Berberine and Palmatine Distribution Across Plant Organs in Berberis darwinii: Basis for Selecting Superior-Producing Accessions
Abstract
:1. Introduction
2. Results
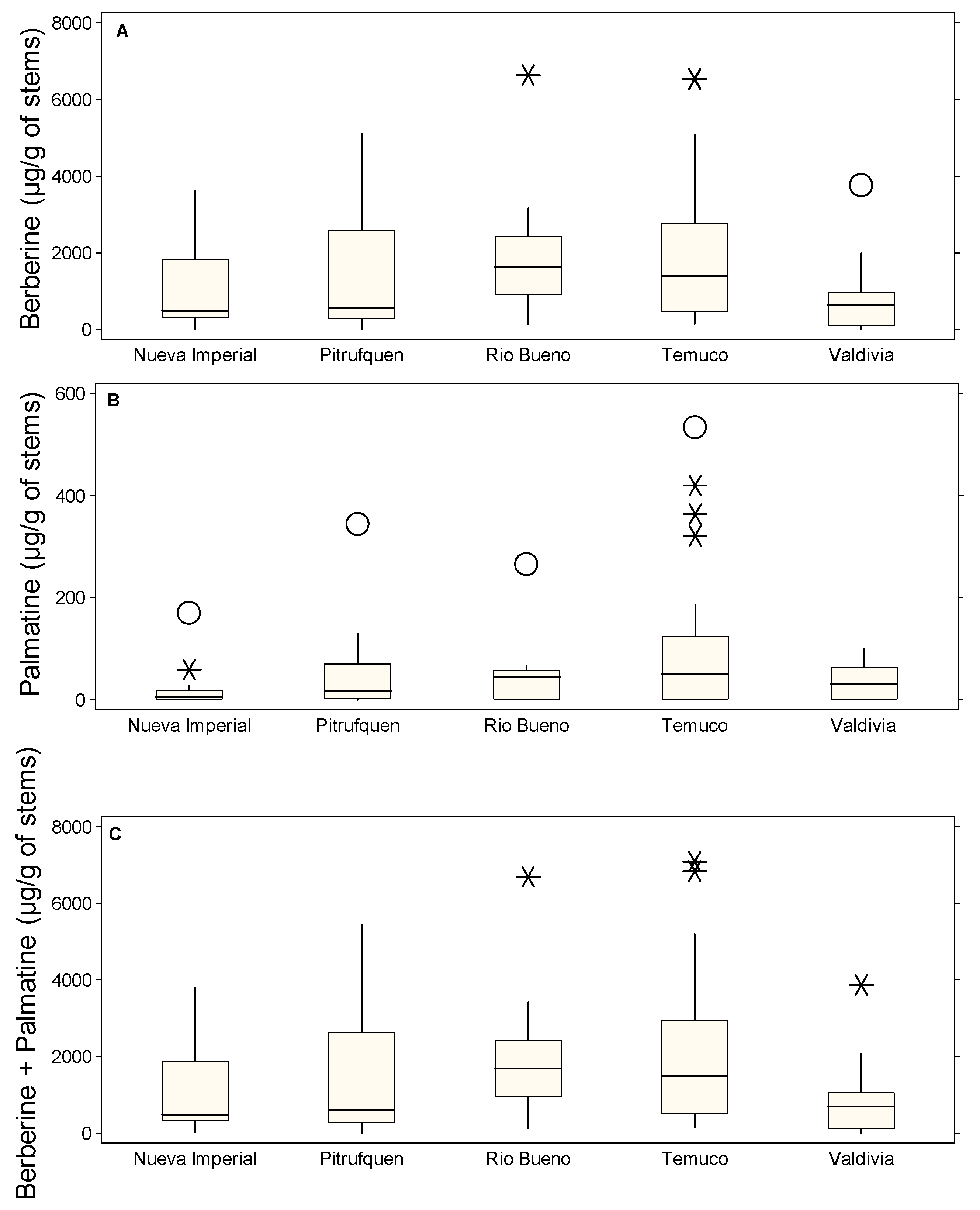
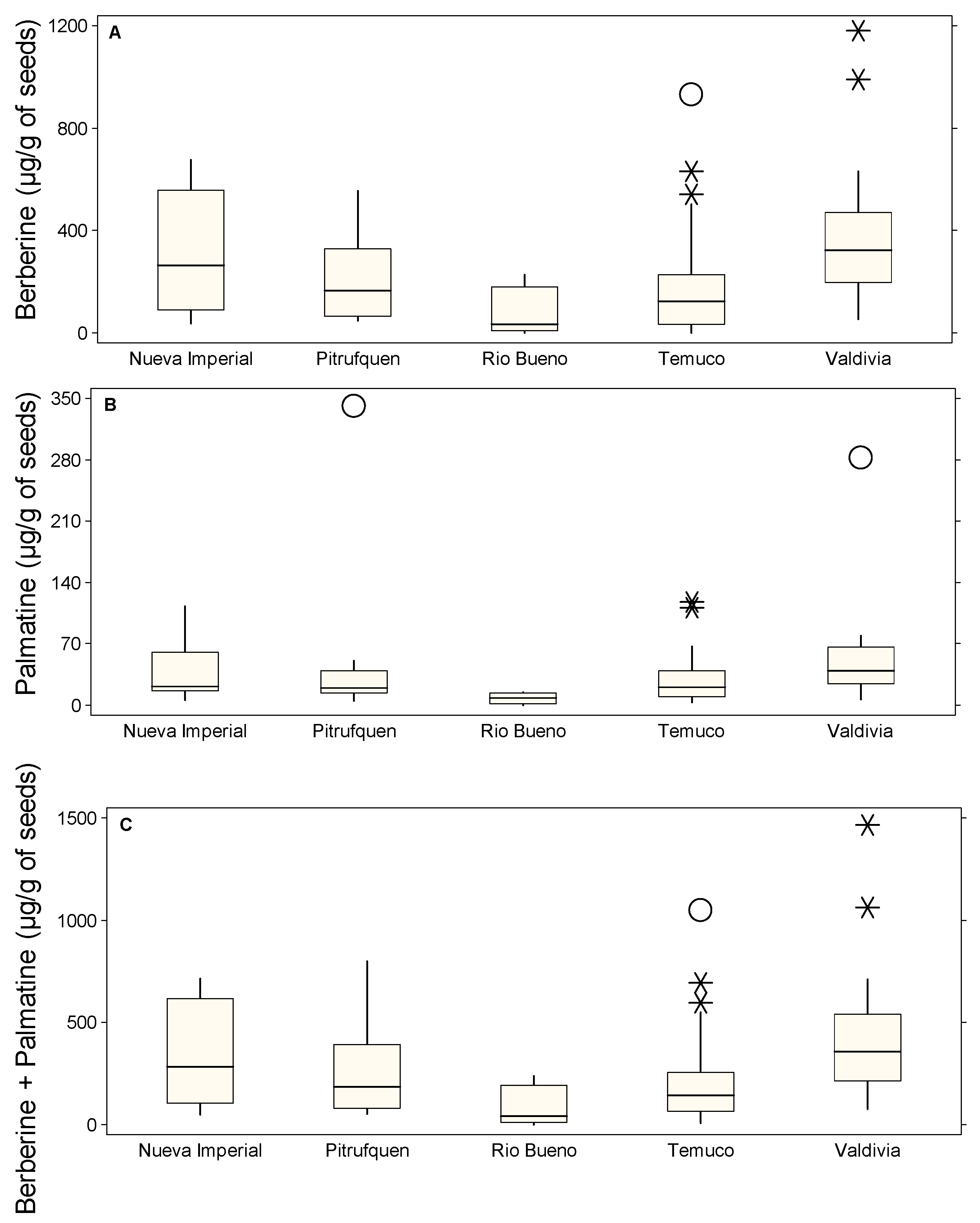
2.1. Regional Variability in Alkaloid Content of B. darwinii: Distinct Patterns of Berberine and Palmatine Accumulation Across Locations
2.2. Tissue-Specific Distribution of Alkaloids in B. darwinii: Roots as the Primary Site of Berberine Accumulation, While Stems Concentrate Palmatine
2.3. Variability of Alkaloid Content Across Plant Organs and Locations
2.4. Outliers Identification
3. Discussion
4. Materials and Methods
4.1. Establishment of Plant Material and Experimental Design
4.2. Sample Collection
4.3. Alkaloid Extraction Procedure
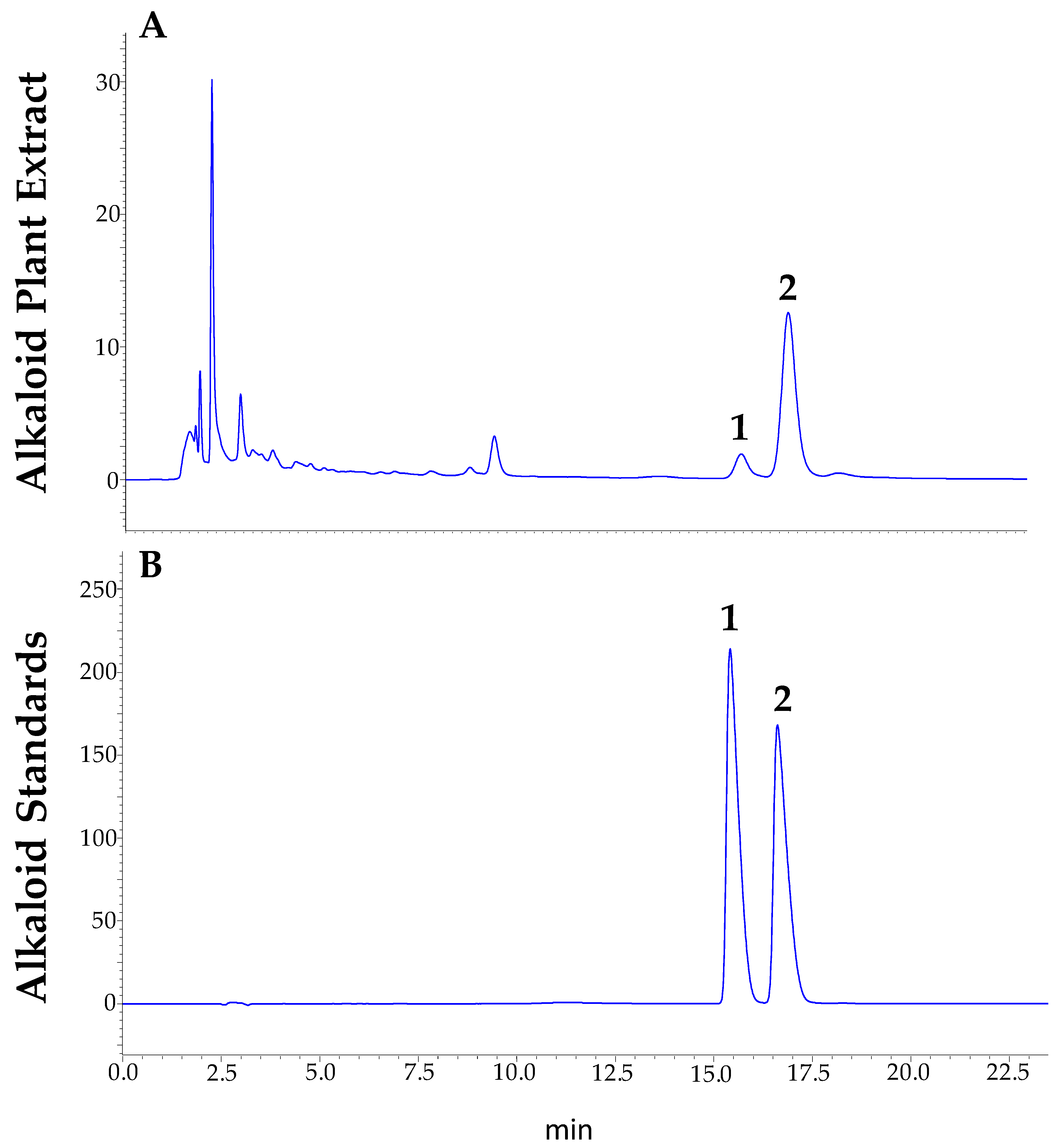
4.4. HPLC-DAD Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orsi, M.C. Berberidaceae. In Flora Patagónica; Colección Científica del Instituto Nacional de Tecnología Agropecuaria, 4 Secc. 4a; Correa, M.N., Ed.; INTA: Buenos Aires, Argentina, 1984; pp. 325–348. [Google Scholar]
- Fajardo Morales, V.; Araya, M.; Manosalva, L. Berberis darwinii Hook. In Medicinal and Aromatic Plants of South America Vol. 2: Argentina, Chile and Uruguay; Springer: Cham, Switzerland, 2021; pp. 127–133. [Google Scholar]
- Chamorro, M.F.; Ladio, A.H. Management of native and exotic plant species with edible fruits in a rural community in a protected area of NW Patagonia. Ethnobiol. Conserv. 2021, 10. [Google Scholar] [CrossRef]
- Chamorro, M.F.; Reiner, G.; Theoduloz, C.; Ladio, A.; Schmeda-Hirschmann, G.; Gómez-Alonso, S.; Jiménez-Aspee, F. Polyphenol composition and (bio) activity of Berberis species and wild strawberry from the Argentinean Patagonia. Molecules 2019, 24, 3331. [Google Scholar] [CrossRef] [PubMed]
- Landrum, L.R. Revision of Berberis (Berberidaceae) in Chile and adjacent southern Argentina. In Annals of the Missouri Botanical Garden; Missouri Botanical Garden Press: St. Louis, MO, USA, 1999; pp. 793–834. [Google Scholar]
- Pino, M.T.; Pérez, R.; Vergara, C.; Zamora, O.; Domínguez, E. Michay: Berry Nativo de Amplia Distribución Con Metabolitos de Interés Para La Industria de Alimentos. Inf. INIA La Platina 2019, 39, 1–4. [Google Scholar]
