Glycerol Handling in Paired Visceral and Subcutaneous Adipose Tissues in Women with Normal Weight and Upper-Body Obesity
Abstract
1. Introduction
2. Results
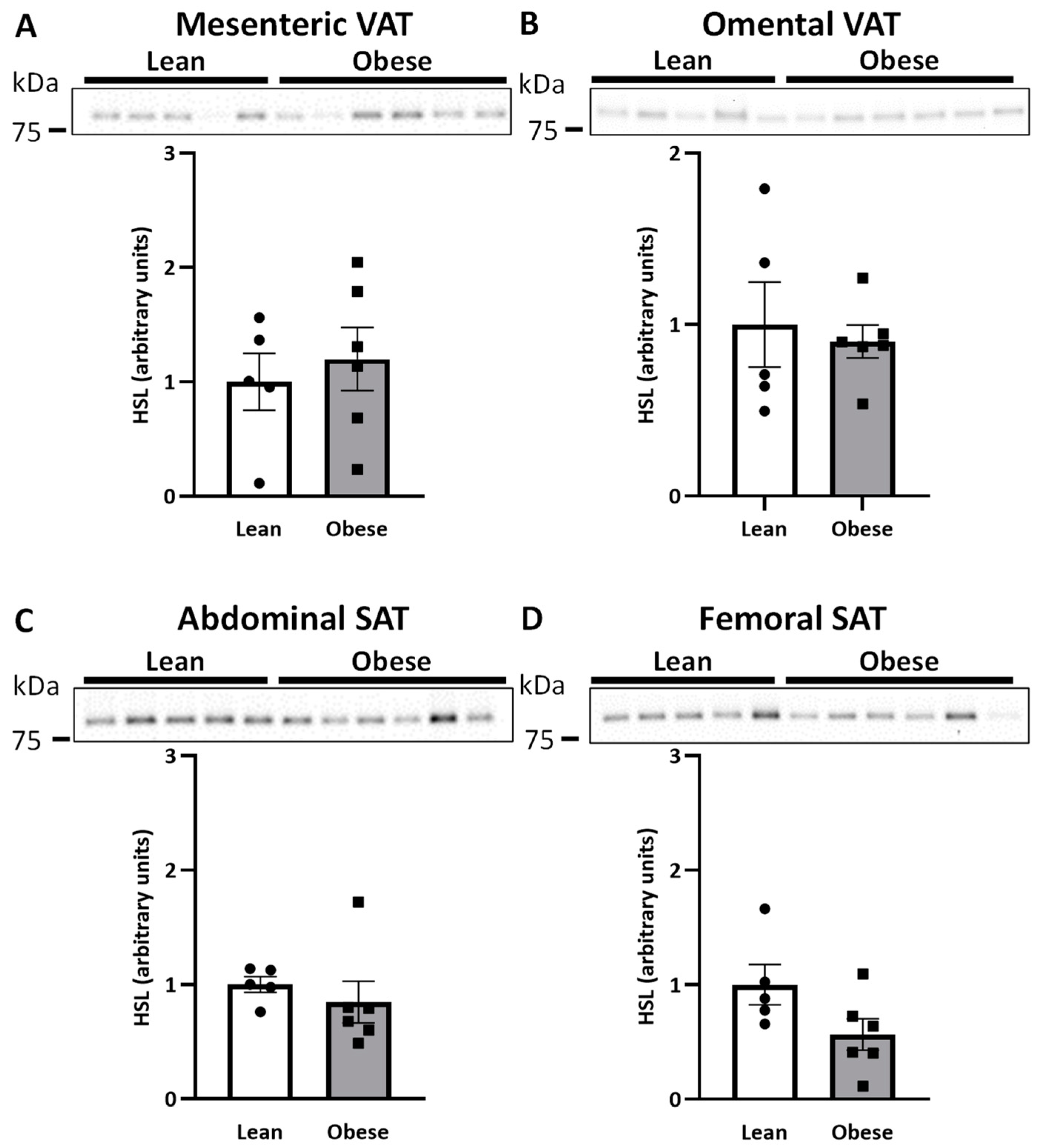
2.1. HSL and AQP7 Expression in Women with Normal Weight or Upper-Body Obesity
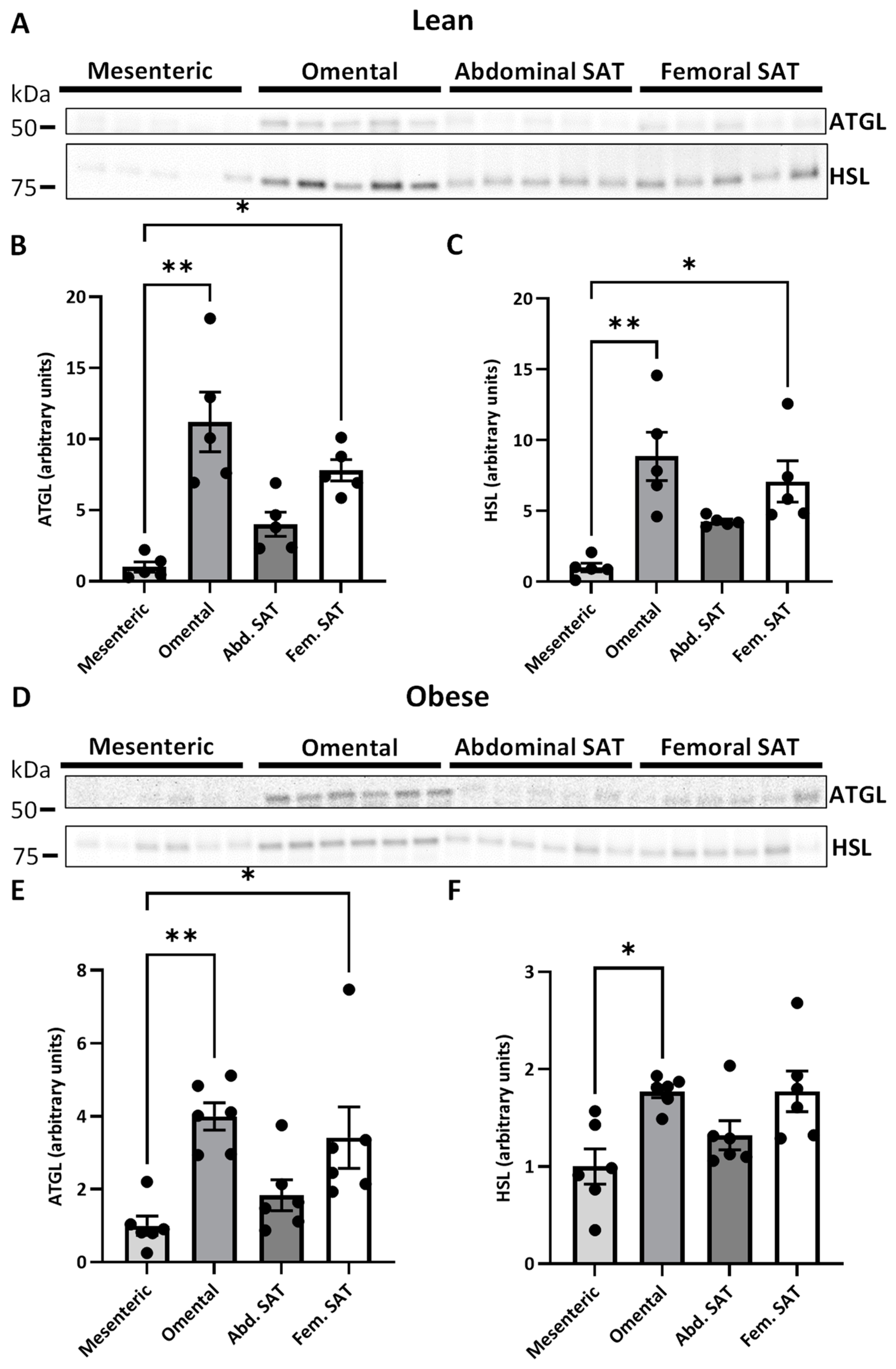
2.2. ATGL and HSL Expression in Paired Visceral and Subcutanous Adiposes Tissue Depots
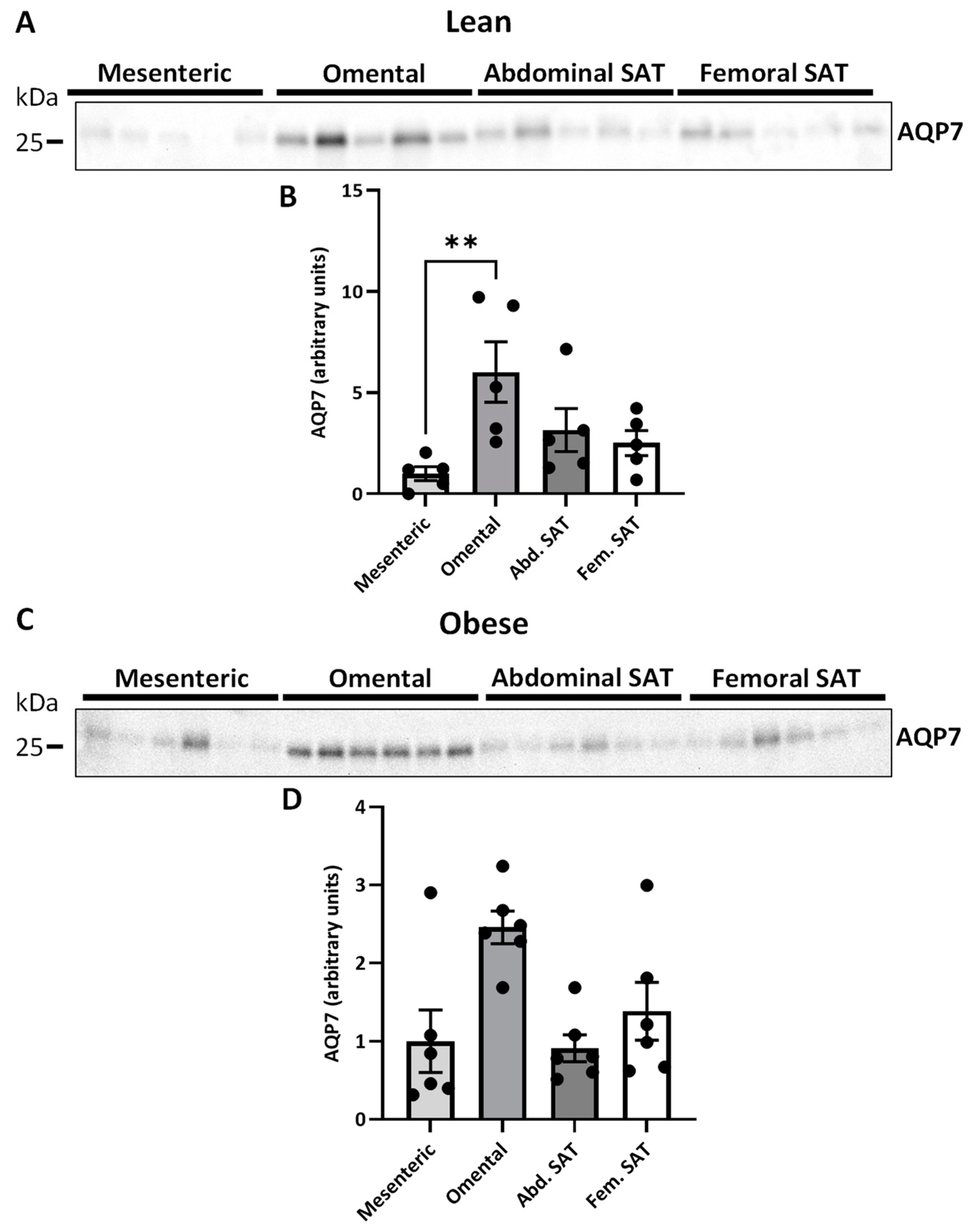
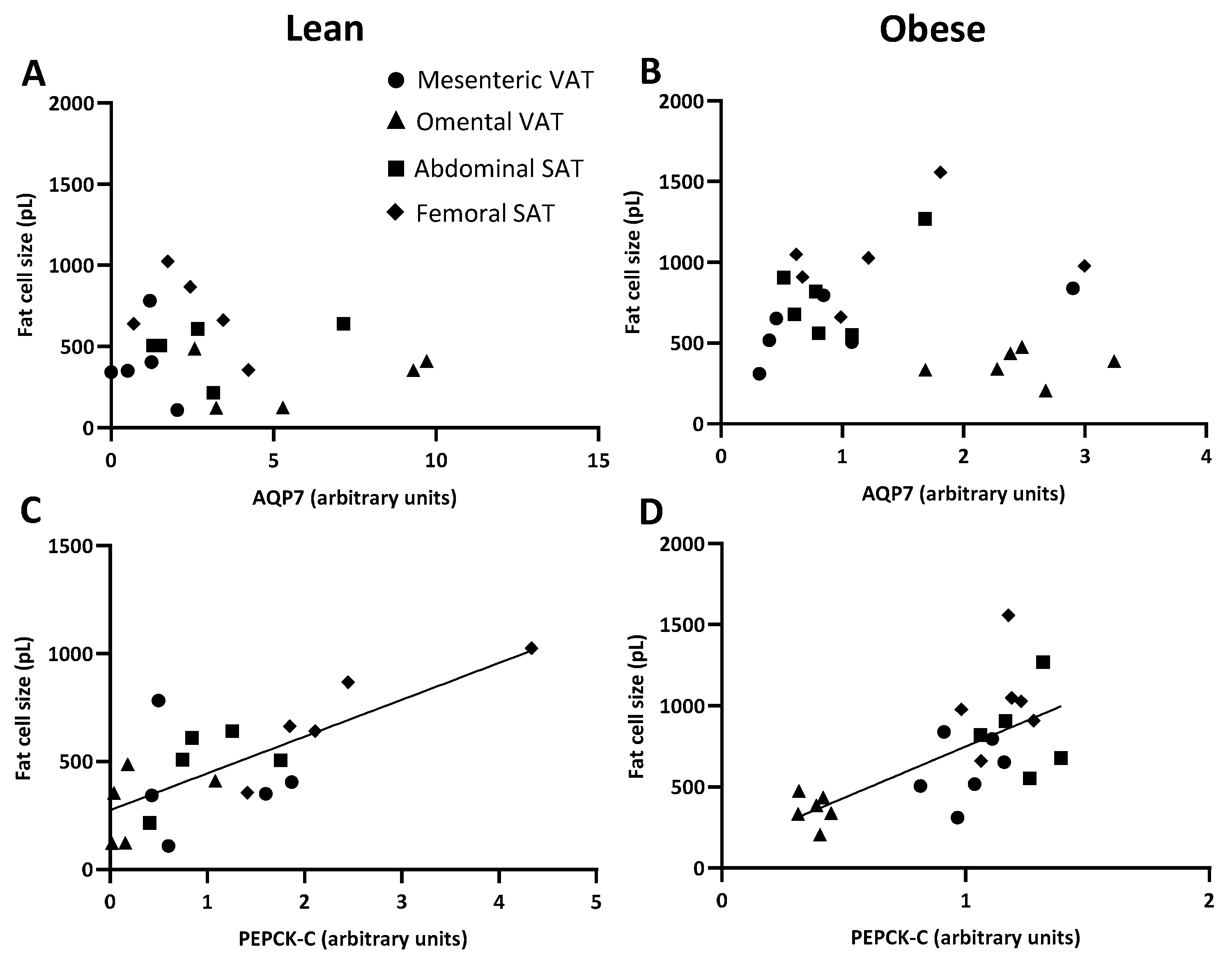
2.3. AQP7 Expression in Paired Visceral and Subcutanous Adiposes Tissue Depots
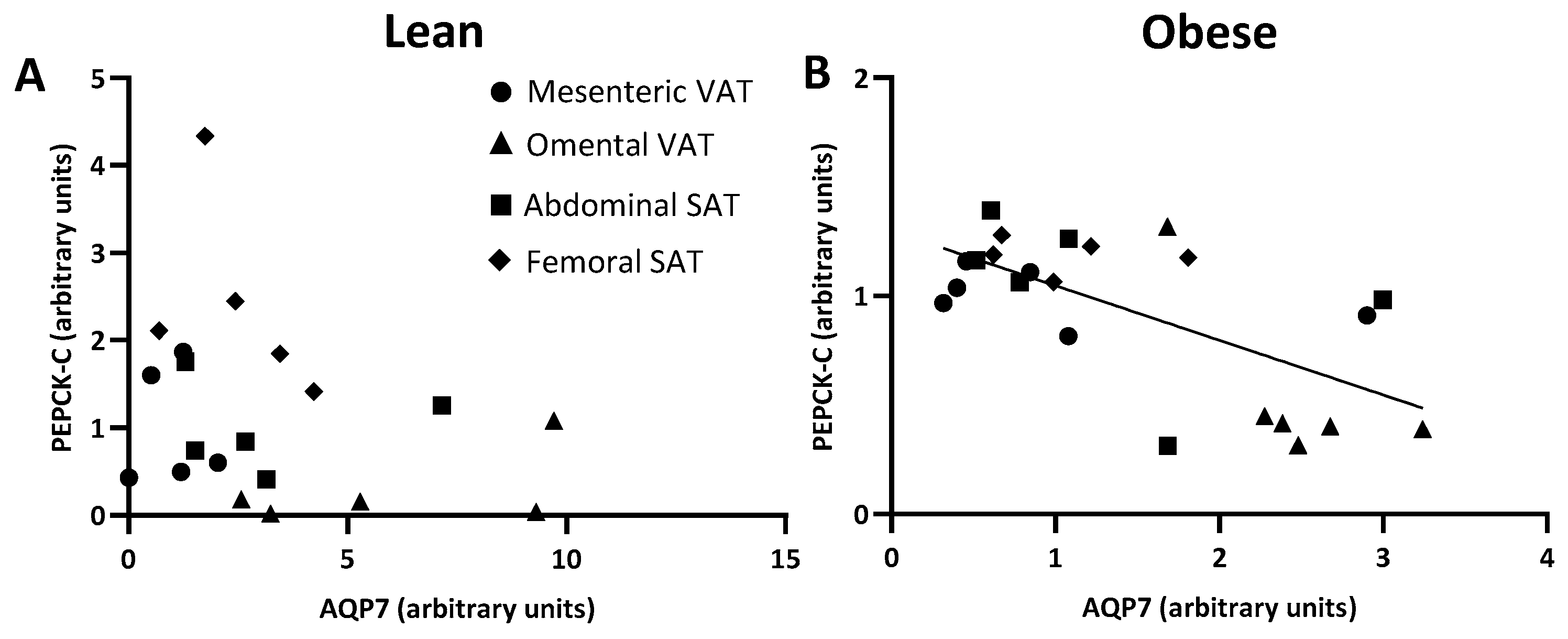
2.4. PEPCK-C Expression in Paired Visceral and Subcutanous Adipose Tissue Depots
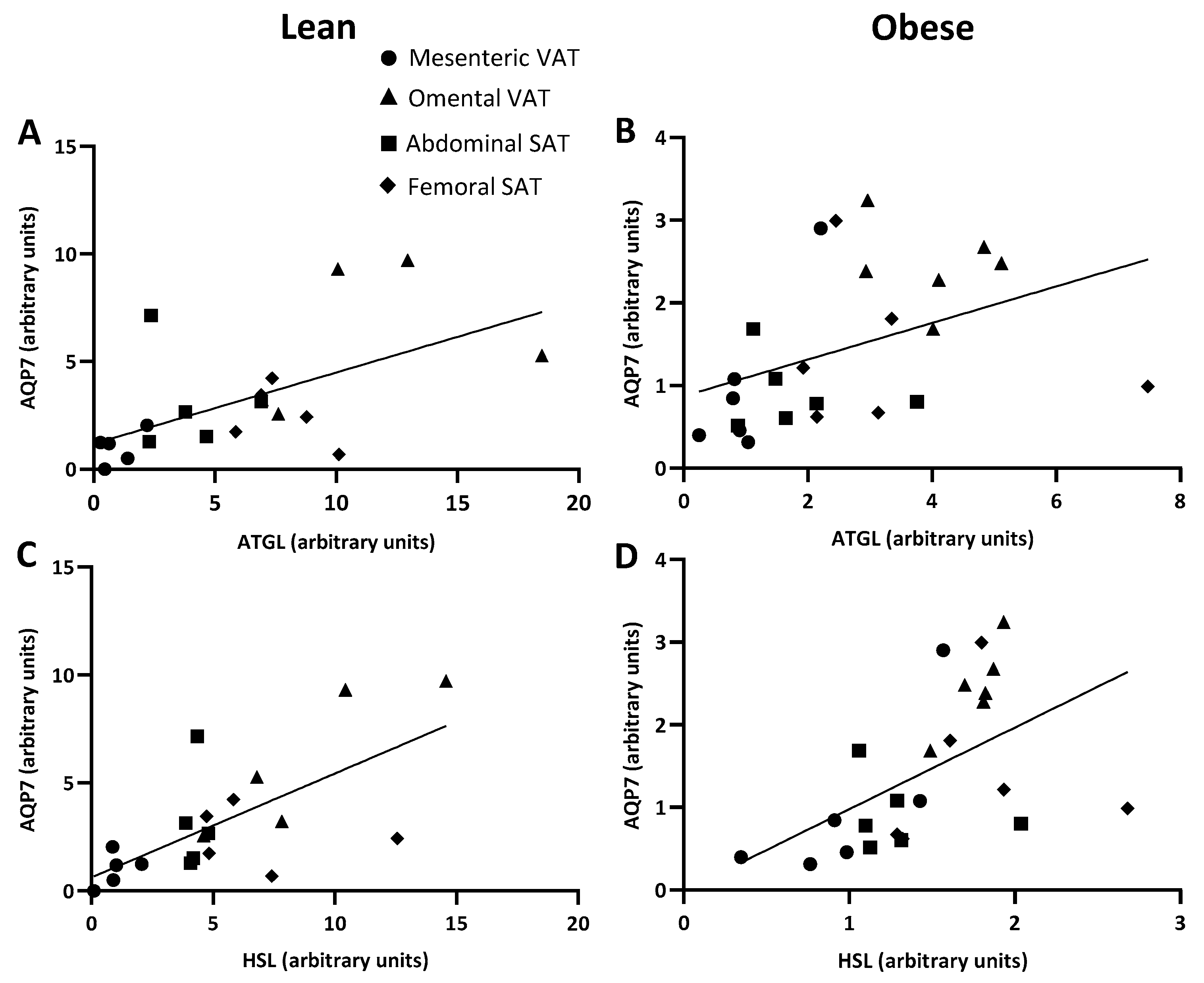
2.5. AQP7 and PEPCK-C Expression and Mean Fat Cell Size
3. Discussion
4. Materials and Methods
4.1. Adipose Tissue Samples
4.2. Semiquantitative Immunoblotting
4.3. Presentation of Data and Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reshef, L.; Olswang, Y.; Cassuto, H.; Blum, B.; Croniger, C.M.; Kalhan, S.C.; Tilghman, S.M.; Hanson, R.W. Glyceroneogenesis and the triglyceride/fatty acid cycle. J. Biol. Chem. 2003, 278, 30413–30416. [Google Scholar] [CrossRef]
- Xue, L.L.; Chen, H.H.; Jiang, J.G. Implications of glycerol metabolism for lipid production. Prog. Lipid Res. 2017, 68, 12–25. [Google Scholar] [CrossRef]
