Silver Nanoparticles Derived by Artemisia arborescens Reveal Anticancer and Apoptosis-Inducing Effects
Abstract
:1. Introduction
2. Results
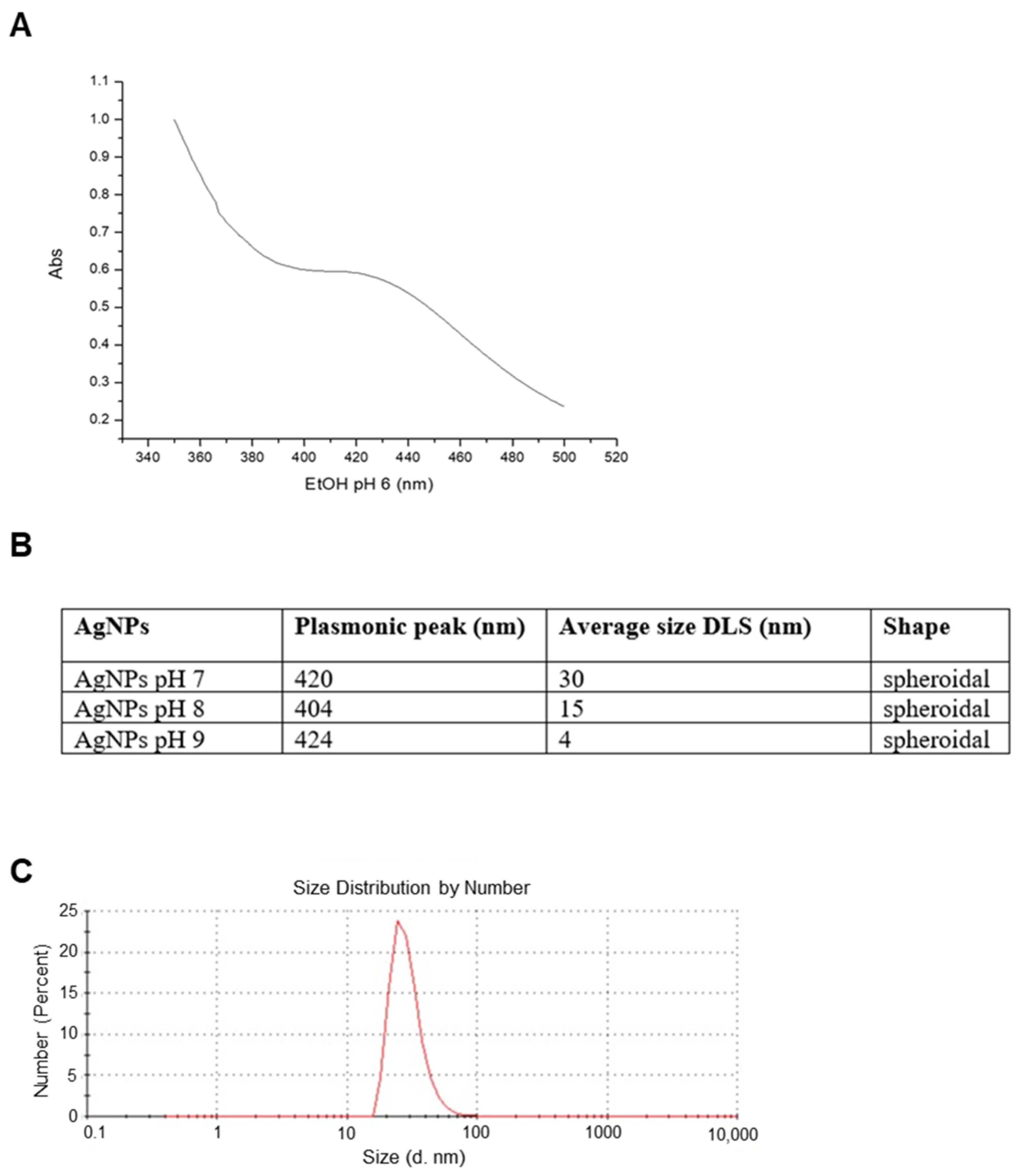
2.1. Nanoparticles Characterization and XTT Analysis
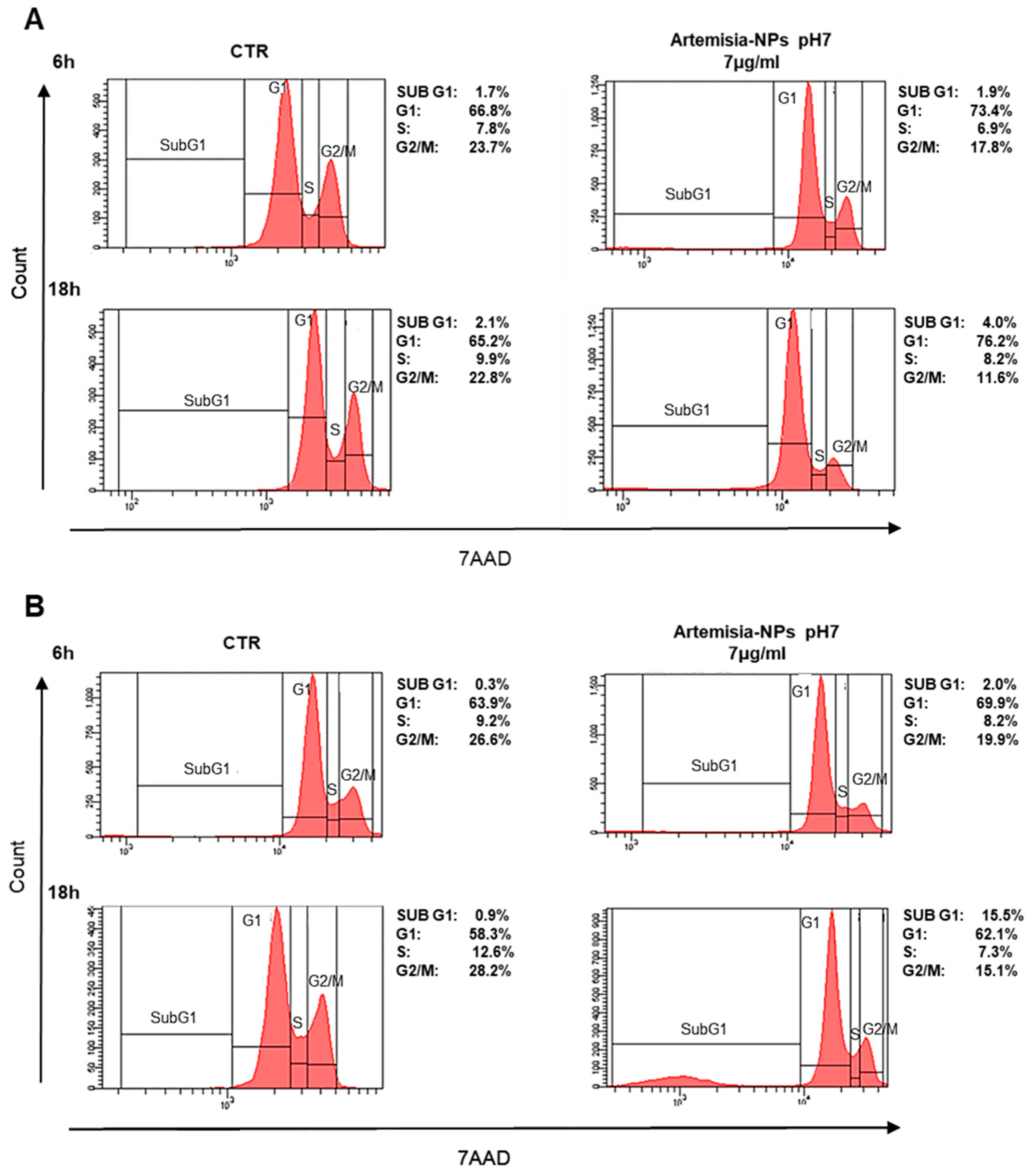
2.2. Cell Cycle Impact
2.3. Artemisia–AgNPs Induce Apoptosis and Inhibit Colony Formation in Cancer Cells
2.4. Artemisia–AgNPs Impact on Gene Expression
3. Discussion
4. Materials and Methods
4.1. Artemisa-AgNPs Synthesis and Characterization
4.2. Cell Cultures
4.3. Proliferation Assay (XTT)
4.4. Cell Cycle Analysis
4.5. Necrosis and Apoptosis Evaluation
4.6. Colony Assay
4.7. mRNA Extraction and Preparation of mRNA-Seq Library
4.8. Quality Control and Gene Analysis
4.9. GO Enrichment and KEGG Pathway Analysis
4.10. Key Modules and Hub Genes Identification
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Dobbelstein, M.; Moll, U. Targeting tumour-supportive cellular machineries in anticancer drug development. Nat. Rev. Drug Discov. 2014, 13, 179–196. [Google Scholar] [CrossRef]
- Arruebo, M.; Vilaboa, N.; Sáez-Gutierrez, B.; Lambea, J.; Tres, A.; Valladares, M.; González-Fernández, Á. Assessment of the evolution of cancer treatment therapies. Cancers 2011, 3, 3279–3330. [Google Scholar] [CrossRef] [Green Version]
- Tran, S.; DeGiovanni, P.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Transl. Med. 2017, 6, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.R.; Liu, C.; Zhang, B.; Yang, F.; Xu, J.; Long, J.; Jin, C.; Fu, D.L.; Ni, Q.X.; Yu, X.J. Carbon nanotubes in cancer diagnosis and therapy. Biochim. Biophys. Acta 2010, 1806, 29–35. [Google Scholar] [CrossRef]
- Gobbo, O.L.; Sjaastad, K.; Radomski, M.W.; Volkov, Y.; Prina-Mello, A. Magnetic Nanoparticles in Cancer Theranostics. Theranostics 2015, 5, 1249–1263. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current trends in the use of liposomes for tumor targeting. Nanomedicine 2013, 8, 1509–1528. [Google Scholar] [CrossRef] [Green Version]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold Nanoparticles in Cancer Treatment. Mol. Pharm. 2019, 16, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Grodzinski, P.; Silver, M.; Molnar, L.K. Nanotechnology for cancer diagnostics: Promises and challenges. Expert Rev. Mol. Diagn. 2006, 6, 307–318. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Crisponi, G.; Nurchi, V.M.; Lachowicz, J.I.; Remelli, M.; Zoroddu, M.A. Silver coordination compounds: A new horizon in medicine. Coord. Chem. Rev. 2016, 327, 349–359. [Google Scholar] [CrossRef]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Dayem, A.A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.M.; Choi, H.Y.; Cho, S.G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajabnia, T.; Meshkini, A. Fabrication of adenosine 5′-triphosphate-capped silver nanoparticles: Enhanced cytotoxicity efficacy and targeting effect against tumor cells. Process Biochem. 2018, 65, 186–196. [Google Scholar] [CrossRef]
