Macrophages and Gut Barrier Function: Guardians of Gastrointestinal Health in Post-Inflammatory and Post-Infection Responses
Abstract
:1. Introduction
2. Research Gap and Objective
3. Role of Macrophages in Maintaining Gut Barrier Function
3.1. Types of Macrophages
3.2. Functions
3.2.1. Barrier Maintenance
3.2.2. Immune Modulation
4. Post-Inflammatory and Post-Infection Responses
4.1. Inflammation Resolution
4.1.1. Macrophage Phenotype Switch
4.1.2. Potential Side Effects of Modulating Macrophage Activity
4.2. Tissue Repair and Regeneration
Wound Healing
5. Microbial Regulation
Microbiota Restoration and Macrophage–Microbiome Interactions
6. Macrophages in Gastrointestinal Diseases
Infections and Inflammatory Conditions
- Pathogen Clearance:
- Irritable Bowel Syndrome (IBS):
- Inflammatory Bowel Disease (IBD):
7. Molecular Mechanisms
Cytokines and Chemokines
8. Signaling Pathways
| Signaling Pathway | Components | Functions | Implications for IBS |
|---|---|---|---|
| NF-κB Pathway | -NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) | -Regulates transcription of inflammatory cytokines. | -Central in regulating inflammatory responses to microbial stimuli. |
| -TNF-α | -Induces expression of genes involved in immune and inflammatory responses. | -Crucial for mounting effective defenses against pathogens [102,103,104]. | |
| -IL-1β | |||
| MAPK Pathway | -ERK (extracellular signal-regulated kinase) | -Modulates cellular responses to extracellular signals. | -Coordinates immune responses. |
| -JNK (c-Jun N-terminal kinase) | -Regulates processes like proliferation, differentiation, stress responses, and immune activation. | -Maintains gut barrier integrity in response to microbial challenges [105]. | |
| -p38 MAPK | -Contributes to cytokine production and antimicrobial peptide synthesis. | ||
| JAK/STAT Pathway | -JAK (Janus kinases) | -Mediates cytokine signaling. | -Influences macrophage activation and polarization. |
| -STAT (Signal transducers and activators of transcription) | -Regulates gene expression related to immune responses and inflammation. | -Impacts inflammation and immune regulation in the gut [98,99]. | |
| PI3K/Akt Pathway | -PI3K (Phosphoinositide 3-kinases) | -Controls cell survival, growth, and metabolism. | -Modulates macrophage function and survival. |
| -Akt (Protein kinase B) | -Regulates inflammatory responses and cytokine production. | -Affects inflammation and tissue repair processes in the gut [100]. | |
| Toll-Like Receptor (TLR) Pathway | -TLRs (Toll-like receptors) | -Recognizes pathogen-associated molecular patterns (PAMPs). | -Critical for recognizing microbial infections. |
| -MyD88 (Myeloid differentiation primary response 88) | -Activates downstream signaling leading to cytokine production and inflammation. | -Drives inflammation and immune responses in the gut [101]. | |
