Abstract
Intestinal dysfunction of farm animals, such as intestinal inflammation and altered gut microbiota, is the critical problem affecting animal welfare, performance and farm profitability. China has prohibited the use of antibiotics to improve feed efficiency and growth performance for farm animals, including poultry, in 2020. With the advantages of maintaining gut homeostasis, enhancing digestion, and absorption and modulating gut microbiota, organic acids are regarded as promising antibiotic alternatives. Butyric and citric acids as presentative organic acids positively impact growth performance, welfare, and intestinal health of livestock mainly by reducing pathogenic bacteria and maintaining the gastrointestinal tract (GIT) pH. This review summarizes the discovery of butyric acid (BA), citric acid (CA) and their salt forms, molecular structure and properties, metabolism, biological functions and their applications in poultry nutrition. The research findings about BA, CA and their salts on rats, pigs and humans are also briefly reviewed. Therefore, this review will fill the knowledge gaps of the scientific community and may be of great interest for poultry nutritionists, researchers and feed manufacturers about these two weak organic acids and their effects on intestinal health and gut microbiota community, with the hope of providing safe, healthy and nutrient-rich poultry products to consumers.
1. Introduction
Organic acids are weak acids having a carboxylic acid group (R-COOH), intermediates in the degradation pathways of carbohydrates, amino acids and fats, and are used as nutritional value and antimicrobial effects in animal feeds [1,2,3].
The use of organic acids in animal feeds started many years ago due to the ban on the use of antibiotics [4]. They are used as an antibiotic alternative that can alter the physiology and lead to the death of pathogenic microorganisms in animals, including poultry [5,6]. Various literatures reveal that antibiotics have better positive effects in modulating metabolism, improving weight gain, feed efficiency and controlling diseases in poultry production [7,8]. However, their continued use in animal nutrition developed antibiotic resistance and drug residues, which resulted in global public health issues and exacerbating poverty in the 21st century [9,10,11]. In this regard, organic acids are selected as a promising feed additive in poultry production due to their ability to maintain gut barrier cellular integrity, modulate intestinal microbiota, improve digestion and nutrient absorption rate and contribute to improved production performance [6,12,13]. BA as a short chain fatty acid (SCFA) and CA as a tricarboxylic acid (TCA) gained considerable attention as representative organic acids in poultry production. They are used as an energy source of prime enterocytes [14] or for the bactericidal efficacy against harmful species (for example, Escherichia coli) and the enhanced bone mineralization and improved function of gut microorganisms [15,16,17]. As organic acids, they are volatile and corrosive in their free forms; thus, they are commercially produced into salt forms [18,19,20] to increase palatability and bioavailability in the gut of birds [21,22]. Previous studies revealed that addition of active ingredients in salt forms into the diet of monogastric and young ruminants [16,23] could improve gut microbiota diversity and intestinal health and reduce microbial infections [24].
Each organic acid has its specific ability against pathogenic bacteria. For example, compared to medium-chain fatty acids (MCFAs), butyrate has a less strong anti-bacterial effect, although it has been widely used in animal production because of its low price [25,26,27]. Furthermore, previous studies largely focused on supplementation of blends of organic acids within short-chain fatty acids (SCFAs) or SCFAs with MCFAs on poultry challenged with Clostridium perfringens [28], Eimeria spp. [27] and salmonella typhimurium-related diseases [29,30]. A meta-analytic study of organic acids confirmed that blends of two or more specific organic acids improved performance, immunity and welfare of birds better than any acid achieved alone [4,8]. For example, Ndelekwute et al. studied the effects of four different acidifiers on gut performance and found that organic acids could be used in diets for broilers [31]. However, studies on blends of BA and CA on performance, nutrient digestion, intestinal health and meat quality of birds have not been found. They can be of great research interest for animal nutritionists and researchers. In addition, both have different modes of action and commonly increase the acidity of gut digesta and are important to keep the digestible nutrients under normal physiological conditions. Therefore, this review briefly summarizes the discovery of BA, CA and their salt forms, molecular structure and properties, metabolism, biological functions and their applications in poultry nutrition.
2. Discovery, Molecular Structure and Properties of BA and CA
BA, a SCFA with a four-carbon (C4) chain length was discovered by Adolf Lieben and Antonio Rossi in 1869. The name BA came from the Latin word, butyrum or buturum, meaning the acid of butter, as it was discovered from rancid butter [32,33]. It has a molecular formula of C4H8O2 and structural formula of CH3CH2CH2COOH [34]. It has synonyms called butanoic acid (CH3CH2CH2CO2H), n-butyric acid (a substance that was isolated from butter in 1869) and n-butanoic acid (International Union of Pure and Applied Chemistry, IUPAC) [35]. BA has a melting point of −7.9 °C, boiling point of 163.5 °C [36], molecular weight of 88.11 g/mol [37] and pKa value of 4.82 [38]. Butyrate and butanoate are also salts and ester forms of BA, respectively [39]. BA has an unpleasant odor, is a colorless liquid, and is potentially volatile and soluble in water, ethanol, and ether property [40]. Naturally, BA is synthesized from dietary fibers by anaerobic bacterial fermentation in the gut of mammals and birds [41,42].
Similarly, CA is a TCA or Krebs cycle acid with a six-carbon (C6) chain length discovered by Swedish chemist Carl Wilhelm Scheele in 1784 by crystallizing it from lemon juice [43,44]. The name CA came from the Latin word citrus, a tree naturally derived from citrus fruits and juices [45]. 3-carboxyl and 1-hydroxyl groups present in CA were recognized by Liebig in 1838, and calcium citrate was prepared from CA in 1860 in the United Kingdom, and in 1880 in France, Germany and the United States of America [46]. It is a weak organic acid with a chemical formula C6H8O7 and IUPAC name 2-hydroxypropane-1,2,3-tricarboxylic acid, also known as β-hydroxy-tricarballylic acid [47]. It has a boiling point of 175 °C, melting point of 153 °C and density of 1.67 g/cm3 [48] and molar mass of 192.12 g/mol [49]. In addition, CA has a molecular weight of 210.14 g/mol, gross energy 10.3 KJ/g [50] and three pKa values (pKa1 = 3.1, pKa2 = 4.7 and pKa3 = 6.4) [51]. It is an odorless, colorless crystal, highly soluble in water, ethanol and a sour taste property [52]. CA is normally used as a feed acidifier, flavoring agent and preservative in foods, beverages, detergents, cosmetics, toiletries and pharmaceuticals [53]. CA is a normal constituent in human and animal diets [54] and an intermediary substance in oxidative metabolism [55]. It is quickly metabolized to CO2 and H2O after ingestion. Supplementing CA in animal feed is safe and poses no risk to the environment [56]. Recently, the demand for commercially produced BA and CA acidifiers have increased worldwide [57,58,59].
3. Metabolism of BA and CA in Poultry
BA, CA and their salt forms play a crucial part in energy metabolism and keep gut homeostasis and epithelial integrity, participating in immune response, suppressing inflammation, and reducing oxidative stress in farm animals, such as poultry and pigs, and humans [60,61,62,63,64,65,66]. When free BA is given orally to poultry, it is rapidly metabolized and absorbed in the crop’s mucosa in the acidic environment of the gizzard and proventriculus [67]. This results in a higher concentration of BA in the foregut and leads to higher proteolytic activity of birds [68]. Therefore, protected butyrate is produced and fully utilized in the colon and cecum, which helps to improve epithelial barrier function, reduce inflammation and limit the invasion of pathogenic bacteria [67,69,70]. Butyrate is also mainly produced from dietary fibers (such as, cereals and grains) to a lesser extent from proteins via bacterial fermentation in the colon of mammals and the cecum of chicken [71,72,73,74]. Bacteroidetes and Firmicutes phyla are the most dominant chicken cecum microbiota (80%). They degrade structural carbohydrates and specific soluble oligosaccharides [69,75] that escape in the upper part of the digestion process [76,77]. In the cecum of chicken, bacterial fermentation coverts dietary fiber (complex polysaccharides) to monosaccharides and then to pyruvate and acetyl-CoA by pentose phosphate and glycolytic pathways [77]. Butyrate, the main energy source, is formed from acetyl-CoA condensation and a stepwise reduction of butyryl-CoA by two metabolic pathways [78] (Figure 1). The first pathway, Butyryl-CoA, an intermediate for the four-step pathway of butyrate production, is transformed to butyryl-phosphate via phosphorylation by the enzyme phospho-transbutyrylase. Butyryl phosphate is then converted to butyrate by the butyrate kinase enzyme [79,80]. In the second pathway, the enzyme butyryl-CoA: acetate CoA transferase, found in most gut bacteria families, transfers the acetyl-CoA moiety of butyryl-CoA to external acetate, leading to the formation of butyrate and acetyl-CoA [81]. Then, butyrate is absorbed in the gut lumen by enterocytes through two mechanisms as described by [82]. First, through simple diffusion of the undissociated form [42], which is used for villus growth and cell turnover. Second, in dissociated form, which is activated by the SCFA transporters such as monocarboxylate transport isoform 1 (MCT1), a H+ coupled transporter and sodium-linked monocarboxylate transport 1 (SMCT1). SMCT1 also known as solute carrier family five-member-eight (SLC5A8), a Na+ coupled transporter found only in the apical membrane of colonic epithelial cells [83]. Orally given butyrate is more efficient and quickly absorbed than naturally produced by bacterial fermentation in the cecum of birds [84]. The type of BA, form and salt or protection structure also affects its absorption rate in the gut. The addition of soluble fibers in the diet [85] and the addition of exogenous enzymes such as xylanase, which converts dietary arabinoxylans (main non-starch carbohydrates in wheat) into xylo-oligosaccharides [86,87], are also the most important strategies to increase BA availability and absorption in the GIT of birds.
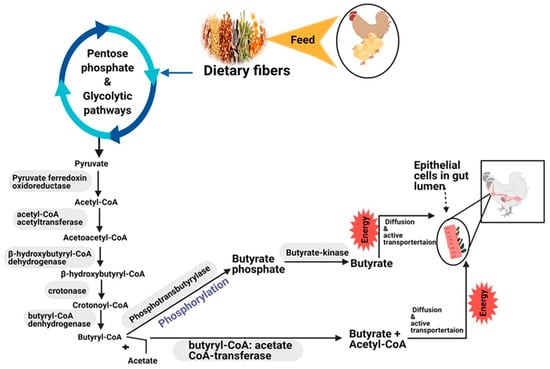
Figure 1.