- Bhunia, A.K.; Samanta, P.K.; Aich, D.; Saha, S.; Kamilya, T. Biocompatibility study of protein capped and uncapped silver nanoparticles on human hemoglobin. J. Phys. 2015, 48, 235305. [Google Scholar] [CrossRef]
- Wang, J.; Xu, C.; Wong, Y.K.; Li, Y.; Liao, F.; Jiang, T.; Tu, Y. Artemisinin, the Magic Drug Discovered from Traditional Chinese Medicine. Engineering 2019, 5, 32–39. [Google Scholar] [CrossRef]
- Efferth, T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin. Cancer Biol. 2017, 46, 65–83. [Google Scholar] [CrossRef]
- Konstat-Korzenny, E.; Ascencio-Aragón, J.A.; Niezen-Lugo, S.; Vázquez-López, R. Artemisinin and Its Synthetic Derivatives as a Possible Therapy for Cancer. Med. Sci. 2018, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xu, G.; Zhang, S.; Wang, D.; Prabha, P.S.; Zuo, Z. Antitumor Research on Artemisinin and Its Bioactive Derivatives. Nat. Prod. Bioprospect. 2018, 8, 303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saddi, M.; Sanna, A.; Cottiglia, F.; Chisu, L.; Casu, L.; Bonsignore, L.; De Logu, A. Antiherpevirus activity of Artemisia arborescens essential oil and inhibition of lateral diffusion in Vero cells. Ann. Clin. Microbiol. Antimicrob. 2007, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; ASabri, N.; Riaz, S.; Naseem, S. Size- and Shape-Dependent Antibacterial Studies of Silver Nanoparticles Synthesized by Wet Chemical Routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Park, J.C.; Jeon, G.E.; Kim, C.S.; Seo, J.H. Effect of the Size and Shape of Silver Nanoparticles on Bacterial Growth and Metabolism by Monitoring Optical Density and Fluorescence Intensity. Biotechnol. Bioprocess Eng. 2017, 22, 210–217. [Google Scholar] [CrossRef]
- Kamali, M.; Ghorashi SA, A.; Asadollahi, M. Controllable Synthesis of Silver Nanoparticles Using Citrate as Complexing Agent: Characterization of Nanopartciles and Effect of pH on Size and Crystallinity. Iran. J. Chem. Chem. Eng. Int. Engl. Ed. 2012, 31, 21–28. [Google Scholar]
- Avitabile, E.; Senes, N.; D’Avino, C.; Tsamesidis, I.; Pinna, A.; Medici, S.; Pantaleo, A. The potential antimalarial efficacy of hemocompatible silver nanoparticles from Artemisia species against P. falciparum parasite. PLoS ONE 2020, 15, e0238532. [Google Scholar] [CrossRef]
