Elucidating Chiral Resolution of Aromatic Amino Acids Using Glycopeptide Selectors: A Combined Molecular Docking and Chromatographic Study
Abstract
1. Introduction
2. Results and Discussion
Modeling Results
3. Materials and Methods
3.1. Procedure
3.2. Molecular Docking Simulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hancu, G.; Papp, L.A.; Szekely-Szentmiklosi, B.; Kelemen, H. The Use of Antibiotics as Chiral Selectors in Capillary Electrophoresis: A Review. Molecules 2022, 27, 3601. [Google Scholar] [CrossRef]
- Hancu, G.; Modroiu, A. Chiral Switch: Between Therapeutical Benefit and Marketing Strategy. Pharmaceuticals 2022, 15, 240. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rodríguez, S.; Martínez-Gómez, A.I.; Rodríguez-Vico, F.; Clemente-Jiménez, J.M.; Las Heras-Vázquez, F.J. Natural Occurrence and Industrial Applications of D-Amino Acids: An Overview. Chem. Biodivers. 2010, 7, 1531–1548. [Google Scholar] [CrossRef]
- Grybinik, S.; Bosakova, Z. An Overview of Chiral Separations of Pharmaceutically Active Substances by HPLC (2018–2020). Monatshefte Für Chem. Chem. Mon. 2021, 152, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Turiel, M.G.; Garrido-González, J.J.; Simón, L.; Sanz, F.; Lithgow, A.M.; Morán, J.R.; Fuentes de Arriba, Á.L.; Alcázar, V. Highly Enantioselective Extraction of Phenylglycine by a Chiral Macrocyclic Receptor Based on Supramolecular Interactions. Org. Lett. 2020, 22, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.A.; He, H.; Pham-Huy, C. Chiral Drugs: An Overview. Int. J. Biomed. Sci. 2006, 2, 85–100. [Google Scholar]
- Lämmerhofer, M. Chiral Recognition by Enantioselective Liquid Chromatography: Mechanisms and Modern Chiral Stationary Phases. J. Chromatogr. A 2010, 1217, 814–856. [Google Scholar] [CrossRef]
- Dalgliesh, C.E. 756. The Optical Resolution of Aromatic Amino-Acids on Paper Chromatograms. J. Chem. Soc. Resumed 1952, 3940–3942. [Google Scholar] [CrossRef]
- Fouad, A.; El-Sayed, D.H.; Salman, B.E.; Bakr, H.H.; Adel, S.E.; Alzarak, T.M.; Mahmoud, A. Macrocyclic Antibiotics as Effective Chiral Selectors in Liquid Chromatography for Enantiomeric Separation of Pharmaceutical Compounds: A Review. Crit. Rev. Anal. Chem. 2023, 1–19. [Google Scholar] [CrossRef]
- Okano, A.; Isley, N.A.; Boger, D.L. Total Syntheses of Vancomycin Related Glycopeptide Antibiotics and Key Analogues. Chem. Rev. 2017, 117, 11952–11993. [Google Scholar] [CrossRef]
- Berthod, A. Chiral Recognition Mechanisms with Macrocyclic Glycopeptide Selectors. Chirality 2009, 21, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Shahnani, M.; Sefidbakht, Y.; Maghari, S.; Mehdi, A.; Rezadoost, H.; Ghassempour, A. Enantioseparation of Mandelic Acid on Vancomycin Column: Experimental and Docking Study. Chirality 2020, 32, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Lekmine, S.; Benslama, O.; Kadi, K.; Brik, A.; Djeffali, O.; Ounissi, M.; Slimani, M.; Ola, M.S.; Eldahshan, O.A.; Martín-García, A.I.; et al. Preliminary Investigation of Astragalus arpilobus subsp. hauarensis: LC-MS/MS Chemical Profiling, In Vitro Evaluation of Antioxidant, Anti-Inflammatory Properties, Cytotoxicity, and In Silico Analysis against COX-2. Antioxidants 2024, 13, 654. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Fu, A.; Zhang, L. Progress in Molecular Docking. Quant. Biol. 2019, 7, 83–89. [Google Scholar] [CrossRef]
- Lekmine, S.; Bendjedid, S.; Benslama, O.; Martín-García, A.I.; Boussekine, S.; Kadi, K.; Akkal, S.; Nieto, G.; Sami, R.; Al-Mushhin, A.A.M.; et al. Ultrasound-Assisted Extraction, LC–MS/MS Analysis, Anticholinesterase, and Antioxidant Activities of Valuable Natural Metabolites from Astragalus armatus Willd.: In Silico Molecular Docking and in Vitro Enzymatic Studies. Antioxidants 2022, 11, 2000. [Google Scholar] [CrossRef]
