Genome-Wide Analysis of the Gibberellin-Oxidases Family Members in Four Prunus Species and a Functional Analysis of PmGA2ox8 in Plant Height
Abstract
1. Introduction
2. Results
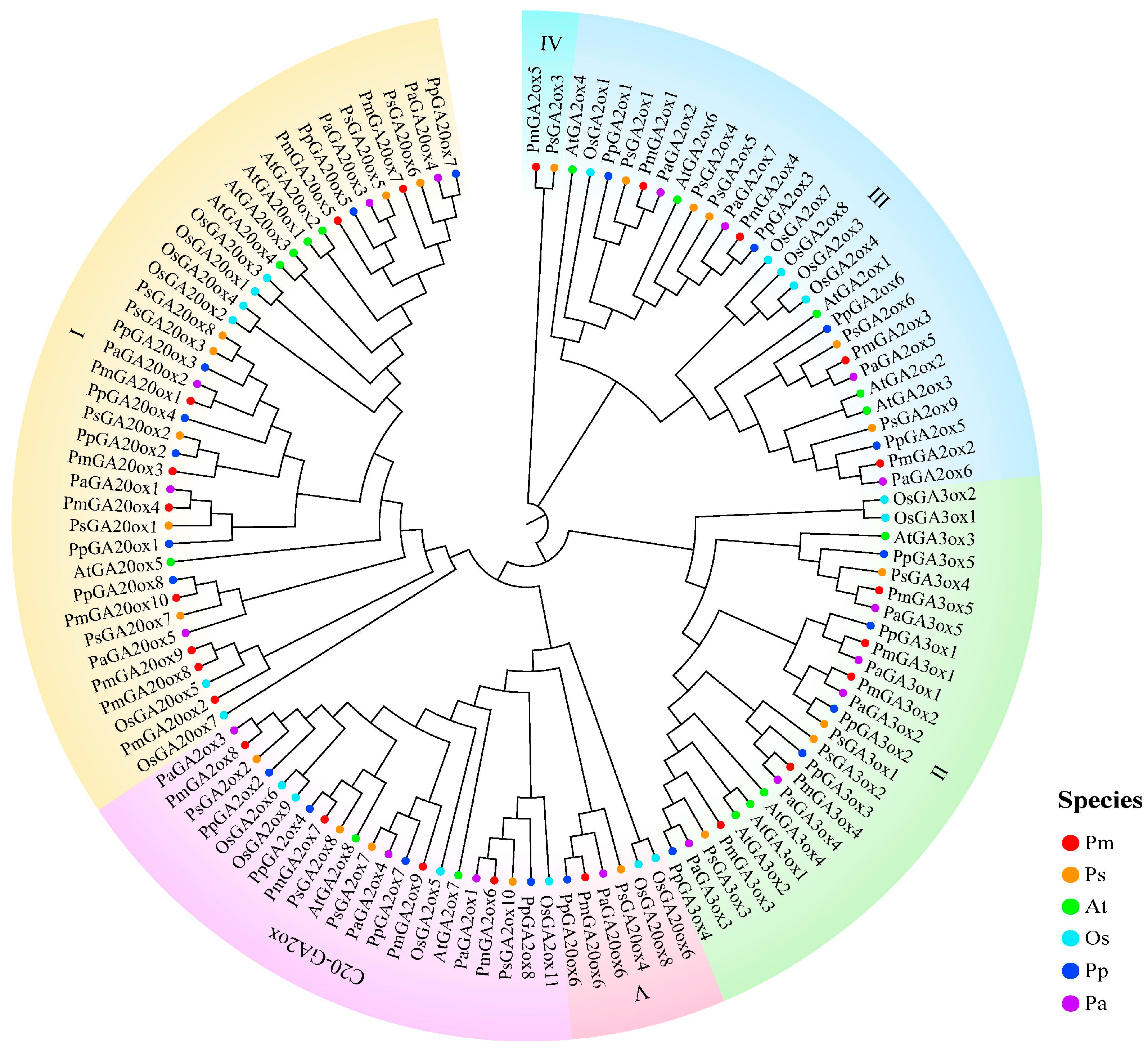
2.1. Genome-Wide Identification and Analysis of GAoxs in Four Prunus Species
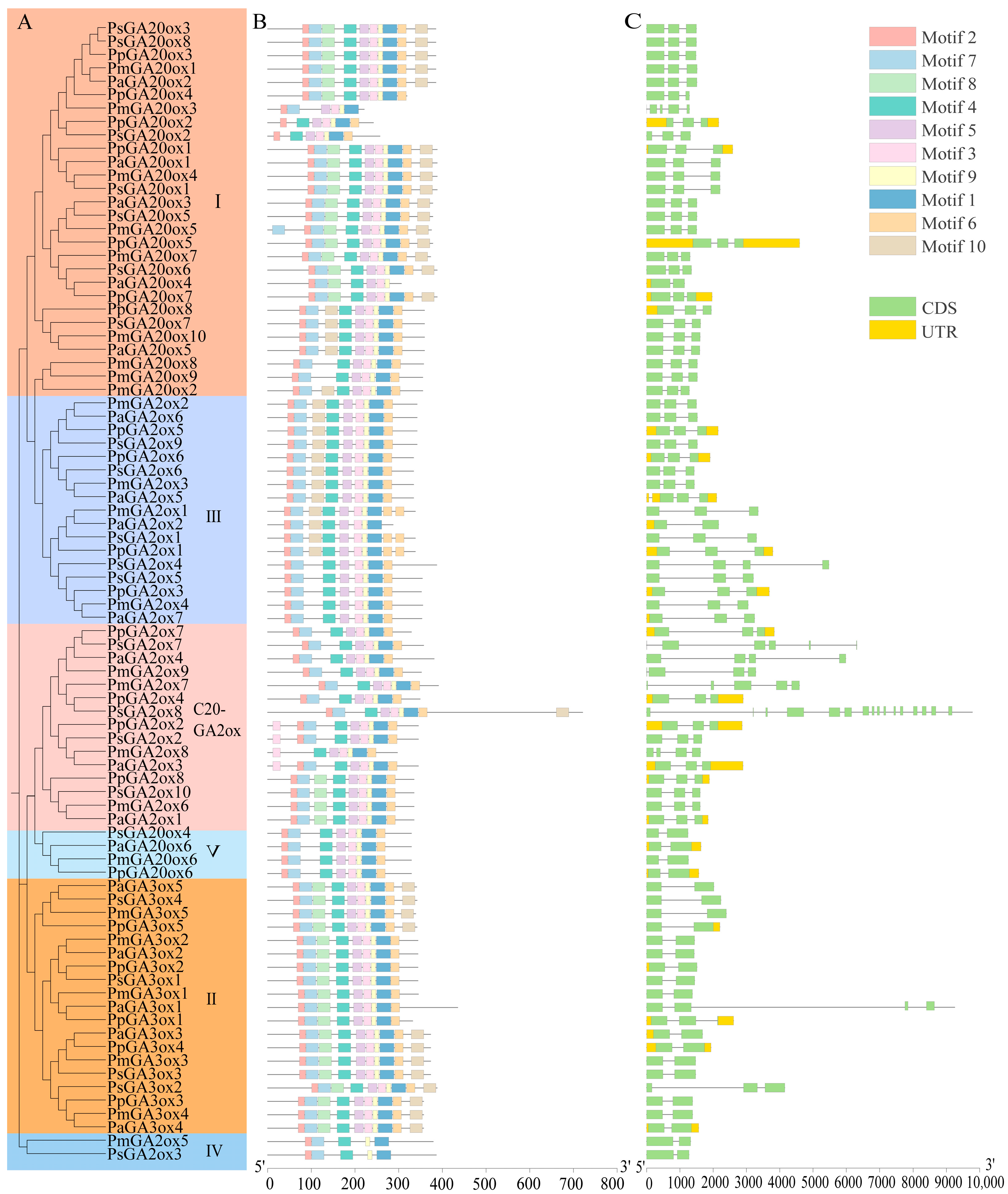
2.2. Gene Structure and Conserved Motif Analysis of GAox in Four Prunus Species
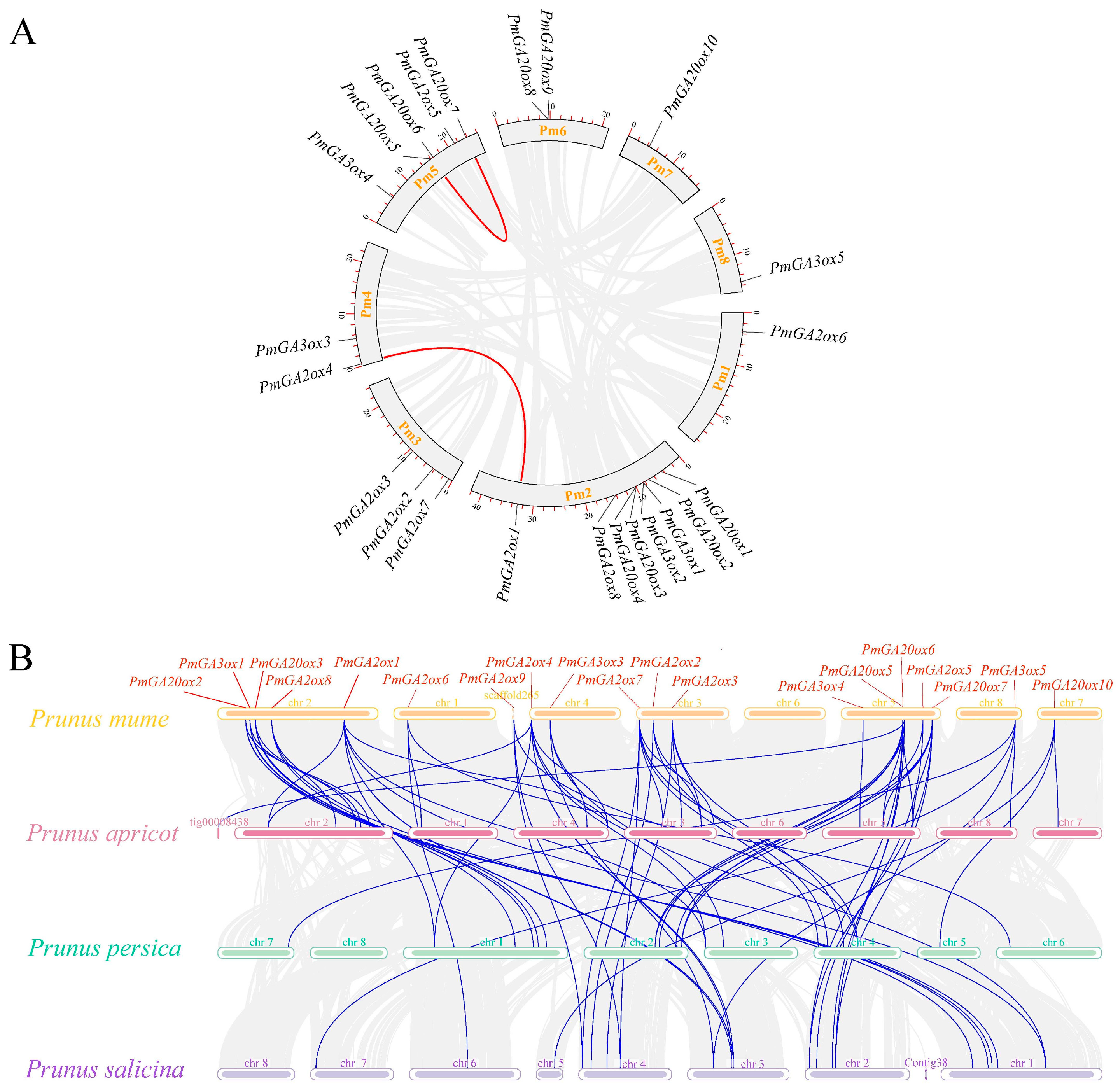
2.3. Synteny Analysis of GAoxs in Four Prunus Species
2.4. Cis-Element Analysis in Promoter of PmGAoxs
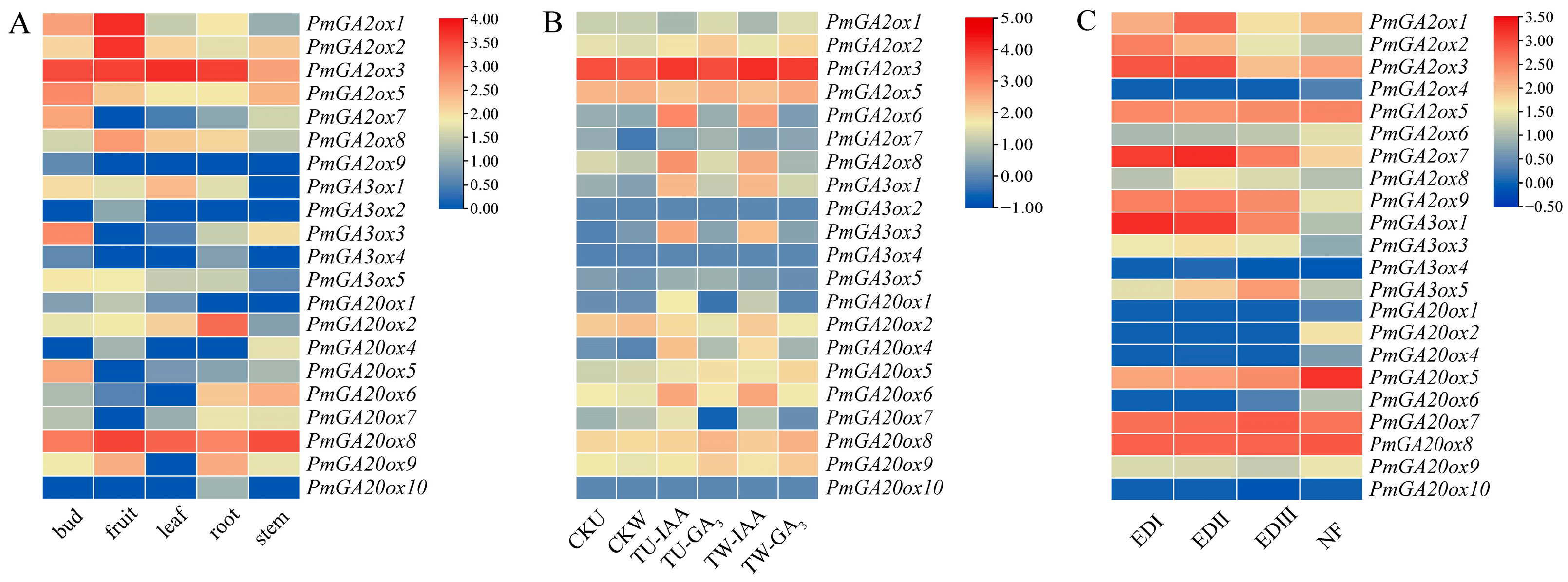
2.5. Expression Pattern Analysis of PmGAoxs
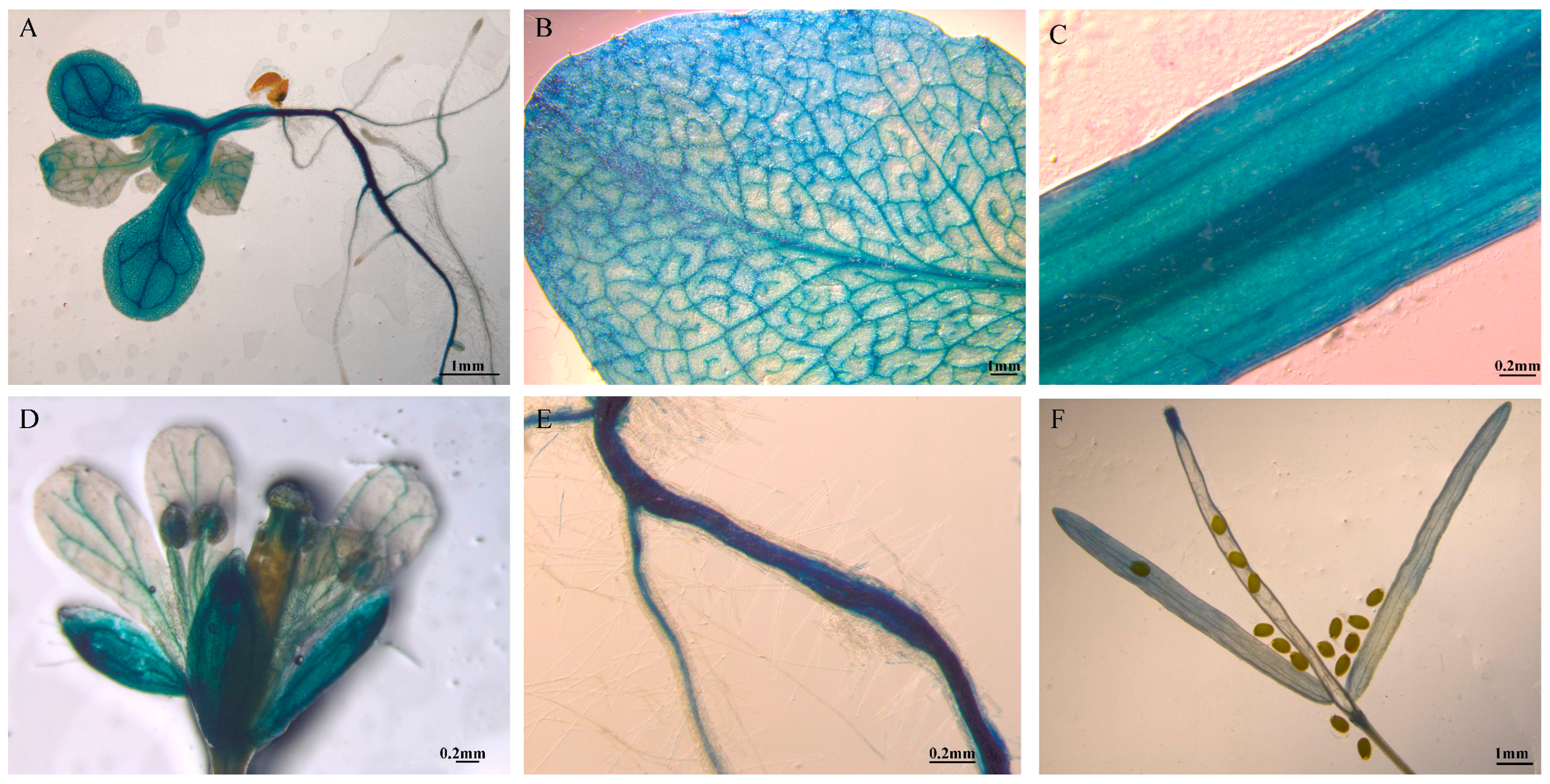
2.6. Transcription Activation Activity of PmGA2ox8 Promoter
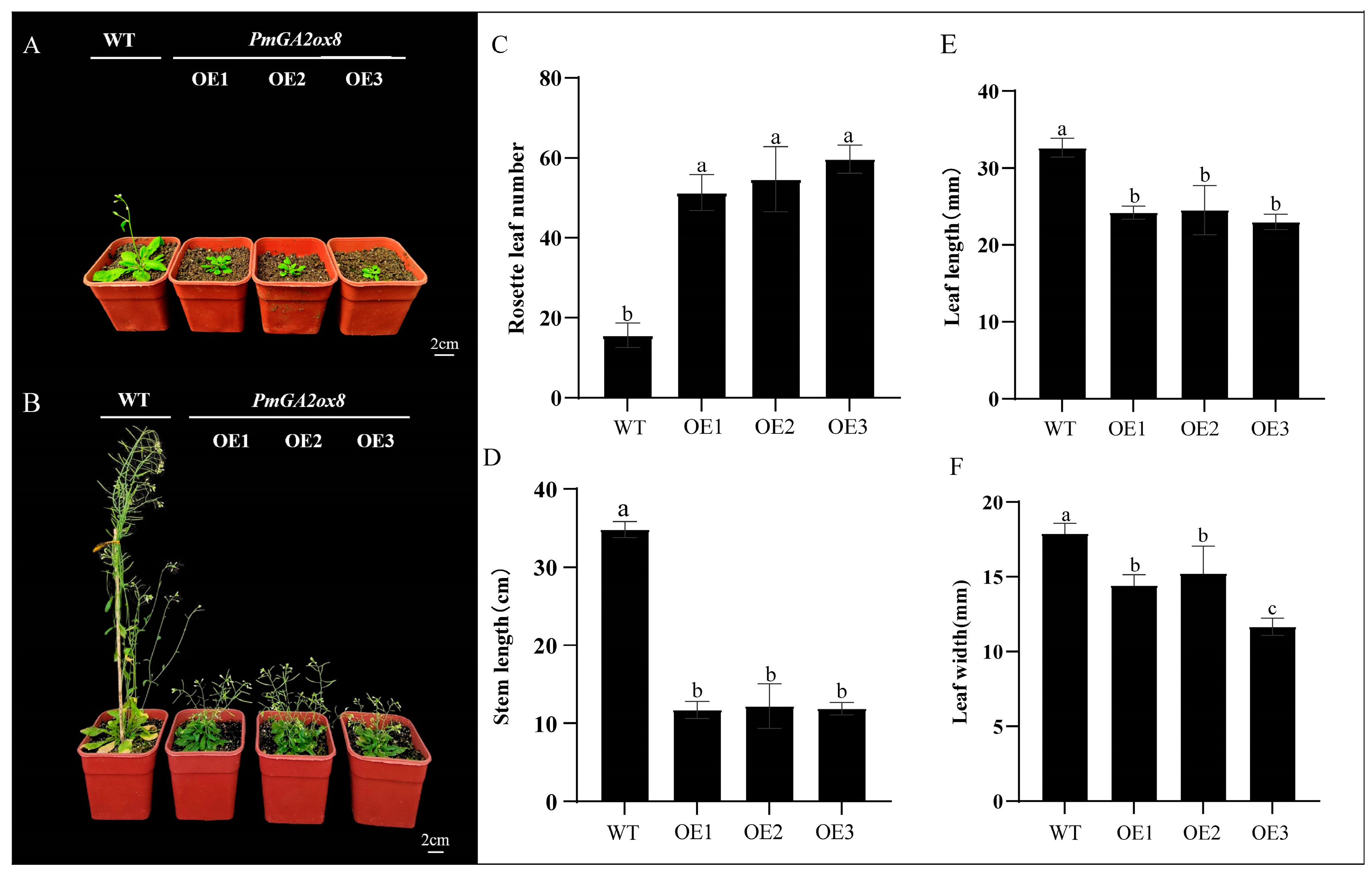
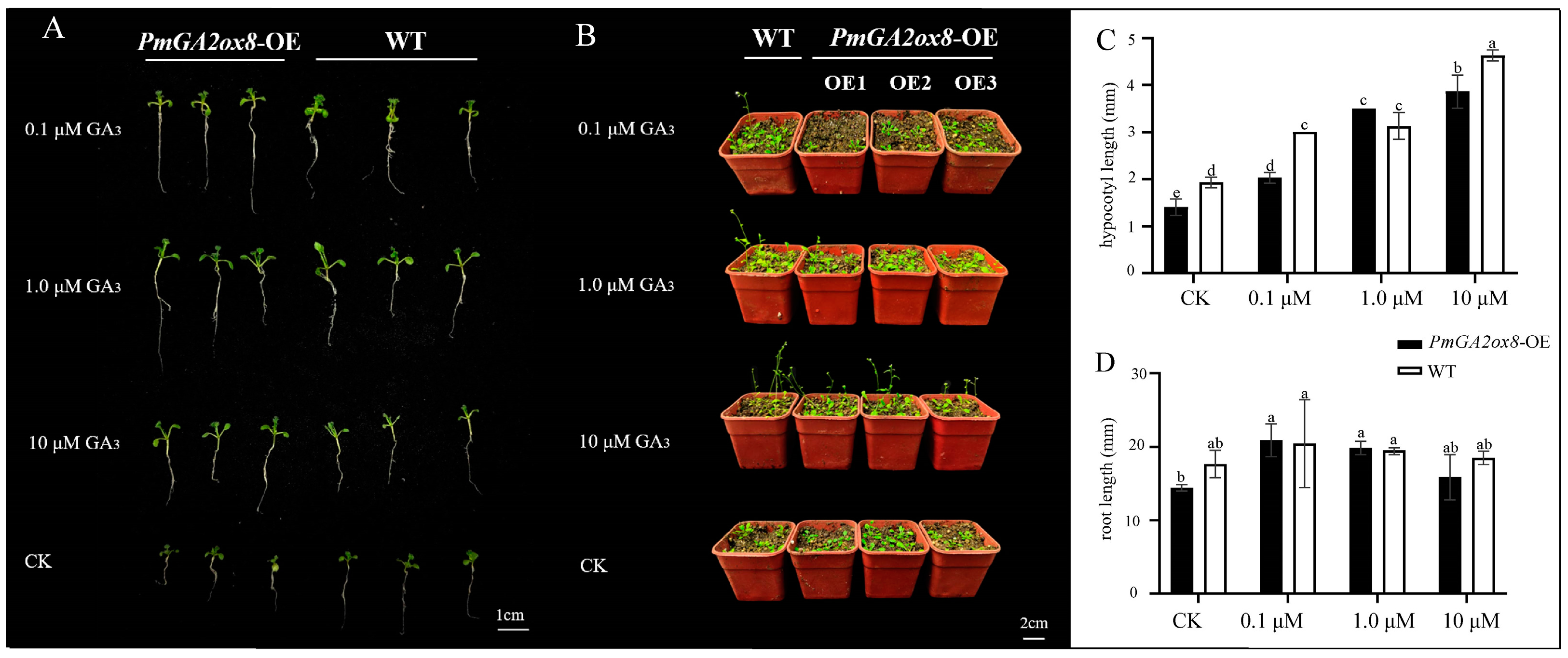
2.7. Overexpression of PmGA2ox8 Inhibited the Growth and Floral Development of Arabidopsis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Identification and Physicochemical Properties Analysis of GAox Genes in Four Prunus Species
4.3. Phylogenetic and Evolution Analysis
4.4. Gene Structure and Protein Motif Identification Analysis
4.5. Syntenic and Cis-Element Analysis of PmGAoxs
4.6. Expression Patterns of PmGAoxs
4.7. Cloning, Vector Construction, and Plants Transformation
4.8. GUS Histological Staining
4.9. Exogenous GA3 Treatment
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gou, J.; Strauss, S.H.; Tsai, C.J.; Fang, K.; Chen, Y.; Jiang, X.; Busov, V.B. Gibberellins Regulate Lateral Root Formation in Populus through Interactions with Auxin and Other Hormones. Plant Cell 2010, 22, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Serrani, J.C.; Sanjuán, R.; Ruiz-Rivero, O.; Fos, M.; García-Martínez, J.L. Gibberellin Regulation of Fruit Set and Growth in Tomato. Plant Physiol. 2007, 145, 246–257. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Kamiya, Y. Gibberellins and Light-Stimulated Seed Germination. J. Plant Growth Regul. 2001, 20, 369–376. [Google Scholar] [CrossRef]