- Hibuse, T.; Maeda, N.; Funahashi, T.; Yamamoto, K.; Nagasawa, A.; Mizunoya, W.; Kishida, K.; Inoue, K.; Kuriyama, H.; Nakamura, T.; et al. Aquaporin 7 deficiency is associated with development of obesity through activation of adipose glycerol kinase. Proc. Natl. Acad. Sci. USA 2005, 102, 10993–10998. [Google Scholar] [CrossRef]
- Prudente, S.; Flex, E.; Morini, E.; Turchi, F.; Capponi, D.; De Cosmo, S.; Tassi, V.; Guida, V.; Avogaro, A.; Folli, F.; et al. A functional variant of the adipocyte glycerol channel aquaporin 7 gene is associated with obesity and related metabolic abnormalities. Diabetes 2007, 56, 1468–1474. [Google Scholar] [CrossRef]
- Iena, F.M.; Jul, J.B.; Vegger, J.B.; Lodberg, A.; Thomsen, J.S.; Bruel, A.; Lebeck, J. Sex-Specific Effect of High-Fat Diet on Glycerol Metabolism in Murine Adipose Tissue and Liver. Front. Endocrinol. 2020, 11, 577650. [Google Scholar] [CrossRef]
- Marrades, M.P.; Milagro, F.I.; Martinez, J.A.; Moreno-Aliaga, M.J. Differential expression of aquaporin 7 in adipose tissue of lean and obese high fat consumers. Biochem. Biophys. Res. Commun. 2006, 339, 785–789. [Google Scholar] [CrossRef]
- Ceperuelo-Mallafre, V.; Miranda, M.; Chacon, M.R.; Vilarrasa, N.; Megia, A.; Gutierrez, C.; Fernandez-Real, J.M.; Gomez, J.M.; Caubet, E.; Fruhbeck, G.; et al. Adipose tissue expression of the glycerol channel aquaporin-7 gene is altered in severe obesity but not in type 2 diabetes. J. Clin. Endocrinol. Metab. 2007, 92, 3640–3645. [Google Scholar] [CrossRef]