- Peluso, P.; Dessì, A.; Dallocchio, R.; Mamane, V.; Cossu, S. Recent Studies of Docking and Molecular Dynamics Simulation for Liquid-Phase Enantioseparations. Electrophoresis 2019, 40, 1881–1896. [Google Scholar] [CrossRef]
- Ma, Q.; Cong, W.; Liu, Y.; Geng, Z.; Lin, Y.; Wang, Z. Experimental and Computational Study on the Enantioseparation of Four Chiral Fluoroquinolones by Capillary Electrophoresis with Sulfated-β-cyclodextrin as Chiral Selector. Chirality 2021, 33, 549–557. [Google Scholar] [CrossRef]
- Dombi, G.; Horváth, P.; Fiser, B.; Mirzahosseini, A.; Dobó, M.; Szabó, Z.-I.; Tóth, G. Enantioselective Human Serum Albumin Binding of Apremilast: Liquid Chromatographic, Fluorescence and Molecular Docking Study. Int. J. Mol. Sci. 2023, 24, 2168. [Google Scholar] [CrossRef]
- Del Rio, A.; Gasteiger, J. Encoding Absolute Configurations with Chiral Enantiophore Descriptors. Application to the Order of Elution of Enantiomers in Liquid Chromatography. QSAR Comb. Sci. 2008, 27, 1326–1336. [Google Scholar] [CrossRef]
- Dobó, M.; Ádám, M.; Fiser, B.; Papp, L.A.; Dombi, G.; Sekkoum, K.; Szabó, Z.-I.; Tóth, G. Enantioseparation and Molecular Docking Study of Selected Chiral Pharmaceuticals on a Commercialized Phenylcarbamate-β-Cyclodextrin Column Using Polar Organic Mode. Sci. Rep. 2023, 13, 14778. [Google Scholar] [CrossRef]
- Saleh, O.A.; Badawey, A.M.; Aboul-Enein, H.Y.; Fouad, M.A. Enantioseparation, Quantification, Molecular Docking and Molecular Dynamics Study of Five β-Adrenergic Blockers on Lux-Cellulose-2 Column. BMC Chem. 2023, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Natalini, B.; Giacchè, N.; Sardella, R.; Ianni, F.; Macchiarulo, A.; Pellicciari, R. Computational Studies for the Elucidation of the Enantiomer Elution Order of Amino Acids in Chiral Ligand-Exchange Chromatography. J. Chromatogr. A 2010, 1217, 7523–7527. [Google Scholar] [CrossRef]
- Tanács, D.; Berkecz, R.; Shahmohammadi, S.; Forró, E.; Armstrong, D.W.; Péter, A.; Ilisz, I. Macrocyclic Glycopeptides- and Derivatized Cyclofructan-Based Chiral Stationary Phases for the Enantioseparation of Fluorinated ß-Phenylalanine Analogs. J. Pharm. Biomed. Anal. 2022, 219, 114912. [Google Scholar] [CrossRef] [PubMed]
- Hellinghausen, G.; Lopez, D.A.; Lee, J.T.; Wang, Y.; Weatherly, C.A.; Portillo, A.E.; Berthod, A.; Armstrong, D.W. Evaluation of the Edman Degradation Product of Vancomycin Bonded to Core-Shell Particles as a New HPLC Chiral Stationary Phase. Chirality 2018, 30, 1067–1078. [Google Scholar] [CrossRef]
- Armstrong, D.W.; Zhou, Y. Use of a Macrocyclic Antibiotic as the Chiral Selector for Enantiomeric Separations by TLC. J. Liq. Chromatogr. 1994, 17, 1695–1707. [Google Scholar] [CrossRef]
- Xiong, W.-Q.; Lv, Y.; Peng, B.; Fu, S.-G.; Duan, A.-H.; Zhang, M.; Yuan, L.-M. Enantioselective Resolutions by High-Performance Liquid Choromatography Using Chiral Inorganic Mesoporous Silica. Sep. Sci. Plus 2021, 4, 77–85. [Google Scholar] [CrossRef]
- Udvarhelyi, P.M.; Watkins, J.C. Direct Resolution of Some Phenylglycines by Liquid Chromatography on a Chiral Crown Ether Phase. Chirality 1990, 2, 200–204. [Google Scholar] [CrossRef]
- Armstrong, D.W.; Tang, Y.; Chen, S.; Zhou, Y.; Bagwill, C.; Chen, J.-R. Macrocyclic Antibiotics as a New Class of Chiral Selectors for Liquid Chromatography. Available online: https://pubs.acs.org/doi/pdf/10.1021/ac00081a019 (accessed on 1 February 2023).