Production pathways and absorption mechanism of butyrate (butyrate metabolism) from dietary fibers in the intestine of monogastric animals.
Similarly, CA is the first intermediate metabolic product formed through the TCA cycle [88] and a crucial element in the metabolic conversion of carbohydrates, fats and proteins to CO2 and H2O [33]. CA is produced by the reaction of oxaloacetate and acetyl-coenzyme A to yield citrate through citrate synthase enzyme [89,90]. In the mitochondria, acetyl-CoA is converted from pyruvate and used for energy production, whereas oxaloacetate is produced from pyruvate and CO2 using the enzyme pyruvate carboxylase in the cytoplasm [91].
Pyruvate is then transferred into the mitochondria and converted to acetyl-CoA by releasing CO2. Energy is then released and captured in the TCA cycle in the form of NADH, FADH2 and ATP [92,93]. Wolffram et al. and Tugnoli et al. summarized the study of intestinal absorption of CA from pig proximal jejunum [94,95], which may provide a reference for poultry. CA is absorbed via a Na-dependent co-transporter on the apical side and metabolized in the enterocyte directly influencing piglet’s intestinal metabolic status.
Literature from previous human studies also reveals that citrate is absorbed from the diet in the small intestine by means of the Na+-dicarboxylate cotransporters (NaDC1 and NaDC2) [96,97,98]. The enterocytes of the small intestine transport citrate out of the cells in the intestinal lumen. When CA is taken in by animals, it usually develops into the form of CA salts in the body. In general, there are limited findings on CA salts such as citrate (Na or K); metabolism in farm animals and human studies can be a baseline for future research work in poultry.
4. Biological Functions of BA and CA
4.1. Antibacterial Function, Acidity, Nutrient Absorption and Performance
Campylobacter sp., Salmonella Typhimurium, Escherichia coli sp., Shigella sp., Clostridium perfringens sp. and other Gram-negative bacteria species are common pathogens found in poultry farming [99,100]. These pathogens damage the villus–crypt units and intestinal mucosa of birds, lower the surface area for nutrient digestion and absorption, then reduce overall performance [101,102]. BA and CA are weak organic acids having different antibacterial actions, depending on the pKa value of the acids and intestinal pH [38,103] that cause the death of harmful bacteria in the gut of animals [16,104]. In BA, the undissociated form will enter and dissociate into butyrate (CH3CH2CH2COO−) and release H+ ions inside pathogenic bacteria cytoplasm [105,106]. This phenomenon lowers the pH value in the stomach/gut of livestock, causing enzymes inactivation [14], destroys DNA replication abnormity, and then disrupts pathogenic bacteria’s normal metabolic function [107]. Besides, the pathogenic bacteria itself consumes energy by activating proton pumps to fight against the lowering of the acid in the cell wall, thus inhibiting pathogenic bacteria growth and colonization in poultry intestines [16], whereas CA’s antibacterial activity creates an acidic condition in the stomach (pH 3.5–4.0), which prevents the growth of Salmonella, Escherichia coli and other acid-intolerant Gram-negative bacteria in the GIT of birds [108]. Its mechanism is to work through the activation of proteolytic enzymes and decrease the risk of sub-clinical infection [17].
Similarly, the acidic environment produced by CA in the stomach promotes lactobacilli growth and prevents pathogenic bacteria multiplications [109]. In addition to the antibacterial function of BA and CA, various studies examined their effects at different dose levels, product forms, and comparisons with antibiotics on nutrient digestibility, growth, and meat yield of poultry. Broilers fed at 0.2% BA significantly increased carcass weight and breast meat yield versus birds fed the control diet [110]. A supportive study on sodium butyrate (SB) at 0.6 and 1.2 g/kg in a broiler significantly increased average daily gain (ADG) (27.6 g) and feed conversion ratio (FCR) (1.8) during 1–21 days [111]. Salt forms of BA are slowly absorbed in the foregut gut (crop, proventriculus and gizzard) and are more effectively absorbed in the hindgut (duodenum, jejunum, ileum and ceca), inhibit the growth of pathogenic bacteria, and they improve the overall performance of birds [22,38]. Song et al. also proved the effects of microencapsulated SB (MESB) orally infected or uninfected with Eimeria species and Clostridium perfringens at 12 days of age followed by an oral inoculation with Clostridium perfringens at 16, 17 and 18 d of age on the growth performance of broilers. Broiler-fed MESB at 800 mg/kg feed challenged with necrotic enteritis showed higher total body weight, daily gain, and FCR at 35 days [112]. In contrast, insignificant changes observed in growth performances of commercial laying hens supplemented with 193, 136 and 198 g/t protected SB (PSB), respectively, whereas the quadratic effect showed maximization of eggshell thickness, percentage and strength sequentially with addition of PSB at 112 days of age [113]. Similarly, the antimicrobial effects of n-butyric acid and its derivatives (Monobutyrin (MB) and a mixture of mono-, di-, and tri-glycerides of BA) at concentrations from 250–7000 mg/kg inoculated either Salmonella typhimurium or Clostridium perfringens. The results showed that n-butyric acid and 50% MB could be used to control Salmonella Typhimurium or Clostridium perfringens in poultry [114].
Similarly, protected calcium butyrate (PCB) feed for broilers was at 0, 0.2, 0.3 and 0.4 g/kg and the results showed chicken-fed PCB at 0.3 g/kg had higher weight gain (125 g) than 0.2 (80) and 0.4 g/kg (83 g), respectively. Moreover, the apparent overall crude fat digestibility, apparent nitrogen corrected metabolic energy and FCR, increased during the entire experimental period [67]. The reason may be that butyrate increases the cell concentration of Ca2+ pancreatic cells, inhibits the growth of bile salt deconjugating bacteria, reduces the utilization of nutrients by microorganisms, and improves the digestibility and absorption rate of nutrients in broilers. Comparatively, Chowdhury et al. examined the effects of CA at 0.5% and avilamycin at 0.001% on broilers and obtained significant growth performance parameters versus avilamycin or control diets at 35 days of age [108]. Similar CA findings in laying hens and broilers showed a positive response to stimulate pepsin activity, support protein digestion, increase apparent digestibility and phosphorus bioavailability [115,116].
In summary, several studies recommended the importance of BA, CA and their salts to reduce the load of pathogenic microorganisms in the intestine, activate digestive enzymes, improve the digestibility and absorption of nutrients, gut microflora function and performance of birds [105,113,117,118,119]. Table 1 also summarizes various findings that support the above summary.

Table 1.
Effects of the different forms of BA and CA on growth, digestibility and carcass yield of broilers.
4.2. Gut Morphology and Barrier Function
Gut morphology, barrier function, and intestinal microbiota community of birds are vulnerable due to many detrimental environmental or nutritional factors [100], which lead to leaky gut, dysbiosis, failed intestinal barrier permeability and intestinal inflammation in poultry [127,128]. The intestinal dysfunction may cause reduced nutrient absorption surface area and growth performance [129]. Supplementation of BA, CA and their salts are among the strategies to modulate gut microbiota and keep poultry intestinal health [130]. They can promote intestinal epithelial cell proliferation and increase villus height (VH), thereby improving the absorptive surface area of the GIT [107]. Broilers supplemented with coated SB (CSB) at 0, 200, 400, 800 or 1000 mg/kg showed improved intestinal integrity by stimulating goblet cells in jejunum and increasing ileal VH at 42 days age [22]. The authors also found that SB at 800 mg/kg can lead to higher total antioxidant capacity (T-AOC) and reduced malondialdehyde (MDA) content in chicken jejunal mucosa. Likewise, Elnesr et al. mentioned the inclusion of SB at 0.5 and 1 g/kg increased villus length (VL) at day 21 (55 and 27%) and day 42 (39 and 18%), respectively, versus the basal diet [106]. Butyrate can also benefit pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α), interleukin-1 (IL-1), interleukin-2 (IL-2), and interleukin-6 (IL-6) which are known to increase epithelial cell permeability in young broiler chicks [131]. Zou et al. conducted confirmatory studies to prove the above statement by supplementing SB at T1: CON, T2: DSS, T3: 150 mg/kg SB and T4: 300 mg/kg levels in female Chinese Yellow broilers. The result revealed 300 mg/kg SB significantly reduced IL-6 and IL-1β levels, whereas it increased IL-10. At the same time, it reduced the lesion score of intestinal bleeding and increased VH and the total mucosa area of the ileum [132]. These cytokines cause a homeorhetic response that changes the portioning of nutrients during inflammatory reactions in the gut of chickens [133]. In other studies, calcium butyrate administration in the colon of rats and BA in humans was found to have anti-inflammatory effects of treating inflammatory colon diseases [134,135]. Yan and Ajuwon also confirmed that butyrate could decrease lipopolysaccharide (LPS) damage on intestinal barrier integrity and tight junction (TJ) permeability and increased the abundance of claudin-3/4 expression [136]. MESB upregulated TJ protein expressions such as claudin-1, claudin-4, occludin, ZO-1, mucin-2, chicken liver-expressed antimicrobial peptides (cLEAP-2), and thus reduced intestinal mucosal barrier damage in Necrotic enteritis infected broilers [112]. A recent study also reported similar positive results on intestine gene expression in coccidia infected broilers supplemented with tributyrin (TB) [137]. The better secretion of mucin-2 by goblet cells prevents the attachment of pathogens to the epithelial tissues by cLEAP-2. Many researchers studied anti-inflammatory and immune-enhancing properties of SB, which influences IL-6, IL-8, IFN-γ, TGF-β and IL-1β inflammatory cytokine expression in broilers and piglets [22,138,139].