| -TRIF (TIR-domain-containing adapter-inducing interferon-β) |
9. MicroRNA Regulation
| miRNA | Target Genes | Function | Implications for IBS and IBD |
|---|---|---|---|
| miR-29 | CLDN1, NKRF | Downregulates target genes, increasing intestinal permeability. | Increased intestinal permeability in IBS-D patients [106]. |
| miR-146a | IRAK1, TRAF6 | Negative regulator of NF-κB signaling, attenuating pro-inflammatory responses, and promoting immune homeostasis. | Reduces inflammation and promotes immune balance [107]. |
| miR-155 | SOCS1, SHIP1 | Enhances macrophage activation, amplifies inflammatory responses, and promotes pathogen clearance. | Elevated levels linked to increased inflammation in IBS-D patients [108]. |
| miR-199 | TRPV1 | Upregulates TRPV1 translation when decreased, enhancing visceral pain. | Associated with increased visceral pain in IBS patients [113]. |
| miR-21 | PTEN | Modulates macrophage polarization by targeting PTEN, leading to enhanced Akt signaling. | Promoting an anti-inflammatory M2 phenotype [114]. |
| miR-331-3p | IL-12/IL-23p40 | Suppresses the expression of IL-12/IL-23p40 in macrophages, thereby reducing the production of these cytokines, which are key mediators of inflammation in IBD. | This miRNA plays a protective role by limiting excessive inflammatory responses in the gut [109,110]. |
| let-7d-5p | LGALS3 | LGALS3 silencing or LPS treatment blocked the TLR4/NF-κB signaling pathway. | Exercising its inhibitory properties in the inflammatory response via inactivation of the LGALS3-dependent TLR4/NF-κB signaling pathway [109,112,118]. |
9.1. Therapeutic Implications
9.2. Microbiota-Based Therapies
9.3. Current Challenges and Future Directions
9.4. Precision Medicine Approaches
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zhou, Q.; Verne, M.L.; Fields, J.Z.; Lefante, J.J.; Basra, S.; Salameh, H.; Verne, G.N. Randomised placebo-controlled trial of dietary glutamine supplements for postinfectious irritable bowel syndrome. Gut 2019, 68, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Bauche, D.; Joyce-Shaikh, B.; Jain, R.; Grein, J.; Ku, K.S.; Blumenschein, W.M.; Ganal-Vonarburg, S.C.; Wilson, D.C.; McClanahan, T.K.; Malefyt, R.W.; et al. LAG3+ Regulatory T Cells Restrain Interleukin-23-Producing CX3CR1+ Gut-Resident Macrophages during Group 3 Innate Lymphoid Cell-Driven Colitis. Immunity 2018, 49, 342–352.e5. [Google Scholar] [CrossRef] [PubMed]
- Coombes, J.L.; Powrie, F. Dendritic cells in intestinal immune regulation. Nat. Rev. Immunol. 2008, 8, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.C.; Mowat, A.M. The monocyte-macrophage axis in the intestine. Cell. Immunol. 2014, 291, 41–48. [Google Scholar] [CrossRef]
- Zhou, Q.; Wesselmann, U.; Walker, L.; Lee, L.; Zeltzer, L.; Verne, G.N. AAPT Diagnostic Criteria for Chronic Abdominal, Pelvic, and Urogenital Pain: Irritable Bowel Syndrome. J. Pain 2018, 19, 257–263. [Google Scholar] [CrossRef]