- Fanali, S.; Crucianelli, M.; De Angelis, F.; Presutti, C. Enantioseparation of Amino Acid Derivatives by Capillary Zone Electrophoresis Using Vancomycin as Chiral Selector. Electrophoresis 2002, 23, 3035–3040. [Google Scholar] [CrossRef] [PubMed]
- Akhter, M. Challenges in Docking: Mini Review. JSM Chem. 2016, 4, 1025. [Google Scholar]
- Mirzaei, H.; Zarbafian, S.; Villar, E.; Mottarella, S.; Beglov, D.; Vajda, S.; Paschalidis, I.C.; Vakili, P.; Kozakov, D. Energy Minimization on Manifolds for Docking Flexible Molecules. J. Chem. Theory Comput. 2015, 11, 1063–1076. [Google Scholar] [CrossRef]
- Gogolishvili, O.S.; Reshetova, E.N. Chromatographic Enantioseparation and Adsorption Thermodynamics of Hydroxy Acids and Their Derivatives on Antibiotic-Based Chiral Stationary Phases as Affected by Eluent pH. Chromatographia 2021, 84, 53–73. [Google Scholar] [CrossRef]
- Pantsar, T.; Poso, A. Binding Affinity via Docking: Fact and Fiction. Molecules 2018, 23, 1899. [Google Scholar] [CrossRef]
- Lekmine, S.; Benslama, O.; Kadi, K.; Martín-García, A.I.; Yilmaz, M.A.; Akkal, S.; Boumegoura, A.; Alhomida, A.S.; Ola, M.S.; Ali, A. LC/MS-MS Analysis of Phenolic Compounds in Hyoscyamus albus L. Extract. In Vitro Antidiabetic Activity, In Silico Molecular Docking, and In Vivo Investigation against STZ-Induced Diabetic Mice. Pharmaceuticals 2023, 16, 1015. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Phyo, Y.Z.; Cravo, S.; Palmeira, A.; Tiritan, M.E.; Kijjoa, A.; Pinto, M.M.; Fernandes, C. Enantiomeric Resolution and Docking Studies of Chiral Xanthonic Derivatives on Chirobiotic Columns. Molecules 2018, 23, 142. [Google Scholar] [CrossRef]