- Rodriguez, A.; Catalan, V.; Gomez-Ambrosi, J.; Garcia-Navarro, S.; Rotellar, F.; Valenti, V.; Silva, C.; Gil, M.J.; Salvador, J.; Burrell, M.A.; et al. Insulin- and Leptin-Mediated Control of Aquaglyceroporins in Human Adipocytes and Hepatocytes Is Mediated via the PI3K/Akt/mTOR Signaling Cascade. J. Clin. Endocrinol. Metab. 2011, 96, E586–E597. [Google Scholar] [CrossRef]
- Miranda, M.; Escote, X.; Ceperuelo-Mallafre, V.; Alcaide, M.J.; Simon, I.; Vilarrasa, N.; Wabitsch, M.; Vendrell, J. Paired subcutaneous and visceral adipose tissue aquaporin-7 expression in human obesity and type 2 diabetes: Differences and similarities between depots. J. Clin. Endocrinol. Metab. 2010, 95, 3470–3479. [Google Scholar] [CrossRef]
- Lebeck, J.; Sondergaard, E.; Nielsen, S. Increased AQP7 abundance in skeletal muscle from obese men with type 2 diabetes. Am. J. Physiol.-Endocrinol. Metab. 2018, 315, E367–E373. [Google Scholar] [CrossRef]
- Sjoholm, K.; Palming, J.; Olofsson, L.E.; Gummesson, A.; Svensson, P.A.; Lystig, T.C.; Jennische, E.; Brandberg, J.; Torgerson, J.S.; Carlsson, B.; et al. A Microarray search for genes predominantly expressed in human omental adipocytes: Adipose tissue as a major production site of serum amyloid A. J. Clin. Endocrinol. Metab. 2005, 90, 2233–2239. [Google Scholar] [CrossRef] [PubMed]