- Ra, Y.E.; Bang, Y.J. Balancing Act of the Intestinal Antimicrobial Proteins on Gut Microbiota and Health. J. Microbiol. 2024, 62, 167–179. [Google Scholar] [CrossRef]
- Horowitz, A.; Chanez-Paredes, S.D.; Haest, X.; Turner, J.R. Paracellular permeability and tight junction regulation in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 417–432. [Google Scholar] [CrossRef]
- Cornick, S.; Tawiah, A.; Chadee, K. Roles and regulation of the mucus barrier in the gut. Tissue Barriers 2015, 3, e982426. [Google Scholar] [CrossRef]
- Bain, C.C.; Scott, C.L.; Uronen-Hansson, H.; Gudjonsson, S.; Jansson, O.; Grip, O.; Guilliams, M.; Malissen, B.; Agace, W.W.; Mowat, A.M. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013, 6, 498–510. [Google Scholar] [CrossRef]
- Chen, S.; Saeed, A.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, e2004433. [Google Scholar] [CrossRef]
- Maciel-Fiuza, M.F.; Muller, G.C.; Campos, D.M.S.; do Socorro Silva Costa, P.; Peruzzo, J.; Bonamigo, R.R.; Veit, T.; Vianna, F.S.L. Role of gut microbiota in infectious and inflammatory diseases. Front. Microbiol. 2023, 14, 1098386. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Ryu, G.; Shin, S.A.; An, J.; Kim, H.; Park, D.; Lee, D.H.; Lee, C.S. Physiological Roles of Apoptotic Cell Clearance: Beyond Immune Functions. Life 2021, 11, 1141. [Google Scholar] [CrossRef] [PubMed]
- Carlini, V.; Noonan, D.M.; Abdalalem, E.; Goletti, D.; Sansone, C.; Calabrone, L.; Albini, A. The multifaceted nature of IL-10: Regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front. Immunol. 2023, 14, 1161067. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Ruder, B.; Becker, C. At the Forefront of the Mucosal Barrier: The Role of Macrophages in the Intestine. Cells 2020, 9, 2162. [Google Scholar] [CrossRef]
- Sawicki, W.; Kucharczyk, K.; Szymanska, K.; Kujawa, M. Lamina propria macrophages of intestine of the guinea pig. Possible role in phagocytosis of migrating cells. Gastroenterology 1977, 73, 1340–1344. [Google Scholar] [CrossRef]
- Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, S.; Zhang, H.; Jo, Y.; Kang, J.S.; Ha, K.T.; Joo, J.; Lee, H.J.; Ryu, D. Gut microbiota-generated metabolites: Missing puzzles to hosts’ health, diseases, and aging. BMB Rep. 2024, 57, 207–215. [Google Scholar] [CrossRef]
- Wickramasuriya, S.S.; Park, I.; Lee, K.; Lee, Y.; Kim, W.H.; Nam, H.; Lillehoj, H.S. Role of Physiology, Immunity, Microbiota, and Infectious Diseases in the Gut Health of Poultry. Vaccines 2022, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Yip, J.L.K.; Balasuriya, G.K.; Spencer, S.J.; Hill-Yardin, E.L. The Role of Intestinal Macrophages in Gastrointestinal Homeostasis: Heterogeneity and Implications in Disease. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Ni, R.; Jiang, L.; Zhang, C.; Liu, M.; Luo, Y.; Hu, Z.; Mou, X.; Zhu, Y. Biologic Mechanisms of Macrophage Phenotypes Responding to Infection and the Novel Therapies to Moderate Inflammation. Int. J. Mol. Sci. 2023, 24, 8358. [Google Scholar] [CrossRef] [PubMed]