CA causes an acidic condition in poultry gut, decreases pathogenic bacteria and improves intestinal morphology and barrier function [140]. CA administration at 3% and 6% significantly increased VL, crypt depth (CD) and goblet cell numbers in the duodenum, jejunum and ileum as well as villus weight (VW) and villus length to crypt depth (VL: CD) ratio in the duodenum of broilers at 42 days of age [141]. A study by Nourmohammadi and Khosravinia also revealed that 30 and 60 g/kg CA in broiler diets significantly improved the weight of proventriculus, gizzard, ileum and length of jejunum and ileum as compared to the control [142]. A similar finding by Khosravinia et al. [126] proved a significant increment in VL, CD and the number of goblet cells in the small intestine of broilers fed 30 g/kg CA with corn-soybean diets. CA decreases pathogenic bacteria colonization and limits toxic metabolite production in the GIT of birds [143]. In general, BA and CA with their salts can be a potential feed additive to maintain gut integrity, improving barrier function and enhancing poultry productivity (Table 2).
4.3. Immune Function and Antioxidation
Additionally, the crucial role of dietary BA and CAs in enhancing the immune system function was mentioned by various authors [16,65,144]. Butyrate also influences the immune response of animals by affecting immune cell migration, adhesion, proliferation and differentiation [60,80], and maintains gut homeostasis in chickens [145]. Many studies suggested that BA as a feed additive for animals decreased oxidative stress and contributed to better nutrient digestibility and growth rate [4,57,146]. It is proven that supplementation of butyrate glyceride can modulate intestinal microflora and serum metabolites to maintain intestinal metabolism homeostasis in broilers [76]. Recent studies showed linear inclusion of SB significantly increased the relative weight of the thymus, the foundation to achieve immune function and antibody titer, which improves the humoral immunity of broilers, protecting against Newcastle disease (NCD) infection [111,147]. Butyrate has powerful effects on several colonic mucous functions, such as inflammatory suppression and carcinogenesis, improving colonic protective barrier elements and reducing oxidative stress [82]. Orally administered butyrate at 0.25 or 1.25 g/kg doses for 5 days caused histone protein H2A hyperacetylation in broilers regardless of the dosage level [148].
Similarly, Moquet found that butyrate supplementation can raise the concentration of digesta butyrate in the gastric, ileum and colon regions. It helped β-oxidation of lipids and showed a positive immune response in chickens [131]. The reason may be the promotion of glycolysis and location impact of butyrate on energy metabolism in the GIT of broilers. Zhou et al. also studied immunomodulatory and protective effects of butyrate on the avian macrophages in the presence or absence of LPS challenged by salmonella typhimurium. The results showed that butyrate inhibited IL-1, IL-6, and IFN-γ expression in LPS-stimulated cells, suggesting that it could be used to regulate inflammation and immune homeostasis in chickens [29]. This is due to the inhibitory action of butyrate on the activation of nuclear factor (NF-κB) via IκB-α and IκB-β stabilization.
Furthermore, Mátis et al. examined the effects of orally given sodium butyrate at 0.25 g/kg body weight on insulin signaling of broilers from 20–24 days. Orally butyrate-treated groups increased glucose plasma and insulin levels versus the control birds [72]. Since oral butyrate administration is more effective and absorbed faster [148], it appears to act as a bioactive molecule in extrahepatic tissues, causing changes in insulin signaling. Besides, butyrate also acts as a ligand for GPCRs such as GPR109A, GPR43, and GPR41, contributing to the immune system, homeostasis, and inflammation by activating anti-inflammatory signaling cascades [83,149].
CA also enhances the density of lymphocytes, a principal constituent of the bird’s immune system, to combat antigens in the lymphoid organs and boost non-specific immunity [107]. CA at 0.5% improved specific and non-specific immunity against NCD vaccinated broilers [150]. Furthermore, the inclusion of CA at 0.5% in a corn–soybean basal starter chicken diet improved tibial ash deposition, lymphocyte organs, and tissue densities to fight against pathogens [151]. Besides, supportive findings showed that broilers supplemented with 30 and 60 g/kg CA increased thymus and bursa fabricius index, respectively, which correlates with the improved immune response of broilers at 42 days [142]. The authors concluded that 60 g/kg CA caused acidic stress, severely reduced performance and disrupted liver function in chickens.
Conversely, Lakshmi and Sunder recommended that 2% CA was the ideal inclusion level and more effective in stimulating humoral immune response and higher antibody titers in broilers at 42 days of age [152]. Similarly, previous research findings stated that the inclusion of CA at 2.5% in the diet of rabbits improved lymphocyte cells, which are the second most common leukocytes that increase the protection mechanism against non-specific pathogens to boost immunity [153]. Based on the various research findings, it can be concluded that the relative increment in the weight of immune organs, inactivation of NF-κB with dietary supplementation of BA or CA with their derivatives improved immune responses and health of broilers (Table 2).

Table 2.
The responses of different forms of BA and CA on histomorphology, immune organs and serum biochemistry of broilers.
Table 2.
The responses of different forms of BA and CA on histomorphology, immune organs and serum biochemistry of broilers.
| Forms of BA/CA | Broiler strain and Trial Duration (Day) | Study Layout and Dosage Levels | Responses Expressed as a Percentage of Respective Controls | Reference |
|---|---|---|---|---|
| BA | Broiler chicks for 42 days | T1: CTR, T2: 20 mg/kg BMD, T3: 3 g/kg BA, T4: 4 g/kg BA | ↑ GLUT5, SGLT1 and PepT1 expression. ↑ humoral, cell-mediated immune responses and serum biochemistry at T4. ↑VL and VD. | [154] |
| SB | M77 Hubbard broiler at d-21 and d-35 | T1: CTR, T2: 0.1 g/kg ZnB, T3: 0.5 g/kg SB, T4: 1.0 g/kg SB | ↑ antibody titer against NCD and SRBCs. ↑ Thymus, spleen and bursa weight. ↑ Duodenum and Jejunum VH. ↑Goblet cells in the SI and ileum. | [147] |
| SB | Cobb 400 broiler for 42 days | T1: CTR, T2: AB (50 ppm), T3: 0.09% CSB, T4: 0.18% CSB, T5: 0.03% UCSB, T6: 0.06% UCSB | Cecal Escherichia coli and Clostridium perfringens count reduced with the addition of CSB. ↑ Jejunum VH, VH: CD ratio and VH: VW ratio with addition of CSB by 0.18%. | [155] |
| SB | Arbor Acres broilers for 45 days | T0: CTR, T1: 0.3, T2: 0.6 and T3: 1.2 g/kg SB | ↑ weight and length of duodenum, jejunum, ileum, SI, pancreas, thymus, and length of caeca. ↑ Antibody titer against NCD. | [111] |
| SB | Broiler chicks | SB with or without Salmonella typhimurium (LPS) challenged disease | SB ↓IL-1, IL-6, IFN-γ, and IL-10 in LPS-stimulated cells. ↓TGF-3 expression in both cases. | [29] |
| ESB | Female Chinese Yellow broilers | T1: CON, T2: DSS, T3: 150 mg/kg SB, T4: 300 mg/kg SB | ↓ Lesion scores of intestinal bleedings. ↑ VH and ileum total mucosa. ↓ D (-)-lactate level, IL-6, and IL-1β. ↑ interleukin-10. | [132] |
| PPSB | Mixed Cobb chicks at 1–14, 15–28 and 29–42 days. | T1: CTR, T2: AB (100,000 IU/kg), T3: 700 ppm PSB | ↑Jejunum and SI length, jejunal villi. T2 produced deepest crypts and lowest VH:CD ratio in all intestinal segments at d-14. | [156] |
| CA | Male Ross 308 broiler for 42 days | Exp. 1: T1: 0, T2: 10, T3: 20, T4: 30 g/kg CA Exp. 2: T1: 0, T2: 30, T3: 60 g/kg CA | Exp.1: ↑ proventriculus weight and IL. ↑ Duodenum, jejunum and ileum, VL. ↑ CD and VL: CD ratio. ↓ epithelial thickness of the Jejunum. Exp. 2: ↑ gizzard weight and IL. ↑ proventriculus, intestine, gizzard, JL and ileum. ↑ VL, CD, and goblet cell count in the hindgut. ↓ Epithelial thickness in the SI. | [126] |
Antibiotic as a growth promotor (oxytetracycline, colistin sulfate/Colival) (AB); antibiotic bacitracin methylene di-salicylate (BMD); dextran sulfate sodium (DSS); Negative control (CON); encapsulated SB (consists 99.9% butyrate salt) (ESB); glucose transporter 5 (GLUT5); ileum length (IL); jejunum length (JL); zinc bacitracin (ZnB); peptide transporter (PepT1); partially protected sodium butyrate (PPSB); sheep red blood cells (SRBCs); sodium-dependent glucose transporter (SGLT1); small intestine (SI); uncoated sodium butyrate (UCSB); villi length (VL); villi width (VD) increased (↑); decreased (↓).
5. Application of BA and CA in Poultry Nutrition
BA and CA are potent feed additives used in livestock nutrition, and we have gained special insights due to gut health enhancer and antimicrobial activity [21,65,142,145,150]. Physical form, flavor property, water solubility and safety concerns of BA and CA are the crucial factors for effective delivery to target gut compartments of animals including birds [95,157,158]. The rate of acid absorption and the compartments of the GIT are closely associated with the various application techniques. These include unprotected butyrate absorbed in the crew, stomach and gizzard, whereas TB is absorbed in the SI and fat-coated butyrate is absorbed throughout the GI tract [159]. BA can be supplemented as straight (natural BA), butyrate (salt forms), coated/encapsulated (lipid shell) salts of butyrate [57,103] and butyrate glycerides (butyrin) [123]. Each form of the product has its advantages and limitations in terms of bioavailability, cost, handling safety, stability, processing temperature/pressure, releasing target in the GIT, and feasibility [38]. In a comparative study to evaluate encapsulated BA at 500 g/t (T2) and protected SB (PSB) at 700 g/t (T3) of feed in laying hens, the results indicated that T2 (41.3 g/kg BW) showed higher (P = 0.005) weight of SI contents than the control diet (35.0 g/kg BW) and T3 (36.5 g/kg BW) group of birds [160].