- Pino, M.T.; Vergara, C. Colorantes y Antioxidantes Naturales en la Industria de Alimentos: Tecnologías de Extracción y Materias Primas Dedicadas; INIA: Santiago, Chile, 2022; p. 188. [Google Scholar]
- Schmeda-Hirschmann, G.; Jimenez-Aspee, F.; Theoduloz, C.; Ladio, A. Patagonian berries as native food and medicine. J. Ethnopharmacol. 2019, 241, 111979. [Google Scholar] [CrossRef]
- Romero-Román, M.E.; Schoebitz, M.; Bastías, R.M.; Fernández, P.S.; García-Viguera, C.; López-Belchi, M.D. Native species facing climate changes: Response of calafate berries to low temperature and UV radiation. Foods 2021, 10, 196. [Google Scholar] [CrossRef]
- Núñez, D.; Balboa, N.; Quilaqueo, N.; Alvear, M.; Paredes, M. Evaluación de la Actividad Inmunomoduladora de Extractos Metanólicos y de Alcaloides de Berberis darwinii H. (Berberidaceae). Int. J. Morphol. 2018, 36, 454–459. [Google Scholar] [CrossRef]
- Núñez, D.; Balboa, A.; Carvajal, F.; Alvear, M.; Paredes, M. Efecto del extracto de alcaloides de Berberis darwinii Hook. sobre respuestas celulares innatas en fagocitos murinos. BLACPMA 2018, 17, 259–269. [Google Scholar]
- Valencia, E.; Weiss, I.; Shamma, M.; Urzua, A.; Fajardo, V. Dihydrorugosinone, a pseudobenzylisoquinoline alkaloid from Berberis darwinii and Berberis actinacantha. J. Nat. Prod. 1984, 47, 1050–1051. [Google Scholar] [CrossRef]
- Valencia, E.; Patra, A.; Freyer, A.; Shamma, M.; Fajardo, V. Santiagonamine: A new aporphinoid alkaloid incorporating a phenanthridine skeleton. Tetrahedron Lett. 1984, 25, 3163–3166. [Google Scholar] [CrossRef]
- Valencia, E.; Freyer, A.; Shamma, M.; Fajardo, V. Nuevamine, an isoindoloisoquinoline alkaloid, and lennoxamine, an isoindolobenzazepine. Tetrahedron Lett. 1984, 25, 599–602. [Google Scholar] [CrossRef]
- Valencia, E.; Fajardo, V.; Freyer, A.; Shamma, M. Magallanesine: An isoindolobenzazocine alkaloid. Tetrahedron Lett. 1985, 26, 993–996. [Google Scholar] [CrossRef]
- Pérez-San Martín, A.; Alvear-Zamora, M.; Carvajal-Caiconte, F.; Paredes, M.; Lagos-Arias, N.; Curaqueo, G.; Mutis, A. Identification and antibacterial activity of benzylisoquinoline alkaloids from Berberis empetrifolia L. and Berberis darwinii H. roots. Nat. Prod. Res. 2025, 1–11. [Google Scholar] [CrossRef]
