Glutenin from the Ancient Wheat Progenitor Is Intrinsically Allergenic as It Can Clinically Sensitize Mice for Systemic Anaphylaxis by Activating Th2 Immune Pathway
Abstract
1. Introduction
2. Results
2.1. Transdermal Exposure to Glutenin Extract from Aegilops tauschii Elicits Robust Specific-IgE Antibody Response in Balb/c Mice
2.2. Systemic Challenge with Aegilops tauschii Glutenin Elicits Hypothermic Shock Responses in Skin-Sensitized Mice
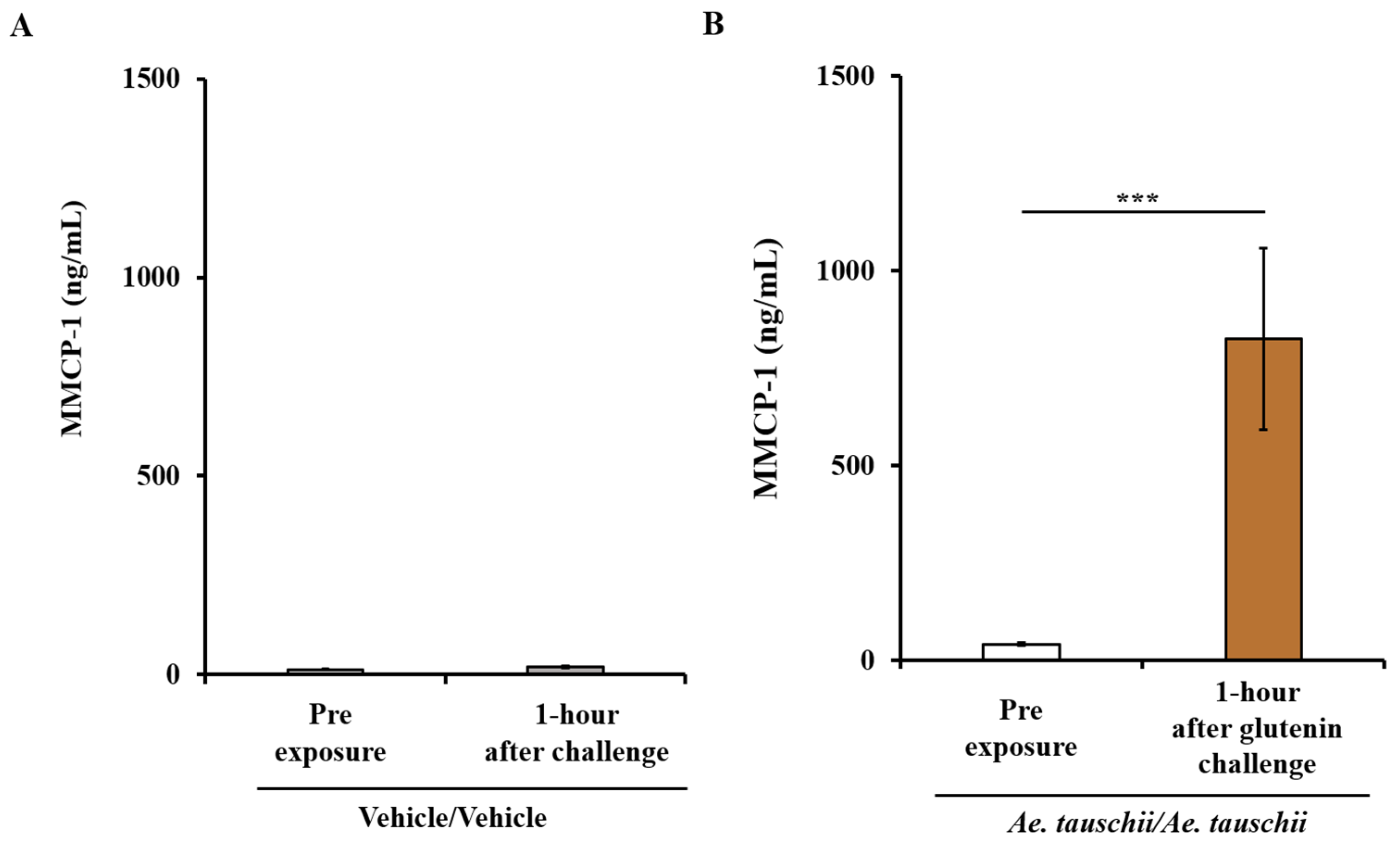
2.3. Sensitized Balb/c Mice Exhibiting Systemic Anaphylaxis Have Strong Mucosal Mast Cell Response (MMCR) as Evidenced by Significant Elevation of MMCP-1 in the Blood
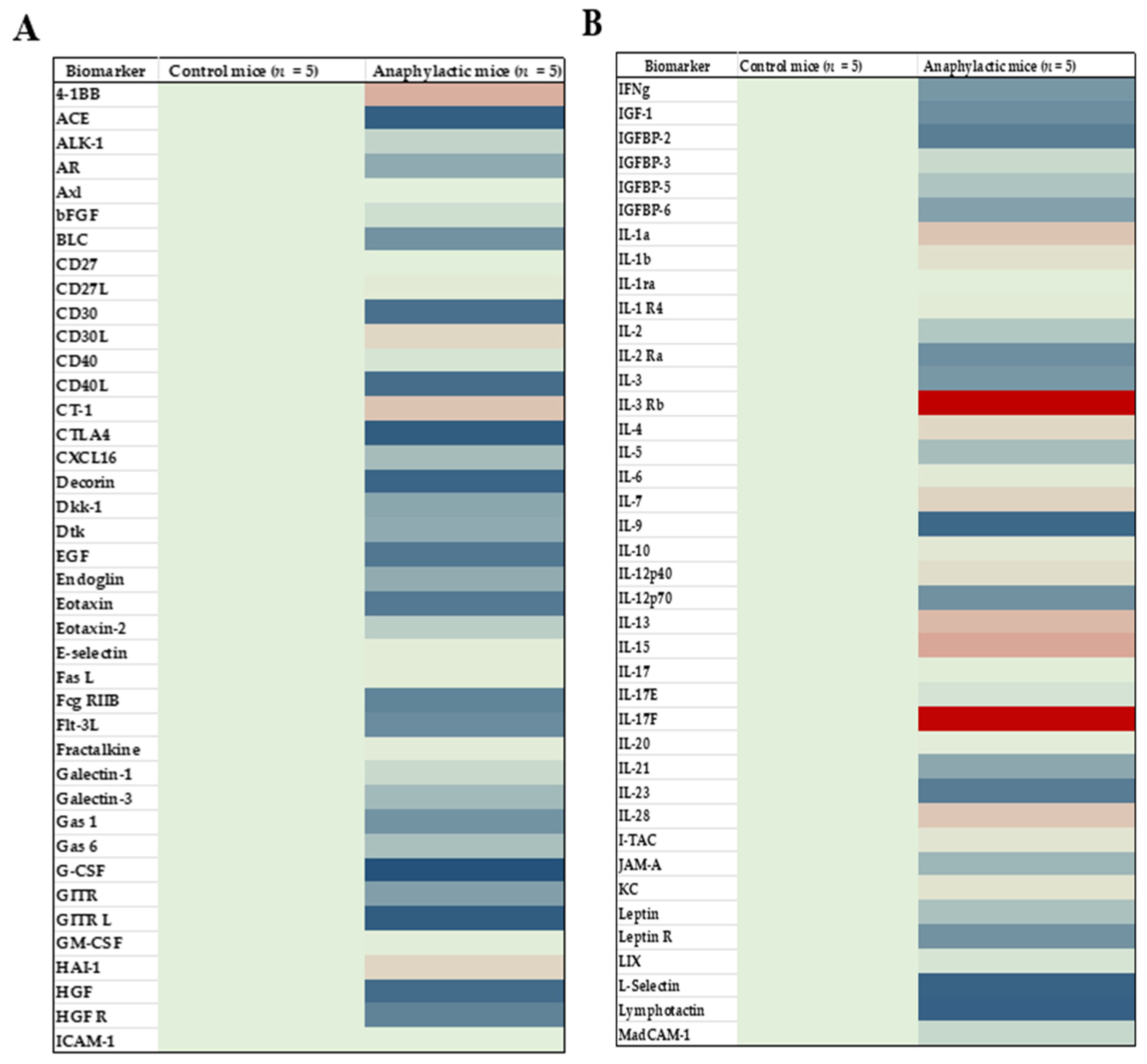
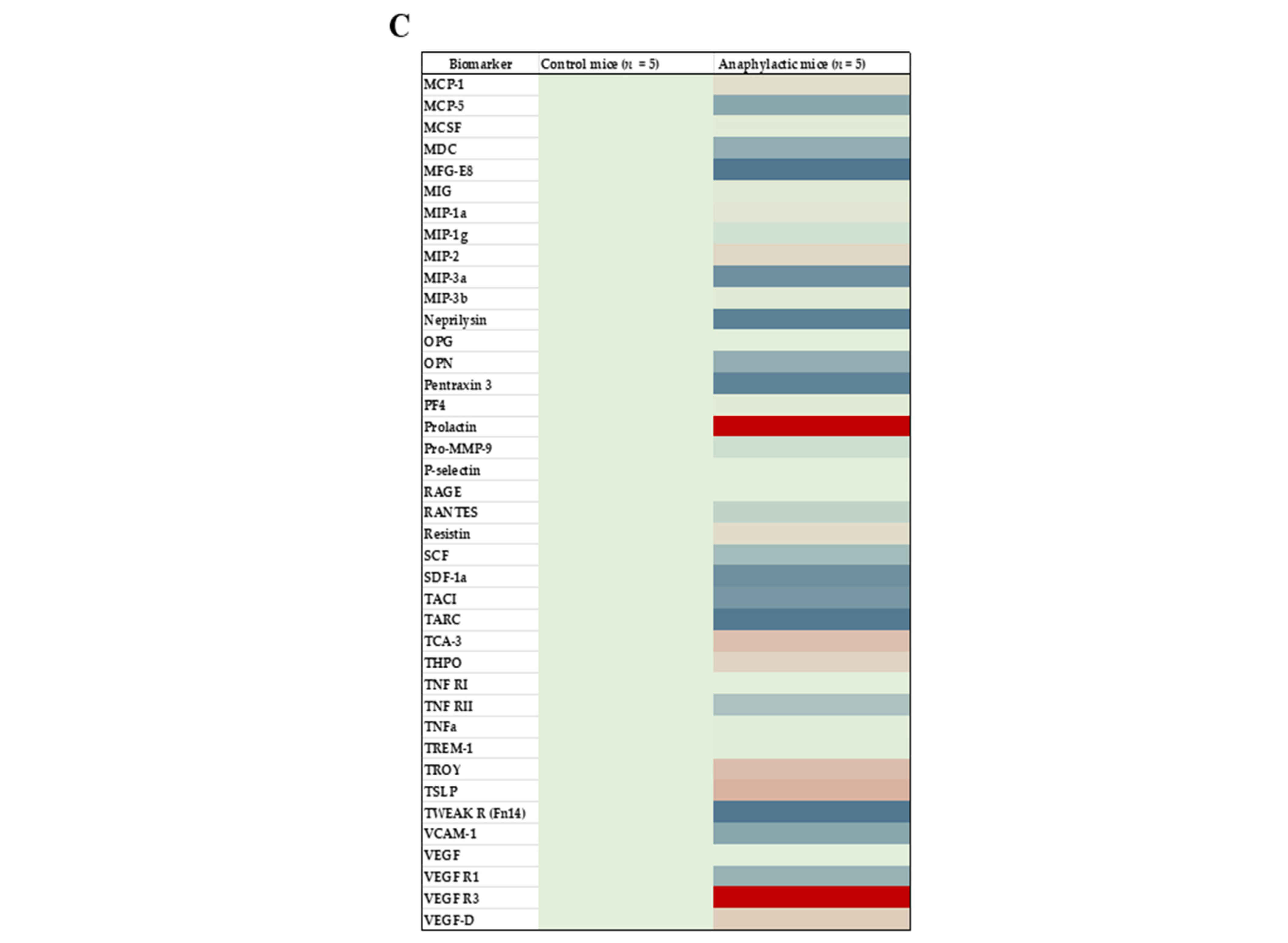
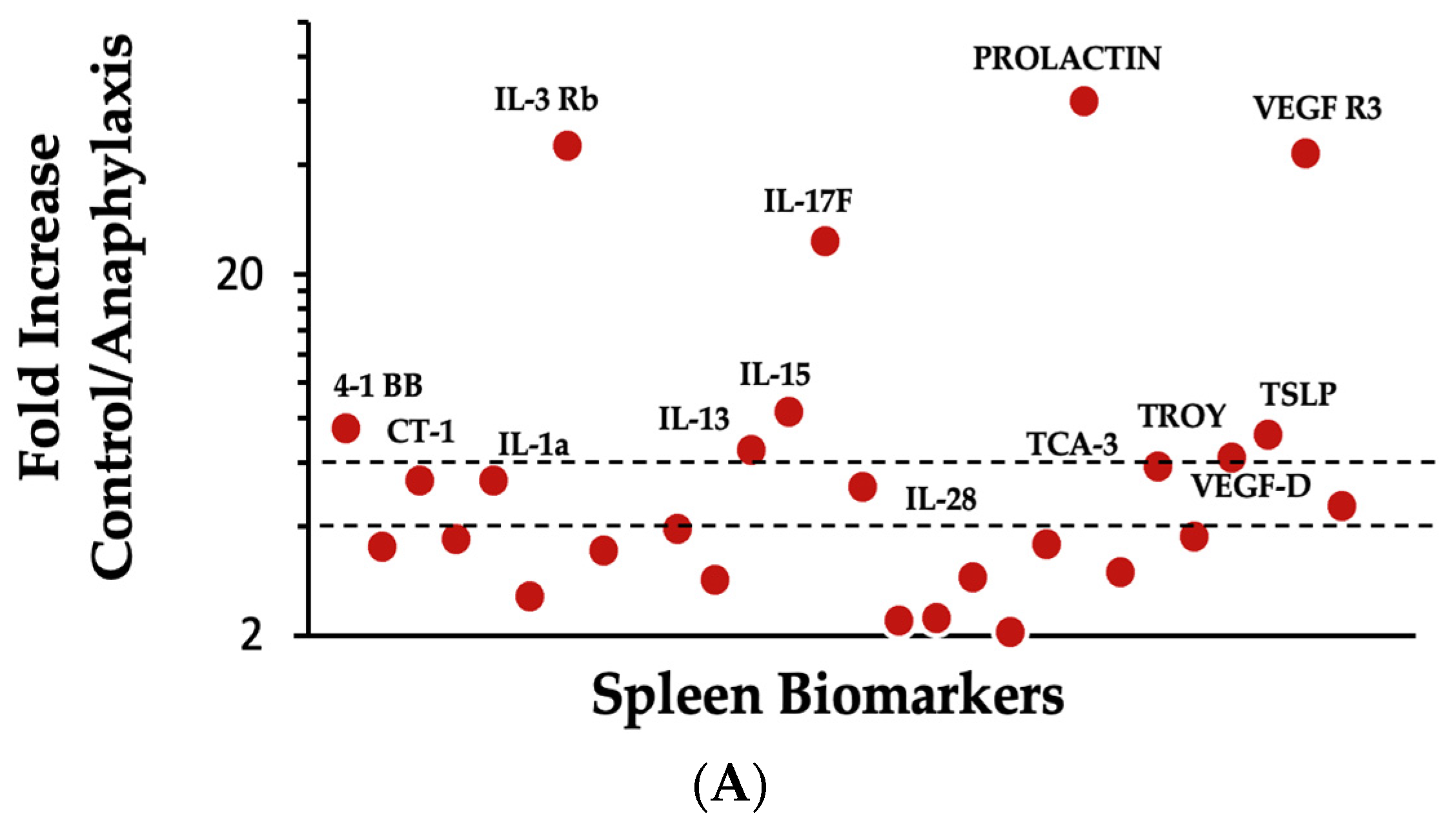
2.4. Proteomic Analysis and Mapping the Differentially Expressed Immune Marker Signatures in the Spleen of Mice Undergoing Systemic Anaphylaxis Elicited by the Ancient Wheat Glutenin
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Mice Breeding and Establishment of a Plant-Protein-Free Mouse Colony
4.3. Preparation of Glutenin Extract from the Ancient Ae. tauschii Wheat
4.4. Skin Sensitization, Bleeding, and Plasma Sample Preparation
4.5. Elicitation of Systemic Anaphylaxis
4.6. Determination of Hypothermic Shock Responses
4.7. Measurement of Specific IgE Antibody Levels
4.8. Quantification of Mucosal Mast Cell Protease-1 (MMCP-1) Level
4.9. Proteomic Analysis of Spleen Immune Markers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cianferoni, A. Wheat Allergy: Diagnosis and Management. J. Asthma Allergy 2016, 9, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wu, Y.; Wang, J.; Wang, Z.; Gao, J.; Yuan, J.; Chen, H. A Meta-Analysis of the Prevalence of Wheat Allergy Worldwide. Nutrients 2023, 15, 1564. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Blumenstock, J.A.; Jiang, J.; Davis, M.M.; Nadeau, K.C. The Public Health Impact of Parent-Reported Childhood Food Allergies in the United States. Pediatrics 2018, 142, e20181235. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Acharya, H.G.; Acharya, D.; Jorgensen, R.; Gao, H.; Secord, J.; Ng, P.K.W.; Gangur, V. Advances in Molecular Mechanisms of Wheat Allergenicity in Animal Models: A Comprehensive Review. Molecules 2019, 24, 1142. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, B. Gluten-Related Disorders: Celiac Disease, Wheat Allergy, and Nonceliac Gluten Sensitivity. Crit. Rev. Food Sci. Nutr. 2020, 60, 2606–2621. [Google Scholar] [CrossRef]
- Gao, H.; Jorgensen, R.; Raghunath, R.; Nagisetty, S.; Ng, P.K.W.; Gangur, V. Creating Hypo-/Nonallergenic Wheat Products Using Processing Methods: Fact or Fiction? Compr. Rev. Food Sci. Food Saf. 2021, 20, 6089–6115. [Google Scholar] [CrossRef]
- Juhász, A.; Belova, T.; Florides, C.G.; Maulis, C.; Fischer, I.; Gell, G.; Birinyi, Z.; Ong, J.; Keeble-Gagnère, G.; Maharajan, A.; et al. Genome Mapping of Seed-Borne Allergens and Immunoresponsive Proteins in Wheat. Sci. Adv. 2018, 4, eaar8602. [Google Scholar] [CrossRef]
- Pastorello, E.A.; Farioli, L.; Conti, A.; Pravettoni, V.; Bonomi, S.; Iametti, S.; Fortunato, D.; Scibilia, J.; Bindslev-Jensen, C.; Ballmer-Weber, B.; et al. Wheat IgE-Mediated Food Allergy in European Patients: α-Amylase Inhibitors, Lipid Transfer Proteins and Low-Molecular-Weight Glutenins—Allergenic Molecules Recognized by Double-Blind, Placebo-Controlled Food Challenge. Int. Arch. Allergy Immunol. 2007, 144, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Obenland, O.A.; Riechers, D.E. Identification of Chromosomes in Triticum Aestivum Possessing Genes That Confer Tolerance to the Synthetic Auxin Herbicide Halauxifen-Methyl. Sci. Rep. 2020, 10, 8713. [Google Scholar] [CrossRef]