- Martínez-Castañón, G.A.; Niño-Martínez, N.; Martínez-Gutierrez, F.; Martínez-Mendoza, J.R.; Ruiz, F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J. Nanoparticle Res. 2008, 10, 1343–1348. [Google Scholar] [CrossRef]
- Qin, Y.; Ji, X.; Jing, J.; Liu, H.; Wu, H.; Yang, W. Size control over spherical silver nanoparticles by ascorbic acid reduction. Colloids Surf. A Phys. Eng Asp. 2010, 372, 172–176. [Google Scholar] [CrossRef]
- Urbańska, K.; Pająk, B.; Orzechowski, A.; Sokołowska, J.; Grodzik, M.; Sawosz, E.; Szmidt, M.; Sysa, P. The effect of silver nanoparticles (AgNPs) on proliferation and apoptosis of in ovo cultured glioblastoma multiforme (GBM) cells. Nanoscale Res. Lett. 2015, 10, 98. [Google Scholar] [CrossRef] [Green Version]
- Loutfy, S.A.; Al-Ansary, N.A.; Abdel-Ghani, N.T.; Hamed, A.R.; Mohamed, M.B.; Craik, J.D.; Eldin, T.A.; Abdellah, A.M.; Hussein, Y.; Hasanin, M.T.; et al. Anti-proliferative Activities of Metallic Nanoparticles in an in Vitro Breast Cancer Model. Asian Pac. J. Cancer Prev. 2015, 16, 6039–6046. [Google Scholar] [CrossRef] [Green Version]
- Sur, I.; Altunbek, M.; Kahraman, M.; Culha, M. Interaction of multi-functional silver nanoparticles with living cells. Nanotechnology 2010, 21, 175104. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Wu, S.H.; Hung, Y.; Mou, C.Y. Size effect on cell uptake in well-suspended, uniform mesoporous silica nanoparticle. Small 2009, 5, 1408–1413. [Google Scholar] [CrossRef]
- Khorrami, S.; Zarrabi, A.; Khaleghi, M.; Danaei, M.; Mozafari, M.R. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nanomed. 2018, 13, 8013–8024. [Google Scholar] [CrossRef] [Green Version]
- Al-Sheddi, E.S.; Farshori, N.N.; Al-Oqail, M.M.; Al-Massarani, S.M.; Saquib, Q.; Wahab, R.; Musarrat, J.; Al-Khedhairy, A.A.; Siddiqui, M.A. Anticancer Potential of Green Synthesized Silver Nanoparticles Using Extract of Nepeta deflersiana against Human Cervical Cancer Cells (HeLA). Bioinorg. Chem. Appl. 2018, 2018, 9390784. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.S.; Kim, D.W.; Lee, Y.H.; Oh, J.H.; Yoon, S.; Choi, M.S.; Lee, S.K.; Kim, J.W.; Lee, K.; Song, C.W. Silver nanoparticles induce apoptosis and G2/M arrest via PKCζ-dependent signaling in A549 lung cells. Arch. Toxicol. 2011, 85, 1529. [Google Scholar] [CrossRef] [PubMed]
- Kovács, D.; Igaz, N.; Keskeny, C.; Bélteky, P.; Tóth, T.; Gáspár, R.; Madarász, D.; Rázga, Z.; Kónya, Z.; Boros, I.M.; et al. Silver nanoparticles defeat p53-positive and p53-negative osteosarcoma cells by triggering mitochondrial stress and apoptosis. Sci. Rep. 2016, 6, 27902. [Google Scholar] [CrossRef] [Green Version]