- Gherdaoui, D.; Yahoum, M.M.; Toumi, S.; Tahraoui, H.; Bouazza, F.; Lefnaoui, S.; Zeghdaoui, A.; Amrane, A.; Jaouadi, B.; Zhang, J. The Effect of the Stationary Phase on Resolution in the HPLC-Based Separation of Racemic Mixtures Using Vancomycin as a Chiral Selector: A Case Study with Profen Nonsteroidal Anti-Inflammatory Drugs. Symmetry 2023, 15, 2154. [Google Scholar] [CrossRef]
- Gherdaoui, D.; Bekdouche, H.; Zerkout, S.; Fegas, R.; Righezza, M. Chiral Separation of Ketoprofen on an Achiral NH2 Column by HPLC Using Vancomycin as Chiral Mobile Phase Additive. J. IRAN Chem. Soc. 2016, 13, 2319–2323. [Google Scholar] [CrossRef]
- Benslama, O.; Lekmine, S.; Mansouri, N. Phytochemical Constituents of Astragalus monspessulanus and Integrative Analysis for Its Antioxidant, Photoprotective, and Antityrosinase Activities: Experimental and Computational Investigation. Eur. J. Integr. Med. 2023, 60, 102247. [Google Scholar] [CrossRef]
- Belhocine, Y.; Rahali, S.; Allal, H.; Assaba, I.M.; Ghoniem, M.G.; Ali, F.A.M. A Dispersion Corrected DFT Investigation of the Inclusion Complexation of Dexamethasone with β-Cyclodextrin and Molecular Docking Study of Its Potential Activity against COVID-19. Molecules 2021, 26, 7622. [Google Scholar] [CrossRef]
