Differential Expression of PACAP/VIP Receptors in the Post-Mortem CNS White Matter of Multiple Sclerosis Donors
Abstract
:1. Introduction
2. Results
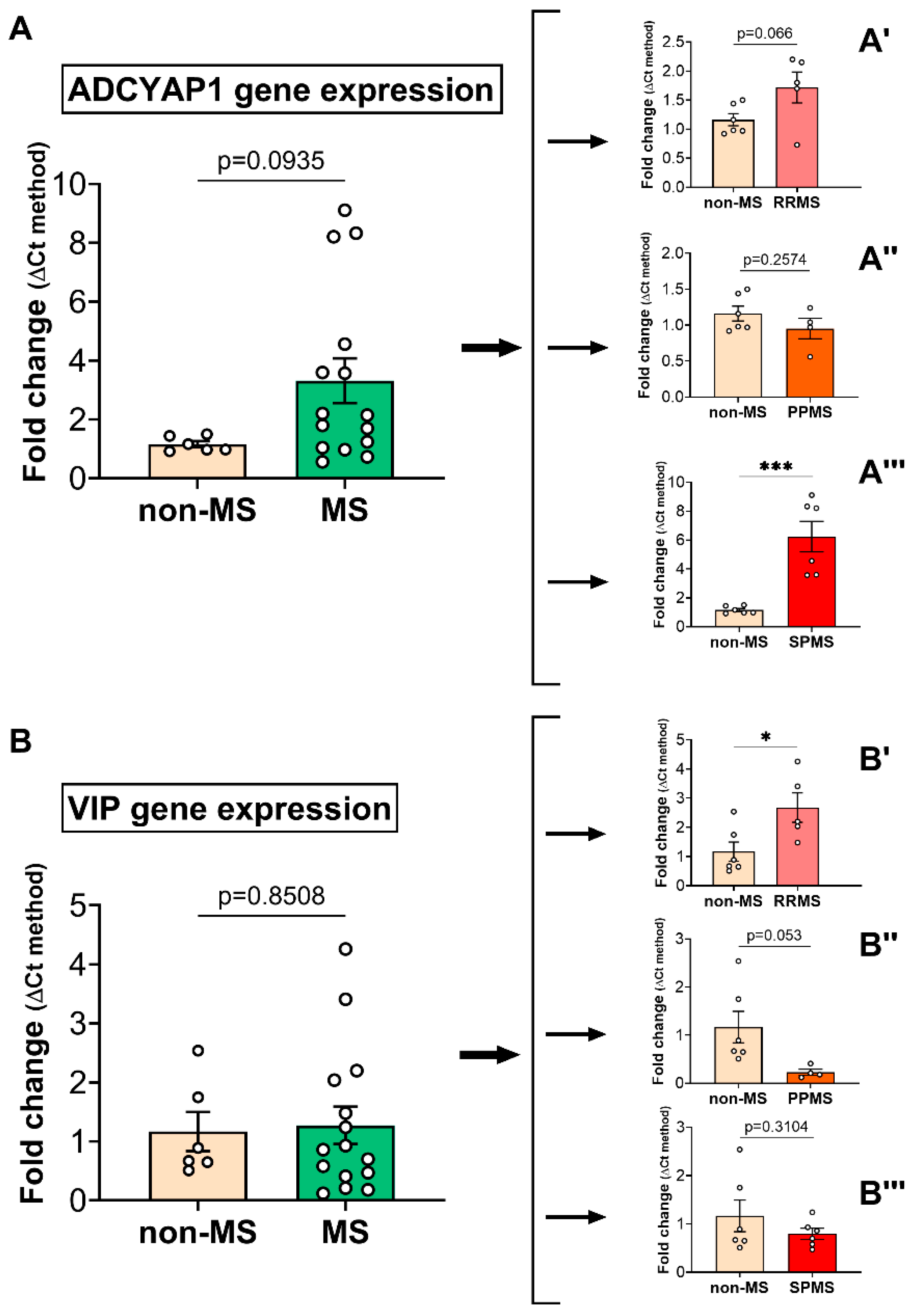
2.1. PACAP and VIP Gene Expression in the Normal-Appearing White Matter of Multiple Sclerosis Cases Reveals Subtype-Specific Changes
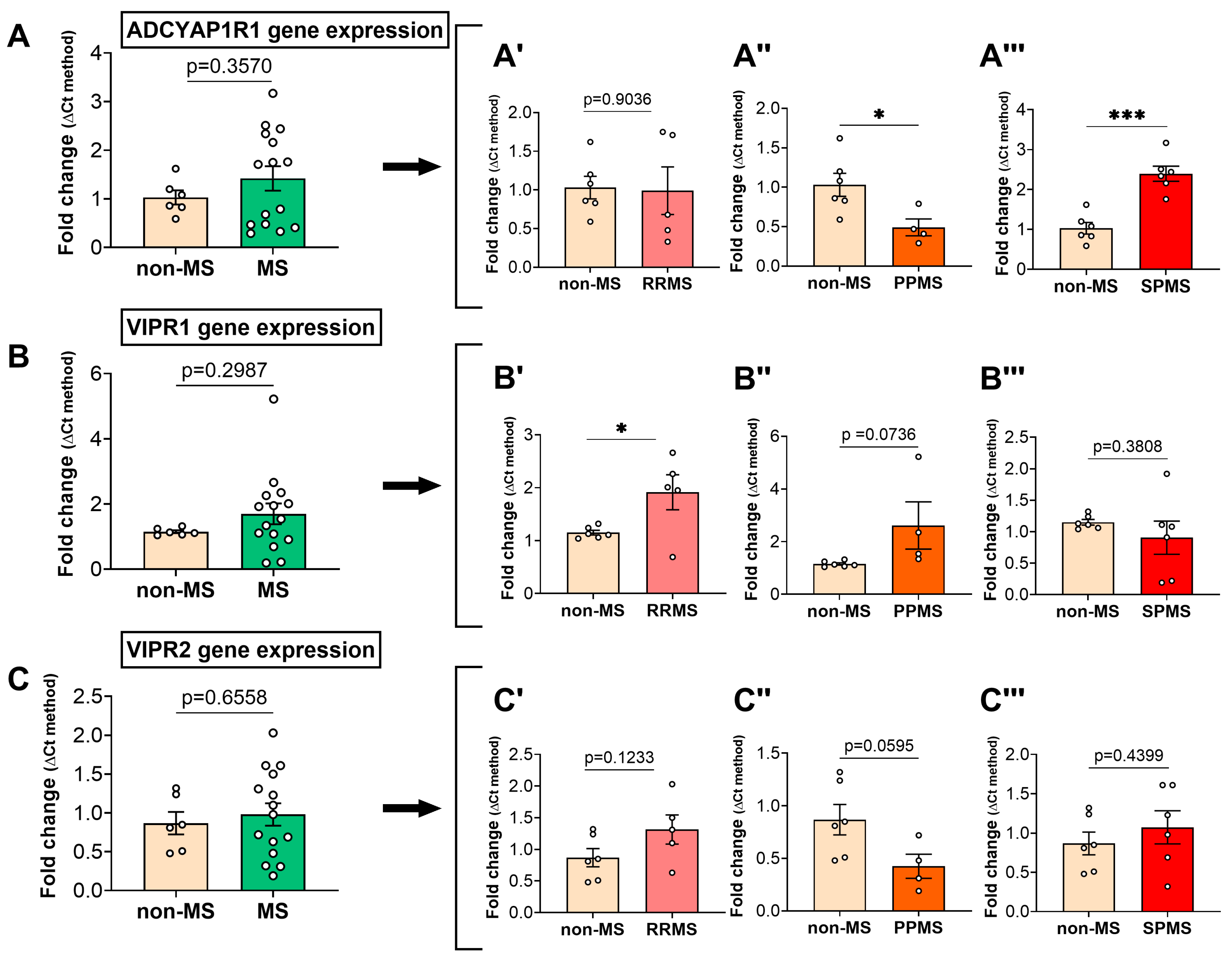
2.2. Differential Gene Expression Levels of the PACAP/VIP Receptors in the NAWM of MS Patients
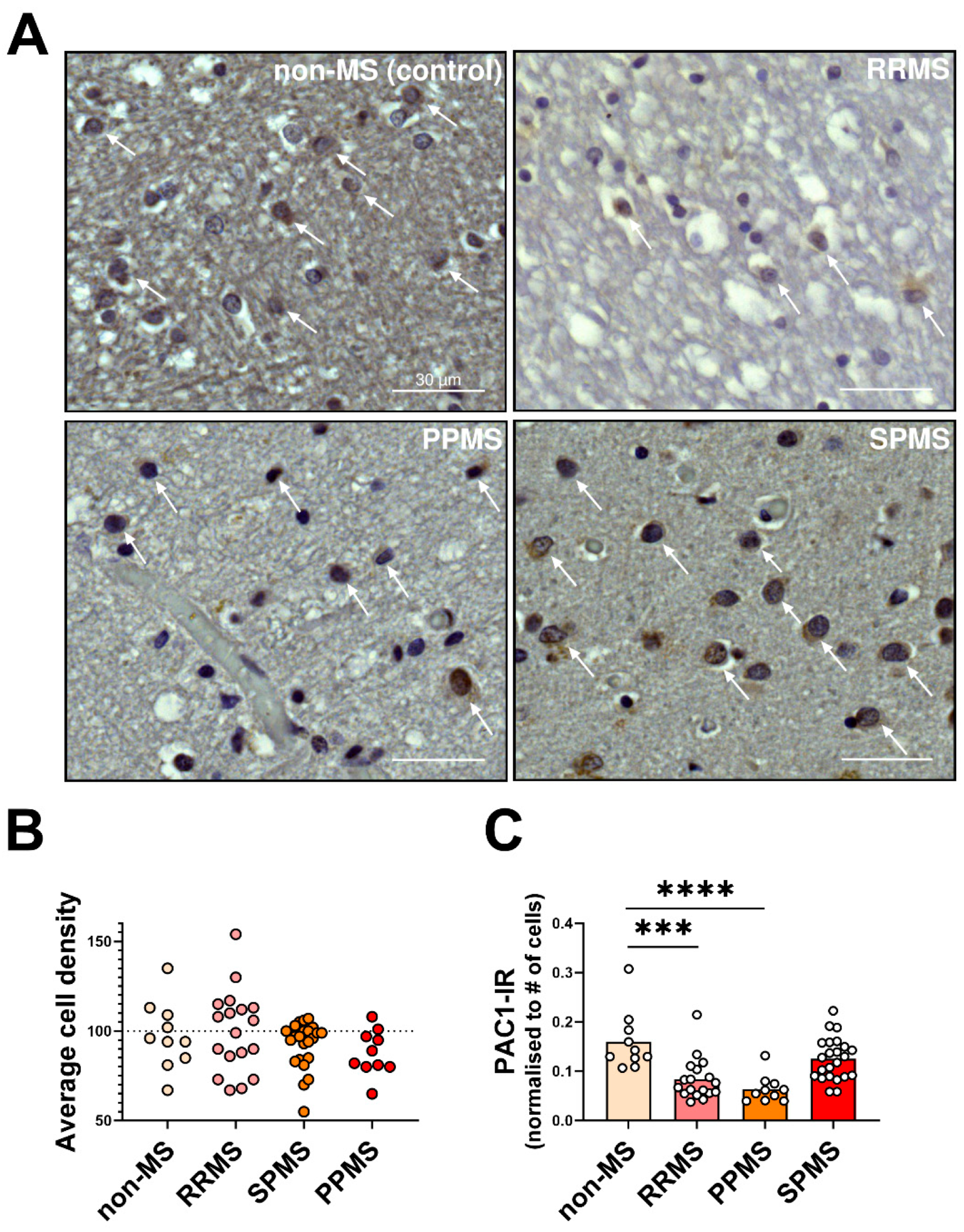
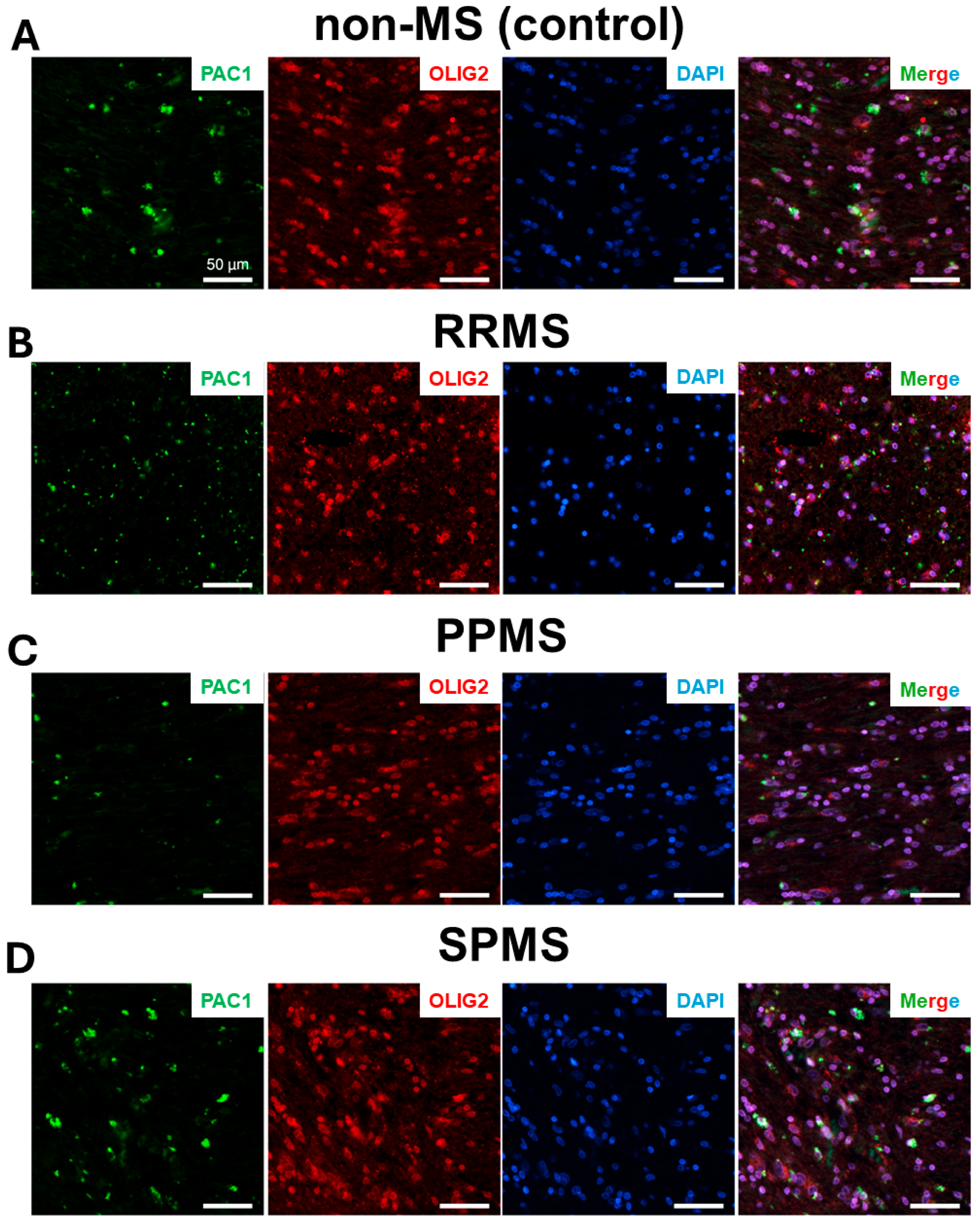
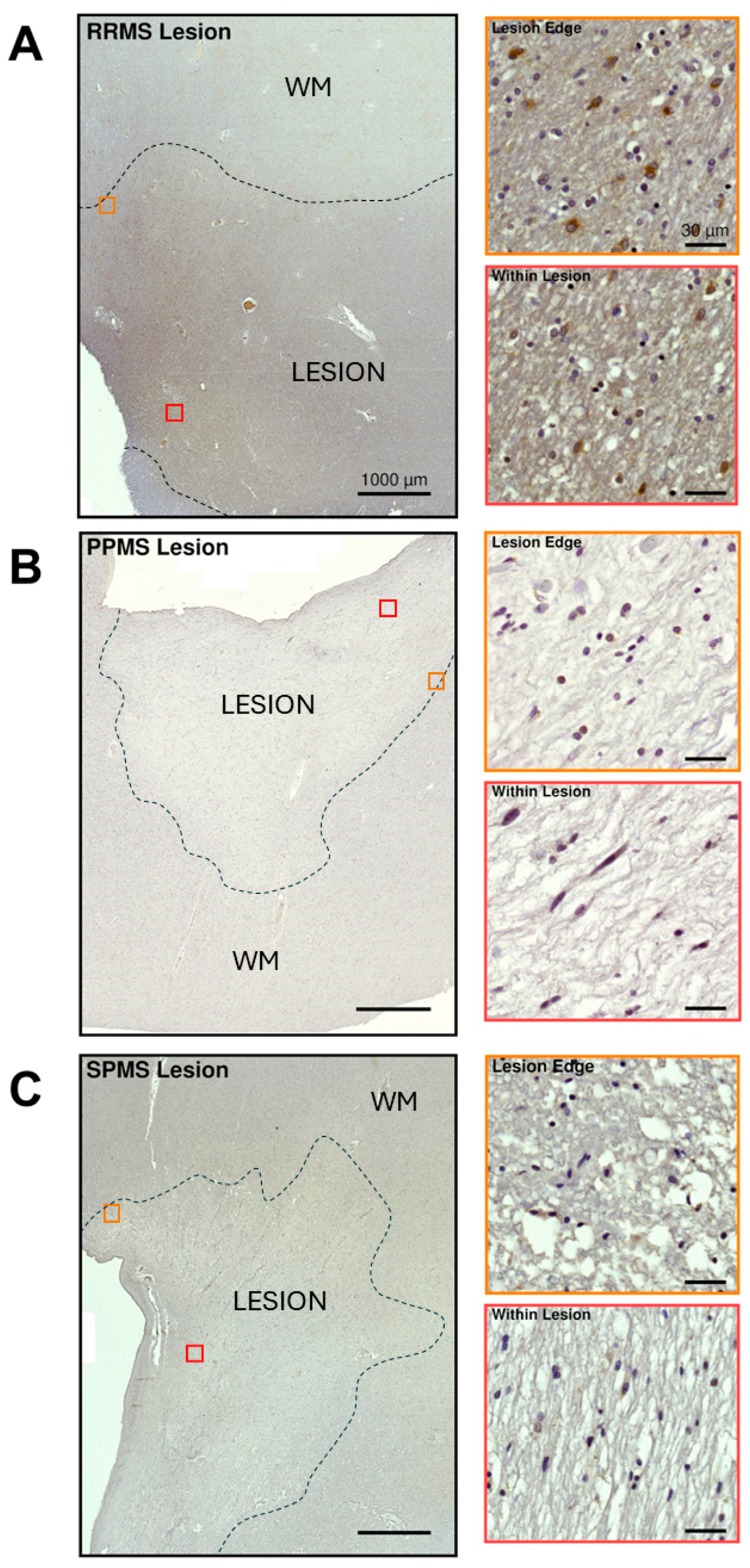
2.3. Differential Expression and Distribution of PAC1 Receptors in the NAWM and Lesions of MS Donors
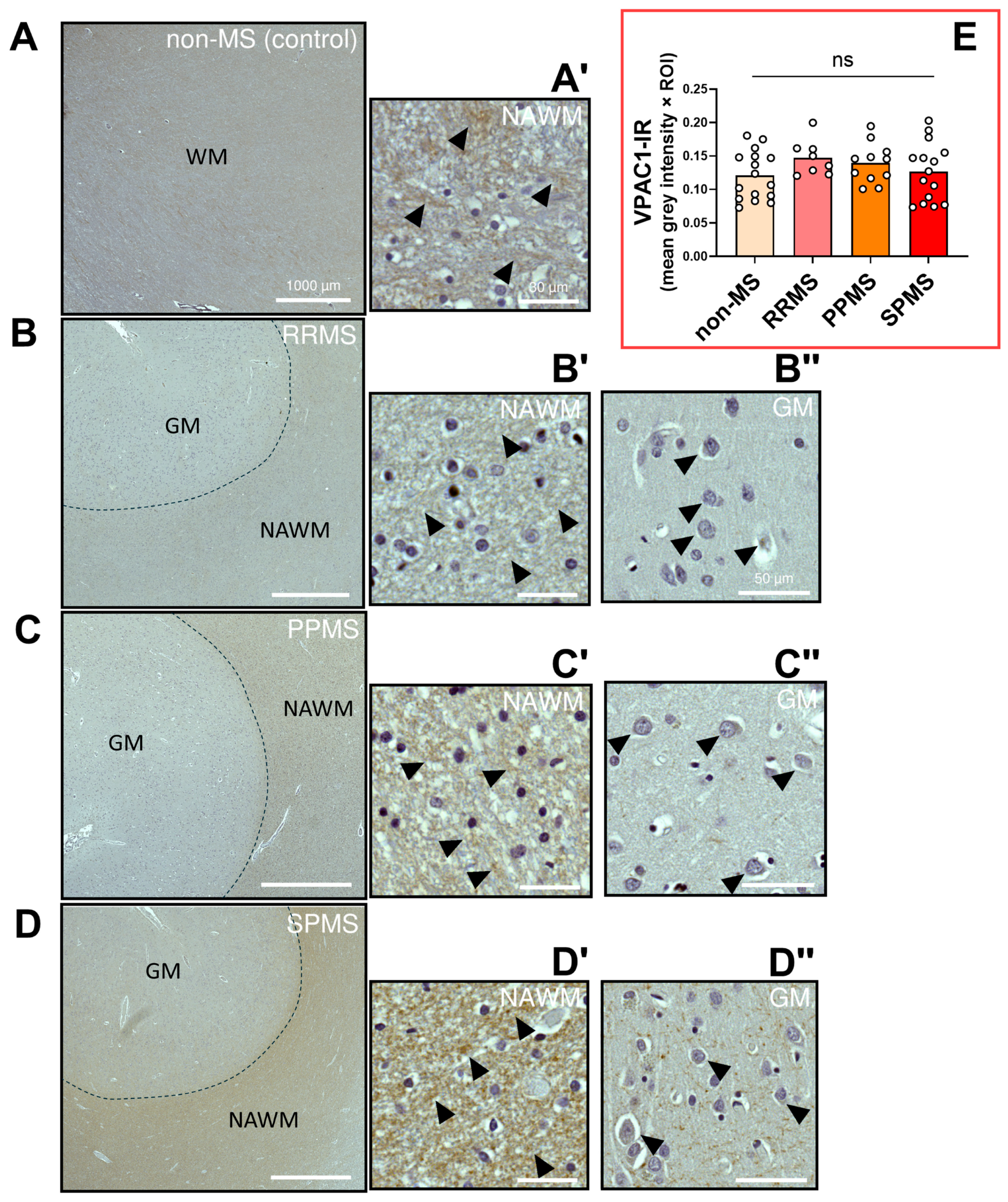
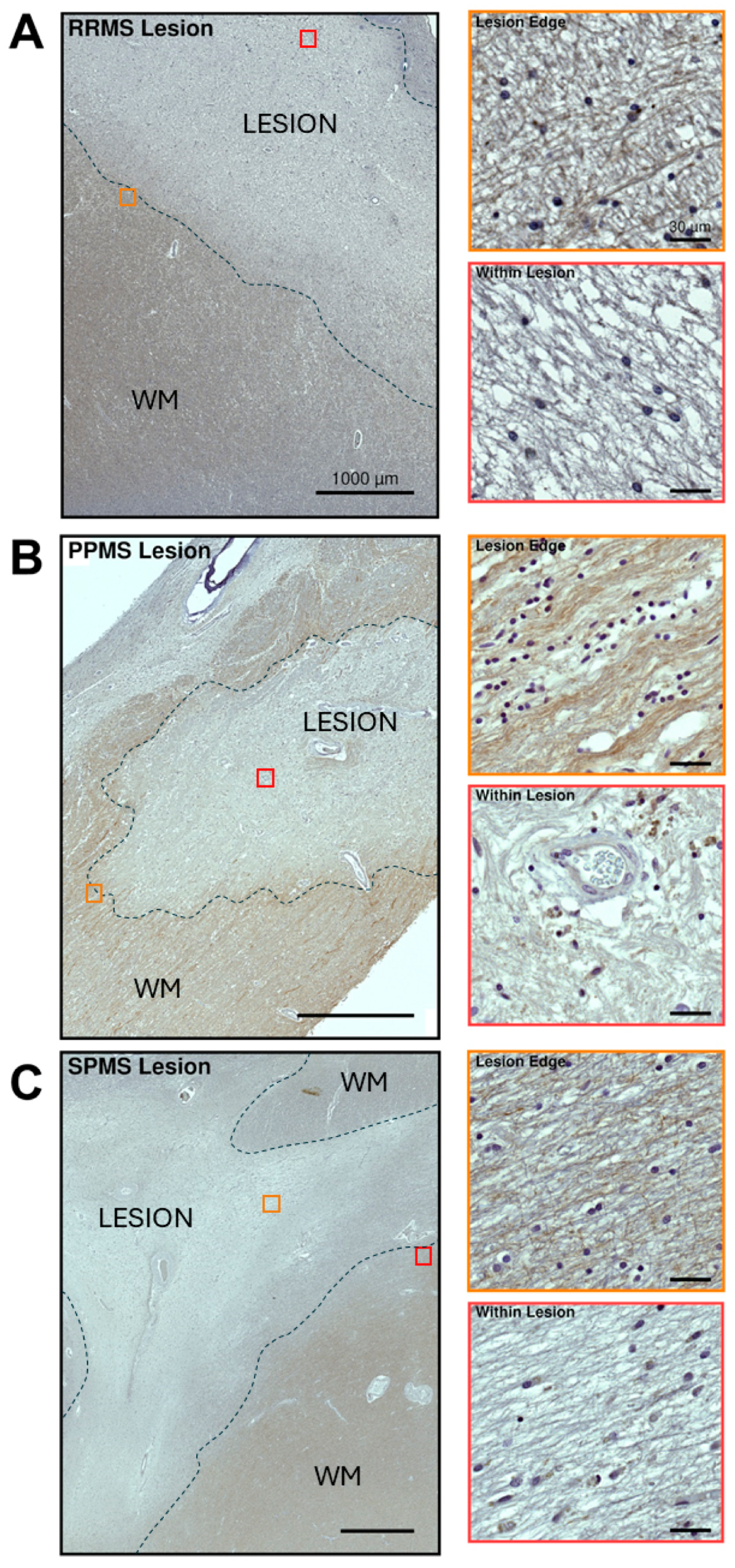
2.4. Differential Expression and Distribution of VPAC1 Receptors in the NAWM and Lesions of MS Donors
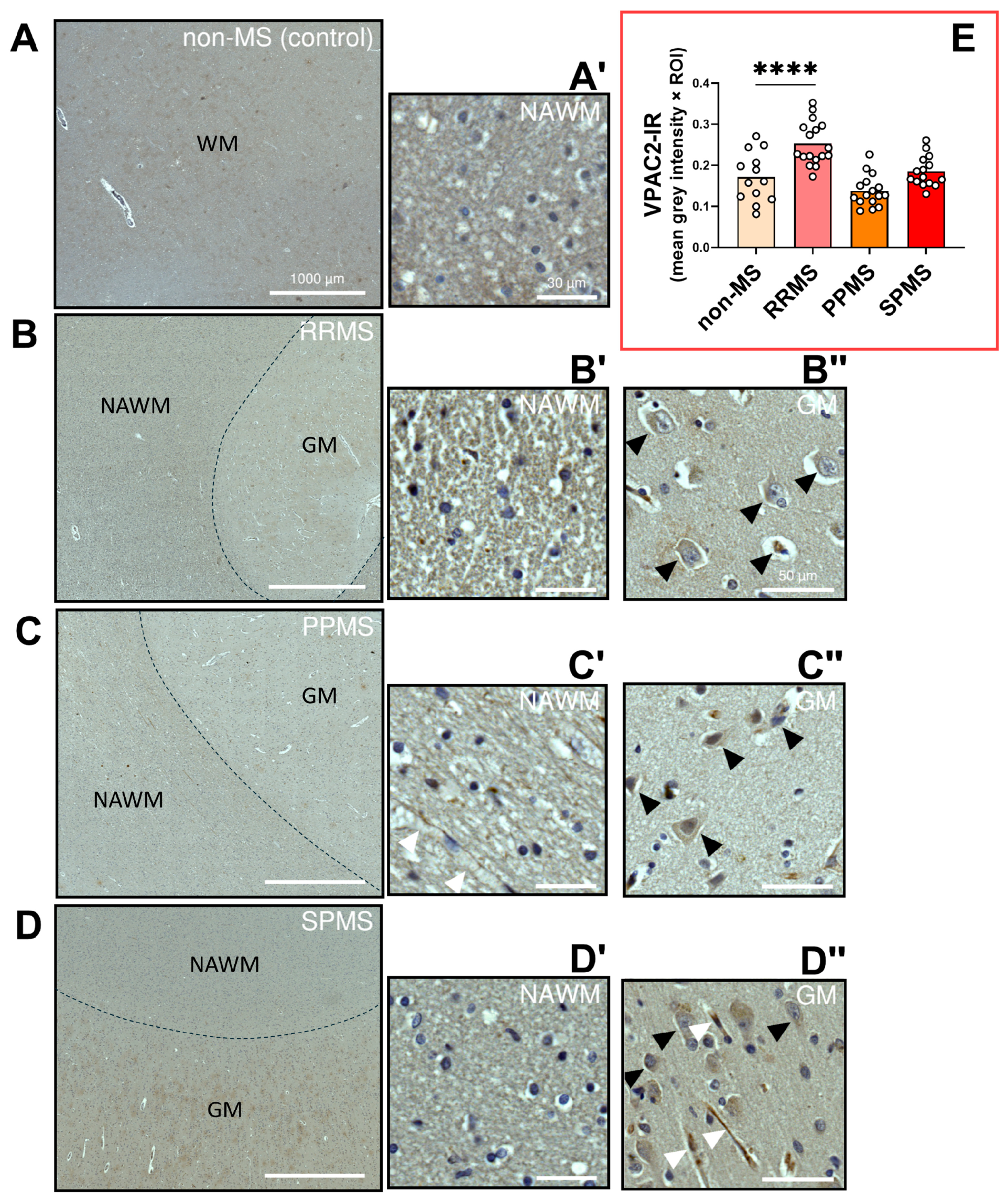
2.5. Differential Expression and Distribution of VPAC2 Receptors in the NAWM and Lesions of MS Donors
3. Discussion
4. Materials and Methods
4.1. Human Postmortem Brain Tissue
4.2. Normal-Appearing White Matter Dissection and RNA Extraction
4.3. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
4.4. Immunohistochemistry
4.5. Immunofluorescence
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef] [PubMed]
- Dargahi, N.; Katsara, M.; Tselios, T.; Androutsou, M.E.; de Courten, M.; Matsoukas, J.; Apostolopoulos, V. Multiple Sclerosis: Immunopathology and Treatment Update. Brain Sci. 2017, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. 2017, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Baranzini, S.E.; Oksenberg, J.R. The Genetics of Multiple Sclerosis: From 0 to 200 in 50 Years. Trends Genet. 2017, 33, 960–970. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, C.; Lucas, R.; Taylor, B. Environmental risk factors for multiple sclerosis: A review with a focus on molecular mechanisms. Int. J. Mol. Sci. 2012, 13, 11718–11752. [Google Scholar] [CrossRef] [PubMed]
- Weiner, H.L. A shift from adaptive to innate immunity: A potential mechanism of disease progression in multiple sclerosis. J. Neurol. 2008, 255 (Suppl. S1), 3–11. [Google Scholar] [CrossRef]