- Mowat, A.M.; Bain, C.C. Mucosal macrophages in intestinal homeostasis and inflammation. J. Innate Immun. 2011, 3, 550–564. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, D.; Iida, T.; Nakase, H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef]
- Ross, E.A.; Devitt, A.; Johnson, J.R. Macrophages: The Good, the Bad, and the Gluttony. Front. Immunol. 2021, 12, 708186. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Lo, B.C.; Chen, G.Y.; Nunez, G.; Caruso, R. Gut microbiota and systemic immunity in health and disease. Int. Immunol. 2021, 33, 197–209. [Google Scholar] [CrossRef]
- Grainger, J.R.; Konkel, J.E.; Zangerle-Murray, T.; Shaw, T.N. Macrophages in gastrointestinal homeostasis and inflammation. Pflugers Arch. 2017, 469, 527–539. [Google Scholar] [CrossRef]
- Yip, J.L.K.; Balasuriya, G.K.; Hill-Yardin, E.L.; Spencer, S.J. The gut-brain and gut-macrophage contribution to gastrointestinal dysfunction with systemic inflammation. Brain Behav. Immun. 2024, 119, 867–877. [Google Scholar] [CrossRef]
- Wang, S.; Ye, Q.; Zeng, X.; Qiao, S. Functions of Macrophages in the Maintenance of Intestinal Homeostasis. J. Immunol. Res. 2019, 2019, 1512969. [Google Scholar] [CrossRef]
- Mosser, D.M.; Hamidzadeh, K.; Goncalves, R. Macrophages and the maintenance of homeostasis. Cell. Mol. Immunol. 2021, 18, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Mischopoulou, M.; D’Ambrosio, M.; Bigagli, E.; Luceri, C.; Farrugia, G.; Cipriani, G. Role of Macrophages and Mast Cells as Key Players in the Maintenance of Gastrointestinal Smooth Muscle Homeostasis and Disease. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 1849–1862. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yang, J.; Wei, Y.; Wei, X. Epigenetic regulation of macrophages: From homeostasis maintenance to host defense. Cell Mol. Immunol. 2020, 17, 36–49. [Google Scholar] [CrossRef]
- D’Angelo, F.; Bernasconi, E.; Schafer, M.; Moyat, M.; Michetti, P.; Maillard, M.H.; Velin, D. Macrophages promote epithelial repair through hepatocyte growth factor secretion. Clin. Exp. Immunol. 2013, 174, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Acciani, T.H.; Suzuki, T.; Trapnell, B.C.; Le Cras, T.D. Epidermal growth factor receptor signalling regulates granulocyte-macrophage colony-stimulating factor production by airway epithelial cells and established allergic airway disease. Clin. Exp. Allergy 2016, 46, 317–328. [Google Scholar] [CrossRef]
- Morhardt, T.L.; Hayashi, A.; Kao, J.Y.; Kamada, N. Regional control of regulatory immune cells in the intestine. Curr. Pathobiol. Rep. 2018, 6, 29–34. [Google Scholar] [CrossRef]
