Transcriptome Analysis Reveals Cross-Talk between the Flagellar Transcriptional Hierarchy and Secretion System in Plesiomonas shigelloides
Abstract
:1. Introduction
2. Results
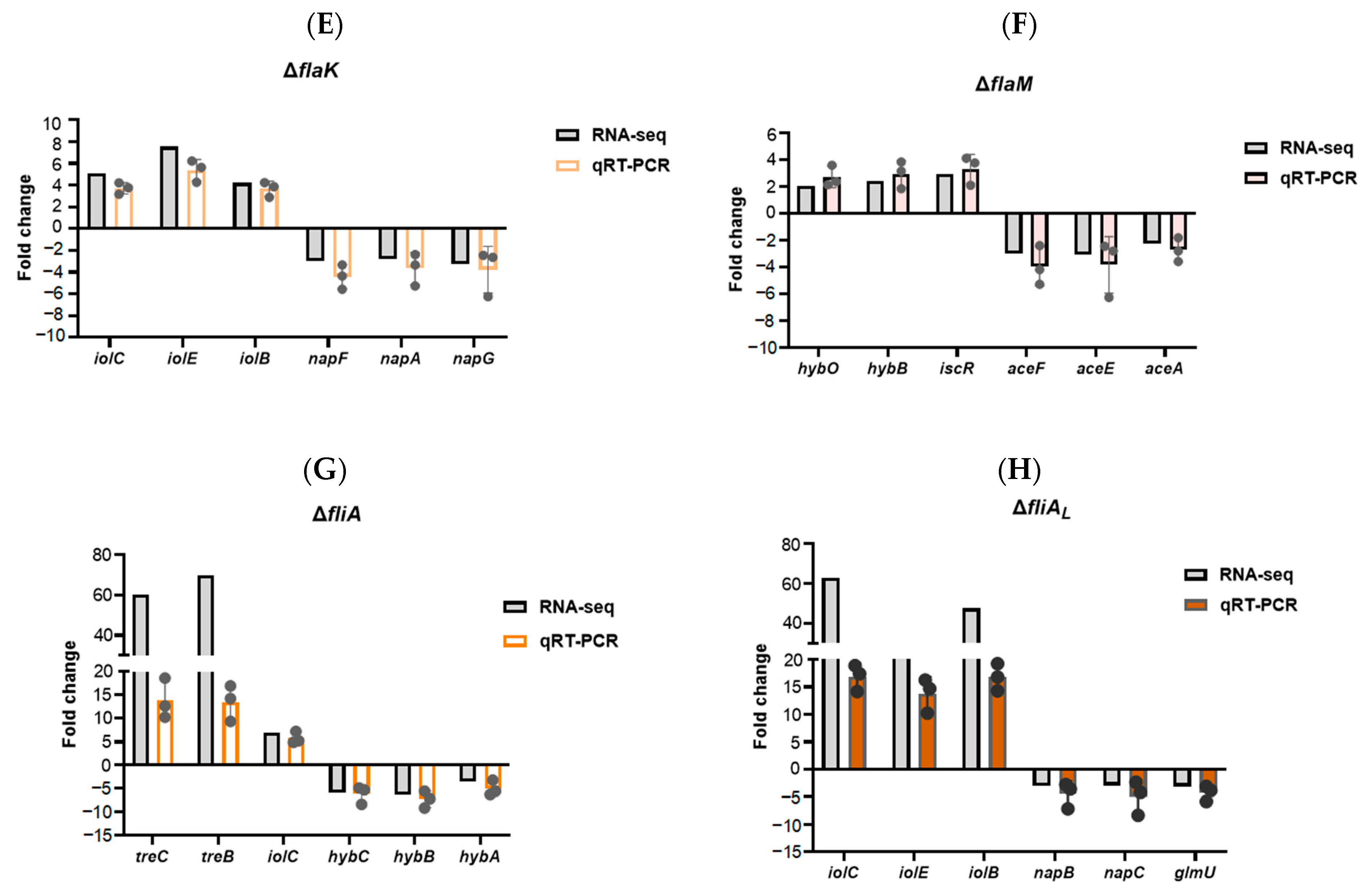
2.1. RNA Sequencing of Flagellar Regulatory Mutants
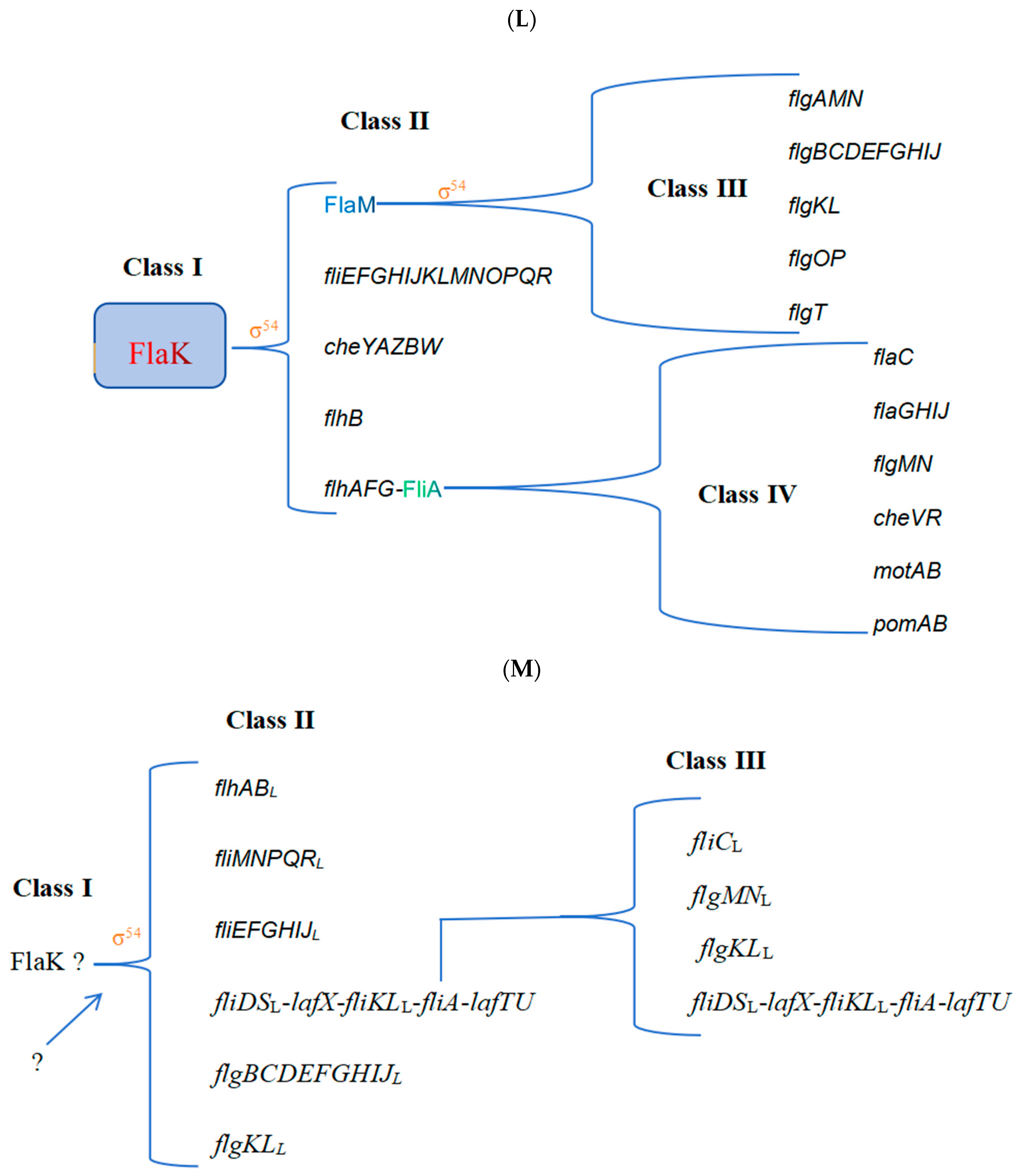
2.2. Refinement of the Flagellar Transcription Hierarchy
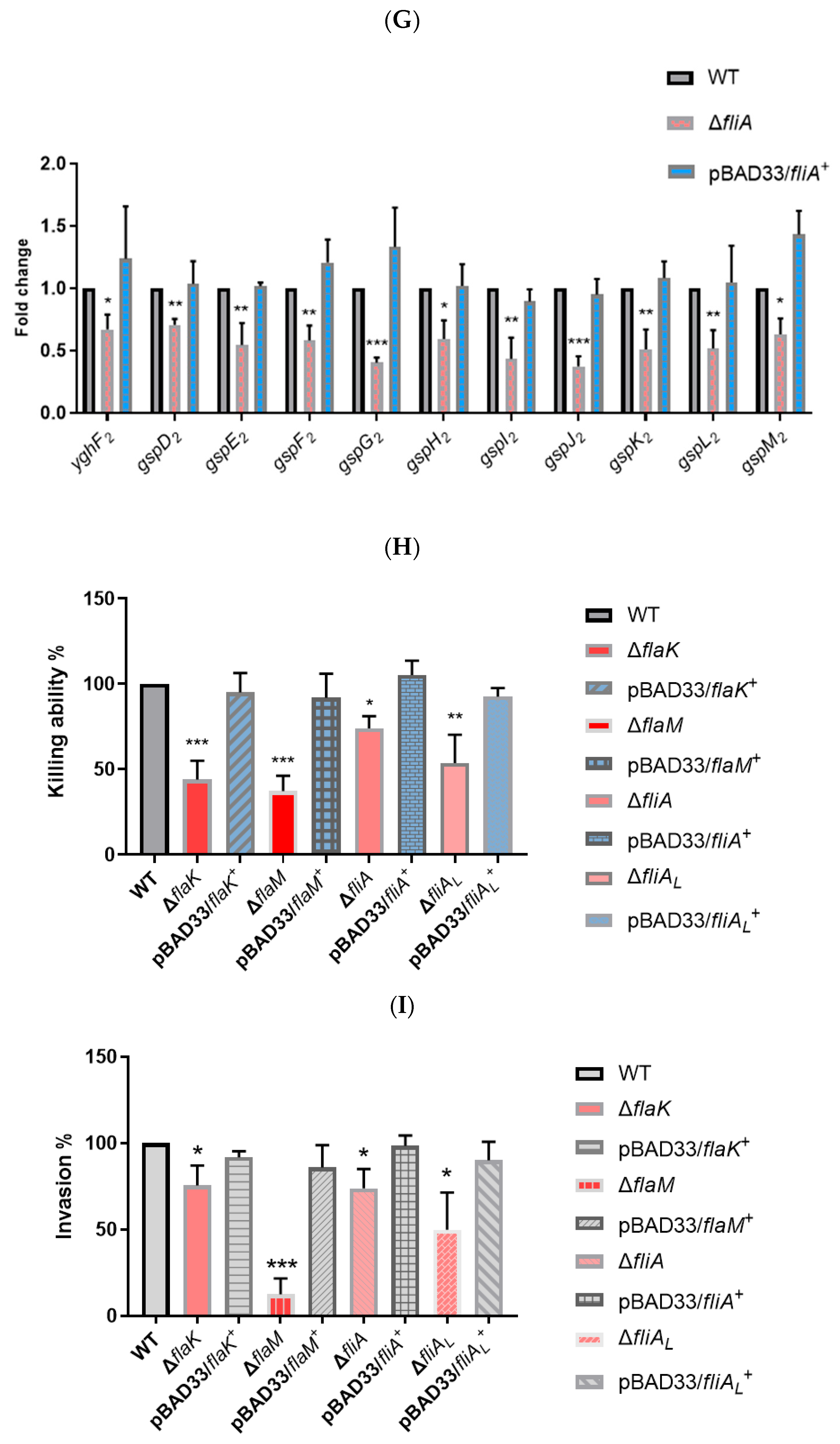
2.3. Flagellar Regulatory Mutants Reduce the Killing Ability and Virulence of P. shigelloides to Varying Degrees
2.4. Cross-Talk between the Flagellar System and P. shigelloides’ Physiological Metabolism
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Plasmids, and Growth Conditions
4.2. Construction of Gene Deletion Strains and Complementation
4.3. RNA Isolation and Transcriptome Sequencing
4.4. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.5. Motility Assay and Transmission Electron Microscopy (TEM) of Flagella
4.6. Luminescence Screening Assay
4.7. Expression and Purification of Proteins and Electrophoretic Mobility Shift Assays (EMSAs)
4.8. Killing Assay
4.9. Growth Assay
4.10. Invasion Assay
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Puah, S.M.; Puthucheary, S.D.A.; Chua, K.H. Virulence Profiles among Gastrointestinal and Extraintestinal Clinical Isolates of Plesiomonas shigelloides. Jpn. J. Infect. Dis. 2022, 75, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.K.; Whale, K.; Morson, B.C. Acute colitis due to Plesiomonas shigelloides. Br. Med. J. (Clin. Res. Ed.) 1982, 285, 1539–1540. [Google Scholar] [CrossRef] [PubMed]
- McNeeley, D.; Ivy, P.; Craft, J.C.; Cohen, I. Plesiomonas: Biology of the organism and diseases in children. Pediatr. Infect. Dis. 1984, 3, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, T.; Kinoshita, Y.; Shimada, T.; Sakazaki, R. Two epidemics of diarrhoeal disease possibly caused by Plesiomonas shigelloides. J. Hyg. 1978, 80, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Billiet, J.; Kuypers, S.; Van Lierde, S.; Verhaegen, J. Plesiomonas shigelloides meningitis and septicaemia in a neonate: Report of a case and review of the literature. J. Infect. 