- Lombardo, C.; Bolla, M.; Chignola, R.; Senna, G.; Rossin, G.; Caruso, B.; Tomelleri, C.; Cecconi, D.; Brandolini, A.; Zoccatelli, G. Study on the Immunoreactivity of Triticum Monococcum (Einkorn) Wheat in Patients with Wheat-Dependent Exercise-Induced Anaphylaxis for the Production of Hypoallergenic Foods. J. Agric. Food Chem. 2015, 63, 8299–8306. [Google Scholar] [CrossRef]
- Jorgensen, R.; Gao, H.; Selvan, T.; Arasan, A.; Van Antwerp, C.; Sundar, V. Is Wheat Glutenin Extract Intrinsically Allergenic? Evaluation Using a Novel Adjuvant-Free Mouse Model of Systemic Anaphylaxis. Int. J. Mol. Sci. 2023, 24, 17247. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, R.; Gao, H.; Chandra, S.; Sundar, V.; Loy, J.; Van Antwerp, C.; Ng, P.K.W.; Gangur, V. Chronic Application of Alcohol-Soluble Gluten Extract over Undamaged Skin Causes Clinical Sensitization for Life-Threatening Anaphylaxis via Activation of Systemic Th2 Immune Responses in Mice. Front. Allergy 2023, 4, 1214051. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, J.; Xue, L.; Liu, J.; Nie, C.; Fan, M.; Qian, H.; Zhang, D.; Ying, H.; Li, Y.; et al. L-Arabinose Attenuates Gliadin-Induced Food Allergy via Regulation of Th1/Th2 Balance and Upregulation of Regulatory T Cells in Mice. J. Agric. Food Chem. 2021, 69, 3638–3646. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Chen, C.; Xie, Q.; Gu, S.; Tao, S.; Xue, W. Pediococcus Acidilactici Strain Alleviates Gluten-Induced Food Allergy and Regulates Gut Microbiota in Mice. Front. Cell. Infect. Microbiol. 2022, 12, 845142. [Google Scholar] [CrossRef]
- Kohno, K.; Takahashi, H.; Endo, T.R.; Matsuo, H.; Shiwaku, K.; Morita, E. Characterization of a Hypoallergenic Wheat Line Lacking ω-5 Gliadin. Allergol. Int. 2016, 65, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.C.; Gu, Y.Q.; Puiu, D.; Wang, H.; Twardziok, S.O.; Deal, K.R.; Huo, N.; Zhu, T.; Wang, L.; Wang, Y.; et al. Genome Sequence of the Progenitor of the Wheat D Genome Aegilops Tauschii. Nature 2017, 551, 498–502. [Google Scholar] [CrossRef]