- Klineova, S.; Lublin, F.D. Clinical Course of Multiple Sclerosis. Cold Spring Harbor Perspect. Med. 2018, 8, a028928. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Gaitan, M.I.; Ysrraelit, M.C.; Fiol, M.P. Progressive multiple sclerosis: From pathogenic mechanisms to treatment. Brain 2017, 140, 527–546. [Google Scholar] [CrossRef]
- Newsome, S.D.; Thrower, B.; Hendin, B.; Danese, S.; Patterson, J.; Chinnapongse, R. Symptom burden, management and treatment goals of people with MS spasticity: Results from SEEN-MSS, a large-scale, self-reported survey. Mult. Scler. Relat. Disord. 2022, 68, 104376. [Google Scholar] [CrossRef]
- De Santis, S.; Granberg, T.; Ouellette, R.; Treaba, C.A.; Herranz, E.; Fan, Q.; Mainero, C.; Toschi, N. Evidence of early microstructural white matter abnormalities in multiple sclerosis from multi-shell diffusion MRI. Neuroimage Clin. 2019, 22, 101699. [Google Scholar] [CrossRef] [PubMed]
- Holley, J.E.; Newcombe, J.; Whatmore, J.L.; Gutowski, N.J. Increased blood vessel density and endothelial cell proliferation in multiple sclerosis cerebral white matter. Neurosci. Lett. 2010, 470, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Delgado, P.; James, R.; Browne, E.; Meng, J.; Umashankar, S.; Tan, L.; Picon, C.; Mazarakis, N.D.; Faisal, A.A.; Howell, O.W.; et al. Neuroinflammation in the normal-appearing white matter (NAWM) of the multiple sclerosis brain causes abnormalities at the nodes of Ranvier. PLoS Biol. 2020, 18, e3001008. [Google Scholar] [CrossRef] [PubMed]
- Allen, I.V.; McQuaid, S.; Mirakhur, M.; Nevin, G. Pathological abnormalities in the normal-appearing white matter in multiple sclerosis. Neurol. Sci. 2001, 22, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Kirk, J.; Plumb, J.; Mirakhur, M.; McQuaid, S. Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood–brain barrier leakage and active demyelination. J. Pathol. 2003, 201, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Zeis, T.; Graumann, U.; Reynolds, R.; Schaeren-Wiemers, N. Normal-appearing white matter in multiple sclerosis is in a subtle balance between inflammation and neuroprotection. Brain 2008, 131, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, T.; Nakamachi, T.; Shioda, S. Discovery of PACAP and its receptors in the brain. J. Headache Pain 2018, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Waschek, J.A. VIP and PACAP: Neuropeptide modulators of CNS inflammation, injury, and repair. Br. J. Pharmacol. 2013, 169, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Tao, Z.; Chen, S.; Zhang, H.; Yu, R. Positive allosteric regulation of PAC1-R up-regulates PAC1-R and its specific ligand PACAP. Acta Biochim. Biophys. Sin. 2022, 54, 657–672. [Google Scholar] [CrossRef]