In contrast, higher total SCFAs and BA concentration in the cecal digesta were observed in T3 versus T1 and T2 groups. However, the addition of the two BA sources (T2 and T3) increased cecal microflora activity of enzymes in birds and noted beneficial effects on eggshell quality, the tibia, and selected GIT parameters [160]. Similarly, Xiong et al. proved that CSB was less effective than TB in improving gut morphology in LPS-challenged broilers [30] because CSB rapidly absorbs in the upper intestine, whereas TB is slowly reached and is more absorbed in the SI. A supportive study for the above findings was conducted by van den Borne et al. on feeding uncoated vs. fat-coated CB on stomach passage time in the GIT of broilers. The results showed that 80% of uncoated CB is oxidized and absorbed from the upper digestive tract. In comparison, fat-coated CB extended-release pattern time of more than six hours indicated that the coating process delayed release more in the lower intestinal segments [161]. Pires et al. also examined the effects of PSB at 0 or 0.105 g/kg on the percentage of broken and dirty eggs at commercial chicken farms. The number of dirty and broken eggs was reduced in laying hens fed 0.105 g/kg PSB versus the control diet [113]. Likewise, PSB significantly increased carcass weight versus unprotected SB or without butyrate in broilers diet, which indicates the role of butyrate in increasing poultry meat production [84]. Therefore, the above findings showed that targeted delivery of organic acids through a coating or encapsulation process effectively exerts the antimicrobial function in the GIT. This reduces coliform counts both in the distal jejunum, cecum and SI, as the main sites of bacterial activity in chicken [95,162]. There is currently a novel application technique called in ovo administration of BA, that occurs in the early life stages of chickens to establish a healthy intestinal microflora environment in the early post-hatch stage of chicks [163,164]. At the early age of birds, butyrate is used as a direct energy source to the gut epithelium and can reduce intestinal inflammation by promoting mucus secretion [57]. In general, and as a witness for its contribution, the global animal feed industry reported protected BA to be the second top (30%) product as a replacement of antibiotics, followed by probiotics (50%), to improve the gut health of broilers.
Similarly, CA as a potential growth promoter and potent chelator of calcium can produce a favorable environment for endogenous and exogenous microbial enzymes such as phytase, which improves the hydrolysis of phytate available in poultry feedstuffs [17,141]. Fazayeli-Rad et al. showed that 30 g/kg CA improved growth performance and nutrient retention of male broilers at 42 days due to improved phosphorus availability [165]. Islam et al. also studied the effects of dietary CA at 0%, 0.25%, 0.75% and 1.25% on broiler performance and mineral metabolism for 35 days. Bodyweight, feed conversion efficiency (FCE), carcass weight, and graded carcasses increased sequentially with increasing CA levels. In addition, mineral digestibility, bone ash content and mineral density, and strength also significantly increased up to 0.75% level of CA [125]. A supportive study on growing rabbits at 0%, 0.5%, 1.0% and 1.5% CA supplementation was examined for 56 days. The growth rate and growth velocity increased linearly with the increasing CA addition and concluded that 1.5% was an effective dosage for rabbits’ positive growth performance [166]. In summary, the type of product, inclusion level, bird type and age, diet composition and particle size, methodology, experimental duration, LPS challenges, and environmental stress are the main factors determining the effectiveness of BA and CA on broiler performance and intestinal development (Table 1 and Table 2).
6. Conclusions and Future Research Directions
The goal of market-oriented modern poultry production depends on high feed efficiency and the health of the gut. The withdrawal of antibiotics in the diet of poultry brought numerous options as antibiotics alternatives, among which organic acids acquire significant attention. In animal nutrition, organic acids and their salts are cost-effective, performance-enhancing options and exert antibacterial, pH reduction effects with the function of energy supply. BA and CAs, as representatives of organic acids, significantly impact their biological functions of antimicrobial activity as a gut health enhancer. They are used as the main energy source of metabolic activities and reduce pathogenic bacterial load in the GIT of birds. The supplementation results in poultry are not consistent due to different conditions such as the type of product (application methods), inclusion levels, target release of the products in the GIT, age, and sex strains of birds. BA and CA are promising antibiotic replacers and are significant concerns in future research work on poultry nutrition. In addition, blends of organic acids or simple monocarboxylic acids/SCFA (e.g., BA) and Krebs cycle acids/TCA/carboxylic acids bearing a hydroxyl group (e.g., CA) are not common and need further investigation about their interactive effects on intestinal microbiota composition, diversity, gut health, and immunity as well as growth performance in poultry.
Author Contributions
M.M. wrote the full manuscript; R.Z., H.H. and F.W., visualization and technical support; B.Y. reviewed and edited the manuscript; H.Z., supervision, project administration and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Major Scientific Research Tasks for Scientific and Technological Innovation Projects of the Chinese Academy of Agricultural Sciences, grant number CAAS-ZDRW202006-02, National Natural Science Foundation of China, grant number 31702119, National Key Research and Development Program of China, grant number 2016YFD0500501, China Agriculture Research System, grant number CARS-41, State Key Laboratory of Animal Nutrition, grant number 2004DA125184G2102 and Central Public-interest Scientific Institution Basal Research Fund, grant number Y2021GH01-4.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The Chinese Government Scholarship for supporting this study, the Graduate School of Chinese Academy of Agricultural Science and Institute of Animal Science for administrative and technical support are also appreciated.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Peng, M.; Salaheen, S.; Biswas, D. Animal Health: Global Antibiotic Issues. In Encyclopedia of Agriculture and Food Systems; Van Alfen, N.K., Ed.; Academic Press: Oxford, UK, 2014; pp. 346–357. [Google Scholar]
- French, D. Chapter Five—Advances in Clinical Mass Spectrometry. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: San Francisco, CA, USA, 2017; Volume 79, pp. 153–198. [Google Scholar]
- Chahardoli, A.; Jalilian, F.; Memariani, Z.; Farzaei, M.H.; Shokoohinia, Y. Analysis of organic acids. In Recent Advances in Natural Products Analysis; Sanches Silva, A., Nabavi, S.F., Saeedi, M., Nabavi, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 26; pp. 767–823. [Google Scholar]
- Polycarpo, G.V.; Andretta, I.; Kipper, M.; Cruz-Polycarpo, V.C.; Dadalt, J.C.; Rodrigues, P.H.M.; Albuquerque, R. Meta-Analytic Study of Organic Acids as an Alternative Performance-Enhancing Feed Additive to Antibiotics for Broiler Chickens. Poult. Sci. 2017, 96, 3645–3653. [Google Scholar] [CrossRef] [PubMed]
- Broom, L.J. Organic Acids for Improving Intestinal Health of Poultry. Worlds Poult. Sci. J. 2015, 71, 630–642. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Kim, I.H. Protected Organic Acids Improved Growth Performance, Nutrient Digestibility and Decreased Gas Emission in Broilers. Animals 2020, 10, 416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehdi, Y.; Létourneau-Montminy, M.-P.; Gaucher, M.-L.; Chorfi, Y.; Suresh, G.; Rouissi, T.; Brar, S.K.; Côté, C.; Ramirez, A.A.; Godbout, S. Use of Antibiotics in Broiler Production: Global Impacts and Alternatives. Anim. Nutr. 2018, 4, 170–178. [Google Scholar] [CrossRef]
- Gao, C.Q.; Shi, H.Q.; Xie, W.Y.; Zhao, L.H.; Zhang, J.Y.; Ji, C.; Ma, Q.G. Dietary Supplementation with Acidifiers Improves the Growth Performance, Meat Quality and Intestinal Health of Broiler Chickens. Anim. Nutr. 2021, 7, 762–769. [Google Scholar] [CrossRef]
- Landers, T.F.; Cohen, B.; Wittum, T.E.; Larson, E.L. A Review of Antibiotic Use in Food Animals: Perspective, Policy and Potential. Public Health Rep. 2012, 127, 4–22. [Google Scholar] [CrossRef] [Green Version]
- Fair, R.J.; Tor, Y. Antibiotics and Bacterial Resistance in the 21st Century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirchhelle, C. Pharming Animals: A Global History of Antibiotics in Food Production (1935–2017). Palgrave Commun. 2018, 4, 96. [Google Scholar] [CrossRef] [Green Version]
- Dibner, J.J.; Buttin, P. Use of Organic Acids as a Model to Study the Impact of Gut Microflora on Nutrition and Metabolism. J. Appl. Poult. Res. 2002, 11, 453–463. [Google Scholar] [CrossRef]
- Dai, D.; Qiu, K.; Zhang, H.; Wu, S.; Han, Y.; Wu, Y.; Qi, G.; Wang, J. Organic Acids as Alternatives for Antibiotic Growth Promoters Alter the Intestinal Structure and Microbiota and Improve the Growth Performance in Broilers. Front. Microbiol. 2020, 11, 618144. [Google Scholar] [CrossRef]
- Hosna, H. Application of Organic Acids in Poultry Nutrition. Int. J. Avian Wildl. Biol. 2018, 3, 324–329. [Google Scholar] [CrossRef] [Green Version]
- Panda, A.K.; Rao, S.V.R.; Raju, M.V.L.N.; Sunder, G.S. Effect of Butyric Acid on Performance, Gastrointestinal Tract Health and Carcass Characteristics in Broiler Chickens. Asian Australas. J. Anim. Sci. 2009, 22, 1026–1031. [Google Scholar] [CrossRef]
- Deepa, K.; Purushothaman, M.R.; Vasanthakumar, P.; Sivakumar, K. Butyric Acid as an Antibiotic Substitute for Broiler Chicken—A Review. Adv. Anim. Vet. Sci. 2018, 6, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.Z.H.; Afzal, M.; Fatima, M. Prospects of Using Citric Acid as Poultry Feed Supplements. J. Anim. Plant. Sci. 2018, 28, 12. [Google Scholar]
- Huyghebaert, G.; Ducatelle, R.; Immerseel, F.V. An Update on Alternatives to Antimicrobial Growth Promoters for Broilers. Vet. J. 2011, 187, 182–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, W.K.; Koh, C.B. The Utilization and Mode of Action of Organic Acids in the Feeds of Cultured Aquatic Animals. Rev. Aquac. 2017, 9, 342–368. [Google Scholar] [CrossRef]