- Lebeck, J.; Ostergard, T.; Rojek, A.; Fuchtbauer, E.M.; Lund, S.; Nielsen, S.; Praetorius, J. Gender-specific effect of physical training on AQP7 protein expression in human adipose tissue. Acta Diabetol. 2012, 49 (Suppl. S1), S215–S226. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Jin, B.; Fu, X.; Zhu, J.; Guo, X.; Xu, W.; Mou, X.; Wang, Z.; Jiang, F.; Zhou, Y.; et al. Identification of functional estrogen response elements in glycerol channel Aquaporin-7 gene. Climacteric 2019, 22, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Chen, X.; Xing, L.; Xu, W.; Fu, X.; Zhu, J.; Mou, X.; Wang, Z.; Shu, J. Tissue-specific effects of estrogen on glycerol channel aquaporin 7 expression in an ovariectomized mouse model of menopause. Climacteric 2017, 20, 385–390. [Google Scholar] [CrossRef]
- Chen, L.; Chen, H.; Liu, X.; Li, J.; Gao, Q.; Shi, S.; Wang, T.; Ye, X.; Lu, Y.; Zhang, D.; et al. AQP7 mediates post-menopausal lipogenesis in adipocytes through FSH-induced transcriptional crosstalk with AP-1 sites. Reprod. Biomed. Online 2020, 41, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F. Sex differences in energy metabolism: Natural selection, mechanisms and consequences. Nat. Rev. Nephrol. 2024, 20, 56–69. [Google Scholar] [CrossRef]
- Large, V.; Arner, P.; Reynisdottir, S.; Grober, J.; Van Harmelen, V.; Holm, C.; Langin, D. Hormone-sensitive lipase expression and activity in relation to lipolysis in human fat cells. J. Lipid Res. 1998, 39, 1688–1695. [Google Scholar] [CrossRef]