- Zhang, N.; Xie, Y.; Guo, H.; Zhao, L.; Xiong, H.; Gu, J.; Li, J.; Kong, F.; Sui, L.; Zhao, Z.; et al. Gibberellins Regulate the Stem Elongation Rate without Affecting the Mature Plant Height of a Quick Development Mutant of Winter Wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2016, 107, 228–236. [Google Scholar] [CrossRef]
- Shan, C.; Mei, Z.; Duan, J.; Chen, H.; Feng, H.; Cai, W. OsGA2ox5, a Gibberellin Metabolism Enzyme, is Involved in Plant Growth, the Root Gravity Response and Salt Stress. PLoS ONE 2014, 9, e87110. [Google Scholar] [CrossRef]
- Weller, J.L.; Ross, J.J.; Reid, J.B. Gibberellins and Phytochrome Regulation of Stem Elongation in Pea. Planta 1994, 192, 489–496. [Google Scholar] [CrossRef]
- Hedden, P.; Thomas, S.G. Gibberellin Biosynthesis and its Regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Macmillan, J.; Takahashi, N. Proposed Procedure for the Allocation of Trivial Names to the Gibberellins. Nature 1968, 217, 170–171. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, N.; Sun, T.P.; Gubler, F. Gibberellin Signaling: Biosynthesis, Catabolism, and Response Pathways. Plant Cell 2002, 14, S61. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P.; Phillips, A.L. Gibberellin Metabolism: New Insights Revealed by The Genes. Trends Plant Sci. 2000, 5, 523–530. [Google Scholar] [CrossRef]
- Lange, T.G.U.G. Purification and Partial Amino-acid Sequence of Gibberellin 20-oxidase from Cucurbita maxima L. endosperm. Planta 1994, 195, 108–115. [Google Scholar] [CrossRef]
- Lange, T.; Hedden, P.; Graebe, J.E. Expression Cloning of a Gibberellin 20-oxidase, a Multifunctional Enzyme Involved in Gibberellin Biosynthesis. Proc. Natl. Acad. Sci. USA 1994, 91, 8552–8556. [Google Scholar] [CrossRef]
- Thomas, S.G.; Phillips, A.L.; Hedden, P. Molecular Cloning and Functional Expression of Gibberellin 2-oxidases, Multifunctional Enzymes Involved in Gibberellin Deactivation. Proc. Natl. Acad. Sci. USA 1999, 96, 4698–4703. [Google Scholar] [CrossRef]