- Górka, P.; Kowalski, Z.M.; Zabielski, R.; Guilloteau, P. Use of Butyrate to Promote Gastrointestinal Tract Development in Calves. J. Dairy Sci. 2018, 101, 4785–4800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.H.; Iqbal, J. Recent Advances in the Role of Organic Acids in Poultry Nutrition. J. Appl. Anim. Res. 2016, 44, 359–369. [Google Scholar] [CrossRef]
- Wu, W.; Xiao, Z.; An, W.; Dong, Y.; Zhang, B. Dietary Sodium Butyrate Improves Intestinal Development and Function by Modulating the Microbial Community in Broilers. PLoS ONE 2018, 13, e0197762. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.M.; Aragona, K.M.; Moreland, S.C.; Erickson, P.S. Supplementation of Sodium Butyrate to Post Weaned Heifer Diets: Effects on Growth Performance, Nutrient Digestibility and Health. J. Dairy Sci. 2019, 102, 3121–3130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maki, J.J.; Klima, C.L.; Sylte, M.J.; Looft, T. The Microbial Pecking Order: Utilization of Intestinal Microbiota for Poultry Health. Microorganisms 2019, 7, 376. [Google Scholar] [CrossRef] [Green Version]
- Van Immerseel, F.; Russell, J.B.; Flythe, M.D.; Gantois, I.; Timbermont, L.; Pasmans, F.; Haesebrouck, F.; Ducatelle, R. The Use of Organic Acids to Combat Salmonella in Poultry: A Mechanistic Explanation of the Efficacy. Avian Pathol. 2006, 35, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Baldevraj, R.S.M.; Jagadish, R.S. Incorporation of chemical antimicrobial agents into polymeric films for food packaging. In Multifunctional and Nanoreinforced Polymers for Food Packaging; Lagarón, J.M., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 368–420. [Google Scholar]
- Nguyen, D.H.; Lee, K.Y.; Mohammadigheisar, M.; Kim, I.H. Evaluation of the Blend of Organic Acids and Medium-Chain Fatty Acids in Matrix Coating as Antibiotic Growth Promoter Alternative on Growth Performance, Nutrient Digestibility, Blood Profiles, Excreta Microflora, and Carcass Quality in Broilers. Poult. Sci. 2018, 97, 4351–4358. [Google Scholar] [CrossRef]
- Pereira, R.; Menten, J.F.M.; Bortoluzzi, C.; Napty, G.S.; Longo, F.A.; Vittori, J.; Lourenço, M.C.; Santin, E. Organic Acid Blend in Diets of Broiler Chickens Challenged with Clostridium Perfringens. J. Appl. Poult. Res. 2015, 24, 387–393. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Packialakshmi, B.; Makkar, S.K.; Dridi, S.; Rath, N.C. Effect of Butyrate on Immune Response of a Chicken Macrophage Cell Line. Vet. Immunol. Immunopathol. 2014, 162, 24–32. [Google Scholar] [CrossRef]
- Xiong, J.; Qiu, H.; Bi, Y.; Zhou, H.; Guo, S.; Ding, B. Effects of Dietary Supplementation with Tributyrin and Coated Sodium Butyrate on Intestinal Morphology, Disaccharidase Activity and Intramuscular Fat of Lipopolysaccharide-Challenged Broilers. Braz. J. Poult. Sci. 2018, 20, 707–716. [Google Scholar] [CrossRef]
- Ndelekwute, E.K.; Unah, U.L.; Udoh, U.H. Effect of Dietary Organic Acids on Nutrient Digestibility, Faecal Moisture, Digesta PH and Viscosity of Broiler Chickens. MOJ Anat. Physiol. 2019, 6, 40–43. [Google Scholar]
- Myers, R.L. Butyric and Fatty Acids. In The 100 Most Important Chemical Compounds: A Reference Guide; Greenwood Press: Santa Barbara, CA, USA, 2007; pp. 52–54. [Google Scholar]
- Goldberg, I.; Rokem, J.S. Organic and Fatty Acid Production, Microbial. In Encyclopedia of Microbiology; Academic Press: Oxford, UK, 2009; pp. 421–442. [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 264, Butyric Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/264 (accessed on 6 July 2021).
- Karimi, G.; Vahabzadeh, M. Butyric Acid. In Encyclopedia of Toxicology; Academic Press: Oxford, UK, 2014; pp. 597–601. [Google Scholar]
- William, H.B.; Butyric Acid. Encyclopedia Britannica. Available online: https://www.britannica.com/science/butyric-acid (accessed on 9 June 2020).
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Ahsan, U.; Cengiz, Ö.; Raza, I.; Kuter, E.; Chacher, M.F.A.; Iqbal, Z.; Umar, S.; Çakir, S. Sodium Butyrate in Chicken Nutrition: The Dynamics of Performance, Gut Microbiota, Gut Morphology and Immunity. Worlds Poult. Sci. J. 2016, 72, 265–275. [Google Scholar] [CrossRef] [Green Version]
- Miguel, A.S.; Salgado, M.T.; Rodríguez, M.S.M.; Pachón, J.; Sánchez, M.A.; Lobatoa, C.; Pastor, M.R. Role of Butyric Acid in Food and Intestinal Health. Immunol. Infect. 2018, 1, 5. [Google Scholar]
- Xu, Z.; Jiang, L. 3.20—Butyric Acid. In Comprehensive Biotechnology; Moo-Young, M., Ed.; Academic Press: Burlington, MA, USA, 2011; pp. 207–215. [Google Scholar]
- Den Besten, G.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [Green Version]
- Kulcsár, A.; Mátis, G.; Molnár, A.; Petrilla, J.; Wágner, L.; Fébel, H.; Husvéth, F.; Dublecz, K.; Neogrády, Z. Nutritional Modulation of Intestinal Drug-Metabolizing Cytochrome P450 by Butyrate of Different Origin in Chicken. Res. Vet. Sci. 2017, 113, 25–32. [Google Scholar] [CrossRef]
- Kirimura, K.; Honda, Y.; Hattori, T. Citric Acid. In Comprehensive Biotechnology; Academic Press: Tokyo, Japan, 2011; Volume 3, pp. 135–142. [Google Scholar]
- Eli̇Uz, E. Antimicrobial Activity of Citric Acid against Escherichia Coli, Staphylococcus Aureus and Candida Albicans as a Sanitizer Agent. Eurasian J. For. Sci. 2020, 8, 295–301. [Google Scholar] [CrossRef]
- Karaffa, L.; Kubicek, C.P. Citric Acid and Itaconic Acid Accumulation: Variations of the Same Story? Appl. Microbiol. Biotechnol. 2019, 103, 2889–2902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhoff, F.H.; Bauweleers, H. Citric Acid. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 1–11. [Google Scholar]
- Sweis, I.E.; Cressey, B.C. Potential Role of the Common Food Additive Manufactured Citric Acid in Eliciting Significant Inflammatory Reactions Contributing to Serious Disease States: A Series of Four Case Reports. Toxicol. Rep. 2018, 5, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Show, P.L.; Oladele, K.O.; Siew, Q.Y.; Aziz Zakry, F.A.; Lan, J.C.W.; Ling, T.C. Overview of Citric Acid Production from Aspergillus Niger. Front. Life Sci. 2015, 8, 271–283. [Google Scholar] [CrossRef] [Green Version]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 311, Citric Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/311 (accessed on 18 September 2021).
- Lückstädt, C. Acidifiers in Animal Nutrition. A Guide for Feed Preservation and Acidification to Promote Animal Performance; Nottingham University Press: Nottingham, UK, 2008. [Google Scholar]
- Ajala, A.S.; Adeoye, A.O.; Olaniyan, S.A.; Fasonyin, O.T. A Study on Effect of Fermentation Conditions on Citric Acid Production from Cassava Peels. Sci. Afr. 2020, 8, e00396. [Google Scholar] [CrossRef]
- Ciriminna, R.; Meneguzzo, F.; Delisi, R.; Pagliaro, M. Citric Acid: Emerging Applications of Key Biotechnology Industrial Product. Chem. Cent. J. 2017, 11, 22. [Google Scholar] [CrossRef] [Green Version]
- Tanpong, S.; Cherdthong, A.; Tengjaroenkul, B.; Tengjaroenkul, U.; Wongtangtintharn, S. Evaluation of Physical and Chemical Properties of Citric Acid Industrial Waste. Trop. Anim. Health Prod. 2019, 51, 2167–2174. [Google Scholar] [CrossRef]
- Apelblat, A. Properties of citric acid and its solutions. In Citric Acid; Springer: Cham, Switzerland, 2014; pp. 13–141. [Google Scholar]
- Abdel-Salam, O.M.E.; Youness, E.R.; Mohammed, N.A.; Morsy, S.M.Y.; Omara, E.A.; Sleem, A.A. Citric Acid Effects on Brain and Liver Oxidative Stress in Lipopolysaccharide-Treated Mice. J. Med. Food 2014, 17, 588–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Food Safety Authority. Scientific Opinion on the Safety and Efficacy of Citric Acid When Used as a Technological Additive (Preservative) for All Animal Species. EFSA 2015, 13, 4009. [Google Scholar]
- Bedford, A.; Gong, J. Implications of Butyrate and Its Derivatives for Gut Health and Animal Production. Anim. Nutr. 2018, 4, 151–159. [Google Scholar] [CrossRef]
- Hu, W.; Li, W.; Yang, H.; Chen, J. Current Strategies and Future Prospects for Enhancing Microbial Production of Citric Acid. Appl. Microbiol. Biotechnol. 2019, 103, 201–209. [Google Scholar] [CrossRef]
- Hesham, A.E.L.; Mostafa, Y.S.; AlSharqi, L.E.O. Optimization of Citric Acid Production by Immobilized Cells of Novel Yeast Isolates. Mycobiology 2020, 48, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Abdelqader, A.; Al-Fataftah, A.R. Effect of Dietary Butyric Acid on Performance, Intestinal Morphology, Microflora Composition and Intestinal Recovery of Heat-Stressed Broilers. Livest. Sci. 2016, 183, 78–83. [Google Scholar] [CrossRef]
- Papatsiros, V.; Katsoulos, P.; Koutoulis, K.; Karatzia, M.; Dedousi, A.; Christodoulopoulos, G. Alternatives to Antibiotics for Farm Animals. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2013, 8, 1–15. [Google Scholar] [CrossRef]
- Vital, M.; Howe, A.; Tiedje, J. Revealing the Bacterial Butyrate Synthesis Pathways by Analyzing (Meta) Genomic Data. mBio 2014, 5, e00889-14. [Google Scholar] [CrossRef] [Green Version]
- Coutzac, C.; Jouniaux, J.-M.; Paci, A.; Schmidt, J.; Mallardo, D.; Seck, A.; Asvatourian, V.; Cassard, L.; Saulnier, P.; Lacroix, L.; et al. Systemic Short Chain Fatty Acids Limit Antitumor Effect of CTLA-4 Blockade in Hosts with Cancer. Nat. Commun. 2020, 11, 2168. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Wu, X.P.; Zhang, K.Y.; Ding, X.M.; Bai, S.P.; Wang, J.P.; Zeng, Q.F. The Effect of Citric Acid Acidification of Drinking Water on Growth Performance, Cecal PH, and Cecal Microflora of Meat Duck. Livest. Sci. 2018, 209, 54–59. [Google Scholar] [CrossRef]
- Pearlin, B.V.; Muthuvel, S.; Govidasamy, P.; Villavan, M.; Alagawany, M.; Farag, M.R.; Dhama, K.; Gopi, M. Role of Acidifiers in Livestock Nutrition and Health: A Review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 558–569. [Google Scholar] [CrossRef] [Green Version]
- Kallam, N.R.K.; Sejian, V. Gut Health and Immunity in Improving Poultry Production. In Advances in Poultry Nutrition Research; Patra, A.K., Ed.; IntechOpen: London, UK, 2021. [Google Scholar]
- Kaczmarek, S.A.; Barri, A.; Hejdysz, M.; Rutkowski, A. Effect of Different Doses of Coated Butyric Acid on Growth Performance and Energy Utilization in Broilers. Poult. Sci. 2016, 95, 851–859. [Google Scholar] [CrossRef]
- Holl, E. Improving Broiler Gut Health by Making the Most of Butyric Acid. Available online: https://www.poultryworld.net/Nutrition/Partner/2021/8/Improving-broiler-gut-health-by-making-the-most-of-butyric-acid-785270E/ (accessed on 3 September 2021).