- Shewry, P.R. Do Ancient Types of Wheat Have Health Benefits Compared with Modern Bread Wheat? J. Cereal Sci. 2018, 79, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, B.B.; Adamidi, C.; Lozano, R.M.; Yee, B.C.; Momma, M.; Kobrehel, K.; Ermel, R.; Frick, O.L. Thioredoxin-Linked Mitigation of Allergic Responses to Wheat. Proc. Natl. Acad. Sci. USA 1997, 94, 5372–5377. [Google Scholar] [CrossRef]
- Frick, O.L.; Teuber, S.S.; Buchanan, B.B.; Morigasaki, S.; Umetsu, D.T. Allergen Immunotherapy with Heat-Killed Listeria Monocytogenes Alleviates Peanut and Food-Induced Anaphylaxis in Dogs. Allergy Eur. J. Allergy Clin. Immunol. 2005, 60, 243–250. [Google Scholar] [CrossRef]
- Yamada, Y.; Yokooji, T.; Ninomiya, N.; Taogoshi, T.; Morita, E.; Matsuo, H. Evaluation of the Allergenicity of Ω5-Gliadin-Deficient Hokushin Wheat (1BS-18) in a Wheat Allergy Rat Model. Biochem. Biophys. Rep. 2019, 20, 100702. [Google Scholar] [CrossRef]
- Ballegaard, A.S.R.; Madsen, C.B.; Bøgh, K.L. An Animal Model for Wheat Allergy Skin Sensitisation: A Comparative Study in Naive versus Tolerant Brown Norway Rats. Int. Arch. Allergy Immunol. 2019, 178, 106–118. [Google Scholar] [CrossRef]
- Adachi, R.; Nakamura, R.; Sakai, S.; Fukutomi, Y.; Teshima, R. Sensitization to Acid-Hydrolyzed Wheat Protein by Transdermal Administration to BALB/c Mice, and Comparison with Gluten. Allergy Eur. J. Allergy Clin. Immunol. 2012, 67, 1392–1399. [Google Scholar] [CrossRef]
- Kroghsbo, S.; Andersen, N.B.; Rasmussen, T.F.; Jacobsenf, S.; Madsen, C.B. Acid Hydrolysis of Wheat Gluten Induces Formation of New Epitopes but Does Not Enhance Sensitizing Capacity by the Oral Route: A Study in “Gluten Free” Brown Norway Rats. PLoS ONE 2014, 9, e107137. [Google Scholar] [CrossRef]
- Dearman, R.J.; Kimber, I. Animal Models of Protein Allergenicity: Potential Benefits, Pitfalls and Challenges. Clin. Exp. Allergy 2009, 39, 458–468. [Google Scholar] [CrossRef]
- Dearman, R.J.; Kimber, I. A Mouse Model for Food Allergy Using Intraperitoneal Sensitization. Methods 2007, 41, 91–98. [Google Scholar] [CrossRef]