- Shen, S.; Gehlert, D.R.; Collier, D.A. PACAP and PAC1 receptor in brain development and behavior. Neuropeptides 2013, 47, 421–430. [Google Scholar] [CrossRef]
- Abad, C.; Waschek, J.A. Immunomodulatory roles of VIP and PACAP in models of multiple sclerosis. Curr. Pharm. Des. 2011, 17, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.V.; Waschek, J.A. Targeting VIP and PACAP receptor signalling: New therapeutic strategies in multiple sclerosis. ASN Neuro 2011, 3, AN20110024. [Google Scholar] [CrossRef] [PubMed]
- Al-Keilani, M.S.; Almomani, B.A.; Al-Sawalha, N.A.; Al Qawasmeh, M.; Jaradat, S.A. Significance of serum VIP and PACAP in multiple sclerosis: An exploratory case-control study. Neurol. Sci. 2022, 43, 2621–2630. [Google Scholar] [CrossRef]
- Baranowska-Bik, A.; Kochanowski, J.; Uchman, D.; Wolinska-Witort, E.; Kalisz, M.; Martynska, L.; Baranowska, B.; Bik, W. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) in humans with multiple sclerosis. J. Neuroimmunol. 2013, 263, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.V.; Abad, C.; Wang, Y.; Lopez, R.; Waschek, J.A. Pituitary adenylate cyclase activating peptide deficient mice exhibit impaired thymic and extrathymic regulatory T cell proliferation during EAE. PLoS ONE 2013, 8, e61200. [Google Scholar] [CrossRef] [PubMed]
- Abad, C.; Jayaram, B.; Becquet, L.; Wang, Y.; O’Dorisio, M.S.; Waschek, J.A.; Tan, Y.V. VPAC1 receptor (Vipr1)-deficient mice exhibit ameliorated experimental autoimmune encephalomyelitis, with specific deficits in the effector stage. J. Neuroinflamm. 2016, 13, 169. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.V.; Abad, C.; Wang, Y.; Lopez, R.; Waschek, J. VPAC2 (vasoactive intestinal peptide receptor type 2) receptor deficient mice develop exacerbated experimental autoimmune encephalomyelitis with increased Th1/Th17 and reduced Th2/Treg responses. Brain Behav. Immun. 2015, 44, 167–175. [Google Scholar] [CrossRef]