1989, 19, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Chakraborty, T.; Hof, H.; Kirchner, T.; Wamsler, O. Pseudoappendicitis caused by Plesiomonas shigelloides. J. Clin. Microbiol. 1988, 26, 2675–2677. [Google Scholar] [CrossRef] [PubMed]
- Pennycook, K.M.; Pennycook, K.B.; McCready, T.A.; Kazanowski, D. Severe cellulitis and bacteremia caused by Plesiomonas shigelloides following a traumatic freshwater injury. IDCases 2019, 19, e00637. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, N.; Wolfgang, M.C.; Goodman, A.L.; Arora, S.K.; Jyot, J.; Lory, S.; Ramphal, R. A four-tiered transcriptional regulatory circuit controls flagellar biogenesis in Pseudomonas aeruginosa. Mol. Microbiol. 2003, 50, 809–824. [Google Scholar] [CrossRef] [PubMed]
- Fenchel, T. Microbial behavior in a heterogeneous world. Science 2002, 296, 1068–1071. [Google Scholar] [CrossRef]
- Tsang, J.; Hoover, T.R. Basal Body Structures Differentially Affect Transcription of RpoN- and FliA-Dependent Flagellar Genes in Helicobacter pylori. J. Bacteriol. 2015, 197, 1921–1930. [Google Scholar] [CrossRef]
- Wilhelms, M.; Molero, R.; Shaw, J.G.; Tomás, J.M.; Merino, S. Transcriptional hierarchy of Aeromonas hydrophila polar-flagellum genes. J. Bacteriol. 2011, 193, 5179–5190. [Google Scholar] [CrossRef] [PubMed]
- Kutsukake, K. Autogenous and global control of the flagellar master operon, flhD, in Salmonella typhimurium. Mol. Genet. Genom. 1997, 254, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Correa, N.E.; Peng, F.; Klose, K.E. Roles of the regulatory proteins FlhF and FlhG in the Vibrio cholerae flagellar transcription hierarchy. J. Bacteriol. 2005, 187, 6324–6332. [Google Scholar] [CrossRef] [PubMed]
- Klose, K.E.; Mekalanos, J.J. Distinct roles of an alternative sigma factor during both free-swimming and colonizing phases of the Vibrio cholerae pathogenic cycle. Mol. Microbiol. 1998, 28, 501–520. [Google Scholar] [CrossRef] [PubMed]