- Large, V.; Reynisdottir, S.; Langin, D.; Fredby, K.; Klannemark, M.; Holm, C.; Arner, P. Decreased expression and function of adipocyte hormone-sensitive lipase in subcutaneous fat cells of obese subjects. J. Lipid Res. 1999, 40, 2059–2065. [Google Scholar] [CrossRef]
- Lofgren, P.; Hoffstedt, J.; Ryden, M.; Thorne, A.; Holm, C.; Wahrenberg, H.; Arner, P. Major gender differences in the lipolytic capacity of abdominal subcutaneous fat cells in obesity observed before and after long-term weight reduction. J. Clin. Endocrinol. Metab. 2002, 87, 764–771. [Google Scholar] [CrossRef][Green Version]
- Steinberg, G.R.; Kemp, B.E.; Watt, M.J. Adipocyte triglyceride lipase expression in human obesity. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E958–E964. [Google Scholar] [CrossRef]
- Jocken, J.W.; Langin, D.; Smit, E.; Saris, W.H.; Valle, C.; Hul, G.B.; Holm, C.; Arner, P.; Blaak, E.E. Adipose triglyceride lipase and hormone-sensitive lipase protein expression is decreased in the obese insulin-resistant state. J. Clin. Endocrinol. Metab. 2007, 92, 2292–2299. [Google Scholar] [CrossRef] [PubMed]
- Mairal, A.; Langin, D.; Arner, P.; Hoffstedt, J. Human adipose triglyceride lipase (PNPLA2) is not regulated by obesity and exhibits low in vitro triglyceride hydrolase activity. Diabetologia 2006, 49, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, M.T.; Lebeck, J.; Rojek, A.; Praetorius, J.; Fuchtbauer, E.M.; Frokiaer, J.; Nielsen, S. AQP7 is localized in capillaries of adipose tissue, cardiac and striated muscle: Implications in glycerol metabolism. Am. J. Physiol.