- Kato, H.; Ito, A.; Kawanokuchi, J.; Jin, S.; Mizuno, T.; Ojika, K.; Ueda, R.; Suzumura, A. Pituitary adenylate cyclase-activating polypeptide (PACAP) ameliorates experimental autoimmune encephalomyelitis by suppressing the functions of antigen presenting cells. Mult. Scler. 2004, 10, 651–659. [Google Scholar] [CrossRef]
- Gonzalez-Rey, E.; Fernandez-Martin, A.; Chorny, A.; Martin, J.; Pozo, D.; Ganea, D.; Delgado, M. Therapeutic effect of vasoactive intestinal peptide on experimental autoimmune encephalomyelitis: Down-regulation of inflammatory and autoimmune responses. Am. J. Pathol. 2006, 168, 1179–1188. [Google Scholar] [CrossRef]
- Happel, P.; Möller, K.; Schwering, N.K.; Dietzel, I.D. Migrating oligodendrocyte progenitor cells swell prior to soma dislocation. Sci. Rep. 2013, 3, 1806. [Google Scholar] [CrossRef]
- Sugita, Y.; Terasaki, M.; Shigemori, M.; Sakata, K.; Morimatsu, M. Acute focal demyelinating disease simulating brain tumors: Histopathologic guidelines for an accurate diagnosis. Neuropathology 2001, 21, 25–31. [Google Scholar] [CrossRef]
- Joo, K.M.; Chung, Y.H.; Kim, M.K.; Nam, R.H.; Lee, B.L.; Lee, K.H.; Cha, C.I. Distribution of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC1, VPAC2, and PAC1 receptor) in the rat brain. J. Comp. Neurol. 2004, 476, 388–413. [Google Scholar] [CrossRef]
- Jolivel, V.; Basille, M.; Aubert, N.; de Jouffrey, S.; Ancian, P.; Le Bigot, J.F.; Noack, P.; Massonneau, M.; Fournier, A.; Vaudry, H.; et al. Distribution and functional characterization of pituitary adenylate cyclase-activating polypeptide receptors in the brain of non-human primates. Neuroscience 2009, 160, 434–451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hernandez, V.S.; Gerfen, C.R.; Jiang, S.Z.; Zavala, L.; Barrio, R.A.; Eiden, L.E. Behavioral role of PACAP signaling reflects its selective distribution in glutamatergic and GABAergic neuronal subpopulations. Elife 2021, 10, e61718. [Google Scholar] [CrossRef]
- Lee, M.; Lelièvre, V.; Zhao, P.; Torres, M.; Rodriguez, W.; Byun, J.-Y.; Doshi, S.; Ioffe, Y.; Gupta, G.; de los Monteros, A.E.; et al. Pituitary Adenylyl Cyclase-Activating Polypeptide Stimulates DNA Synthesis but Delays Maturation of Oligodendrocyte Progenitors. J. Neurosci. 2001, 21, 3849–3859. [Google Scholar] [CrossRef]
- Castorina, A.; Waschek, J.A.; Marzagalli, R.; Cardile, V.; Drago, F. PACAP interacts with PAC1 receptors to induce tissue plasminogen activator (tPA) expression and activity in schwann cell-like cultures. PLoS ONE 2015, 10, e0117799. [Google Scholar] [CrossRef] [PubMed]