- Tang, X.; Liu, H.; Yang, S.; Li, Z.; Zhong, J.; Fang, R. Epidermal Growth Factor and Intestinal Barrier Function. Mediat. Inflamm. 2016, 2016, 1927348. [Google Scholar] [CrossRef]
- Tang, X.; Xiong, K. Dietary Epidermal Growth Factor Supplementation Alleviates Intestinal Injury in Piglets with Intrauterine Growth Retardation via Reducing Oxidative Stress and Enhancing Intestinal Glucose Transport and Barrier Function. Animals 2022, 12, 2245. [Google Scholar] [CrossRef]
- Yang, J.; Radulescu, A.; Chen, C.L.; Zhang, H.Y.; James, I.O.; Besner, G.E. Heparin-binding epidermal growth factor-like growth factor improves intestinal barrier function and reduces mortality in a murine model of peritonitis. Surgery 2013, 153, 52–62. [Google Scholar] [CrossRef]
- Kahata, K.; Dadras, M.S.; Moustakas, A. TGF-beta Family Signaling in Epithelial Differentiation and Epithelial-Mesenchymal Transition. Cold Spring Harb. Perspect. Biol. 2018, 10, a022194. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-beta and the TGF-beta Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb. Perspect. Biol. 2016, 8, a021873. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, F.; Balas, I.; Robinson, M.J.; Bakdash, G. Border Control: The Role of the Microbiome in Regulating Epithelial Barrier Function. Cells 2024, 13, 477. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef] [PubMed]
- Oral, H.B.; Kotenko, S.V.; Yilmaz, M.; Mani, O.; Zumkehr, J.; Blaser, K.; Akdis, C.A.; Akdis, M. Regulation of T cells and cytokines by the interleukin-10 (IL-10)-family cytokines IL-19, IL-20, IL-22, IL-24 and IL-26. Eur. J. Immunol. 2006, 36, 380–388. [Google Scholar] [CrossRef]
- Yu, Y.; Yue, Z.; Xu, M.; Zhang, M.; Shen, X.; Ma, Z.; Li, J.; Xie, X. Macrophages play a key role in tissue repair and regeneration. PeerJ 2022, 10, e14053. [Google Scholar] [CrossRef]
- Han, X.; Ding, S.; Jiang, H.; Liu, G. Roles of Macrophages in the Development and Treatment of Gut Inflammation. Front. Cell Dev. Biol. 2021, 9, 625423. [Google Scholar] [CrossRef]
- Zigmond, E.; Bernshtein, B.; Friedlander, G.; Walker, C.R.; Yona, S.; Kim, K.W.; Brenner, O.; Krauthgamer, R.; Varol, C.; Muller, W.; et al. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity 2014, 40, 720–733. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, M.; Jia, S. Macrophage: Key player in the pathogenesis of autoimmune diseases. Front. Immunol. 2023, 14, 1080310. [Google Scholar] [CrossRef]
- Sun, L.; Su, Y.; Jiao, A.; Wang, X.; Zhang, B. T cells in health and disease. Signal Transduct. Target. Ther. 2023, 8, 235. [Google Scholar]
- Fox, P.C.; Brennan, M.; Di Sun, P. Cytokine expression in human labial minor salivary gland epithelial cells in health and disease. Arch. Oral Biol. 1999, 44 (Suppl. S1), S49–S52. [Google Scholar] [CrossRef] [PubMed]