- Chiang, H.H.; Hwang, I.; Goodman, H.M. Isolation of the Arabidopsis GA4 locus. Plant Cell 1995, 7, 195–201. [Google Scholar] [CrossRef]
- Dong, F.; Fan, S.; Ma, X.; Meng, Y.; Zuo, X.; Liu, X.; Li, K.; Liu, Z.; Han, M.; Zhang, D. Genome-wide Identification and Expression Analysis of GA2ox, GA3ox and GA20ox in Apple. Acta Hortic. Sin. 2018, 45, 613–626. [Google Scholar]
- He, H.; Liang, G.; Lu, S.; Wang, P.; Liu, T.; Ma, Z.; Zuo, C.; Sun, X.; Chen, B.; Mao, J. Genome-Wide Identification and Expression Analysis of GA2ox, GA3ox, and GA20ox Are Related to Gibberellin Oxidase Genes in Grape (Vitis Vinifera L.). Genes 2019, 10, 680. [Google Scholar] [CrossRef]
- Hu, L.; Wang, P.; Hao, Z.; Lu, Y.; Xue, G.; Cao, Z.; Qu, H.; Cheng, T.; Shi, J.; Chen, J. Gibberellin Oxidase Gene Family in L. chinense: Genome-Wide Identification and Gene Expression Analysis. Int. J. Mol. Sci. 2021, 22, 7167. [Google Scholar] [CrossRef]
- Pan, C.; Tian, K.; Ban, Q.; Wang, L.; Sun, Q.; He, Y.; Yang, Y.; Pan, Y.; Li, Y.; Jiang, J.; et al. Genome-Wide Analysis of the Biosynthesis and Deactivation of Gibberellin-Dioxygenases Gene Family in Camellia sinensis (L.) O. Kuntze. Genes 2017, 8, 235. [Google Scholar] [CrossRef]
- Cheng, J.; Ma, J.; Zheng, X.; Lv, H.; Zhang, M.; Tan, B.; Ye, X.; Wang, W.; Zhang, L.; Li, Z.; et al. Functional Analysis of the Gibberellin 2-oxidase Gene Family in Peach. Front. Plant Sci. 2021, 12, 619158. [Google Scholar] [CrossRef]
- Jeon, H.W.; Cho, J.S.; Park, E.J.; Han, K.H.; Choi, Y.I.; Ko, J.H. Developing Xylem-Preferential Expression of PdGA20ox1, a Gibberellin 20-oxidase 1 from Pinus densiflora, Improves Woody Biomass Production in a Hybrid Poplar. Plant Biotechnol. J. 2016, 14, 1161–1170. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Sabir, I.A.; Sun, W.; Wang, L.; Xu, Y.; Zhang, N.; Liu, H.; Jiu, S.; Liu, L.; et al. PavGA2ox-2L Inhibits the Plant Growth and Development Interacting with PavDWARF in Sweet Cherry (Prunus avium L.). Plant Physiol. Biochem. 2022, 186, 299–309. [Google Scholar] [CrossRef]