- Castorina, A.; Scuderi, S.; D’Amico, A.G.; Drago, F.; D’Agata, V. PACAP and VIP increase the expression of myelin-related proteins in rat schwannoma cells: Involvement of PAC1/VPAC2 receptor-mediated activation of PI3K/Akt signaling pathways. Exp. Cell Res. 2014, 322, 108–121. [Google Scholar] [CrossRef]
- Castorina, A.; Tiralongo, A.; Giunta, S.; Carnazza, M.L.; Rasi, G.; D’Agata, V. PACAP and VIP prevent apoptosis in schwannoma cells. Brain Res. 2008, 1241, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Vincze, A.; Reglodi, D.; Helyes, Z.; Hashimoto, H.; Shintani, N.; Abraham, H. Role of endogenous pituitary adenylate cyclase activating polypeptide (PACAP) in myelination of the rodent brain: Lessons from PACAP-deficient mice. Int. J. Dev. Neurosci. 2011, 29, 923–935. [Google Scholar] [CrossRef]
- Zhang, Y.; Da, R.R.; Hilgenberg, L.G.; Tourtellotte, W.W.; Sobel, R.A.; Smith, M.A.; Olek, M.; Nagra, R.; Sudhir, G.; van den Noort, S.; et al. Clonal expansion of IgA-positive plasma cells and axon-reactive antibodies in MS lesions. J. Neuroimmunol. 2005, 167, 120–130. [Google Scholar] [CrossRef]
- Tan, Y.V.; Abad, C.; Lopez, R.; Dong, H.; Liu, S.; Lee, A.; Gomariz, R.P.; Leceta, J.; Waschek, J.A. Pituitary adenylyl cyclase-activating polypeptide is an intrinsic regulator of Treg abundance and protects against experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2009, 106, 2012–2017. [Google Scholar] [CrossRef] [PubMed]
- Karunia, J.; Niaz, A.; Mandwie, M.; Thomas Broome, S.; Keay, K.A.; Waschek, J.A.; Al-Badri, G.; Castorina, A. PACAP and VIP Modulate LPS-Induced Microglial Activation and Trigger Distinct Phenotypic Changes in Murine BV2 Microglial Cells. Int. J. Mol. Sci. 2021, 22, 10947. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, N.; Li, Z.; Li, L. Knockdown of PAC1 improved inflammatory pain in mice by regulating the RAGE/TLR4/NF-kappaB signaling pathway. Brain Res. Bull. 2023, 197, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.I.; Thomas Broome, S.; Castorina, A. Exploring the Pro-Phagocytic and Anti-Inflammatory Functions of PACAP and VIP in Microglia: Implications for Multiple Sclerosis. Int. J. Mol. Sci. 2022, 23, 4788. [Google Scholar] [CrossRef] [PubMed]