- Dharmasiri, S.; Garrido-Martin, E.M.; Harris, R.J.; Bateman, A.C.; Collins, J.E.; Cummings, J.R.F.; Sanchez-Elsner, T. Human Intestinal Macrophages Are Involved in the Pathology of Both Ulcerative Colitis and Crohn Disease. Inflamm. Bowel Dis. 2021, 27, 1641–1652. [Google Scholar] [CrossRef] [PubMed]
- Al-Qahtani, A.A.; Alhamlan, F.S. Pro-Inflammatory and Anti-Inflammatory Interleukins in Infectious Diseases: A Comprehensive Review. Trop. Med. Infect. Dis. 2024, 9, 13. [Google Scholar] [CrossRef]
- Viola, A.; Munari, F.; Sanchez-Rodriguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Y.; Wang, N.; Li, S.; Hong, M.; Wang, X.; Feng, Y. The Reactive Oxygen Species in Macrophage Polarization: Reflecting Its Dual Role in Progression and Treatment of Human Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 2795090. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, Y.; Yi, J.; Zhao, Z.; Ye, R. Hyperglycemia modulates M1/M2 macrophage polarization via reactive oxygen species overproduction in ligature-induced periodontitis. J. Periodontal Res. 2021, 56, 991–1005. [Google Scholar] [CrossRef]
- Griess, B.; Mir, S.; Datta, K.; Teoh-Fitzgerald, M. Scavenging reactive oxygen species selectively inhibits M2 macrophage polarization and their pro-tumorigenic function in part, via Stat3 suppression. Free Radic. Biol. Med. 2020, 147, 48–60. [Google Scholar] [CrossRef]
- Mendoza-Coronel, E.; Ortega, E. Macrophage Polarization Modulates FcgammaR- and CD13-Mediated Phagocytosis and Reactive Oxygen Species Production, Independently of Receptor Membrane Expression. Front. Immunol. 2017, 8, 303. [Google Scholar] [CrossRef] [PubMed]
- Strizova, Z.; Benesova, I.; Bartolini, R.; Novysedlak, R.; Cecrdlova, E.; Foley, L.K.; Striz, I. M1/M2 macrophages and their overlaps—Myth or reality? Clin. Sci. 2023, 137, 1067–1093. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Bain, C.C.; Mowat, A.M. Macrophages in intestinal homeostasis and inflammation. Immunol. Rev. 2014, 260, 102–117. [Google Scholar] [CrossRef]
- Mamilos, A.; Winter, L.; Schmitt, V.H.; Barsch, F.; Grevenstein, D.; Wagner, W.; Babel, M.; Keller, K.; Schmitt, C.; Gurtler, F.; et al. Macrophages: From Simple Phagocyte to an Integrative Regulatory Cell for Inflammation and Tissue Regeneration—A Review of the Literature. Cells 2023, 12, 276. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Jung, S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 392–404. [Google Scholar] [CrossRef]
- Guo, L.; Akahori, H.; Harari, E.; Smith, S.L.; Polavarapu, R.; Karmali, V.; Otsuka, F.; Gannon, R.L.; Braumann, R.E.; Dickinson, M.H.; et al. CD163+ macrophages promote angiogenesis and vascular permeability accompanied by inflammation in atherosclerosis. J. Clin. Investig. 2018, 128, 1106–1124. [Google Scholar] [CrossRef] [PubMed]