- Moldovan, O.-L.; Sandulea, A.; Lungu, I.-A.; Gâz, Ș.A.; Rusu, A. Identification of Some Glutamic Acid Derivatives with Biological Potential by Computational Methods. Molecules 2023, 28, 4123. [Google Scholar] [CrossRef]
- Lekmine, S.; Benslama, O.; Kadi, K.; Ignacio Martín-García, A.; Shamsul Ola, M.; Abdullah Yilmaz, M.; Ali, A. Therapeutic Potential of Hyoscyamus niger-Derived Compounds: Targeting Ovarian Cancer through Antioxidant Activity and EGFR Tyrosine Kinase Inhibition. J. King Saud. Univ. Sci. 2024, 36, 103103. [Google Scholar] [CrossRef]
- Rizvi, S.; Mohd, D.; Shakil, S.; Haneef, M. A Simple Click by Click Protocol to Perform Docking: AutoDock 4.2 Made Easy for Non-Bioinformaticians. EXCLI J. 2013, 12, 831–857. [Google Scholar]
- Pazhani, J.; Veeraraghavan, V.P.; Jayaraman, S. Molecular Docking Analysis of Proflavin with the Wnt Pathway Targets for OSCC. Bioinformation 2023, 19, 464–466. [Google Scholar] [CrossRef]
- Zhang, B.; Li, H.; Yu, K.; Jin, Z. Molecular Docking-Based Computational Platform for High-Throughput Virtual Screening. CCF Trans. High. Perform. Comput. 2022, 4, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.N.; Nicholls, A. Recommendations for Evaluation of Computational Methods. J. Comput. Aided Mol. Des. 2008, 22, 133–139. [Google Scholar] [CrossRef] [PubMed]

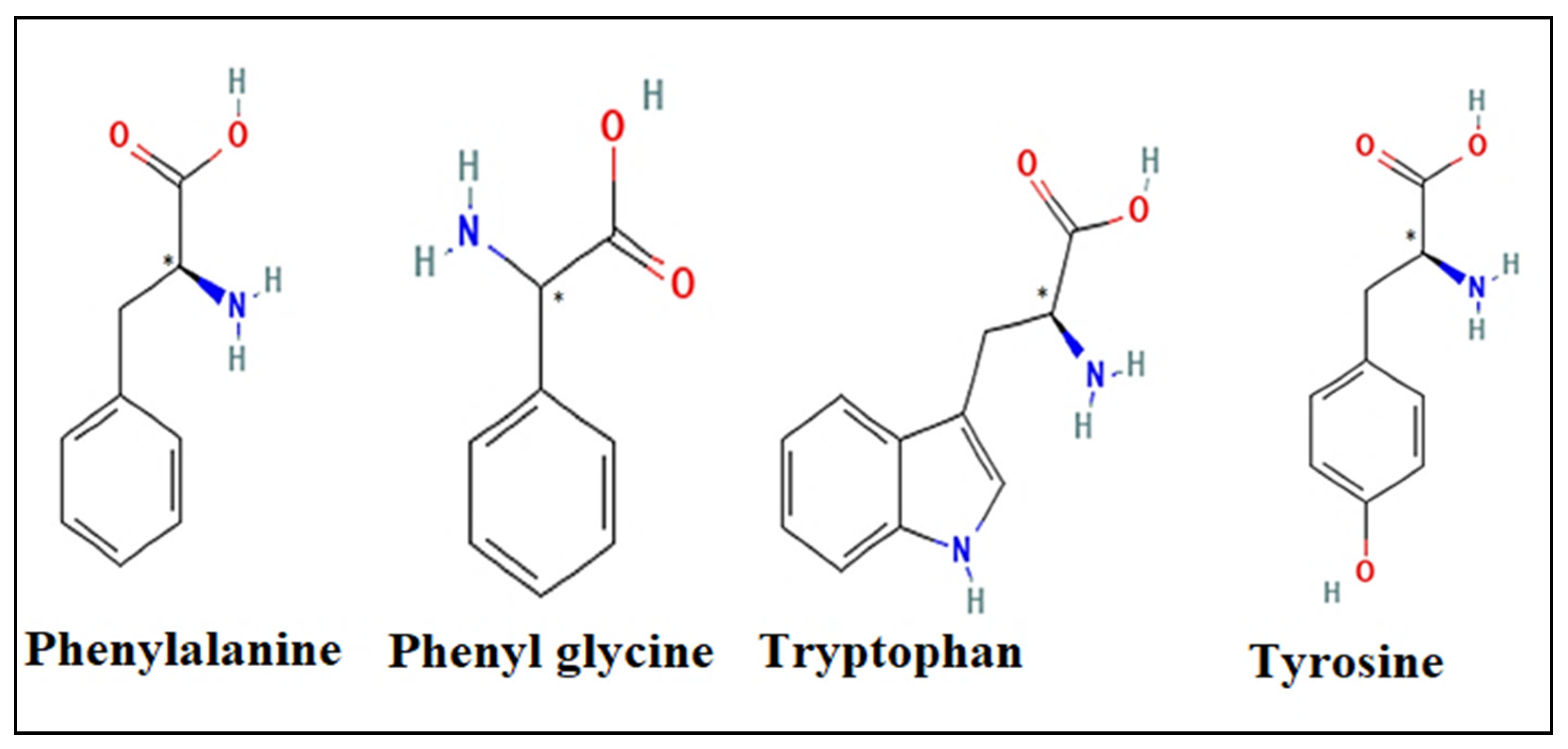

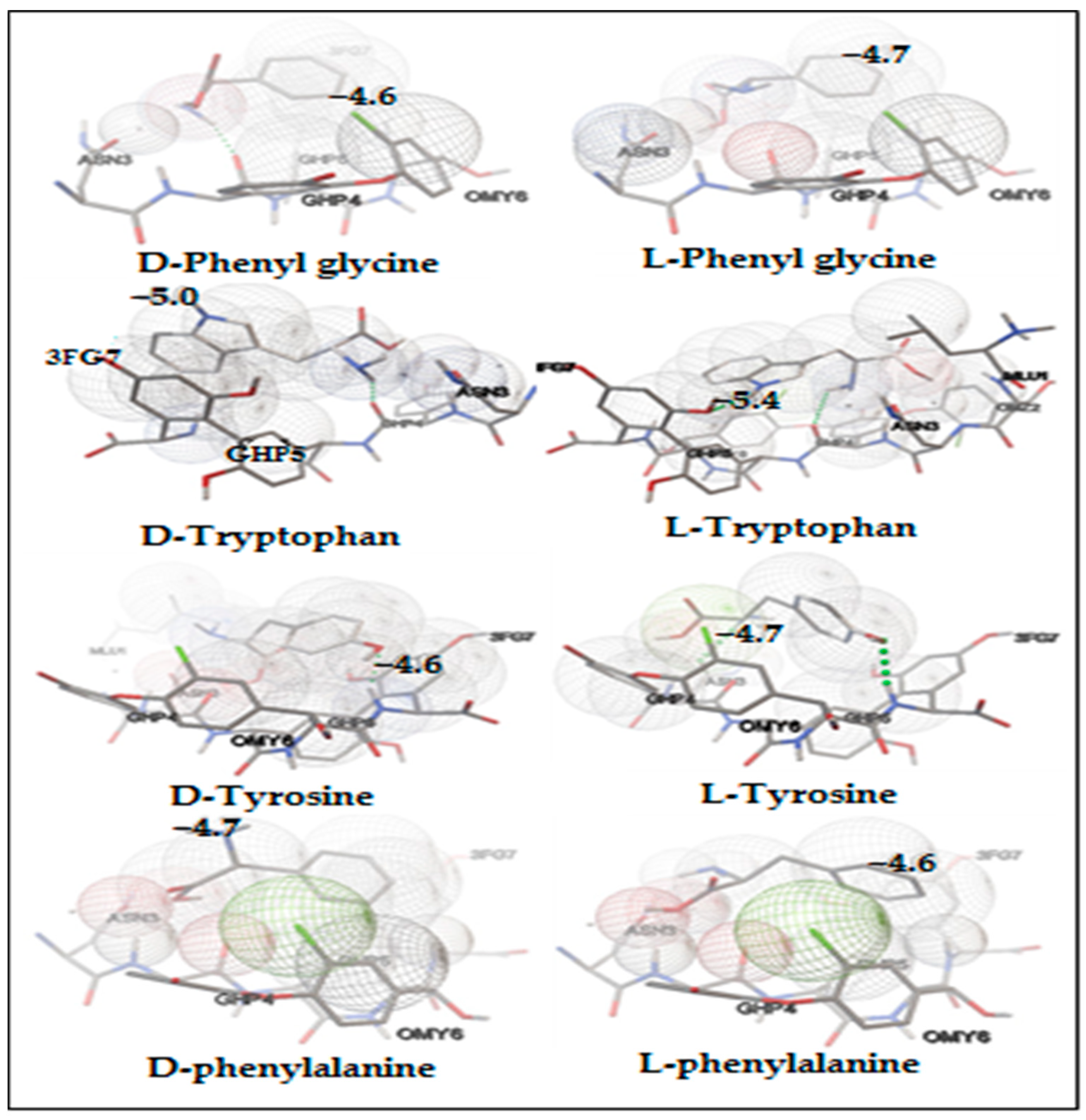

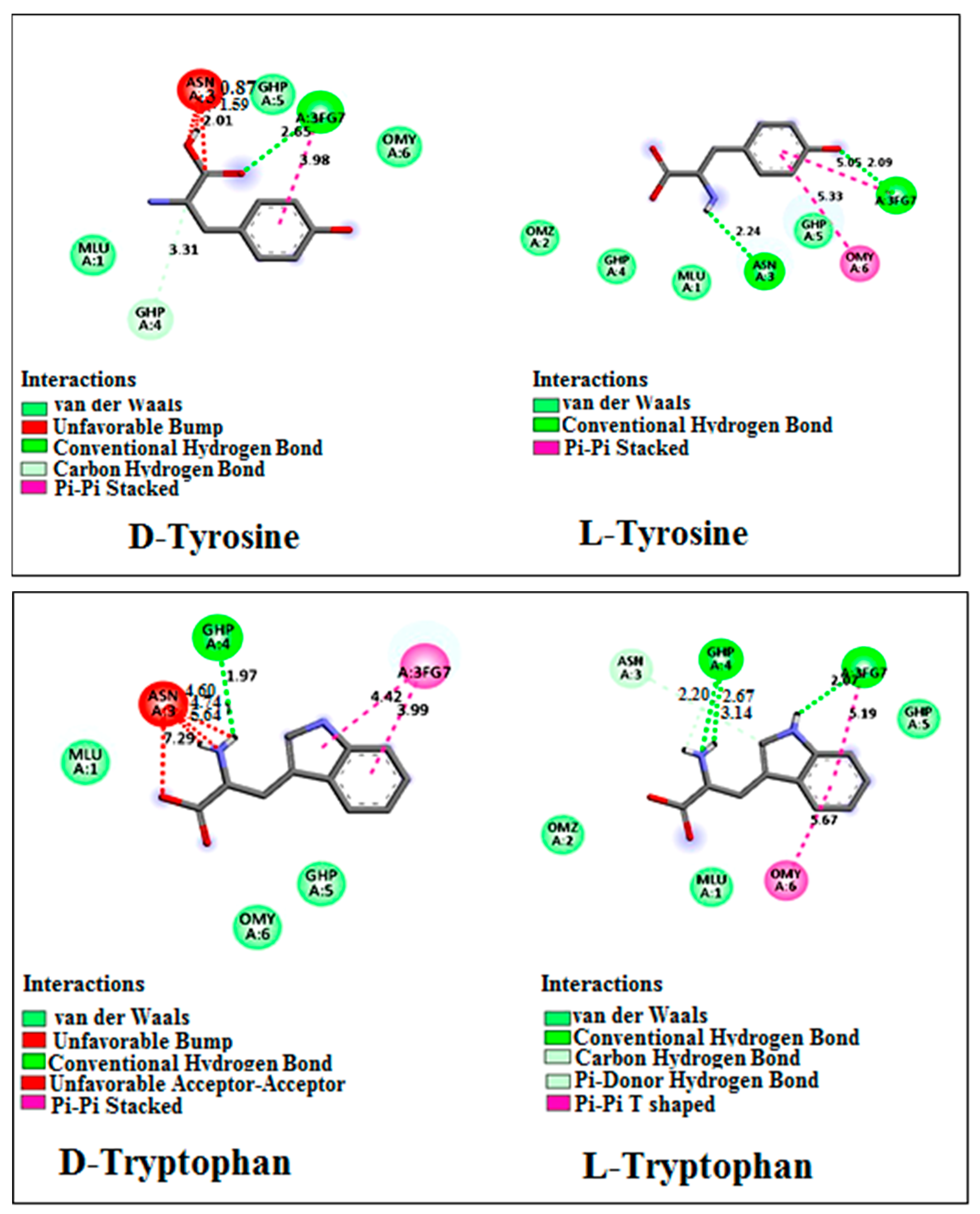