- MacLeod, B.P. Regulation of Benzylisoquinoline Alkaloid Biosynthesis in Opium Poppy. Master’s Thesis, University of Calgary, Calgary, AB, Canada, 2006. [Google Scholar]
- Duan, Y.; You, J.; Wang, J.; Tang, T.; Guo, X.; Wang, F.; Guo, J. Transcriptome analysis reveals the potential mechanism of altering viability, yield, and isoquinoline alkaloids in Coptis chinensis through Cunninghamia lanceolata understory cultivation. Chem. Biol. Technol. Agric. 2024, 11, 24. [Google Scholar] [CrossRef]
- Lee, E.J.; Hagel, J.M.; Facchini, P.J. Role of the phloem in the biochemistry and ecophysiology of benzylisoquinoline alkaloid metabolism. Front. Plant Sci. 2013, 4, 182. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.C.; Wu, Z.F.; Yin, Z.Q.; Lin, L.G.; Wang, R.; Zhang, Q.W. Coptidis rhizoma and its main bioactive components: Recent advances in chemical investigation, quality evaluation and pharmacological activity. Chin. Med. 2018, 13, 1–18. [Google Scholar] [CrossRef]
- Wang, T.; Guo, R.; Zhou, G.; Zhou, X.; Kou, Z.; Sui, F.; Wang, Z. Traditional uses, botany, phytochemistry, pharmacology and toxicology of Panax notoginseng (Burk.) FH Chen: A review. J. Ethnopharmacol. 2016, 188, 234–258. [Google Scholar] [CrossRef] [PubMed]
- Mohi-Ud-Din, R.; Mir, R.H.; Mir, P.A.; Farooq, S.; Raza, S.N.; Raja, W.Y.; Bhat, Z.A. Ethnomedicinal uses, phytochemistry and pharmacological aspects of the genus Berberis linn: A comprehensive review. Comb. Chem. High Throughput Screen. 2021, 24, 624–644. [Google Scholar] [CrossRef]
- Han, B.; Wang, K.; Tu, Y.; Tan, L.; He, C. Low-dose berberine attenuates the anti-breast cancer activity of chemotherapeutic agents via induction of autophagy and antioxidation. Dose-Response 2020, 18, 1559325820939751. [Google Scholar] [CrossRef]
- Zhou, G.; Yan, M.; Guo, G.; Tong, N. Ameliorative effect of berberine on neonatally induced type 2 diabetic neuropathy via modulation of BDNF, IGF-1, PPAR-γ, and AMPK expressions. Dose-Response 2019, 17, 1559325819862449. [Google Scholar] [CrossRef]