- Laviv, Y.; Kasper, B.; Kasper, E.M. Vascular hyperpermeability as a hallmark of phacomatoses: Is the etiology angiogenesis related to or comparable with mechanisms seen in inflammatory pathways? Part II: Angiogenesis- and inflammation-related molecular pathways, tumor-associated macrophages, and possible therapeutic implications: A comprehensive review. Neurosurg. Rev. 2018, 41, 931–944. [Google Scholar]
- Burnett, G.T.; Weathersby, D.C.; Taylor, T.E.; Bremner, T.A. Regulation of inflammation- and angiogenesis-related gene expression in breast cancer cells and co-cultured macrophages. Anticancer Res. 2008, 28, 2093–2099. [Google Scholar]
- Guo, N.; Lv, L.L. Mechanistic insights into the role of probiotics in modulating immune cells in ulcerative colitis. Immun. Inflamm. Dis. 2023, 11, e1045. [Google Scholar] [CrossRef]
- Mass, E.; Nimmerjahn, F.; Kierdorf, K.; Schlitzer, A. Tissue-specific macrophages: How they develop and choreograph tissue biology. Nat. Rev. Immunol. 2023, 23, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Kang, X.; Xue, H.; Yin, H. Toll-like receptor 4 is up-regulated by mTOR activation during THP-1 macrophage foam cells formation. Acta Biochim. Biophys. Sin. 2011, 43, 940–947. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Mei, J.X.; Yu, G.; Lei, L.; Zhang, W.H.; Liu, K.; Chen, X.L.; Kolat, D.; Yang, K.; Hu, J.K. Role of the gut microbiota in anticancer therapy: From molecular mechanisms to clinical applications. Signal Transduct. Target. Ther. 2023, 8, 201. [Google Scholar] [CrossRef]
- Amamou, A.; O’Mahony, C.; Leboutte, M.; Savoye, G.; Ghosh, S.; Marion-Letellier, R. Gut Microbiota, Macrophages and Diet: An Intriguing New Triangle in Intestinal Fibrosis. Microorganisms 2022, 10, 490. [Google Scholar] [CrossRef] [PubMed]
- Ney, L.M.; Wipplinger, M.; Grossmann, M.; Engert, N.; Wegner, V.D.; Mosig, A.S. Short chain fatty acids: Key regulators of the local and systemic immune response in inflammatory diseases and infections. Open Biol. 2023, 13, 230014. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Wang, L.; Huangfu, M.; Li, H. The impact of microbiota-derived short-chain fatty acids on macrophage activities in disease: Mechanisms and therapeutic potentials. Biomed. Pharmacother. 2023, 165, 115276. [Google Scholar] [CrossRef]
- Al-Qadami, G.H.; Secombe, K.R.; Subramaniam, C.B.; Wardill, H.R.; Bowen, J.M. Gut Microbiota-Derived Short-Chain Fatty Acids: Impact on Cancer Treatment Response and Toxicities. Microorganisms 2022, 10, 2048. [Google Scholar] [CrossRef] [PubMed]
- Gravina, A.G.; Pellegrino, R.; Auletta, S.; Palladino, G.; Brandimarte, G.; D’Onofrio, R.; Arboretto, G.; Imperio, G.; Ventura, A.; Cipullo, M.; et al. Hericium erinaceus, a medicinal fungus with a centuries-old history: Evidence in gastrointestinal diseases. World J. Gastroenterol. 2023, 29, 3048–3065. [Google Scholar] [CrossRef]