- Prouty, M.G.; Correa, N.E.; Klose, K.E. The novel sigma54- and sigma28-dependent flagellar gene transcription hierarchy of Vibrio cholerae. Mol. Microbiol. 2001, 39, 1595–1609. [Google Scholar] [CrossRef] [PubMed]
- Stewart, B.J.; McCarter, L.L. Lateral flagellar gene system of Vibrio parahaemolyticus. J. Bacteriol. 2003, 185, 4508–4518. [Google Scholar] [CrossRef] [PubMed]
- Wilhelms, M.; Gonzalez, V.; Tomás, J.M.; Merino, S. Aeromonas hydrophila lateral flagellar gene transcriptional hierarchy. J. Bacteriol. 2013, 195, 1436–1445. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Meng, H.; Li, Y.; Ge, H.; Jiao, X. A GntR Family Transcription Factor (VPA1701) for Swarming Motility and Colonization of Vibrio parahaemolyticus. Pathogens 2019, 8, 235. [Google Scholar] [CrossRef]
- Kim, Y.K.; McCarter, L.L. Cross-regulation in Vibrio parahaemolyticus: Compensatory activation of polar flagellar genes by the lateral flagellar regulator LafK. J. Bacteriol. 2004, 186, 4014–4018. [Google Scholar] [CrossRef]
- Merino, S.; Shaw, J.G.; Tomás, J.M. Bacterial lateral flagella: An inducible flagella system. FEMS Microbiol. Lett. 2006, 263, 127–135. [Google Scholar] [CrossRef]
- Shinoda, S.; Okamoto, K. Formation and function of Vibrio parahaemolyticus lateral flagella. J. Bacteriol. 1977, 129, 1266–1271. [Google Scholar] [CrossRef]
- Petersen, B.D.; Liu, M.S.; Podicheti, R.; Yang, A.Y.; Simpson, C.A.; Hemmerich, C.; Rusch, D.B.; van Kessel, J.C. The Polar Flagellar Transcriptional Regulatory Network in Vibrio campbellii Deviates from Canonical Vibrio Species. J. Bacteriol. 2021, 203, e0027621. [Google Scholar] [CrossRef] [PubMed]
- Gardel, C.L.; Mekalanos, J.J. Alterations in Vibrio cholerae motility phenotypes correlate with changes in virulence factor expression. Infect. Immun. 1996, 64, 2246–2255. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Yamamoto, K.; Kawagishi, I.; Homma, M. The polar flagellar motor of Vibrio cholerae is driven by an Na+ motive force. J. Bacteriol. 1999, 181, 1927–1930. [Google Scholar] [CrossRef] [PubMed]