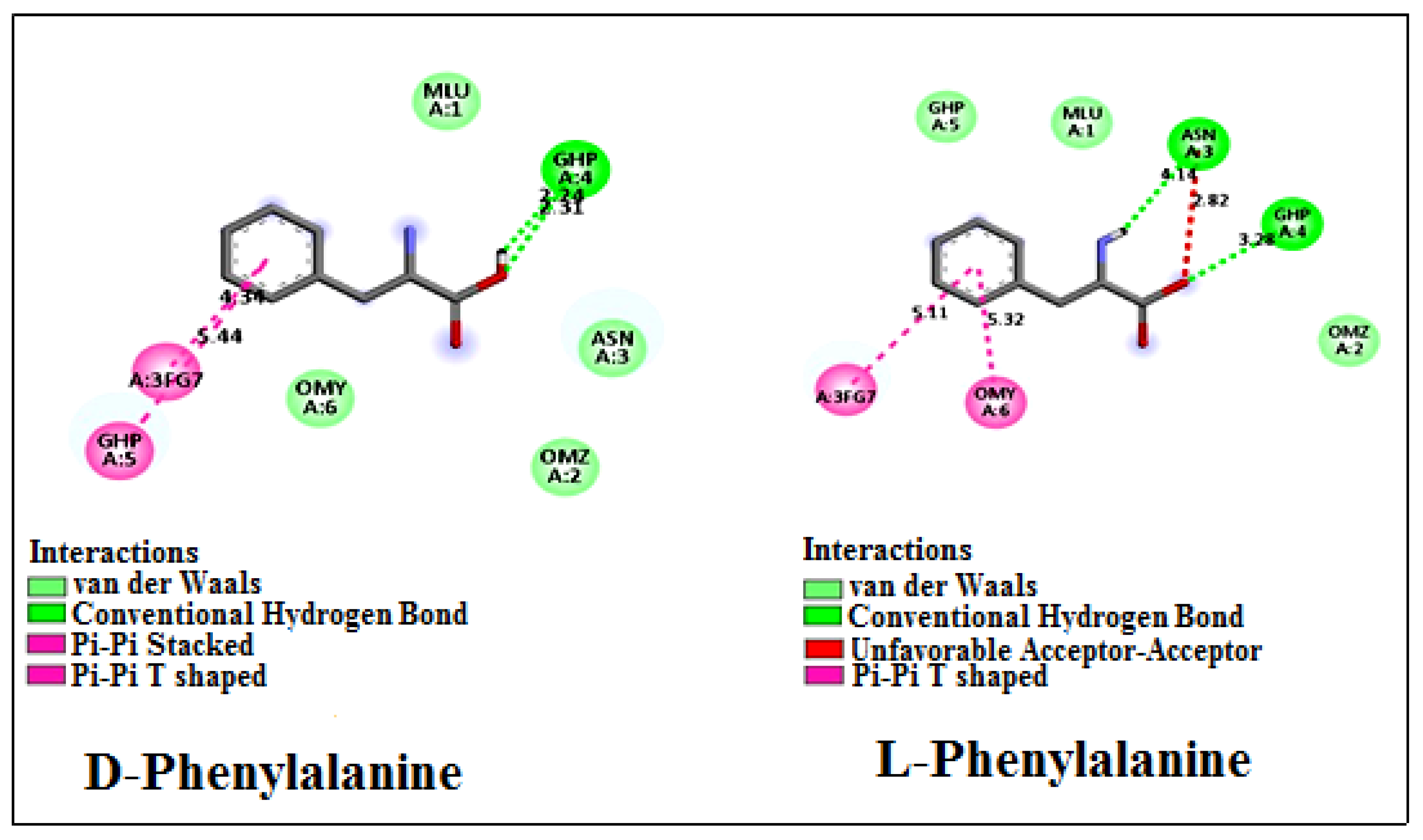
| Amino Acids | Concentration of Chiral Selector (mM) | Retention Factor | Selectivity | Resolution |
|---|---|---|---|---|
| Phenylglycine | 0 | 5.075 | ||
| 0.5 | 5.073 | -------- | -------- | |
| 1.0 | 5.051 | -------- | -------- | |
| 1.5 | 5.023 | -------- | -------- | |
| 2.0 | 5.011 | -------- | -------- | |
| Phenylalanine | 0 | 5.183 | ||
| 0.5 | 5.175 | -------- | -------- | |
| 1.0 | 5.184 | -------- | -------- | |
| 1.5 | 5.197 | -------- | -------- | |
| 2.0 | k1 = 5.153 | 1.01 | -------- | |
| k2 = 5.247 | ||||
| Tryptophan | 0 | 6.99 | ||
| 0.5 | kD = 5.259 | 1.44 | 2.93 | |
| kL = 7.497 | ||||
| 1.0 | kD = 5.201 | 1.45 | 3.09 | |
| kL = 7.552 | ||||
| 1.5 | kD = 5.155 | 1.68 | 3.98 | |
| kL = 8.663 | ||||
| Tyrosine | 0 | 5.035 | -------- | -------- |
| 0.5 | kD = 6.480 | 1.19 | 1.89 | |
| kL = 7.724 | ||||
| 1.0 | kD = 7.180 | 1.25 | 2.04 | |
| kL = 9.041 | ||||
| 1.5 | kD = 8.743 | 1.50 | 2.97 | |
| kL = 13.182 |
| Phenylglycine | Type of Energy (kcal/mol) | ΔGD | ΔGL | |ΔΔG| |
| Estimated Free Energy of Binding | −3.19 | −3.12 | 0.03 | |
| Final Intermolecular Energy | −4.38 | −4.31 | 0.07 | |
| vdW + Hbond + desolv Energy | −4.08 | −3.91 | 0.17 | |
| Electrostatic Energy | −0.30 | −0.40 | 0.10 | |
| Final Total Internal Energy | −1.59 | −1.72 | 0.13 | |
| Torsional Free Energy | +1.19 | +1.19 | ||
| Unbound System’s Energy | −1.59 | −1.72 | 0.13 |
| Tryptophan | Type of Energy (kcal/mol) | ΔGD | ΔGL | |ΔΔG| |