- Cignarella, F.; Filipello, F.; Bollman, B.; Cantoni, C.; Locca, A.; Mikesell, R.; Manis, M.; Ibrahim, A.; Deng, L.; Benitez, B.A.; et al. TREM2 activation on microglia promotes myelin debris clearance and remyelination in a model of multiple sclerosis. Acta Neuropathol. 2020, 140, 513–534. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, S.A.; Spieth, L.; Sun, T.; Hosang, L.; Schlaphoff, L.; Depp, C.; Düking, T.; Winchenbach, J.; Neuber, J.; Ewers, D.; et al. Microglia facilitate repair of demyelinated lesions via post-squalene sterol synthesis. Nat. Neurosci. 2021, 24, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Plemel, J.R.; Manesh, S.B.; Sparling, J.S.; Tetzlaff, W. Myelin inhibits oligodendroglial maturation and regulates oligodendrocytic transcription factor expression. Glia 2013, 61, 1471–1487. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Qian, Z.; Wang, B.; Cao, J.; Zhang, S.; Jiang, F.; Kong, R.; Yu, X.; Cao, X.; Yang, L.; et al. Transplantation of A2 type astrocytes promotes neural repair and remyelination after spinal cord injury. Cell Commun. Signal. 2023, 21, 37. [Google Scholar] [CrossRef] [PubMed]
- Ponath, G.; Park, C.; Pitt, D. The Role of Astrocytes in Multiple Sclerosis. Front. Immunol. 2018, 9, 217. [Google Scholar] [CrossRef]
- Delgado, M.; Ganea, D. Neuroprotective effect of vasoactive intestinal peptide (VIP) in a mouse model of Parkinson’s disease by blocking microglial activation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2003, 17, 944–946. [Google Scholar] [CrossRef]
- Garry, E.M.; Delaney, A.; Blackburn-Munro, G.; Dickinson, T.; Moss, A.; Nakalembe, I.; Robertson, D.C.; Rosie, R.; Robberecht, P.; Mitchell, R.; et al. Activation of p38 and p42/44 MAP kinase in neuropathic pain: Involvement of VPAC2 and NK2 receptors and mediation by spinal glia. Mol. Cell. Neurosci. 2005, 30, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Thomas Broome, S.; Jansen, M.I.; Mandwie, M.; Logan, G.J.; Marzagalli, R.; Musumeci, G.; Castorina, A. Altered Hippocampal and Striatal Expression of Endothelial Markers and VIP/PACAP Neuropeptides in a Mouse Model of Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2023, 24, 11118. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, C.; Yang, Y.; Jia, N.; Sun, Q. Protection of Vasoactive Intestinal Peptide on the Blood-Brain Barrier Dysfunction Induced by Focal Cerebral Ischemia in Rats. J. Stroke Cerebrovasc. Dis. 2022, 31, 106160. [Google Scholar] [CrossRef] [PubMed]
