Wuhan Sequence-Based Recombinant Antigens Expressed in E. coli Elicit Antibodies Capable of Binding with Omicron S-Protein
Abstract
1. Introduction
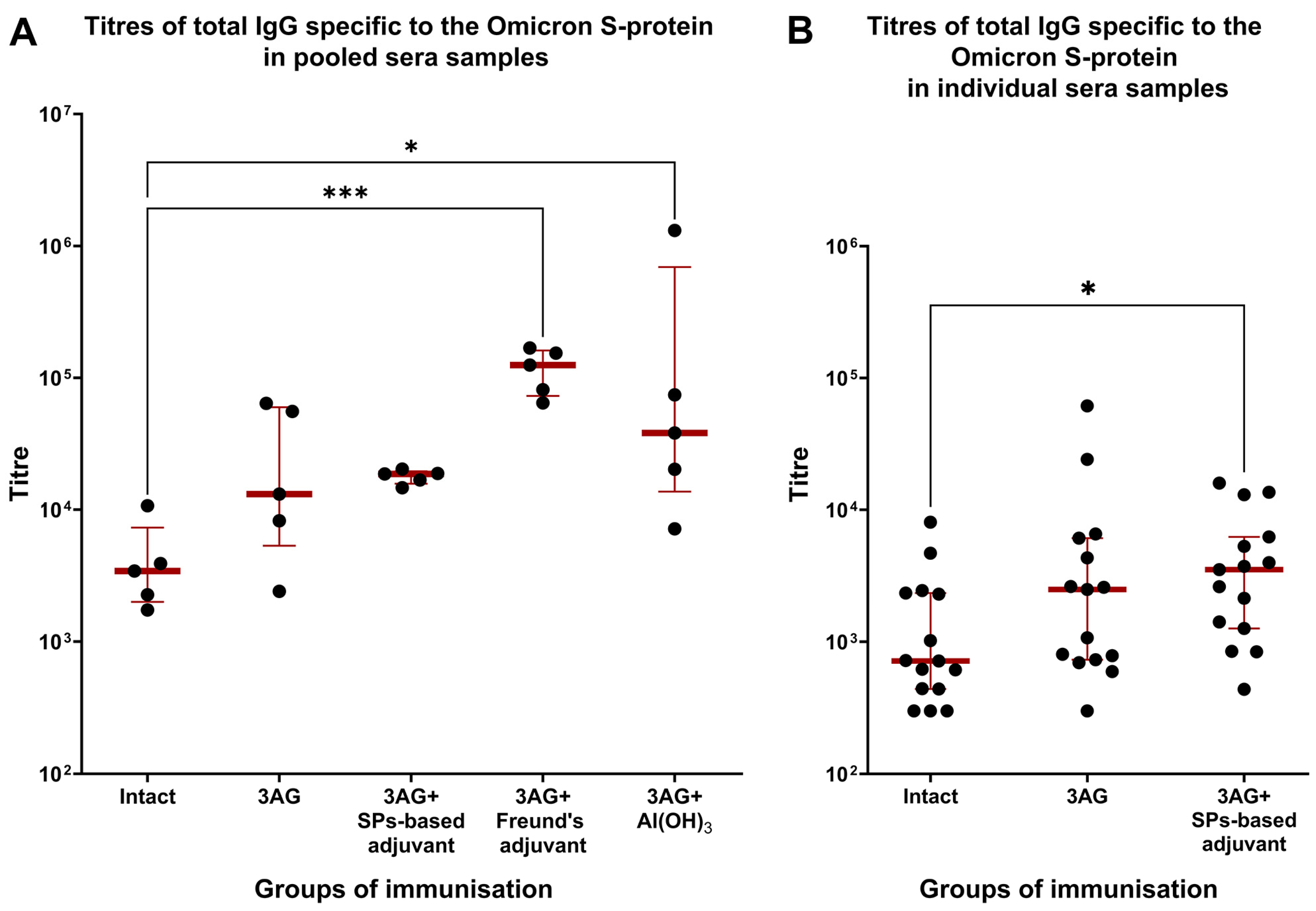
2. Results
3. Discussion
4. Materials and Methods
4.1. Recombinant Antigens and SPs Production
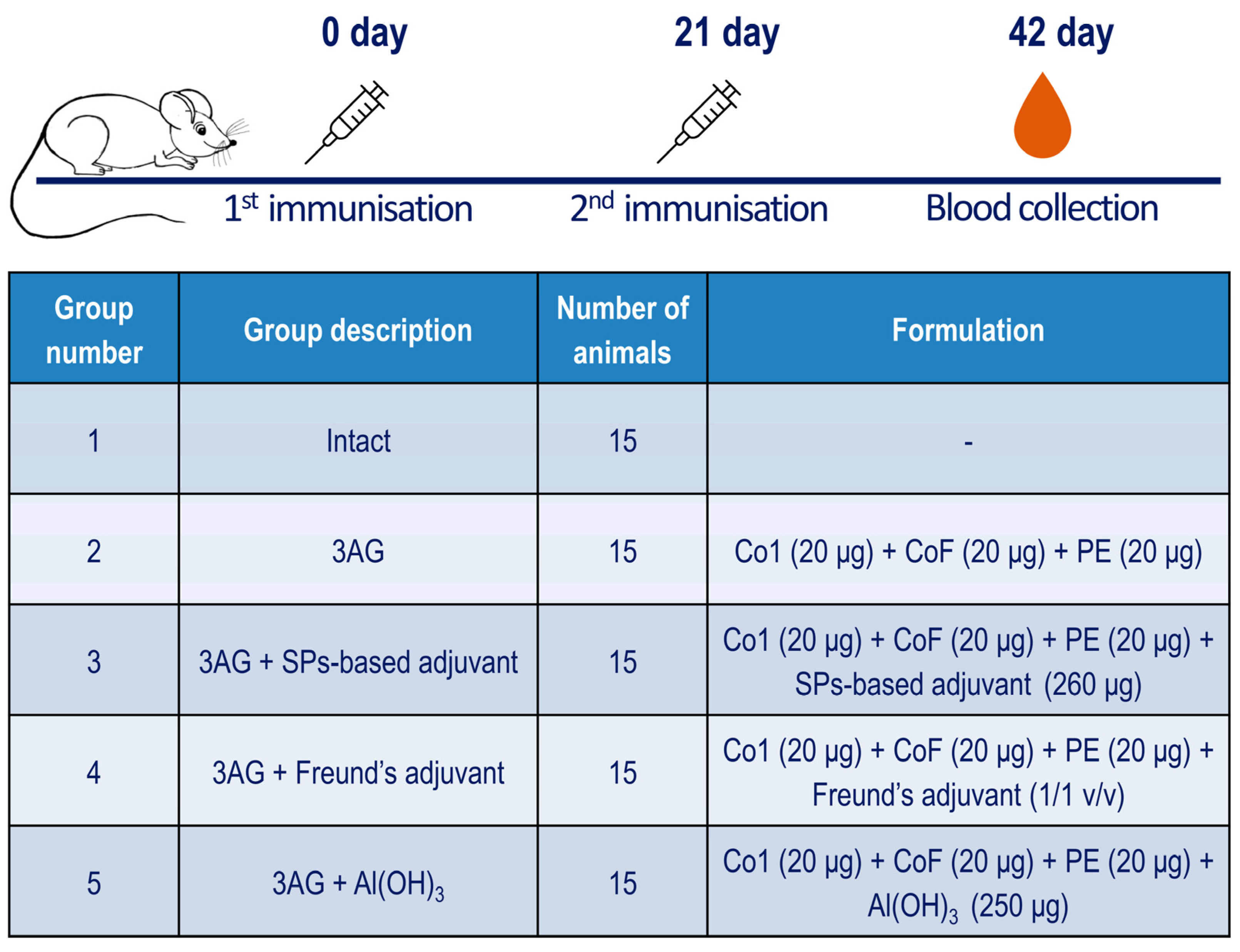
4.2. Immunisation of Mice and Sera Collection
4.3. Ethical Statement
4.4. Western Blot Analysis
4.5. ELISA
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rabaan, A.A.; Al-Ahmed, S.H.; Haque, S.; Sah, R.; Tiwari, R.; Malik, Y.S.; Dhama, K.; Yatoo, M.I.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. SARS-CoV-2, SARS-CoV, and MERS-COV: A comparative overview. Infez. Med. 2020, 28, 174–184. [Google Scholar] [PubMed]
- Padhi, A.K.; Tripathi, T. Can SARS-CoV-2 Accumulate Mutations in the S-Protein to Increase Pathogenicity? ACS Pharmacol. Transl. Sci. 2020, 3, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Flores-Vega, V.R.; Monroy-Molina, J.V.; Jiménez-Hernández, L.E.; Torres, A.G.; Santos-Preciado, J.I.; Rosales-Reyes, R. SARS-CoV-2: Evolution and Emergence of New Viral Variants. Viruses 2022, 14, 653. [Google Scholar] [CrossRef] [PubMed]
- Looi, M.-K. Covid-19: Scientists sound alarm over new BA.2.86 “Pirola” variant. BMJ 2023, 382, 1964. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Covid-19: New “Pirola” variant BA.2.86 continues to spread in UK and US. BMJ 2023, 382, 2097. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Epidemiological Update—29 September 2023, Ed. 159, WHO. Available online: https://www.who.int/publications/m/item/covid-19-epidemiological-update---29-september-2023 (accessed on 28 June 2024).