- Awan, M.J.A.; Rasheed, A.; Saeed, N.A.; Mansoor, S. Aegilops Tauschii Presents a Genetic Roadmap for Hexaploid Wheat Improvement. Trends Genet. 2022, 38, 307–309. [Google Scholar] [CrossRef]
- Gao, H.; Jorgensen, R.; Raghunath, R.; Ng, P.K.W.; Gangur, V. An Adjuvant-Free Mouse Model Using Skin Sensitization Without Tape-Stripping Followed by Oral Elicitation of Anaphylaxis: A Novel Pre-Clinical Tool for Testing Intrinsic Wheat Allergenicity. Front. Allergy 2022, 3, 926576. [Google Scholar] [CrossRef]
- Gao, H.; Jorgensen, R.; Raghunath, R.; Chandra, S.; Othman, A.; Olson, E.; Ng, P.K.W.; Gangur, V. Intrinsic Allergenicity Potential of Salt-Soluble Protein Extracts from the Diploid, Tetraploid and Hexaploid Wheats: Validation Using an Adjuvant-Free Mouse Model. Int. J. Mol. Sci. 2023, 24, 5453. [Google Scholar] [CrossRef]
- de Sousa, T.; Ribeiro, M.; Sabença, C.; Igrejas, G. The 10,000-Year Success Story of Wheat! Foods 2021, 10, 2124. [Google Scholar] [CrossRef]
- Jorgensen, R.; Raghunath, R.; Gao, H.; Olson, E.; Ng, P.K.W.; Gangur, V. A Mouse-Based Method to Monitor Wheat Allergens in Novel Wheat Lines and Varieties Created by Crossbreeding: Proof-of-Concept Using Durum and A. Tauschii Wheats. Int. J. Mol. Sci. 2022, 23, 6505. [Google Scholar] [CrossRef]
- Jorgensen, R.; Devarahalli, S.S.; Shah, Y.; Gao, H.; Arul Arasan, T.S.; Ng, P.K.W.; Gangur, V. Advances in Gluten Hypersensitivity: Novel Dietary-Based Therapeutics in Research and Development. Int. J. Mol. Sci. 2024, 25, 4399. [Google Scholar] [CrossRef] [PubMed]
- Renz, H.; Allen, K.J.; Sicherer, S.H.; Sampson, H.A.; Lack, G.; Beyer, K.; Oettgen, H.C. Food Allergy. Nat. Rev. Dis. Prim. 2018, 4, 17098. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Leung, D.Y.M. Advances in Allergic Skin Disease, Anaphylaxis, and Hypersensitivity Reactions to Foods, Drugs, and Insects in 2014. J. Allergy Clin. Immunol. 2015, 135, 357–367. [Google Scholar] [CrossRef]