- Yang, J. Optimization of Genetic Transformation System and Dwarfing Functional Analysis of GA2ox8 Gene and in Pyrus. Master’s Thesis, Northwest A&F University, Xi’an, China, 2020. [Google Scholar]
- Li, J.; Li, C.; Smith, S.M. Hormone Metabolism and Signaling in Plants; Academic Press: Cambridge, UK, 2017. [Google Scholar]
- Yamaguchi, S. Gibberellin Metabolism and its Regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Ashikari, M.; Sasaki, A.; Ueguchi-Tanaka, M.; Itoh, H.; Nishimura, A.; Datta, S.; Ishiyama, K.; Saito, T.; Kobayashi, M.; Khush, G.S.; et al. Loss-of-function of a Rice Gibberellin Biosynthetic Gene, GA20 oxidase (GA20ox-20), Led to the Rice ‘Green Revolution’. Breed. Sci. 2002, 52, 143–150. [Google Scholar] [CrossRef]
- Spielmeyer, W.; Ellis, M.H.; Chandler, P.M. Semidwarf (sd-1), “Green Revolution” Rice, Contains a Defective Gibberellin 20-oxidase Gene. Proc. Natl. Acad. Sci. USA 2002, 99, 9043–9048. [Google Scholar] [CrossRef]
- Mitchum, M.G.; Yamaguchi, S.; Hanada, A.; Kuwahara, A.; Yoshioka, Y.; Kato, T.; Tabata, S.; Kamiya, Y.; Sun, T.P. Distinct and Overlapping Roles of Two Gibberellin 3-oxidases in Arabidopsis Development. Plant J. 2006, 45, 804–818. [Google Scholar] [CrossRef]
- Min, Z.; Hu, X.; Han, X.; Li, Y.; Li, J.; Wang, D.; Sun, L.; Sun, X. Genetic Mapping and Identification of the Gibberellin 3-oxidase Gene GA3ox Leading to a GA-Deficient Dwarf Phenotype in Pumpkin (Cucurbita moschata D.). Agronomy 2022, 12, 1779. [Google Scholar] [CrossRef]
- Busov, V.B.; Meilan, R.; Pearce, D.W.; Ma, C.; Rood, S.B.; Strauss, S.H. Activation Tagging of a Dominant Gibberellin Catabolism Gene (GA 2-oxidase) from Poplar That Regulates Tree Stature. Plant Physiol. 2003, 132, 1283–1291. [Google Scholar] [CrossRef]
- Huang, J.; Tang, D.; Shen, Y.; Qin, B.; Hong, L.; You, A.; Li, M.; Wang, X.; Yu, H.; Gu, M. Activation of Gibberellin 2-oxidase 6 Decreases Active Gibberellin Levels and Creates a Dominant Semi-Dwarf Phenotype in Rice (Oryza sativa L.). J. Genet. Genom. 