- Satapathy, S.R.; Mohapatra, P.; Preet, R.; Das, D.; Sarkar, B.; Choudhuri, T.; Wyatt, M.D.; Kundu, C.N. Silver-based nanoparticles induce apoptosis in human colon cancer cells mediated through p53. Nanomedicine 2013, 8, 1307. [Google Scholar] [CrossRef]
- Çiftçi, H.; Türk, M.; Tamer, U.; Karahan, S.; Menemen, Y. Silver nanoparticles: Cytotoxic, apoptotic, and necrotic effects on MCF-7 cells. Turk. J. Biol. 2013, 37, 573–581. [Google Scholar] [CrossRef]
- Deng, L.; Meng, T.; Chen, L.; Wei, W.; Wang, P. The role of ubiquitination in tumorigenesis and targeted drug discovery. Signal Transduct. Target. Ther. 2020, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Hartshorn, C.M.; Bradbury, M.S.; Lanza, G.M.; Nel, A.E.; Rao, J.; Wang, A.Z.; Wiesner, U.B.; Yang, L.; Grodzinski, P. Nanotechnology Strategies To Advance Outcomes in Clinical Cancer Care. ACS Nano 2018, 12, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.; Dobrovolskaia, M.A. Addressing barriers to effective cancer immunotherapy with nanotechnology: Achievements, challenges, and roadmap to the next generation of nanoimmunotherapeutics. Adv. Drug Deliv. Rev. 2018, 141, 3–22. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Sun, B.; Liu, S.; Liu, H. Two-Dimensional Nanomaterials for Cancer Nanotheranostics. Small 2017, 13, 10. [Google Scholar] [CrossRef]
- Russell, L.M.; Liu, C.H.; Grodzinski, P. Nanomaterials innovation as an enabler for effective cancer interventions. Biomaterials 2020, 242, 119926. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Hsiao, A.; Mann, A.P.; Landry, M.G.; Meric-Bernstam, F.; Ferrari, M. Nanomedicine in cancer therapy: Innovative trends and prospects. Cancer Sci. 2011, 102, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- da Silva, P.B.; Machado RT, A.; Pironi, A.M.; Alves, R.C.; de Araújo, P.R.; Dragalzew, A.C.; Dalberto, I.; Chorilli, M. Recent Advances in the Use of Metallic Nanoparticles with Antitumoral Action—Review. Curr. Med. Chem. 2019, 26, 2108–2146. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Murtaza, G.; Rashid, F.; Iqbal, J. Eco-friendly green synthesis of silver nanoparticles and their potential applications as antioxidant and anticancer agents. Drug Dev. Ind. Pharm. 2019, 45, 1682–1694. [Google Scholar] [CrossRef] [PubMed]
- Huy, T.Q.; Huyen PT, M.; Le, A.T.; Tonezzer, M. Recent Advances of Silver Nanoparticles in Cancer Diagnosis and Treatment. Anticancer Agents Med. Chem. 2020, 11, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, Y.; Yan, J.; Ingle, T.; Jones, M.Y.; Mei, N.; Boudreau, M.D.; Cunningham, C.K.; Abbas, M.; Paredes, A.M.; et al. Size- and coating-dependent cytotoxicity and genotoxicity of silver nanoparticles evaluated using in vitro standard assays. Nanotoxicology 2016, 10, 1373–1384. [Google Scholar] [CrossRef]