- Kovalenko, A.O.; Ryabchevskaya, E.M.; Evtushenko, E.A.; Manukhova, T.I.; Kondakova, O.A.; Ivanov, P.A.; Arkhipenko, M.V.; Gushchin, V.A.; Nikitin, N.A.; Karpova, O.V. Vaccine Candidate Against COVID-19 Based on Structurally Modified Plant Virus as an Adjuvant. Front. Microbiol. 2022, 13, 845316. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, A.O.; Ryabchevskaya, E.M.; Evtushenko, E.A.; Kondakova, O.A.; Ivanov, P.A.; Arkhipenko, M.V.; Nikitin, N.A.; Karpova, O.V. Dataset on safety and protective efficacy studies of COVID-19 vaccine candidates based on structurally modified plant virus in female hamsters. Data Brief. 2023, 48, 109158. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, N.; Vasiliev, Y.; Kovalenko, A.; Ryabchevskaya, E.; Kondakova, O.; Evtushenko, E.; Karpova, O. Plant Viruses as Adjuvants for Next-Generation Vaccines and Immunotherapy. Vaccines 2023, 11, 1372. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, L.; Kuwahara, K.; Li, L.; Liu, Z.; Li, T.; Zhu, H.; Liu, J.; Xu, Y.; Xie, J.; et al. Immunodominant SARS Coronavirus Epitopes in Humans Elicited both Enhancing and Neutralizing Effects on Infection in Non-human Primates. ACS Infect. Dis. 2016, 2, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Xue, L.; He, Q.; Zhao, Y.; Xu, W.; Wang, Z. Structural insights into SARS-CoV-2 infection and therapeutics development. Stem Cell Res. 2021, 52, 102219. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, Z.; Nazarian, S.; Dorostkar, R.; Sotoodehnejadnematalahi, F.; Amani, J. Recombinant expression of SARS-CoV-2 receptor binding domain (RBD) in Escherichia coli and its immunogenicity in mice. Iran. J. Basic. Med. Sci. 2022, 25, 1110–1116. [Google Scholar] [PubMed]
- Apostólico, J.S.; Lunardelli, V.A.S.; Coirada, F.C.; Boscardin, S.B.; Rosa, D.S. Adjuvants: Classification, Modus Operandi, and Licensing. J. Immunol. Res. 2016, 2016, 1459394. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Marrack, P.; McKee, A.S.; Munks, M.W. Towards an understanding of the adjuvant action of aluminium. Nat. Rev. Immunol. 2009, 9, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Ebensen, T.; Delandre, S.; Prochnow, B.; Guzmán, C.A.; Schulze, K. The Combination Vaccine Adjuvant System Alum/c-di-AMP Results in Quantitative and Qualitative Enhanced Immune Responses Post Immunization. Front. Cell Infect. Microbiol. 2019, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.S.; Tao, X.; Algaissi, A.; Garron, T.; Narayanan, K.; Peng, B.-H.; Couch, R.B.; Tseng, C.-T.K. Immunization with inactivated Middle East Respiratory Syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum. Vaccin. Immunother. 2016, 12, 2351–2356. [Google Scholar] [CrossRef] [PubMed]
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020, 20, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Pollet, J.; Chen, W.-H.; Strych, U. Recombinant protein vaccines, a proven approach against coronavirus pandemics. Adv. Drug Deliv. Rev. 2021, 170, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Swanson, P.A., 2nd; Padilla, M.; Hoyland, W.; McGlinchey, K.; Fields, P.A.; Bibi, S.; Faust, S.N.; McDermott, A.B.; Lambe, T.; Pollard, A.J.; et al. AZD1222/ChAdOx1 nCoV-19 vaccination induces a polyfunctional spike protein-specific TH1 response with a diverse TCR repertoire. Sci. Transl. Med. 2021, 13, eabj7211. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evtushenko, E.; Ryabchevskaya, E.; Kovalenko, A.; Granovskiy, D.; Arkhipenko, M.; Vasiliev, Y.; Nikitin, N.; Karpova, O. Wuhan Sequence-Based Recombinant Antigens Expressed in E. coli Elicit Antibodies Capable of Binding with Omicron S-Protein. Int. J. Mol. Sci. 2024, 25, 9016. https://doi.org/10.3390/ijms25169016
Evtushenko E, Ryabchevskaya E, Kovalenko A, Granovskiy D, Arkhipenko M, Vasiliev Y, Nikitin N, Karpova O. Wuhan Sequence-Based Recombinant Antigens Expressed in E. coli Elicit Antibodies Capable of Binding with Omicron S-Protein. International Journal of Molecular Sciences. 2024; 25(16):9016. https://doi.org/10.3390/ijms25169016
Chicago/Turabian StyleEvtushenko, Ekaterina, Ekaterina Ryabchevskaya, Angelina Kovalenko, Dmitriy Granovskiy, Marina Arkhipenko, Yuri Vasiliev, Nikolai Nikitin, and Olga Karpova. 2024. "Wuhan Sequence-Based Recombinant Antigens Expressed in E. coli Elicit Antibodies Capable of Binding with Omicron S-Protein" International Journal of Molecular Sciences 25, no. 16: 9016. https://doi.org/10.3390/ijms25169016
APA StyleEvtushenko, E., Ryabchevskaya, E., Kovalenko, A., Granovskiy, D., Arkhipenko, M., Vasiliev, Y., Nikitin, N., & Karpova, O. (2024). Wuhan Sequence-Based Recombinant Antigens Expressed in E. coli Elicit Antibodies Capable of Binding with Omicron S-Protein. International Journal of Molecular Sciences, 25(16), 9016. https://doi.org/10.3390/ijms25169016