- Asbaghi, O.; Ghanbari, N.; Reiner, Ž.; Amirani, E.; Hallajzadeh, J.; Mirsafaei, L.; Asemi, Z. The effect of berberine supplementation on obesity parameters, inflammation and liver function enzymes: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2020, 38, 43–49. [Google Scholar] [CrossRef]
- Shen, Y.B.; Piao, X.S.; Kim, S.W.; Wang, L.; Liu, P. The effects of berberine on the magnitude of the acute inflammatory response induced by Escherichia coli lipopolysaccharide in broiler chickens. Poult. Sci. 2010, 89, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Seng, Q.; Wang, H.; Wei, W.; Guo, T.; Wang, Y.; Li, Y.; Song, D. Synthesis and biological evaluation of berberine derivatives as a new class. Bioorganic Chem. 2019, 95, 103490. [Google Scholar]
- Catia, V.D.; Nuno, G.M.; Ines, A.B.; Teresa, L.S.; Ana, B.; Paulo, J.O. Berberine as a promising safe anti-cancer agent-is there a role for mitochondria? Curr. Drug Targets 2011, 12, 850–859. [Google Scholar]
- Jahan Oni, M.I.; Bhuia, M.S.; Chowdhury, R.; Sheikh, S.; Munshi, M.H.; Hasan, M.S.A.; Islam, M.T. Botanical Sources, Pharmacokinetics, and Therapeutic Efficacy of Palmatine and Its Derivatives in the Management of Cancer: A Comprehensive Mechanistic Analysis. J. Food Biochem. 2024, 2024, 8843855. [Google Scholar] [CrossRef]
- Long, J.; Song, J.; Zhong, L.; Liao, Y.; Liu, L.; Li, X. Palmatine: A review of its pharmacology, toxicity and pharmacokinetics. Biochimie 2019, 162, 176–184. [Google Scholar] [CrossRef]
- Fan, T.; Ge, M.; Guo, Z.; He, H.; Zhang, N.; Li, Y.; Song, D. Discovery of 9 O-Substituted Palmatine Derivatives as a New Class of Anti-COL1A1 Agents Via Repressing TGF-β1/Smads and JAK1/STAT3 Pathways. Molecules 2020, 25, 773. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, Z.; Li, D.; Qin, J.; Pan, Z.; Guo, B.; Su, Z. Investigation of the therapeutic effect of total alkaloids of Corydalis saxicola Bunting on CCl4-induced liver fibrosis in rats by LC/MS-Based metabolomics analysis and network pharmacology. Metabolites 2022, 13, 9. [Google Scholar] [CrossRef]
- Qin, J.; Luo, Z.; Wang, Q.; Tang, C.; Meng, M.; Huang, Z.; Su, Z. Integrating metabonomics and metagenomics sequencing to study the anti-liver fibrosis effects of palmatine in Corydalis saxicola Bunting. J. Ethnopharmacol. 2023, 315, 116666. [Google Scholar] [CrossRef]