2010, 37, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, F.M.; Bizzell, C.M.; Lee, D.J.; Zeevaart, J.A.D.; Amasino, R.M. Overexpression of a Novel Class of Gibberellin 2-oxidases Decreases Gibberellin Levels and Creates Dwarf Plants. Plant Cell 2003, 15, 151–163. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.W.; Wong, F.L.; Luk, C.Y.; Chung, C.Y.L.; Yung, W.S.; Wang, Z.; Xie, M.; Song, S.; Chung, G.; et al. Increased Copy Number of Gibberellin 2-oxidase 8 genes Reduced Trailing Growth and Shoot Length During Soybean Domestication. Plant J. 2021, 107, 1739–1755. [Google Scholar] [CrossRef]
- Le Bris, M. Growth Regulation|Hormones in Growth and Development. In Encyclopedia of Rose Science; Roberts, A.V., Ed.; Elsevier: Oxford, UK, 2003; pp. 364–369. [Google Scholar]
- Srivastava, L.M. CHAPTER 7—Gibberellins. In Plant Growth and Development; Srivastava, L.M., Ed.; Academic Press: San Diego, CA, USA, 2002; pp. 171–190. [Google Scholar]
- Han, F.; Zhu, B. Evolutionary Analysis of Three Gibberellin Oxidase Genes in Rice, Arabidopsis, and Soybean. Gene 2011, 473, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Li, P.; Zhuo, X.; Liu, W.; Qiu, L.; Li, L.; Yuan, C.; Sun, L.; Zhang, Z.; Wang, J.; et al. The Chromosome-Level Genome Provides Insight into the Molecular Mechanism Underlying the Tortuous-branch Phenotype of Prunus mume. New Phytol. 2022, 235, 141–156. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, D.P.; Davidson, S.E.; Clarke, V.C.; Yamauchi, Y.; Yamaguchi, S.; Kamiya, Y.; Reid, J.B.; Ross, J.J. Regulation of the Gibberellin Pathway by Auxin and DELLA Proteins. Planta 2010, 232, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.P.O.N. Auxin Regulation of the Gibberellin Pathway in Pea. Plant Physiol. 2002, 130, 1974–1982. [Google Scholar]
- Ouellette, L.; Anh Tuan, P.; Toora, P.K.; Yamaguchi, S.; Ayele, B.T. Heterologous Functional Analysis and Expression Patterns of Gibberellin 2-oxidase Genes of Barley (Hordeum vulgare L.). Gene 2023, 861, 147255. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, H.; Shi, Y.; Huang, S.; Liu, T.; Xiao, C.; Tian, X.; Zhao, P.; Dai, X.; Huang, T.; et al. Genome-Wide Identification, Evolution and Expression Analyses of GA2ox Gene Family in Brassica napus L. Phyton 2023, 92, 815–835. [Google Scholar] [CrossRef]