- Mousavi, B.; Tafvizi, F.; Zaker, S. Green synthesis of silver nanoparticles using Artemisia turcomanica leaf extract and the study of anti-cancer effect and apoptosis induction on gastric cancer cell line (AGS). Artif. Cells Nanomed. Biotechnol. 2018, 46, 499–510. [Google Scholar] [CrossRef] [Green Version]
- Baghbani-Arani, F.; Movagharnia, R.; Sharifian, A.; Salehi, S.; Shandiz, S.A.S. Photo-catalytic, anti-bacterial, and anti-cancer properties of phyto-mediated synthesis of silver nanoparticles from Artemisia tournefortiana Rchb extract. J. Photochem. Photobiol. B 2017, 173, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Fard, N.N.; Noorbazargan, H.; Mirzaie, A.; Hedayati MCh Moghimiyan, Z.; Rahimi, A. Biogenic synthesis of AgNPs using Artemisia oliveriana extract and their biological activities for an effective treatment of lung cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, S1047–S1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.J.; Lee, S.J.; Yun, S.J.; Jang, J.Y.; Kang, H.; Kim, K.; Choi, I.H.; Park, S. Silver nanoparticles affect glucose metabolism in hepatoma cells through production of reactive oxygen species. Int J. Nanomed. 2016, 11, 55–68. [Google Scholar]
- Gurunathan, S.; Park, J.H.; Han, J.W.; Kim, J.H. Comparative assessment of the apoptotic potential of silver nanoparticles synthesized by Bacillus tequilensis and Calocybe indica in MDA-MB-231 human breast cancer cells: Targeting p53 for anticancer therapy. Int. J. Nanomed. 2015, 10, 4203–4222. [Google Scholar] [CrossRef] [Green Version]
- Swanner, J.; Mims, J.; Carroll, D.L.; Akman, S.A.; Furdui, C.M.; Torti, S.V.; Singh, R.N. Differential cytotoxic and radiosensitizing effects of silver nanoparticles on triple-negative breast cancer and non-triple-negative breast cells. Int. J. Nanomed. 2015, 10, 3937–3953. [Google Scholar]
- Webb, G.C.; Baker, R.T.; Coggan, M.; Board, P.G. Localization of the human UBA52 ubiquitin fusion gene to chromosome band 19p13.1-p12. Genomics 1994, 19, 567–569. [Google Scholar] [CrossRef]