- Chacón-Fuentes, M.A.; Lizama, M.G.; Parra, L.J.; Seguel, I.E.; Quiroz, A.E. Insect diversity, community composition and damage index on wild and cultivated murtilla. Cienc. Investig. Agrar. 2016, 43, 57–67. [Google Scholar] [CrossRef]
- Cromwell, B.T. Experiments on the origin and function of berberine in Berberis Darwinii. Biochem. J. 1933, 27, 860. [Google Scholar] [CrossRef]
- Habtemariam, S. Berberine and inflammatory bowel disease: A concise review. Pharmacol. Res. 2016, 113, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Olivoto, T.; Nardino, M.; Carvalho, I.R.; Follmann, D.N.; Szareski, V.J.; Ferrari, M.; de Souza, V.Q. Plant secondary metabolites and its dynamical systems of induction in response to environmental factors: A review. Afr. J. Agric. Res. 2017, 12, 71–84. [Google Scholar]
- Herms, D.A.; Mattson, W.J. The dilemma of plants: To grow or defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef]
- Pang, B.; Zhao, L.H.; Zhou, Q.; Zhao, T.Y.; Wang, H.; Gu, C.J.; Tong, X.L. Application of berberine on treating type 2 diabetes mellitus. Int. J. Endocrinol. 2015, 2015, 905749. [Google Scholar] [CrossRef]
- Kheir, M.M.; Wang, Y.; Hua, L.; Hu, J.; Li, L.; Lei, F.; Du, L. Acute toxicity of berberine and its correlation with the blood concentration in mice. Food Chem. Toxicol. 2010, 48, 1105–1110. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zou, D.; Liu, W.; Yang, J.; Zhu, N.; Ning, G. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J. Clin. Endocrinol. Metab. 2008, 93, 2559–2565. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Piao, X.S.; Zhang, Q.; Li, P.; Yi, J.Q.; Liu, J.D.; Wang, G.Q. The effects of Forsythia suspensa extract and berberine on growth performance, immunity, antioxidant activities, and intestinal microbiota in broilers under high stocking density. Poult. Sci. 2013, 92, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Zhan, X.; Chen, Z.; Chen, R.; Shen, C. Environmental and genetic factors involved in plant protection-associated secondary metabolite biosynthesis pathways. Front. Plant Sci. 2022, 13, 877304. [Google Scholar] [CrossRef]
- Li, X.; Xu, B.; Sahito, Z.A.; Chen, S.; Liang, Z. Transcriptome analysis reveals cadmium exposure enhanced the isoquinoline alkaloid biosynthesis and disease resistance in Coptis chinensis. Ecotoxicol. Environ. Saf. 2024, 271, 115940. [Google Scholar] [CrossRef]