| Estimated Free Energy of Binding | −3.73 | −4.50 | 0.77 | |
| Final Intermolecular Energy | −5.23 | −5.99 | 0.76 | |
| vdW + Hbond + desolv Energy | −4.83 | −5.41 | 0.58 | |
| Electrostatic Energy | −0.40 | −0.58 | 0.18 | |
| Final Total Internal Energy | −2.24 | −1.95 | 0.29 | |
| Torsional Free Energy | +1.49 | +1.49 | ||
| Unbound System’s Energy | −2.24 | −1.95 | 0.29 |
| Tyrosine | Type of Energy (kcal/mol) | ΔGD | ΔGL | |ΔΔG| |
| Estimated Free Energy of Binding | −3.15 | −3.62 | 0.49 | |
| Final Intermolecular Energy | −4.94 | −5.41 | 0.47 | |
| vdW + Hbond + desolv Energy | −4.59 | −5.94 | 0.35 | |
| Electrostatic Energy | −0.35 | −0.47 | 0.12 | |
| Final Total Internal Energy | −2.05 | −1.83 | 0.22 | |
| Torsional Free Energy | +1.49 | +1.49 | ||
| Unbound System’s Energy | −2.05 | −1.83 | 0.22 |
| Phenylalanine | Type of Energy (kcal/mol) | ΔGD | ΔGL | |ΔΔG| |
| Estimated Free Energy of Binding | −3.00 | −3.24 | 0.24 | |
| Final Intermolecular Energy | −4.49 | −4.73 | 0.24 | |
| vdW + Hbond + desolv Energy | −3.68 | −4.34 | 0.66 | |
| Electrostatic Energy | −0.81 | −0.39 | 0.42 | |
| Final Total Internal Energy | −2.15 | −1.92 | 0.23 | |
| Torsional Free Energy | +1.49 | +1.49 | ||
| Unbound System’s Energy | −2.15 | −1.92 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gherdaoui, D.; Yahoum, M.M.; Toumi, S.; Lekmine, S.; Lefnaoui, S.; Benslama, O.; Bouallouche, R.; Tahraoui, H.; Ola, M.S.; Ali, A.; et