- Bortoluzzi, C.; Pedroso, A.A.; Mallo, J.J.; Puyalto, M.; Kim, W.K.; Applegate, T.J. Sodium Butyrate Improved Performance While Modulating the Cecal Microbiota and Regulating the Expression of Intestinal Immune-Related Genes of Broiler Chickens. Poult. Sci. 2017, 96, 3981–3993. [Google Scholar] [CrossRef]
- Bortoluzzi, C.; Rothrock, M.J.; Vieira, B.S.; Mallo, J.J.; Puyalto, M.; Hofacre, C.; Applegate, T.J. Supplementation of Protected Sodium Butyrate Alone or in Combination with Essential Oils Modulated the Cecal Microbiota of Broiler Chickens Challenged With Coccidia and Clostridium Perfringens. Front. Sustain. Food Syst. 2018, 2, 72. [Google Scholar] [CrossRef]
- Lei, F.; Yin, Y.; Wang, Y.; Deng, B.; Yu, H.D.; Li, L.; Xiang, C.; Wang, S.; Zhu, B.; Wang, X. Higher-Level Production of Volatile Fatty Acids In Vitro by Chicken Gut Microbiotas than by Human Gut Microbiotas as Determined by Functional Analyses. Appl. Environ. Microbiol. 2012, 78, 5763–5772. [Google Scholar] [CrossRef] [Green Version]
- Mátis, G.; Kulcsár, A.; Turowski, V.; Fébel, H.; Neogrády, Z.; Huber, K. Effects of Oral Butyrate Application on Insulin Signaling in Various Tissues of Chickens. Domest. Anim. Endocrinol. 2015, 50, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D. Dietary Fiber and Prebiotics and the Gastrointestinal Microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Mishra, P. Dietary Fiber in Poultry Nutrition and Their Effects on Nutrient Utilization, Performance, Gut Health, and on the Environment: A Review. J. Anim. Sci. Biotechnol. 2021, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Eeckhaut, V.; Van Immerseel, F.; Croubels, S.; De Baere, S.; Haesebrouck, F.; Ducatelle, R.; Louis, P.; Vandamme, P. Butyrate Production in Phylogenetically Diverse Firmicutes Isolated from the Chicken Caecum: Butyrate-Producing Bacteria from the Chicken Caecum. Microb. Biotechnol. 2011, 4, 503–512. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Yin, F.; Yang, Y.; Lepp, D.; Yu, H.; Ruan, Z.; Yang, C.; Yin, Y.; Hou, Y.; Leeson, S.; et al. Dietary Butyrate Glycerides Modulate Intestinal Microbiota Composition and Serum Metabolites in Broilers. Sci. Rep. 2018, 8, 4940. [Google Scholar] [CrossRef] [Green Version]
- Xiao, S.; Jiang, S.; Qian, D.; Duan, J. Modulation of Microbially Derived Short-Chain Fatty Acids on Intestinal Homeostasis, Metabolism, and Neuropsychiatric Disorder. Appl. Microbiol. Biotechnol. 2020, 104, 589–601. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Louis, P.; Flint, H.J. Formation of Propionate and Butyrate by the Human Colonic Microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Trachsel, J.; Bayles, D.O.; Looft, T.; Levine, U.Y.; Allen, H.K. Function and Phylogeny of Bacterial Butyryl Coenzyme A: Acetate Transferases and Their Diversity in the Proximal Colon of Swine. Appl. Environ. Microbiol. 2016, 82, 6788–6798. [Google Scholar] [CrossRef] [Green Version]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.-J. The Role of Butyrate on Colonic Function. Aliment. Pharmacol. Ther. 2007, 27, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Sivaprakasam, S.; Bhutia, Y.D.; Yang, S.; Ganapathy, V. Short Chain Fatty Acid Transporters: Role in Colonic Homeostasis. Compr. Physiol. 2017, 8, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Mátis, G.; Petrilla, J.; Kulcsár, A.; van den Bighelaar, H.; Boomsma, B.; Neogrády, Z.; Fébel, H. Effects of Dietary Butyrate Supplementation and Crude Protein Level on Carcass Traits and Meat Composition of Broiler Chickens. Arch. Anim. Breed. 2019, 62, 527–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maesschalck, C.; Eeckhaut, V.; Maertens, L.; De Lange, L.; Marchal, L.; Nezer, C.; De Baere, S.; Croubels, S.; Daube, G.; Dewulf, J.; et al. Effects of Xylo-Oligosaccharides on Broiler Chicken Performance and Microbiota. Appl. Environ. Microbiol. 2015, 81, 5880–5888. [Google Scholar] [CrossRef] [Green Version]
- Bautil, A.; Verspreet, J.; Buyse, J.; Goos, P.; Bedford, M.R.; Courtin, C.M. Age-Related Arabinoxylan Hydrolysis and Fermentation in the Gastrointestinal Tract of Broilers Fed Wheat-Based Diets. Poult. Sci. 2019, 98, 4606–4621. [Google Scholar] [CrossRef]
- Bautil, A.; Verspreet, J.; Buyse, J.; Goos, P.; Bedford, M.R.; Courtin, C.M. Arabinoxylan-Oligosaccharides Kick-Start Arabinoxylan Digestion in the Aging Broiler. Poult. Sci. 2020, 99, 2555–2565. [Google Scholar] [CrossRef]
- Guo, L.; Liu, Y.; Luo, L.; Hussain, S.B.; Bai, Y.; Alam, S.M. Comparative Metabolites and Citrate-Degrading Enzymes Activities in Citrus Fruits Reveal the Role of Balance between ACL and Cyt-ACO in Metabolite Conversions. Plants 2020, 9, 350. [Google Scholar] [CrossRef] [Green Version]
- Peretó, J. Citric Acid Cycle. In Encyclopedia of Astrobiology; Amils, R., Gargaud, M., Cernicharo Quintanilla, J., Cleaves, H.J., Irvine, W.M., Pinti, D., Viso, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–3. [Google Scholar]
- Kumari, A. Chapter 2—Citric Acid Cycle. In Sweet Biochemistry; Academic Press: Haryana, India, 2018; pp. 7–11. [Google Scholar]
- Tong, Z.; Zheng, X.; Tong, Y.; Shi, Y.-C.; Sun, J. Systems Metabolic Engineering for Citric Acid Production by Aspergillus Niger in the Post-Genomic Era. Microb. Cell Factories 2019, 18, 28. [Google Scholar] [CrossRef] [Green Version]
- Bender, D.A. Tricarboxylic Acid Cycle. In Encyclopedia of Food Sciences and Nutrition; Academic Press: Oxford, UK, 2003; pp. 5851–5856. [Google Scholar]
- Cole, L.; Kramer, P.R. Sugars, Fatty Acids, and Energy Biochemistry. In Human Physiology, Biochemistry and Basic Medicine; Cole, L., Kramer, P.R., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 17–30. [Google Scholar]
- Wolffram, S.; Hagemann, C.; Grenacher, B.; Scharrer, E. Characterization of the Transport of Tri- and Dicarboxylates by Pig Intestinal Brush-Border Membrane Vesicles. Comp. Biochem. Physiol. A Physiol. 1992, 101, 759–767. [Google Scholar] [CrossRef]
- Tugnoli, B.; Giovagnoni, G.; Piva, A.; Grilli, E. From Acidifiers to Intestinal Health Enhancers: How Organic Acids Can Improve Growth Efficiency of Pigs. Animals 2020, 10, 134. [Google Scholar] [CrossRef] [Green Version]
- Renata, C. Citrate and Mineral Metabolism Kidney Stones and Bone Disease. Front. Biosci. 2003, 8, s1084–s1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pajor, A.M.; Sun, N.N. Single Nucleotide Polymorphisms in the Human Na+-Dicarboxylate Cotransporter Affect Transport Activity and Protein Expression. Am. J. Physiol. Ren. Physiol. 2010, 299, F704–F711. [Google Scholar] [CrossRef] [Green Version]
- Granchi, D.; Baldini, N.; Ulivieri, F.M.; Caudarella, R. Role of Citrate in Pathophysiology and Medical Management of Bone Diseases. Nutrients 2019, 11, 2576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adedokun, S.A.; Olojede, O.C. Optimizing Gastrointestinal Integrity in Poultry: The Role of Nutrients and Feed Additives. Front. Vet. Sci. 2019, 5, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrasco, J.M.D.; Casanova, N.A.; Miyakawa, M.E.F. Microbiota, Gut Health and Chicken Productivity: What Is the Connection? Microorganisms 2019, 7, 374. [Google Scholar] [CrossRef] [Green Version]
- Pan, D.; Yu, Z. Intestinal Microbiome of Poultry and Its Interaction with Host and Diet. Gut Microbes 2014, 5, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Chen, Y.; Yu, L.; Wang, J.; Huang, M.; Zhu, N. Effects of Lactobacillus Plantarum on Intestinal Integrity and Immune Responses of Egg-Laying Chickens Infected with Clostridium Perfringens under the Free-Range or the Specific Pathogen Free Environment. BMC Vet. Res. 2020, 16, 47. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.H.; Seok, W.J.; Kim, I.H. Organic Acids Mixture as a Dietary Additive for Pigs—A Review. Animals 2020, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- Su, L.-C.; Xie, Z.; Zhang, Y.; Nguyen, K.T.; Yang, J. Study on the Antimicrobial Properties of Citrate-Based Biodegradable Polymers. Front. Bioeng. Biotechnol. 2014, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.Y.; Kil, D.Y.; Oh, H.K.; Han, I.K. Acidifier as an Alternative Material to Antibiotics in Animal Feed. Asian Australas. J. Anim. Sci. 2005, 18, 1048–1060. [Google Scholar] [CrossRef]