- Sun, H.; Pang, B.; Yan, J.; Wang, T.; Wang, L.; Chen, C.; Li, Q.; Ren, Z. Comprehensive Analysis of Cucumber Gibberellin Oxidase Family Genes and Functional Characterization of CsGA20ox1 in Root Development in Arabidopsis. Int. J. Mol. Sci. 2018, 19, 3135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Construction of High-Density Genetic Map and QTL Analysis of Ornamental Traits in Mei. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2016. [Google Scholar]
- Li, S.; Zheng, T.; Zhuo, X.; Li, Z.; Li, L.; Li, P.; Qiu, L.; Pan, H.; Wang, J.; Cheng, T.; et al. Transcriptome Profiles Reveal that Gibberellin-related Genes Regulate Weeping Traits in Crape Myrtle. Hortic. Res. 2020, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, M.; Alabadí, D.; Pérez-Gómez, J.; García-Cárcel, L.; Phillips, A.L.; Hedden, P.; Blázquez, M.A. Transcriptional Regulation of Gibberellin Metabolism Genes by Auxin Signaling in Arabidopsis. Plant Physiol. 2006, 142, 553–563. [Google Scholar] [CrossRef]
- Jasinski, S.; Piazza, P.; Craft, J.; Hay, A.; Woolley, L.; Rieu, I.; Phillips, A.; Hedden, P.; Tsiantis, M. KNOX Action in Arabidopsis Is Mediated by Coordinate Regulation of Cytokinin and Gibberellin Activities. Curr. Biol. 2005, 15, 1560–1565. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Zheng, T.; Zhuo, X.; Li, P.; Qiu, L.; Liu, W.; Wang, J.; Cheng, T.; Zhang, Q. Comparative Gene Expression Analysis Reveals that Multiple Mechanisms Regulate the Weeping Trait in Prunus mume. Sci. Rep. 2021, 11, 2675. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Chen, J.; Lu, Z.; Zhang, X.; Liu, G.; Lian, B.; Chen, Y.; Zhong, F.; Yu, C.; Zhang, J. Crape myrtle LiGAoxs displaying activities of gibberellin oxidases respond to branching architecture. Plant Physiol. Biochem. 