al. Elucidating Chiral Resolution of Aromatic Amino Acids Using Glycopeptide Selectors: A Combined Molecular Docking and Chromatographic Study. Int. J. Mol. Sci. 2024, 25, 9120. https://doi.org/10.3390/ijms25169120
Gherdaoui D, Yahoum MM, Toumi S, Lekmine S, Lefnaoui S, Benslama O, Bouallouche R, Tahraoui H, Ola MS, Ali A, et al. Elucidating Chiral Resolution of Aromatic Amino Acids Using Glycopeptide Selectors: A Combined Molecular Docking and Chromatographic Study. International Journal of Molecular Sciences. 2024; 25(16):9120. https://doi.org/10.3390/ijms25169120
Chicago/Turabian StyleGherdaoui, Dehbiya, Madiha Melha Yahoum, Selma Toumi, Sabrina Lekmine, Sonia Lefnaoui, Ouided Benslama, Rachida Bouallouche, Hichem Tahraoui, Mohammad Shamsul Ola, Ahmad Ali, and et al. 2024. "Elucidating Chiral Resolution of Aromatic Amino Acids Using Glycopeptide Selectors: A Combined Molecular Docking and Chromatographic Study" International Journal of Molecular Sciences 25, no. 16: 9120. https://doi.org/10.3390/ijms25169120
APA StyleGherdaoui, D., Yahoum, M. M., Toumi, S., Lekmine, S., Lefnaoui, S., Benslama, O., Bouallouche, R., Tahraoui, H., Ola, M. S., Ali, A., Zhang, J., & Amrane, A. (2024). Elucidating Chiral Resolution of Aromatic Amino Acids Using Glycopeptide Selectors: A Combined Molecular Docking and Chromatographic Study. International Journal of Molecular Sciences, 25(16), 9120. https://doi.org/10.3390/ijms25169120