- Warner, J.R.; McIntosh, K.B. How common are extraribosomal functions of ribosomal proteins? Mol. Cell 2009, 34, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Han, X.J.; Lee, M.J.; Yu, G.R.; Lee, Z.W.; Bae, J.Y.; Bae, Y.C.; Kang, S.H.; Kim, D.G. Altered dynamics of ubiquitin hybrid proteins during tumor cell apoptosis. Cell Death Dis. 2012, 3, e255. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Xie, B.; Kong, Y.; Tao, Y.; Yang, G.; Gao, M.; Xu, H.; Zhan, F.; Shi, J.; Zhang, Y.; et al. Overexpression of RPS27a contributes to enhanced chemoresistance of CML cells to imatinib by the transactivated STAT3. Oncotarget 2016, 7, 18638–18650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.W.; Kim, S.M.; Jin, D.H.; Kim, Y.S.; Hur, D.Y. RPS27a enhances EBV-encoded LMP1-mediated proliferation and invasion by stabilizing of LMP1. Biochem. Biophys. Res. Commun. 2017, 491, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.Y.; Lee, J.Y.; Kim, J. RpS3, a DNA repair endonuclease and ribosomal protein, is involved in apoptosis. FEBS Lett. 2004, 560, 81–85. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Xu, Q.; Wang, X.; Wang, J.; Mu, X.; Cai, Y.; Qian, Y.; Shao, W.; Shao, Z. RPLP1 promotes tumor metastasis and is associated with a poor prognosis in triple-negative breast cancer patients. Cancer Cell Int. 2018, 18, 170. [Google Scholar] [CrossRef]
- Teller, A.; Jechorek, D.; Hartig, R.; Adolf, D.; Reißig, K.; Roessner, A.; Franke, S. Dysregulation of apoptotic signaling pathways by interaction of RPLP0 and cathepsin X/Z in gastric cancer. Pathol. Res. Pract. 2015, 211, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Luo, G.; Wei, J.; Zheng, L.; Wang, H.; Yu, M.; Xu, N. Apolipoprotein M promotes growth and inhibits apoptosis of colorectal cancer cells through upregulation of ribosomal protein S27a. EXCLI J. 2021, 20, 145–159. [Google Scholar]
- Alam, E.; Maaliki, L.; Nasr, Z. Ribosomal protein S3 selectively affects colon cancer growth by modulating the levels of p53 and lactate dehydrogenase. Mol. Biol. Rep. 2020, 47, 6083–6090. [Google Scholar] [CrossRef]
- Wang, C.H.; Wang, L.K.; Wu, C.C.; Chen, M.L.; Lee, M.C.; Lin, Y.Y.; Tsai, F.M. The Ribosomal Protein RPLP0 Mediates PLAAT4-induced Cell Cycle Arrest and Cell Apoptosis. Cell Biochem. Biophys. 2019, 77, 253–260. [Google Scholar] [CrossRef]
- Pertile, P.; Baldi, A.; De Luca, A.; Bagella, L.; Virgilio, L.; Pisano, M.M.; Giordano, A. Molecular cloning, expression, and developmental characterization of the murine retinoblastoma-related gene Rb2/p130. Cell Growth Differ. 1995, 6, 1659–1664. [Google Scholar]
- Nieddu, V.; Pinna, G.; Marchesi, I.; Sanna, L.; Asproni, B.; Pinna, G.A.; Bagella, L.; Murineddu, G. Synthesis and Antineoplastic Evaluation of Novel Unsymmetrical 1,3,4-Oxadiazoles. J. Med. Chem. 2016, 59, 10451–10469. [Google Scholar] [CrossRef]
- Alam-Faruque, Y.; Dimmer, E.C.; Huntley, R.P.; O’Donovan, C.; Scambler, P.; Apweiler, R. The Renal Gene Ontology Annotation Initiative. Organogenesis 2010, 6, 71. [Google Scholar] [CrossRef] [Green Version]