- Häse, C.C.; Mekalanos, J.J. Effects of changes in membrane sodium flux on virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 1999, 96, 3183–3187. [Google Scholar] [CrossRef] [PubMed]
- Coster, T.S.; Killeen, K.P.; Waldor, M.K.; Beattie, D.T.; Spriggs, D.R.; Kenner, J.R.; Trofa, A.; Sadoff, J.C.; Mekalanos, J.J.; Taylor, D.N. Safety, immunogenicity, and efficacy of live attenuated Vibrio cholerae O139 vaccine prototype. Lancet 1995, 345, 949–952. [Google Scholar] [PubMed]
- Yang, Q.; Defoirdt, T. Quorum sensing positively regulates flagellar motility in pathogenic Vibrio harveyi. Environ. Microbiol. 2015, 17, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.; Wang, X.; Shawky, R.M.; Emara, M.; Aldridge, P.D.; Rao, C.V. Synergistic action of SPI-1 gene expression in Salmonella enterica serovar typhimurium through transcriptional crosstalk with the flagellar system. BMC Microbiol. 2019, 19, 211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.H.; Shaw, J.G. Cross-Talk between the Aeromonas hydrophila Type III Secretion System and Lateral Flagella System. Front. Microbiol. 2016, 7, 1434. [Google Scholar] [CrossRef]
- Montie, T.C.; Doyle-Huntzinger, D.; Craven, R.C.; Holder, I.A. Loss of virulence associated with absence of flagellum in an isogenic mutant of Pseudomonas aeruginosa in the burned-mouse model. Infect. Immun. 1982, 38, 1296–1298. [Google Scholar] [CrossRef]
- Fleiszig, S.M.; Arora, S.K.; Van, R.; Ramphal, R. FlhA, a component of the flagellum assembly apparatus of Pseudomonas aeruginosa, plays a role in internalization by corneal epithelial cells. Infect. Immun. 2001, 69, 4931–4937. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, K.; Braun, D.; Kleigrewe, K.; Jung, K. Motility-activating mutations upstream of flhDC reduce acid shock survival of Escherichia coli. Microbiol. Spectr. 2024, 12, e0054424. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.M.; Noorian, P.; Espinoza-Vergara, G.; Adhikary, S.; To, J.; Rice, S.A.; McDougald, D. Increased iron utilization and oxidative stress tolerance in a Vibrio cholerae flrA mutant confers resistance to amoeba predation. Appl. Environ. Microbiol. 2023, 89, e0109523. [Google Scholar] [CrossRef] [PubMed]
- Sinha-Ray, S.; Ali, A. Mutation in flrA and mshA Genes of Vibrio cholerae Inversely Involved in vps-Independent Biofilm Driving Bacterium Toward Nutrients in Lake Water. Front. Microbiol. 2017, 8, 1770. [Google Scholar] [CrossRef]
- Rodríguez-Herva, J.J.; Duque, E.; Molina-Henares, M.A.; Navarro-Avilés, G.; Van Dillewijn, P.; De La Torre, J.; Molina-Henares, A.J.; La Campa, A.S.; Ran, F.A.; Segura, A.; et al. Physiological and transcriptomic characterization of a fliA mutant of Pseudomonas putida KT2440. Environ. Microbiol. Rep. 2010, 2, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zong, W.; Zhang, Z.; Lv, W.; Ji, X.; Zhu, D.; Du, X.; Wang, S. Effects and molecular mechanism of flagellar gene flgK on the motility, adhesion/invasion, and desiccation resistance of Cronobacter sakazakii. Food Res. Int. 2023, 164, 112418. [Google Scholar] [CrossRef] [PubMed]
- Jubelin, G.; Lanois, A.; Severac, D.; Rialle, S.; Longin, C.; Gaudriault, S.; Givaudan, A. FliZ is a global regulatory protein affecting the expression of flagellar and virulence genes in individual Xenorhabdus nematophila bacterial cells. PLoS Genet. 2013, 9, e1003915. [Google Scholar] [CrossRef] [PubMed]
- Lanois, A.; Jubelin, G.; Givaudan, A. FliZ, a flagellar regulator, is at the crossroads between motility, haemolysin expression and virulence in the insect pathogenic bacterium Xenorhabdus. Mol. Microbiol. 2008, 68, 516–533. [Google Scholar] [CrossRef]
- Merino, S.; Aquilini, E.; Fulton, K.M.; Twine, S.M.; Tomás, J.M. The polar and lateral flagella from Plesiomonas shigelloides are glycosylated with legionaminic acid. Front. Microbiol. 2015, 6, 649. [Google Scholar] [CrossRef]