- Platts-Mills, T.A.E. The Allergy Epidemics: 1870–2010. J. Allergy Clin. Immunol. 2015, 136, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Finkelman, F.D.; Rothenberg, M.E.; Brandt, E.B.; Morris, S.C.; Strait, R.T. Molecular Mechanisms of Anaphylaxis: Lessons from Studies with Murine Models. J. Allergy Clin. Immunol. 2005, 115, 449–457. [Google Scholar] [CrossRef]
- Gouel-Chéron, A.; Dejoux, A.; Lamanna, E.; Bruhns, P. Animal Models of IgE Anaphylaxis. Biology 2023, 12, 931. [Google Scholar] [CrossRef] [PubMed]
- Gonipeta, B.; Kim, E.; Gangur, V. Mouse Models of Food Allergy: How Well Do They Simulate the Human Disorder? Crit. Rev. Food Sci. Nutr. 2015, 55, 437–452. [Google Scholar] [CrossRef]
- Gonipeta, B.; Para, R.; He, Y.; Srkalovic, I.; Ortiz, T.; Kim, E.; Parvataneni, S.; Gangur, V. Cardiac MMCP-4+ Mast Cell Expansion and Elevation of IL-6, and CCR1/3 and CXCR2 Signaling Chemokines in an Adjuvant-Free Mouse Model of Tree Nut Allergy. Immunobiology 2015, 220, 663–672. [Google Scholar] [CrossRef]
- Tsujimura, Y.; Obata, K.; Mukai, K.; Shindou, H.; Yoshida, M.; Nishikado, H.; Kawano, Y.; Minegishi, Y.; Shimizu, T.; Karasuyama, H. Basophils Play a Pivotal Role in Immunoglobulin-G-Mediated but Not Immunoglobulin-E-Mediated Systemic Anaphylaxis. Immunity 2008, 28, 581–589. [Google Scholar] [CrossRef]
- Miyajima, I.; Dombrowicz, D.; Martin, T.R.; Ravetch, J.V.; Kinet, J.P.; Galli, S.J. Systemic Anaphylaxis in the Mouse Can Be Mediated Largely through IgG1 and FcyRIII. J. Clin. Investig. 1997, 99, 901–914. [Google Scholar] [CrossRef] [PubMed]
- Khodoun, M.V.; Strait, R.; Armstrong, L.; Yanase, N.; Finkelman, F.D. Identification of Markers That Distinguish IgE- from IgG-Mediated Anaphylaxis. Proc. Natl. Acad. Sci. USA 2011, 108, 12413–12418. [Google Scholar] [CrossRef]