-Ren. Physiol. 2007, 292, P956–P965. [Google Scholar] [CrossRef] [PubMed]
- Franckhauser, S.; Munoz, S.; Pujol, A.; Casellas, A.; Riu, E.; Otaegui, P.; Su, B.; Bosch, F. Increased fatty acid re-esterification by PEPCK overexpression in adipose tissue leads to obesity without insulin resistance. Diabetes 2002, 51, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Iena, F.M.; Kalucka, J.; Nielsen, L.; Sondergaard, E.; Nielsen, S.; Lebeck, J. Localization of aquaglyceroporins in human and murine white adipose tissue. Histochem. Cell Biol. 2022, 157, 623–639. [Google Scholar] [CrossRef]
- Kishida, K.; Kuriyama, H.; Funahashi, T.; Shimomura, I.; Kihara, S.; Ouchi, N.; Nishida, M.; Nishizawa, H.; Matsuda, M.; Takahashi, M.; et al. Aquaporin adipose, a putative glycerol channel in adipocytes. J. Biol. Chem. 2000, 275, 20896–20902. [Google Scholar] [CrossRef]
- Hansen, J.S.; Krintel, C.; Hernebring, M.; Haataja, T.J.K.; de Mare, S.; Wasserstrom, S.; Kosinska-Eriksson, U.; Palmgren, M.; Holm, C.; Stenkula, K.G.; et al. Perilipin 1 binds to aquaporin 7 in human adipocytes and controls its mobility via protein kinase A mediated phosphorylation. Metab. Clin. Exp. 2016, 65, 1731–1742. [Google Scholar] [CrossRef]