- Chilcott, G.S.; Hughes, K.T. Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar typhimurium and Escherichia coli. Microbiol. Mol. Biol. Rev. 2000, 64, 694–708. [Google Scholar] [CrossRef]
- Ferguson, W.W.; Henderson, N.D. Description of Strain C27: A Motile Organism with the Major Antigen of Shigella sonnei Phase I. J. Bacteriol. 1947, 54, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.M.; Abbott, S.L.; McIver, C.J. Plesiomonas shigelloides Revisited. Clin. Microbiol. Rev. 2016, 29, 349–374. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.S.; McLaughlin, R.W.; Li, J.; Wan, X.; Liu, Y.; Xie, H.; Hao, Y.; Zheng, J. Putative virulence factors of Plesiomonas shigelloides. Antonie Van Leeuwenhoek 2019, 112, 1815–1826. [Google Scholar] [CrossRef] [PubMed]
- Ekundayo, T.C.; Okoh, A.I. A global bibliometric analysis of Plesiomonas-related research (1990–2017). PLoS ONE 2018, 13, e0207655. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Guo, X.; Li, J.; Li, Y.; Sun, H.; Li, A.; Cao, B. RpoN is required for the motility and contributes to the killing ability of Plesiomonas shigelloides. BMC Microbiol. 2022, 22, 299. [Google Scholar] [CrossRef]
- Canals, R.; Altarriba, M.; Vilches, S.; Horsburgh, G.; Shaw, J.G.; Tomás, J.M.; Merino, S. Analysis of the lateral flagellar gene system of Aeromonas hydrophila AH-3. J. Bacteriol. 2006, 188, 852–862. [Google Scholar] [CrossRef]
- Speare, L.; Zhao, L.; Pavelsky, M.N.; Jackson, A.; Smith, S.; Tyagi, B.; Sharpe, G.C.; Woo, M.; Satkowiak, L.; Bolton, T.; et al. Flagella are required to coordinately activate competition and host colonization factors in response to a mechanical signal. bioRxiv 2024. [Google Scholar] [CrossRef]
- Huang, Z.; Yu, K.; Lan, R.; Glenn Morris, J.; Xiao, Y.; Ye, J.; Zhang, L.; Luo, L.; Gao, H.; Bai, X.; et al. Vibrio metschnikovii as an emergent pathogen: Analyses of phylogeny and O-antigen and identification of possible virulence characteristics. Emerg. Microbes Infect. 2023, 12, 2252522. [Google Scholar] [CrossRef]