- Guo, X.; Li, Y.; Li, C.; Luo, H.; Wang, L.; Qian, J.; Chen, S. Analysis of the Dendrobium officinale transcriptome reveals putative alkaloid biosynthetic genes and genetic markers. Gene 2013, 527, 131–138. [Google Scholar] [CrossRef]
- Yu, Y.; Kleuter, M.; Dinani, S.T.; Trindade, L.M.; van der Goot, A.J. The role of plant age and leaf position on protein extraction and phenolic compounds removal from tomato (Solanum lycopersicum) leaves using food-grade solvents. Food Chem. 2023, 406, 135072. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Chen, T.; Wang, X.; Cao, J.; Li, X.; Xu, X.; Zhang, L. Physiological and expressional regulation on photosynthesis, starch and sucrose metabolism response to waterlogging stress in peanut. Front. Plant Sci. 2021, 12, 601771. [Google Scholar]
- Uddin, A.S.M.M.; Gomasta, J.; Islam, M.T.; Islam, M.; Kayesh, E.; Karim, M.R. Gibberellic Acid Spray modulates Fruiting, Yield, Quality, and Shelf Life of Rambutan (Nephelium lappaceum L). J. Hortic. Res. 2024, 32, 51–66. [Google Scholar] [CrossRef]
- Zhang, G.; Mao, Z.; Maillard, P.; Brancheriau, L.; Gérard, B.; Engel, J.; Stokes, A. Not all sweetness and light: Non-structural carbohydrate storage capacity in tree stems is decoupled from leaf but not from root economics. Funct. Ecol. 2024, 38, 668–678. [Google Scholar] [CrossRef]
- Manosalva, L.; Mutis, A.; Palma, R.; Fajardo, V.; Quiroz, A. Antifeedant activity of alkaloid extracts from calafate (Berberis microphylla, G. Forst 2019, 1789) against diamondback moth larvae (Plutella xylostella, Linnaeus, 1758). In Anales del Instituto de la Patagonia; Universidad de Magallanes: Punta Arenas, Chile, 2019; Volume 47, pp. 17–23. [Google Scholar]
- Manosalva, L.; Mutis, A.; Urzúa, A.; Fajardo, V.; Quiroz, A. Antibacterial activity of alkaloid fractions from Berberis microphylla G. Forst and study of synergism with ampicillin and cephalothin. Molecules 2016, 21, 76. [Google Scholar] [CrossRef]