- Laforenza, U.; Scaffino, M.F.; Gastaldi, G. Aquaporin-10 Represents an Alternative Pathway for Glycerol Efflux from Human Adipocytes. PLoS ONE 2013, 8, e54474. [Google Scholar] [CrossRef]
- Sondergaard, E.; Nellemann, B.; Sorensen, L.P.; Gormsen, L.C.; Christiansen, J.S.; Ernst, E.; Dueholm, M.; Nielsen, S. Similar VLDL-TG storage in visceral and subcutaneous fat in obese and lean women. Diabetes 2011, 60, 2787–2791. [Google Scholar] [CrossRef] [PubMed]
- Di Girolamo, M.; Mendlinger, S.; Fertig, J.W. A simple method to determine fat cell size and number in four mammalian species. Am. J. Physiol. 1971, 221, 850–858. [Google Scholar] [CrossRef]
- Perez-Perez, R.; Lopez, J.A.; Garcia-Santos, E.; Camafeita, E.; Gomez-Serrano, M.; Ortega-Delgado, F.J.; Ricart, W.; Fernandez-Real, J.M.; Peral, B. Uncovering suitable reference proteins for expression studies in human adipose tissue with relevance to obesity. PLoS ONE 2012, 7, e30326. [Google Scholar] [CrossRef] [PubMed]


| Lean | Obese | p-Value | |
|---|---|---|---|
| n | 5 | 6 | |
| Age (years) | 39 (33–43) | 35 (28–40) | 0.301 |
| BMI (kg/m2) | 23 (22–25) | 32 (28–37) * | 0.004 |
| Total VAT (g) | 1071 ± 258 | 2052 ± 298 | 0.052 |
| Total upper-body SAT (g) | 8904 ± 978 | 16,007 ± 874 * | 0.004 |
| Total femoral SAT (g) | 10,237 ± 638 | 14,579 ± 692 * | 0.004 |
| Mesenteric fat cell size (pL) | 398 ± 109 | 603 ± 81 | 0.157 |
| Omental fat cell size (pL) | 299 ± 75 | 363 ± 39 | 0.792 |