- Andersson, M.K.; Pemberton, A.D.; Miller, H.R.P.; Hellman, L. Extended Cleavage Specificity of MMCP-1, the Major Mucosal Mast Cell Protease in Mouse-High Specificity Indicates High Substrate Selectivity. Mol. Immunol. 2008, 45, 2548–2558. [Google Scholar] [CrossRef]
- Dunne, M.R.; Byrne, G.; Chirdo, F.G.; Feighery, C. Coeliac Disease Pathogenesis: The Uncertainties of a Well-Known Immune Mediated Disorder. Front. Immunol. 2020, 11, 1374. [Google Scholar] [CrossRef]
- Han, X.; Krempski, J.W.; Nadeau, K. Advances and Novel Developments in Mechanisms of Allergic Inflammation. Allergy Eur. J. Allergy Clin. Immunol. 2020, 75, 3100–3111. [Google Scholar] [CrossRef]
- Nguyen, S.M.T.; Rupprecht, C.P.; Haque, A.; Pattanaik, D.; Yusin, J.; Krishnaswamy, G. Mechanisms Governing Anaphylaxis: Inflammatory Cells, Mediators, Endothelial Gap Junctions and Beyond. Int. J. Mol. Sci. 2021, 22, 7785. [Google Scholar] [CrossRef]
- Zhang, H.; Dhalla, N.S. The Role of Pro-Inflammatory Cytokines in the Pathogenesis of Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 1082. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Vickery, B.P.; Wood, R.A. The Use of Biologics in Food Allergy. Clin. Exp. Allergy 2021, 51, 1006–1018. [Google Scholar] [CrossRef]
- Abadie, V.; Kim, S.M.; Lejeune, T.; Palanski, B.A.; Ernest, J.D.; Tastet, O.; Voisine, J.; Discepolo, V.; Marietta, E.V.; Hawash, M.B.F.; et al. IL-15, Gluten and HLA-DQ8 Drive Tissue Destruction in Coeliac Disease. Nature 2020, 578, 600–604. [Google Scholar] [CrossRef]
- Chen, C.H.; Bushuk, W. Nature of proteins in triticale and its parental species: I. solubility characteristics and amino acid composition of endosperm proteins. Can. J. Plant Sci. 1970, 50, 9–14. [Google Scholar] [CrossRef]
- Jin, Y.; Gao, H.; Jorgensen, R.; Salloum, J.; Jian, D.I.; Ng, P.K.W.; Gangur, V. Mechanisms of Wheat Allergenicity in Mice: Comparison of Adjuvant-Free vs. Alum-Adjuvant Models. Int. J. Mol. Sci. 2020, 21, 3205. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ebaugh, S.; Martens, A.; Gao, H.; Olson, E.; Ng, P.K.W.; Gangur, V. A Mouse Model of Anaphylaxis and Atopic Dermatitis to Salt-Soluble Wheat Protein Extract. Int. Arch. Allergy Immunol. 2017, 174, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Jin, Y.; Jian, D.I.; Olson, E.; Ng, P.K.W.; Gangur, V. Development and Validation of a Mouse-Based Primary Screening Method for Testing Relative Allergenicity of Proteins from Different Wheat Genotypes. J. Immunol. Methods 2019, 464, 95–104. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jorgensen, R.; Arul Arasan, T.S.; Srkalovic, M.B.; Van Antwerp, C.; Ng, P.K.W.; Gangur, V. Glutenin from the Ancient Wheat Progenitor Is Intrinsically Allergenic as It Can Clinically Sensitize Mice for Systemic Anaphylaxis by Activating Th2 Immune Pathway. Int. J. Mol. Sci. 2024, 25, 7324. https://doi.org/10.3390/ijms25137324
Jorgensen R, Arul Arasan TS, Srkalovic MB, Van Antwerp C, Ng PKW, Gangur V. Glutenin from the Ancient Wheat Progenitor Is Intrinsically Allergenic as It Can Clinically Sensitize Mice for Systemic Anaphylaxis by Activating Th2 Immune Pathway. International Journal of Molecular Sciences. 2024; 25(13):7324. https://doi.org/10.3390/ijms25137324
Chicago/Turabian StyleJorgensen, Rick, Tamil Selvan Arul Arasan, Maya Blanka Srkalovic, Chris Van Antwerp, Perry K. W. Ng, and Venu Gangur. 2024. "Glutenin from the Ancient Wheat Progenitor Is Intrinsically Allergenic as It Can Clinically Sensitize Mice for Systemic Anaphylaxis by Activating Th2 Immune Pathway" International Journal of Molecular Sciences 25, no. 13: 7324. https://doi.org/10.3390/ijms25137324
APA StyleJorgensen, R., Arul Arasan, T. S., Srkalovic, M. B., Van Antwerp, C., Ng, P. K. W., & Gangur, V. (2024). Glutenin from the Ancient Wheat Progenitor Is Intrinsically Allergenic as It Can Clinically Sensitize Mice for Systemic Anaphylaxis by Activating Th2 Immune Pathway. International Journal of Molecular Sciences, 25(13), 7324. https://doi.org/10.3390/ijms25137324