- Lu, T.; Liang, Y.; Song, J.; Xie, L.; Wang, G.J.; Liu, X.D. Simultaneous determination of berberine and palmatine in rat plasma by HPLC–ESI-MS after oral administration of traditional Chinese medicinal preparation Huang-Lian-Jie-Du decoction and the pharmacokinetic application of the method. J. Pharm. Biomed. Anal. 2006, 40, 1218–1224. [Google Scholar] [CrossRef]





| Collection Zone | Mean (µg/g) | Standard Deviation | Coefficient of Variation | Minimum | Maximum | |
|---|---|---|---|---|---|---|
| Nueva Imperial | Leaf | |||||
| Berberine | 102.88 | 135.56 | 131.76 | 0.0001 | 511.02 | |
| Palmatine | 4.79 | 7.04 | 147.07 | 0.0001 | 24.78 | |
| Pitrufquén | ||||||
| Berberine | 44.54 | 72.71 | 163.23 | 0.0001 | 233.82 | |
| Palmatine | 3.72 | 5.01 | 134.72 | 0.0001 | 18.71 | |
| Rio Bueno | ||||||
| Berberine | 109.51 | 153.51 | 140.19 | 3.8800 | 493.10 | |
| Palmatine | 9.17 | 9.80 | 106.90 | 1.0200 | 30.23 | |
| Temuco | ||||||
| Berberine | 82.52 | 106.89 | 129.52 | 0.0001 | 455.63 | |
| Palmatine | 9.98 | 13.83 | 138.58 | 0.0001 | 62.57 | |
| Valdivia | ||||||
| Berberine | 20.88 | 25.71 | 123.17 | 0.0001 | 89.36 | |
| Palmatine | 2.88 | 2.83 | 98.07 | 0.0001 | 9.12 | |
| Nueva Imperial | Stem | |||||
| Berberine | 1059.00 | 1102.90 | 104.14 | 13.800 | 3619.80 | |
| Palmatine | 19.89 | 40.21 | 202.10 | 0.0001 | 170.03 | |
| Pitrufquén | ||||||
| Berberine | 1540.90 | 1678.90 | 108.96 | 0.0001 | 5101.30 | |
| Palmatine | 55.37 | 91.65 | 165.51 | 0.0001 | 342.78 | |
| Rio Bueno | ||||||
| Berberine | 2020.10 | 1740.60 | 86.16 | 136.00 | 6639.60 | |
| Palmatine | 51.16 | 75.47 | 147.52 | 0.0001 | 265.32 | |
| Temuco | ||||||
| Berberine | 1934.60 | 1761.80 | 91.06 | 145.73 | 6544.20 | |
| Palmatine | 100.95 | 137.21 | 135.92 | 0.0001 | 533.41 | |
| Valdivia | ||||||
| Berberine | 828.20 | 954.60 | 115.26 | 0.0001 | 3766.30 | |
| Palmatine | 34.02 | 34.52 | 101.48 | 0.0001 | 99.11 | |
| Nueva Imperial | Root | |||||
| Berberine | 1721.20 | 3153.50 | 183.21 | 0.0001 | 13,448.00 | |
| Palmatine | 50.77 | 83.11 | 163.69 | 0.0001 | 279.53 | |
| Pitrufquén | ||||||
| Berberine | 2404.00 | 2604.60 | 108.34 | 111.77 | 9627.00 | |
| Palmatine | 186.93 | 336.03 | 179.76 | 0.0001 | 1257.90 | |
| Rio Bueno | ||||||
| Berberine | 2389.50 | 4394.30 | 183.90 | 0.0001 | 15,910.00 | |
| Palmatine | 105.89 | 205.85 | 194.40 | 0.0001 | 674.80 | |
| Temuco | ||||||