- Elnesr, S.S.; Alagawany, M.; Elwan, H.A.M.; Fathi, M.A.; Farag, M.R. Effect of Sodium Butyrate on Intestinal Health of Poultry—A Review. Ann. Anim. Sci. 2020, 20, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Waseem, M.M.; Mukhtar, N.; Rehman, Z. Use of Organic Acids as Potential Feed Additives in Poultry Production. J. World’s Poult. Res. 2016, 6, 105–116. [Google Scholar]
- Chowdhury, R.; Islam, K.M.S.; Khan, M.J.; Karim, M.R.; Haque, M.N.; Khatun, M.; Pesti, G.M. Effect of Citric Acid, Avilamycin, and Their Combination on the Performance, Tibia Ash, and Immune Status of Broilers. Poult. Sci. 2009, 88, 1616–1622. [Google Scholar] [CrossRef]
- Archana, K.; Zuyie, R.; Sharma, K.G.; Vidyarthi, V.K. Organic Acid Supplementation in the Diet of Broiler Chicken—A Review. Livest. Res. Int. 2016, 4, 112–119. [Google Scholar]
- Leeson, S.; Namkung, H.; Antongiovanni, M.; Lee, E.H. Effect of Butyric Acid on the Performance and Carcass Yield of Broiler Chickens. Poult. Sci. 2005, 84, 1418–1422. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.X.; Li, S.Q.; Zhao, Z.; An, L.L. Sodium Butyrate as an Effective Feed Additive to Improve Growth Performance and Gastrointestinal Development in Broilers. Vet. Med. Sci. 2020, 6, 491–499. [Google Scholar] [CrossRef] [Green Version]
- Song, B.; Li, H.; Wu, Y.; Zhen, W.; Wang, Z.; Xia, Z.; Guo, Y. Effect of Microencapsulated Sodium Butyrate Dietary Supplementation on Growth Performance and Intestinal Barrier Function of Broiler Chickens Infected with Necrotic Enteritis. Anim. Feed Sci. Technol. 2017, 232, 6–15. [Google Scholar] [CrossRef]
- Pires, M.F.; Leandro, N.S.M.; Jacob, D.V.; Carvalho, F.B.; Oliveira, H.F.; Stringhini, J.H.; Pires, S.F.; Mello, H.H.C.; Carvalho, D.P. Performance and Egg Quality of Commercial Laying Hens Fed with Various Levels of Protected Sodium Butyrate. South Afr. J. Anim. Sci. 2020, 50, 759–765. [Google Scholar]
- Namkung, H.; Yu, H.; Gong, J.; Leeson, S. Antimicrobial Activity of Butyrate Glycerides toward Salmonella Typhimurium and Clostridium Perfringens. Poult. Sci. 2011, 90, 2217–2222. [Google Scholar] [CrossRef] [PubMed]
- Al-Harthi, M.A.; Attia, Y.A. Effect of Citric Acid on the Utilization of Olive Cake Diets for Laying Hens. Ital. J. Anim. Sci. 2015, 14, 3966. [Google Scholar] [CrossRef]
- Haq, Z.; Rastogi, A.; Sharma, R.K.; Khan, N. Advances in Role of Organic Acids in Poultry Nutrition: A Review. J. Appl. Nat. Sci. 2017, 9, 2152–2157. [Google Scholar] [CrossRef] [Green Version]
- Dehghani-Tafti, N.; Jahanian, R. Effect of Supplemental Organic Acids on Performance, Carcass Characteristics, and Serum Biochemical Metabolites in Broilers Fed Diets Containing Different Crude Protein Levels. Anim. Feed Sci. Technol. 2016, 211, 109–116. [Google Scholar] [CrossRef]
- Feye, K.M.; Swaggerty, C.L.; Kogut, M.H.; Ricke, S.C.; Piva, A.; Grilli, E. The Biological Effects of Microencapsulated Organic Acids and Botanicals Induces Tissue-Specific and Dose-Dependent Changes to the Gallus Gallus Microbiota. BMC Microbiol. 2020, 20, 332. [Google Scholar] [CrossRef]
- Stamilla, A.; Messina, A.; Sallemi, S.; Condorelli, L.; Antoci, F.; Puleio, R.; Loria, G.R.; Cascone, G.; Lanza, M. Effects of Microencapsulated Blends of Organics Acids and Essential Oils as a Feed Additive for Broiler Chicken. A Focus on Growth Performance, Gut Morphology and Microbiology. Animals 2020, 10, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, A.W.; Kessler, J.W.; Fuller, L.; Williams, S.; Mathis, G.F.; Lumpkins, B.; Valdez, F. Effect of Feeding an Encapsulated Source of Butyric Acid (ButiPEARL) on the Performance of Male Cobb Broilers Reared to 42 d of Age. Poult. Sci. 2015, 94, 1864–1870. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Ahmed, S.; Ditta, Y.A.; Mehmood, S.; Khan, M.I.; Gillani, S.S.; Rasool, Z.; Sohail, M.L.; Mushtaq, A.; Umar, S. Effect of Microencapsulated Butyric Acid Supplementation on Growth Performance, Ileal Digestibility of Protein, Duodenal Morphology and Immunity in Broilers. J. Hell. Vet. Med. Soc. 2018, 69, 1109. [Google Scholar] [CrossRef] [Green Version]
- Lum, J.; Sygall, R.; Ros Felip, J.M. Comparison of Tributyrin and Coated Sodium Butyrate as Sources of Butyric Acid for Improvement of Growth Performance in Ross 308 Broilers. Int. J. Poult. Sci. 2018, 17, 290–294. [Google Scholar] [CrossRef] [Green Version]
- Bedford, A.; Yu, H.; Squires, E.J.; Leeson, S.; Gong, J. Effects of Supplementation Level and Feeding Schedule of Butyrate Glycerides on the Growth Performance and Carcass Composition of Broiler Chickens. Poult. Sci. 2017, 96, 3221–3228. [Google Scholar] [CrossRef]
- Archana, K.; Zuyie, R.; Vidyarthi, V.K. Effects of Dietary Addition of Organic Acid on Performance of Broiler Chicken. Livest. Res. Int. 2019, 7, 71–76. [Google Scholar]
- Islam, K.M.S.; Schaeublin, H.; Wenk, C.; Wanner, M.; Liesegang, A. Effect of Dietary Citric Acid on the Performance and Mineral Metabolism of Broiler. J. Anim. Physiol. Anim. Nutr. 2012, 96, 808–817. [Google Scholar] [CrossRef]
- Khosravinia, H.; Nourmohammadi, R.; Afzali, N. Productive Performance, Gut Morphometry, and Nutrient Digestibility of Broiler Chicken in Response to Low and High Dietary Levels of Citric Acid. J. Appl. Poult. Res. 2015, 24, 470–480. [Google Scholar] [CrossRef]
- De Meyer, F.; Eeckhaut, V.; Ducatelle, R.; Dhaenens, M.; Daled, S.; Dedeurwaerder, A.; De Gussem, M.; Haesebrouck, F.; Deforce, D.; Van Immerseel, F. Host Intestinal Biomarker Identification in a Gut Leakage Model in Broilers. Vet. Res. 2019, 50, 46. [Google Scholar] [CrossRef] [Green Version]
- Barekatain, R.; Howarth, G.S.; Willson, N.-L.; Cadogan, D.; Wilkinson, S. Excreta Biomarkers in Response to Different Gut Barrier Dysfunction Models and Probiotic Supplementation in Broiler Chickens. PLoS ONE 2020, 15, e0237505. [Google Scholar] [CrossRef] [PubMed]
- Pascual, A.; Trocino, A.; Birolo, M.; Cardazzo, B.; Bordignon, F.; Ballarin, C.; Carraro, L.; Xiccato, G. Dietary Supplementation with Sodium Butyrate: Growth, Gut Response at Different Ages, and Meat Quality of Female and Male Broiler Chickens. Ital. J. Anim. Sci. 2020, 19, 1135–1146. [Google Scholar] [CrossRef]
- Yadav, S.; Jha, R. Strategies to Modulate the Intestinal Microbiota and Their Effects on Nutrient Utilization, Performance and Health of Poultry. J. Anim. Sci. Biotechnol. 2019, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Moquet, P.C.A. Butyrate in Broiler Diets: Impact of Butyrate Presence in Distinct Gastrointestinal Tract Segments on Digestive Function, Microbiota Composition and Immune Responses. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2018. [Google Scholar]
- Zou, X.; Ji, J.; Qu, H.; Wang, J.; Shu, D.M.; Wang, Y.; Liu, T.F.; Li, Y.; Luo, C.L. Effects of Sodium Butyrate on Intestinal Health and Gut Microbiota Composition during Intestinal Inflammation Progression in Broilers. Poult. Sci. 2019, 98, 4449–4456. [Google Scholar] [CrossRef]
- Zhang, J.M.; Sun, Y.S.; Zhao, L.Q.; Chen, T.T.; Fan, M.N.; Jiao, H.C.; Zhao, J.P.; Wang, X.J.; Li, F.C.; Li, H.F.; et al. SCFAs-Induced GLP-1 Secretion Links the Regulation of Gut Microbiome on Hepatic Lipogenesis in Chickens. Front. Microbiol. 2019, 10, 2176. [Google Scholar] [CrossRef] [Green Version]