- Pitt, D.; Lo, C.H.; Gauthier, S.A.; Hickman, R.A.; Longbrake, E.; Airas, L.M.; Mao-Draayer, Y.; Riley, C.; De Jager, P.L.; Wesley, S.; et al. Toward Precision Phenotyping of Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e200025. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H. Pathogenic Mechanisms Associated with Different Clinical Courses of Multiple Sclerosis. Front. Immunol. 2018, 9, 3116. [Google Scholar] [CrossRef] [PubMed]
- Kutzelnigg, A.; Lucchinetti, C.F.; Stadelmann, C.; Brück, W.; Rauschka, H.; Bergmann, M.; Schmidbauer, M.; Parisi, J.E.; Lassmann, H. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 2005, 128, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.I.; Castorina, A. Identification of Key Genes and Regulatory Pathways in Multiple Sclerosis Brain Samples: A Meta-Analysis of Micro-Array Datasets. Int. J. Mol. Sci. 2023, 24, 9361. [Google Scholar] [CrossRef]
- Thomas Broome, S.; Fisher, T.; Faiz, A.; Keay, K.A.; Musumeci, G.; Al-Badri, G.; Castorina, A. Assessing the Anti-Inflammatory Activity of the Anxiolytic Drug Buspirone Using CRISPR-Cas9 Gene Editing in LPS-Stimulated BV-2 Microglial Cells. Cells 2021, 10, 1312. [Google Scholar] [CrossRef]
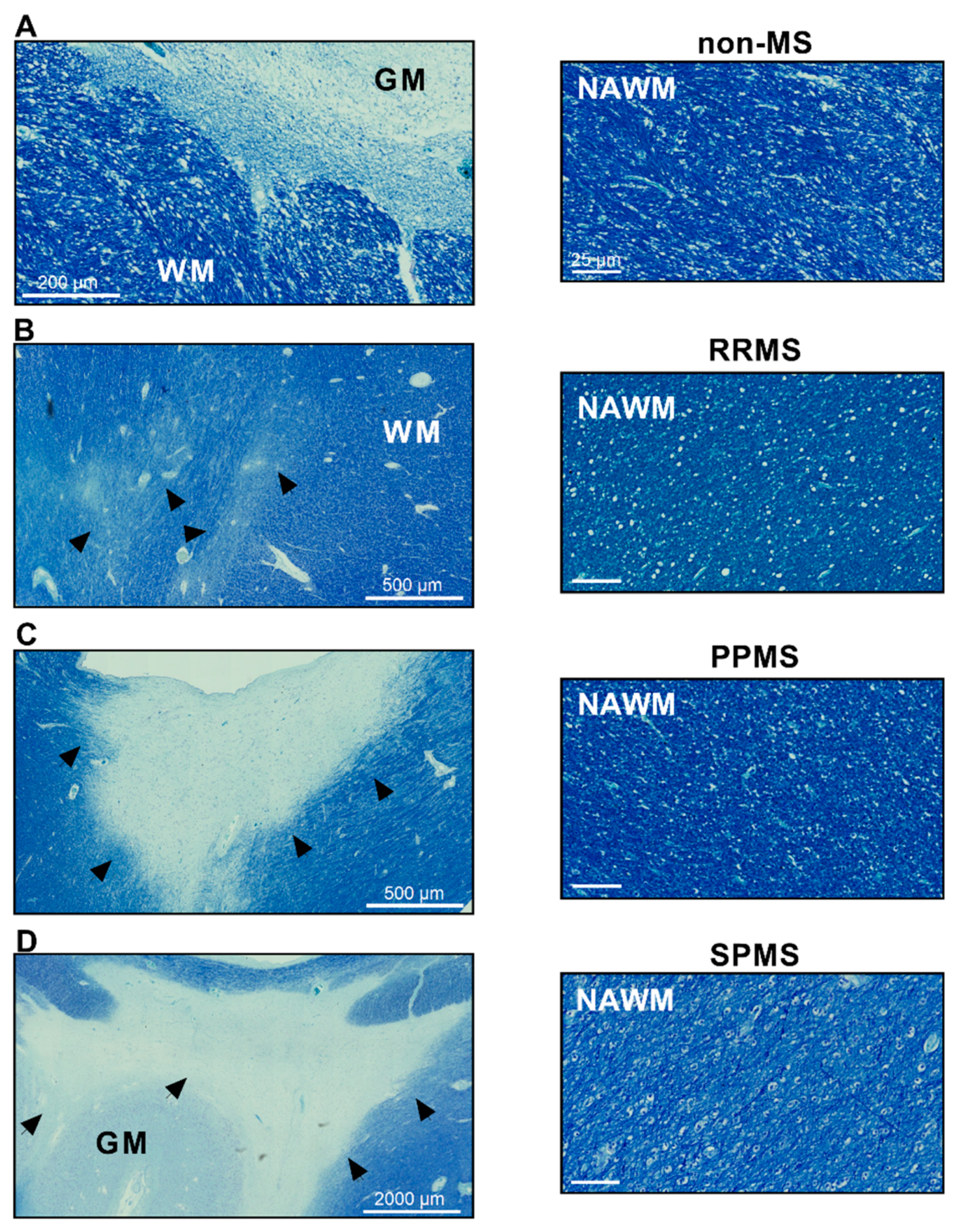

| Group | Age (Years) | Place of Birth | Sex | PMI (Hours) | MS Duration (Years) | Lesion Type |
|---|---|---|---|---|---|---|
| Control | 79 | Australia | Female | 59 | N/A | N/A |
| Control | 82 | England | Female | 25 | N/A | N/A |
| Control | 83 | Australia | Male | 27 | N/A | N/A |
| Control | 73 | Australia | Male | 22 | N/A | N/A |
| Control | 73 | Australia | Female | 26.5 | N/A | N/A |
| RRMS | 70 | Australia | Male | 21 | 43 | Chronic active |
| RRMS | 80 | Australia | Male | 14 | 21.3 | Chronic active |
| RRMS | 40 | Australia | Male | 5 | 8 | Chronic active |
| RRMS | 72 | Australia | Female | 31 | 20 | Chronic active |
| RRMS | 79 | New Zealand | Female | 24 | 29.5 | Chronic active |
| RRMS | 82 | Australia | Female | 19 | 33.1 | Chronic active—minimal regeneration |
| SPMS | 57 | Australia | Female | 26.8 | 17.9 | Chronic active—minimal regeneration |
| SPMS | 68 | Australia | Female | 15 | 33.5 | Chronic active—minimal regeneration |