| Berberine | 3871.00 | 6995.40 | 180.71 | 0.0001 | 26,482.00 | |
| Palmatine | 385.91 | 1680.60 | 435.50 | 0.0001 | 9978.30 | |
| Valdivia | ||||||
| Berberine | 4262.20 | 5553.90 | 130.31 | 0.0001 | 18,296.00 | |
| Palmatine | 165.04 | 237.87 | 144.13 | 0.0001 | 807.56 | |
| Nueva Imperial | Seed | |||||
| Berberine | 313.03 | 240.17 | 76.72 | 35.840 | 674.99 | |
| Palmatine | 39.38 | 32.74 | 83.13 | 5.6300 | 113.24 | |
| Pitrufquén | ||||||
| Berberine | 214.49 | 174.50 | 81.35 | 46.270 | 554.25 | |
| Palmatine | 54.19 | 101.65 | 187.57 | 4.6500 | 340.89 | |
| Rio Bueno | ||||||
| Berberine | 81.53 | 94.74 | 116.20 | 0.0001 | 226.95 | |
| Palmatine | 7.84 | 6.40 | 81.69 | 0.0001 | 14.57 | |
| Temuco | ||||||
| Berberine | 194.11 | 227.18 | 117.04 | 1.2600 | 930.76 | |
| Palmatine | 29.76 | 30.40 | 102.17 | 3.2300 | 118.11 | |
| Valdivia | ||||||
| Berberine | 409.03 | 321.31 | 78.555 | 52.350 | 1181.80 | |
| Palmatine | 58.68 | 67.56 | 115.12 | 6.8300 | 281.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chacón-Fuentes, M.; Burgos-Díaz, C.; Opazo-Navarrete, M.; Mercado, A.; Westermeyer, F. Berberine and Palmatine Distribution Across Plant Organs in Berberis darwinii: Basis for Selecting Superior-Producing Accessions. Molecules 2025, 30, 1849. https://doi.org/10.3390/molecules30081849
Chacón-Fuentes M, Burgos-Díaz C, Opazo-Navarrete M, Mercado A, Westermeyer F. Berberine and Palmatine Distribution Across Plant Organs in Berberis darwinii: Basis for Selecting Superior-Producing Accessions. Molecules. 2025; 30(8):1849. https://doi.org/10.3390/molecules30081849
Chicago/Turabian StyleChacón-Fuentes, Manuel, César Burgos-Díaz, Mauricio Opazo-Navarrete, Alan Mercado, and Fernando Westermeyer. 2025. "Berberine and Palmatine Distribution Across Plant Organs in Berberis darwinii: Basis for Selecting Superior-Producing Accessions" Molecules 30, no. 8: 1849. https://doi.org/10.3390/molecules30081849
APA StyleChacón-Fuentes, M., Burgos-Díaz, C., Opazo-Navarrete, M., Mercado, A., & Westermeyer, F. (2025). Berberine and Palmatine Distribution Across Plant Organs in Berberis darwinii: Basis for Selecting Superior-Producing Accessions. Molecules, 30(8), 1849. https://doi.org/10.3390/molecules30081849









