Rajić, Z.; Živković, Z.; Bajić, M.; Glavaš-Obrovac, L. Design, Synthesis, Antitumor, and Antiplasmodial Evaluation of New 7-Chloroquinoline–Benzimidazole Hybrids. Molecules 2024, 29, 2997. https://doi.org/10.3390/molecules29132997
Krstulović L, Rastija V, Pessanha de Carvalho L, Held J, Rajić Z, Živković Z, Bajić M, Glavaš-Obrovac L. Design, Synthesis, Antitumor, and Antiplasmodial Evaluation of New 7-Chloroquinoline–Benzimidazole Hybrids. Molecules. 2024; 29(13):2997. https://doi.org/10.3390/molecules29132997
Chicago/Turabian StyleKrstulović, Luka, Vesna Rastija, Lais Pessanha de Carvalho, Jana Held, Zrinka Rajić, Zorislava Živković, Miroslav Bajić, and Ljubica Glavaš-Obrovac. 2024. "Design, Synthesis, Antitumor, and Antiplasmodial Evaluation of New 7-Chloroquinoline–Benzimidazole Hybrids" Molecules 29, no. 13: 2997. https://doi.org/10.3390/molecules29132997
APA StyleKrstulović, L., Rastija, V., Pessanha de Carvalho, L., Held, J., Rajić, Z., Živković, Z., Bajić, M., & Glavaš-Obrovac, L. (2024). Design, Synthesis, Antitumor, and Antiplasmodial Evaluation of New 7-Chloroquinoline–Benzimidazole Hybrids. Molecules, 29(13), 2997. https://doi.org/10.3390/molecules29132997