| Abdominal fat cell size (pL) | 496 ± 75 | 798 ± 110 | 0.059 |
| Femoral fat cell size (pL) | 710 ± 113 a | 1030 ± 120 a,b | 0.089 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nørholm, A.; Kjær, I.G.; Søndergaard, E.; Nellemann, B.; Nielsen, S.; Lebeck, J. Glycerol Handling in Paired Visceral and Subcutaneous Adipose Tissues in Women with Normal Weight and Upper-Body Obesity. Int. J. Mol. Sci. 2024, 25, 9008. https://doi.org/10.3390/ijms25169008
Nørholm A, Kjær IG, Søndergaard E, Nellemann B, Nielsen S, Lebeck J. Glycerol Handling in Paired Visceral and Subcutaneous Adipose Tissues in Women with Normal Weight and Upper-Body Obesity. International Journal of Molecular Sciences. 2024; 25(16):9008. https://doi.org/10.3390/ijms25169008
Chicago/Turabian StyleNørholm, Anne, Ida Guldbrandt Kjær, Esben Søndergaard, Birgitte Nellemann, Søren Nielsen, and Janne Lebeck. 2024. "Glycerol Handling in Paired Visceral and Subcutaneous Adipose Tissues in Women with Normal Weight and Upper-Body Obesity" International Journal of Molecular Sciences 25, no. 16: 9008. https://doi.org/10.3390/ijms25169008
APA StyleNørholm, A., Kjær, I. G., Søndergaard, E., Nellemann, B., Nielsen, S., & Lebeck, J. (2024). Glycerol Handling in Paired Visceral and Subcutaneous Adipose Tissues in Women with Normal Weight and Upper-Body Obesity. International Journal of Molecular Sciences, 25(16), 9008. https://doi.org/10.3390/ijms25169008