2024, 212, 108738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhuo, X.; Zhao, K.; Zheng, T.; Han, Y.; Yuan, C.; Zhang, Q. Transcriptome Profiles Reveal the Crucial Roles of Hormone and Sugar in the Bud Dormancy of Prunus mume. Sci. Rep. 2018, 8, 5090. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, X.; Zhang, L.; Lin, S.; Liu, D.; Wang, Q.; Cai, S.; El-Tanbouly, R.; Gan, L.; Wu, H.; et al. Identification and characterization of tomato gibberellin 2-oxidases (GA2oxs) and effects of fruit-specific SlGA2ox1 overexpression on fruit and seed growth and development. Hortic. Res. 2016, 3, 16059. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Ren, L.; Ma, J.; Li, Y.; Xu, H.; Yao, H.; Dai, Y.; Wang, L.; Li, S.; Li, Z.; et al. Sweet Potato Gibberellin 2-oxidase Genes in the Dwarf Phenotype. Sci. Hortic. 2023, 313, 111921. [Google Scholar] [CrossRef]
- Xiao, Z.; Fu, R.; Li, J.; Fan, Z.; Yin, H. Overexpression of the Gibberellin 2-oxidase Gene from Camellia lipoensis Induces Dwarfism and Smaller Flowers in Nicotiana tabacum. Plant Mol. Biol. Rep. 2016, 34, 182–191. [Google Scholar] [CrossRef]
- Xing, M.Q.; Chen, S.H.; Zhang, X.F.; Xue, H.W. Rice OsGA2ox9 Regulates Seed GA Metabolism and Dormancy. Plant Biotechnol. J. 2023, 21, 2411–2413. [Google Scholar] [CrossRef]
- Bolser, D.M.; Staines, D.M.; Perry, E.; Kersey, P.J. Ensembl Plants: Integrating Tools for Visualizing, Mining, and Analyzing Plant Genomic Data; Springer: New York, NY, USA, 2017; Volume 1533, pp. 1–31. [Google Scholar]
- Swarbreck, D.; Wilks, C.; Lamesch, P.; Berardini, T.Z.; Garcia-Hernandez, M.; Foerster, H.; Li, D.; Meyer, T.; Muller, R.; Ploetz, L.; et al. The Arabidopsis Information Resource (TAIR): Gene Structure and Function Annotation. Nucleic Acids Res. 2007, 36, D1009–D1014. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S. Gibberellin Biosynthesis in Arabidopsis. Phytochem. Rev. 2006, 5, 39–47. [Google Scholar] [CrossRef]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER Web Server: Interactive Sequence Similarity Searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef]