- Bouteiller, M.; Gallique, M.; Bourigault, Y.; Kosta, A.; Hardouin, J.; Massier, S.; Konto-Ghiorghi, Y.; Barbey, C.; Latour, X.; Chane, A.; et al. Crosstalk between the Type VI Secretion System and the Expression of Class IV Flagellar Genes in the Pseudomonas fluorescens MFE01 Strain. Microorganisms 2020, 8, 622. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Luan, Y.; Yang, J.; Chen, X.; Wu, T.; Li, Y.; Munang’andu, H.M.; Shao, G.; Chen, X. Comparative secretome analysis reveals cross-talk between type III secretion system and flagella assembly in Pseudomonas plecoglossicida. Heliyon 2023, 9, e22669. [Google Scholar] [CrossRef]
- Soria-Bustos, J.; Saldaña-Ahuactzi, Z.; Samadder, P.; Yañez-Santos, J.A.; Laguna, Y.M.; Cedillo-Ramírez, M.L.; Girón, J.A. The Assembly of Flagella in Enteropathogenic Escherichia coli Requires the Presence of a Functional Type III Secretion System. Int. J. Mol. Sci. 2022, 23, 13705. [Google Scholar] [CrossRef] [PubMed]
- Xi, D.; Jing, F.; Liu, Q.; Cao, B. Plesiomonas shigelloides sipD mutant, generated by an efficient gene transfer system, is less invasive. J. Microbiol. Methods 2019, 159, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Feng, L.; Yang, B.; Zhang, W.; Wang, P.; Jiang, X.; Wang, L. Signal transduction pathway mediated by the novel regulator LoiA for low oxygen tension induced Salmonella Typhimurium invasion. PLoS Pathog. 2017, 13, e1006429. [Google Scholar] [CrossRef]
- Wilhelms, M.; Fulton, K.M.; Twine, S.M.; Tomás, J.M.; Merino, S. Differential glycosylation of polar and lateral flagellins in Aeromonas hydrophila AH-3. J. Biol. Chem. 2012, 287, 27851–27862. [Google Scholar] [CrossRef]
- Evans, M.R.; Fink, R.C.; Vazquez-Torres, A.; Porwollik, S.; Jones-Carson, J.; McClelland, M.; Hassan, H.M. Analysis of the ArcA regulon in anaerobically grown Salmonella enterica sv. Typhimurium. BMC Microbiol. 2011, 11, 58. [Google Scholar] [CrossRef]