- Weidong, L.; Sanna, L.; Bordoni, V.; Tiansheng, Z.; Chengxun, L.; Murineddu, G.; Pinna, G.A.; Kelvin, D.J.; Bagella, L. Target identification of a novel unsymmetrical 1,3,4-oxadiazole derivative with antiproliferative properties. J. Cell Physiol. 2021, 236, 3789–3799. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M. The KEGG Database. Novartis Found. Symp. 2002, 247, 91. [Google Scholar] [PubMed]







| Category | Term | Description | Gene Count | p Value |
|---|---|---|---|---|
| Biological Process (GO) | GO:0010629 | Negative regulation of gene expression | 12 | 2.33 × 10−12 |
| Biological Process (GO) | GO:0090304 | Nucleic acid metabolic process | 12 | 5.05 × 10−8 |
| Biological Process (GO) | GO:00427969 | DNA demage response, detection of DNA demage | 4 | 9.62 × 10−8 |
| Biological Process (GO) | GO:0042276 | Error-prone translesion synthesis | 4 | 2.46 × 10−6 |
| Biological Process (GO) | GO:0006297 | Nucleotide-excision repair, DNA gap filling | 3 | 3.52 × 10−6 |
| Biological Process (GO) | GO:0070911 | Global genome nucleotide-excision repair | 3 | 4.76 × 10−6 |
| Biological Process (GO) | GO:0000122 | Negative regulation of transcription by RNA polymerase II | 5 | 1.05 × 10−3 |
| KEGG_PATHWAY | Hsa03010 | Ribosome | 11 | 2.96 × 10−20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordoni, V.; Sanna, L.; Lyu, W.; Avitabile, E.; Zoroddu, S.; Medici, S.; Kelvin, D.J.; Bagella, L. Silver Nanoparticles Derived by Artemisia arborescens Reveal Anticancer and Apoptosis-Inducing Effects. Int. J. Mol. Sci. 2021, 22, 8621. https://doi.org/10.3390/ijms22168621
Bordoni V, Sanna L, Lyu W, Avitabile E, Zoroddu S, Medici S, Kelvin DJ, Bagella L. Silver Nanoparticles Derived by Artemisia arborescens Reveal Anticancer and Apoptosis-Inducing Effects. International Journal of Molecular Sciences. 2021; 22(16):8621. https://doi.org/10.3390/ijms22168621
Chicago/Turabian StyleBordoni, Valentina, Luca Sanna, Weidong Lyu, Elisabetta Avitabile, Stefano Zoroddu, Serenella Medici, David J. Kelvin, and Luigi Bagella. 2021. "Silver Nanoparticles Derived by Artemisia arborescens Reveal Anticancer and Apoptosis-Inducing Effects" International Journal of Molecular Sciences 22, no. 16: 8621. https://doi.org/10.3390/ijms22168621
APA StyleBordoni, V., Sanna, L., Lyu, W., Avitabile, E., Zoroddu, S., Medici, S., Kelvin, D. J., & Bagella, L. (2021). Silver Nanoparticles Derived by Artemisia arborescens Reveal Anticancer and Apoptosis-Inducing Effects. International Journal of Molecular Sciences, 22(16), 8621. https://doi.org/10.3390/ijms22168621