- Sossai, P. Butyric Acid: What Is the Future for This Old Substance? Swiss Med. Wkly. 2012, 142, w13596. [Google Scholar] [CrossRef]
- Celasco, G.; Moro, L.; Aiello, C.; Mangano, K.; Milasi, A.; Quattrocchi, C.; Di Marco, R. Calcium Butyrate: Anti-Inflammatory Effect on Experimental Colitis in Rats and Antitumor Properties. Biomed. Rep. 2014, 2, 559–563. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Ajuwon, K.M. Butyrate Modifies Intestinal Barrier Function in IPEC-J2 Cells through a Selective Upregulation of Tight Junction Proteins and Activation of the Akt Signaling Pathway. PLoS ONE 2017, 12, e0179586. [Google Scholar] [CrossRef]
- Hansen, V.L.; Kahl, S.; Proszkowiec-Weglarz, M.; Jiménez, S.C.; Vaessen, S.F.C.; Schreier, L.L.; Jenkins, M.C.; Russell, B.; Miska, K.B. The Effects of Tributyrin Supplementation on Weight Gain and Intestinal Gene Expression in Broiler Chickens during Eimeria Maxima-Induced Coccidiosis. Poult. Sci. 2021, 100, 100984. [Google Scholar] [CrossRef]
- Sunkara, L.T.; Achanta, M.; Schreiber, N.B.; Bommineni, Y.R.; Dai, G.; Jiang, W.; Lamont, S.; Lillehoj, H.S.; Beker, A.; Teeter, R.G.; et al. Butyrate Enhances Disease Resistance of Chickens by Inducing Antimicrobial Host Defense Peptide Gene Expression. PLoS ONE 2011, 6, e27225. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, X.; Yu, S.; Su, Y.; Zhu, W. Effects of Early Intervention with Sodium Butyrate on Gut Microbiota and the Expression of Inflammatory Cytokines in Neonatal Piglets. PLoS ONE 2016, 11, e0162461. [Google Scholar] [CrossRef] [PubMed]
- Demirel, G.; Pekel, A.Y.; Alp, M.; Kocabağlı, N. Effects of Dietary Supplementation of Citric Acid, Copper, and Microbial Phytase on Growth Performance and Mineral Retention in Broiler Chickens Fed a Low Available Phosphorus Diet. J. Appl. Poult. Res. 2012, 21, 335–347. [Google Scholar] [CrossRef]
- Nourmohammadi, R.; Afzali, N. Effect of Citric Acid and Microbial Phytase on Small Intestinal Morphology in Broiler Chicken. Ital. J. Anim. Sci. 2013, 12, e7. [Google Scholar] [CrossRef]
- Nourmohammadi, R.; Khosravinia, H. Acidic Stress Caused by Dietary Administration of Citric Acid in Broiler Chickens. Arch. Anim. Breed. 2015, 58, 309–315. [Google Scholar] [CrossRef] [Green Version]
- Suiryanrayna, M.V.A.N.; Ramana, J.V. A Review of the Effects of Dietary Organic Acids Fed to Swine. J. Anim. Sci. Biotechnol. 2015, 6, 45. [Google Scholar] [CrossRef] [Green Version]
- Sabour, S.; Tabeidian, S.A.; Sadeghi, G. Dietary Organic Acid and Fiber Sources Affect Performance, Intestinal Morphology, Immune Responses and Gut Microflora in Broilers. Anim. Nutr. 2019, 5, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Bansal, M.; Liyanage, R.; Upadhyay, A.; Rath, N.; Donoghue, A.; Sun, X. Sodium Butyrate Modulates Chicken Macrophage Proteins Essential for Salmonella Enteritidis Invasion. PLoS ONE 2021, 16, e0250296. [Google Scholar] [CrossRef] [PubMed]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the Gut to the Peripheral Tissues: The Multiple Effects of Butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikandar, A.; Zaneb, H.; Younus, M.; Masood, S.; Aslam, A.; Khattak, F.; Ashraf, S.; Yousaf, M.S.; Rehman, H. Effect of Sodium Butyrate on Performance, Immune Status, Microarchitecture of Small Intestinal Mucosa and Lymphoid Organs in Broiler Chickens. Asian Australas. J. Anim. Sci. 2017, 30, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Mátis, G.; Neogrády, Z.; Csikó, G.; Kulcsár, A.; Kenéz, Á.; Huber, K. Effects of Orally Applied Butyrate Bolus on Histone Acetylation and Cytochrome P450 Enzyme Activity in the Liver of Chicken–a Randomized Controlled Trial. Nutr. Metab. 2013, 10, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, K.M.S. Use of Citric Acid in Broiler Diets. Worlds Poult. Sci. J. 2012, 68, 104–118. [Google Scholar] [CrossRef]
- Haque, M.N.; Islam, K.M.S.; Akbar, M.A.; Chowdhury, R.; Khatun, M.; Karim, M.R.; Kemppainen, B.W. Effect of Dietary Citric Acid, Flavomycin and Their Combination on the Performance, Tibia Ash and Immune Status of Broiler. Can. J. Anim. Sci. 2010, 90, 57–63. [Google Scholar] [CrossRef]
- Lakshmi, K.V.; Sunder, G.S. Supplementation of Lactic Acid and Citric Acid in Diets Replacing Antibiotic and Its Influence on Broiler Performance, Meat Yield and Immune Response up to 42 Days of Age. Int. J. Sci. Res. 2013, 4, 1007–1011. [Google Scholar]
- Debi, M.; Islam, K.; Akbar, M.; Ullha, B.; Das, S. Response of Growing Rabbits to Different Levels of Dietary Citric Acid. Bangladesh J. Anim. Sci. 1970, 39, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Raza, M.; Biswas, A.; Mir, N.A.; Mandal, A.B. Butyric Acid as a Promising Alternative to Antibiotic Growth Promoters in Broiler Chicken Production. J. Agric. Sci. 2019, 157, 55–62. [Google Scholar] [CrossRef]
- Deepa, K.; Purushothaman, M.; Vasanthakumar, P.; Sivakumar, K. Effect of Dietary Supplementation of Sodium Butyrate on Ceacal Microflora and Villi Morphology in Broiler Chicken. Int. J. Chem. Stud. 2020, 8, 44–48. [Google Scholar] [CrossRef]
- Chamba, F.; Puyalto, M.; Ortiz, A.; Torrealba, H.; Mallo, J.J.; Riboty, R. Effect of Partially Protected Sodium Butyrate on Performance, Digestive Organs, Intestinal Villi and E. Coli Development in Broilers Chickens. Int. J. Poult. Sci. 2014, 13, 390–396. [Google Scholar] [CrossRef] [Green Version]
- Grilli, E. Microencapsulation Allows Slow Release of Organic Acids in the GI Tract of Broilers. In Proceedings of the 16th European Poultry Nutrition Symposium, Strasbourg, France, 26–30 August 2007. [Google Scholar]
- Moquet, P.C.A.; Onrust, L.; Van Immerseel, F.; Ducatelle, R.; Hendriks, W.H.; Kwakkel, R.P. Importance of Release Location on the Mode of Action of Butyrate Derivatives in the Avian Gastrointestinal Tract. Worlds Poult. Sci. J. 2016, 72, 61–80. [Google Scholar] [CrossRef]
- Moquet, P.C.A.; Salami, S.A.; Onrust, L.; Hendriks, W.H.; Kwakkel, R.P. Butyrate Presence in Distinct Gastrointestinal Tract Segments Modifies Differentially Digestive Processes and Amino Acid Bioavailability in Young Broiler Chickens. Poult. Sci. 2018, 97, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Alicja, S.; Kozłowski, K. Effect of Dietary Supplementation with Butyric Acid or Sodium Butyrate on Egg Production and Physiological Parameters in Laying Hens. Eur. Poult. Sci. 2016, 80. [Google Scholar] [CrossRef]
- van den Borne, J.J.G.C.; Heetkamp, M.J.W.; Buyse, J.; Niewold, T.A. Fat Coating of Ca Butyrate Results in Extended Butyrate Release in the Gastrointestinal Tract of Broilers. Livest. Sci. 2015, 175, 96–100. [Google Scholar] [CrossRef]
- Mohammadi Gheisar, M.; Hosseindoust, A.; Kim, I.H. Evaluating the Effect of Microencapsulated Blends of Organic Acids and Essential Oils in Broiler Chickens Diet. J. Appl. Poult. Res. 2015, 24, 511–519. [Google Scholar] [CrossRef]
- Salahi, A. Effect of in Ovo Administration of Butyric Acid into Broiler Breeder Eggs on Chicken Small Intestine PH and Morphology. Slovak J. Anim. Sci. 2015, 18, 8–15. [Google Scholar]
- Oladokun, S.; Adewole, D.I. In Ovo Delivery of Bioactive Substances: An Alternative to the Use of Antibiotic Growth Promoters in Poultry Production—A Review. J. Appl. Poult. Res. 2020, 29, 744–763. [Google Scholar] [CrossRef]
- Fazayeli-Rad, A.R.; Nazarizadeh, H.; Vakili, M.; Afzali, N.; Nourmohammadi, R. Effect of Citric Acid on Performance, Nutrient Retention and Tissue Biogenic Amine Contents in Breast and Thigh Meat from Broiler Chickens. Eur. Poult. Sci. 2014, 78, 9. [Google Scholar] [CrossRef]
- Uddin, M.; Islam, K.; Reza, A.; Chowdhury, R. Citric Acid as Feed Additive in Diet of Rabbit- Effect on Growth Performance. J. Bangladesh Agric. Univ. 2014, 12, 87–90. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).