- Li, Y.; Yan, J.; Guo, X.; Wang, X.; Liu, F.; Cao, B. The global regulators ArcA and CytR collaboratively modulate Vibrio cholerae motility. BMC Microbiol. 2022, 22, 22. [Google Scholar] [CrossRef]
- Borgeaud, S.; Metzger, L.C.; Scrignari, T.; Blokesch, M. The type VI secretion system of Vibrio cholerae fosters horizontal gene transfer. Science 2015, 347, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, Y.; Guo, X.; Wang, X.; Liu, F.; Li, A.; Cao, B. The effect of ArcA on the growth, motility, biofilm formation, and virulence of Plesiomonas shigelloides. BMC Microbiol. 2021, 21, 266. [Google Scholar] [CrossRef] [PubMed]
- Schubert, R.H.; Holz-Bremer, A. Cell adhesion of Plesiomonas shigelloides. Zentralblatt Hyg. Umweltmed. 1999, 202, 383–388. [Google Scholar] [CrossRef]
- Yang, S.; Xi, D.; Wang, X.; Li, Y.; Li, Y.; Yan, J.; Cao, B. Vibrio cholerae VC1741 (PsrA) enhances the colonization of the pathogen in infant mice intestines in the presence of the long-chain fatty acid, oleic acid. Microb. Pathog. 2020, 147, 104443. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Zhang, Z.; Shi, H.; Xue, X.; Li, A.; Ding, P.; Guo, X.; Wang, J.; Wang, Y.; Cao, B. Transcriptome Analysis Reveals Cross-Talk between the Flagellar Transcriptional Hierarchy and Secretion System in Plesiomonas shigelloides. Int. J. Mol. Sci. 2024, 25, 7375. https://doi.org/10.3390/ijms25137375
Yan J, Zhang Z, Shi H, Xue X, Li A, Ding P, Guo X, Wang J, Wang Y, Cao B. Transcriptome Analysis Reveals Cross-Talk between the Flagellar Transcriptional Hierarchy and Secretion System in Plesiomonas shigelloides. International Journal of Molecular Sciences. 2024; 25(13):7375. https://doi.org/10.3390/ijms25137375
Chicago/Turabian StyleYan, Junxiang, Zixu Zhang, Hongdan Shi, Xinke Xue, Ang Li, Peng Ding, Xi Guo, Jinzhong Wang, Ying Wang, and Boyang Cao. 2024. "Transcriptome Analysis Reveals Cross-Talk between the Flagellar Transcriptional Hierarchy and Secretion System in Plesiomonas shigelloides" International Journal of Molecular Sciences 25, no. 13: 7375. https://doi.org/10.3390/ijms25137375