| SPMS | 69 | New Zealand | Female | 8.5 | 38 | Chronic active—minimal regeneration |
| SPMS | 84 | Australia | Female | 15 | 42 | Chronic active—minimal regeneration |
| SPMS | 47 | Australia | Female | 20.8 | 25.8 | Chronic active—minimal regeneration |
| SPMS | 55 | Australia | Male | 7 | 40.1 | Chronic active—minimal regeneration |
| PPMS | 36 | Australia | Female | 24 | 13 | Chronic active |
| PPMS | 83 | Australia | Female | 16 | 16 | Chronic active |
| PPMS | 73 | Australia | Male | 25 | 15.6 | Chronic active—moderate regeneration |
| PPMS | 73 | England | Male | 24 | 41 | Chronic active—minimal regeneration |
| Accession Number | Gene | Primer Sequences (5′-3′) | Product Size (bp) |
|---|---|---|---|
| NM_001099733.2 | Pituitary adenylate-cyclase-activating peptide (PACAP; ADCYAP1) | TAACGAGGCCTACCGCAAAG GTGAAGATCCCGTCCGAGTG | 150 |
| NM_003381.4 | Vasoactive intestinal peptide (VIP) | AATAAGGCCCAGCTCCTTGTG TGTCACCCAACCTGAGAGCA | 106 |
| NM_001199635.2 | Pituitary adenylate-cyclase-activating peptide receptor 1 (PAC1; ADCYAP1R1) | TTGGCATTATCGTCATCCTTGT AATGGTGGACAGTTCTGACATC | 152 |
| NM_004624.4 | Vasoactive intestinal peptide receptor 1 (VPAC1; VIPR1) | TAAGCCTGAAGTGAAGATGGTC CATTGAGGAAGCAGTAGAGGAT | 86 |
| NM_003382.5 | Vasoactive intestinal peptide receptor 2 (VPAC2; VIPR2) | CTCGCCCCCGTGAACAG GCACGTGATGTTGTCCCAGA | 141 |
| NM_022551.3 | Ribosomal protein S18 (RPS18) | GAGGATGAGGTGGAACGTGT GGACCTGGCTGTATTTTCCA | 115 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansen, M.I.; Musumeci, G.; Castorina, A. Differential Expression of PACAP/VIP Receptors in the Post-Mortem CNS White Matter of Multiple Sclerosis Donors. Int. J. Mol. Sci. 2024, 25, 8850. https://doi.org/10.3390/ijms25168850
Jansen MI, Musumeci G, Castorina A. Differential Expression of PACAP/VIP Receptors in the Post-Mortem CNS White Matter of Multiple Sclerosis Donors. International Journal of Molecular Sciences. 2024; 25(16):8850. https://doi.org/10.3390/ijms25168850
Chicago/Turabian StyleJansen, Margo Iris, Giuseppe Musumeci, and Alessandro Castorina. 2024. "Differential Expression of PACAP/VIP Receptors in the Post-Mortem CNS White Matter of Multiple Sclerosis Donors" International Journal of Molecular Sciences 25, no. 16: 8850. https://doi.org/10.3390/ijms25168850