- He, Y.; Liu, W.; Huang, Z.; Huang, J.; Xu, Y.; Zhang, Q.; Hu, J. Genome-Wide Analysis of the Rice Gibberellin Dioxygenases Family Genes. Agronomy 2022, 12, 1627. [Google Scholar] [CrossRef]
- Xie, J.; Chen, Y.; Cai, G.; Cai, R.; Hu, Z.; Wang, H. Tree Visualization by One Table (tvBOT): A Web Application for Visualizing, Modifying and Annotating Phylogenetic Trees. Nucleic Acids Res. 2023, 51, W587–W592. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, W.; Sun, L.; Zhao, F.; Huang, B.; Yang, W.; Tao, Y.; Wang, J.; Yuan, Z.; Fan, G.; et al. The Genome of Prunus mume. Nat. Commun. 2012, 3, 1318. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhang, J.; Guo, X.; Qiu, L.; Chen, K.; Wang, J.; Cheng, T.; Zhang, Q.; Zheng, T. Genome-Wide Analysis of the Gibberellin-Oxidases Family Members in Four Prunus Species and a Functional Analysis of PmGA2ox8 in Plant Height. Int. J. Mol. Sci. 2024, 25, 8697. https://doi.org/10.3390/ijms25168697
Li X, Zhang J, Guo X, Qiu L, Chen K, Wang J, Cheng T, Zhang Q, Zheng T. Genome-Wide Analysis of the Gibberellin-Oxidases Family Members in Four Prunus Species and a Functional Analysis of PmGA2ox8 in Plant Height. International Journal of Molecular Sciences. 2024; 25(16):8697. https://doi.org/10.3390/ijms25168697
Chicago/Turabian StyleLi, Xue, Jie Zhang, Xiaoyu Guo, Like Qiu, Ke Chen, Jia Wang, Tangren Cheng, Qixiang Zhang, and Tangchun Zheng. 2024. "Genome-Wide Analysis of the Gibberellin-Oxidases Family Members in Four Prunus Species and a Functional Analysis of PmGA2ox8 in Plant Height" International Journal of Molecular Sciences 25, no. 16: 8697. https://doi.org/10.3390/ijms25168697
APA StyleLi, X., Zhang, J., Guo, X., Qiu, L., Chen, K., Wang, J., Cheng, T., Zhang, Q., & Zheng, T. (2024). Genome-Wide Analysis of the Gibberellin-Oxidases Family Members in Four Prunus Species and a Functional Analysis of PmGA2ox8 in Plant Height. International Journal of Molecular Sciences, 25(16), 8697. https://doi.org/10.3390/ijms25168697