- Priori, E.C.; Ratto, D.; De Luca, F.; Sandionigi, A.; Savino, E.; Giammello, F.; Romeo, M.; Brandalise, F.; Roda, E.; Rossi, P. Hericium erinaceus Extract Exerts Beneficial Effects on Gut-Neuroinflammaging-Cognitive Axis in Elderly Mice. Biology 2023, 13, 18. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. Control of Phagocytosis by Microbial Pathogens. Front. Immunol. 2017, 8, 1368. [Google Scholar] [CrossRef]
- Shiner, M.; Fuhrman, B.; Aviram, M. Macrophage paraoxonase 2 (PON2) expression is up-regulated by pomegranate juice phenolic anti-oxidants via PPAR gamma and AP-1 pathway activation. Atherosclerosis 2007, 195, 313–321. [Google Scholar] [CrossRef]
- Zhou, Q.; Verne, G.N. New insights into visceral hypersensitivity—Clinical implications in IBS. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 349–355. [Google Scholar] [CrossRef]
- Verne, G.N.; Price, D.D.; Callam, C.S.; Zhang, B.; Peck, J.; Zhou, Q. Viscerosomatic facilitation in a subset of IBS patients, an effect mediated by N-methyl-D-aspartate receptors. J. Pain 2012, 13, 901–909. [Google Scholar] [CrossRef]
- Li, M.; Hou, Q.; Zhong, L.; Zhao, Y.; Fu, X. Macrophage Related Chronic Inflammation in Non-Healing Wounds. Front. Immunol. 2021, 12, 681710. [Google Scholar] [CrossRef]
- Yang, J.; Ran, Y.; Yang, Y.; Song, S.; Wu, Y.; Qi, Y.; Gao, Y.; Li, G. 4-Hydroxyisoleucine Alleviates Macrophage-Related Chronic Inflammation and Metabolic Syndrome in Mice Fed a High-Fat Diet. Front. Pharmacol. 2020, 11, 606514. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Cristiani, C.M.; Ilari, S.; Passacatini, L.C.; Malafoglia, V.; Viglietto, G.; Maiuolo, J.; Oppedisano, F.; Palma, E.; Tomino, C.; et al. Fibromyalgia and Irritable Bowel Syndrome Interaction: A Possible Role for Gut Microbiota and Gut-Brain Axis. Biomedicines 2023, 11, 1701. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Ruan, G.; Chen, L.; Ying, S.; Li, G.; Xu, F.; Xiao, Z.; Tian, Y.; Lv, L.; Ping, Y.; et al. Neurotransmitter and Intestinal Interactions: Focus on the Microbiota-Gut-Brain Axis in Irritable Bowel Syndrome. Front. Endocrinol. 2022, 13, 817100. [Google Scholar] [CrossRef]
- Hillestad, E.M.R.; van der Meeren, A.; Nagaraja, B.H.; Bjorsvik, B.R.; Haleem, N.; Benitez-Paez, A.; Sanz, Y.; Hausken, T.; Lied, G.A.; Lundervold, A.; et al. Gut bless you: The microbiota-gut-brain axis in irritable bowel syndrome. World J. Gastroenterol. 2022, 28, 412–431. [Google Scholar] [CrossRef]
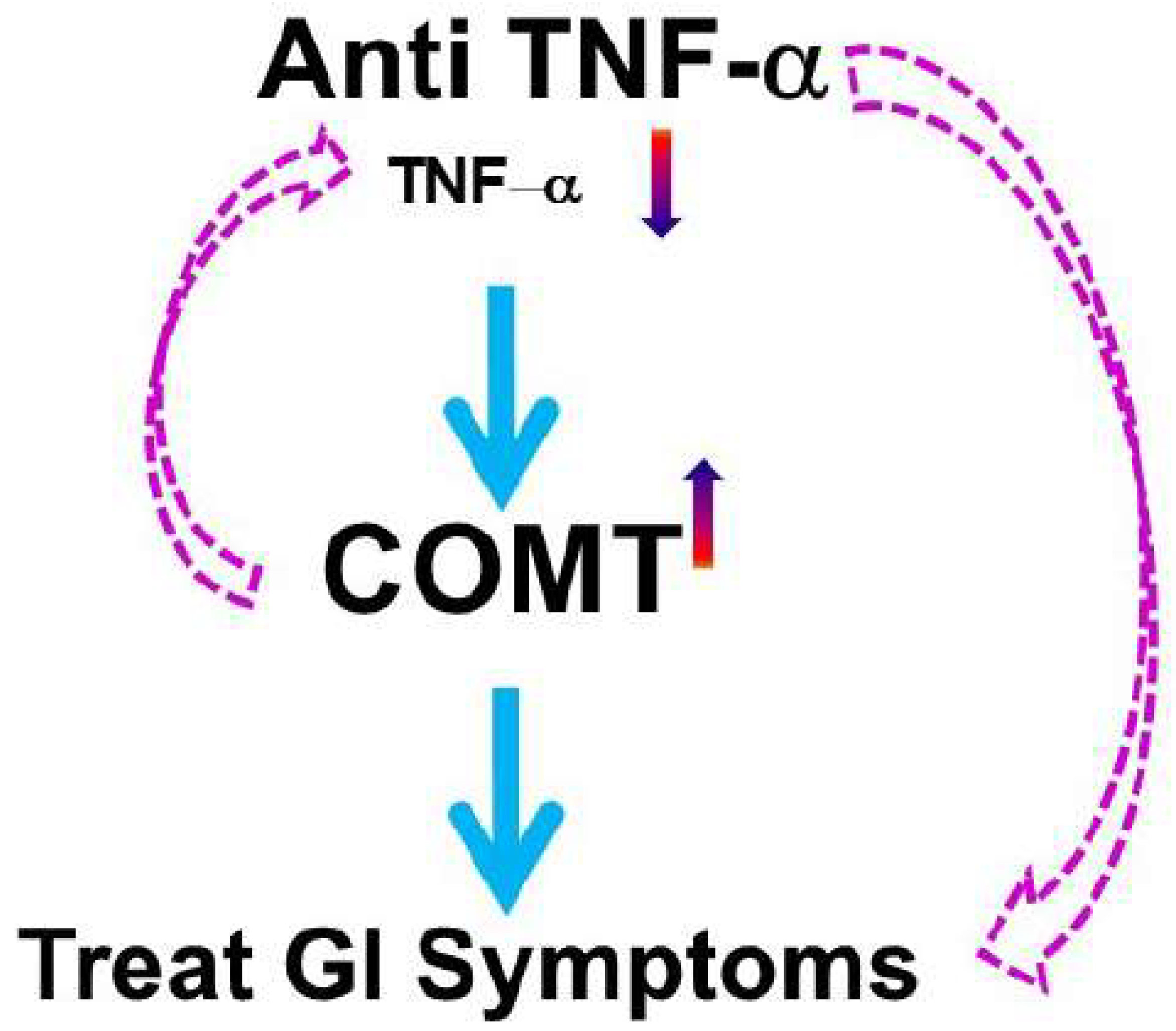
- Zhou, Q.; Yang, L.; Verne, M.L.; Zhang, B.B.; Fields, J.; Verne, G.N. Catechol-O-Methyltransferase Loss Drives Cell-Specific Nociceptive Signaling via the Enteric Catechol-O-Methyltransferase/microRNA-155/Tumor Necrosis Factor alpha Axis. Gastroenterology 2023, 164, 630–641.e34. [Google Scholar] [CrossRef] [PubMed]
- Saez, A.; Herrero-Fernandez, B.; Gomez-Bris, R.; Sanchez-Martinez, H.; Gonzalez-Granado, J.M. Pathophysiology of Inflammatory Bowel Disease: Innate Immune System. Int. J. Mol. Sci. 2023, 24, 1526. [Google Scholar] [CrossRef]
- Padoan, A.; Musso, G.; Contran, N.; Basso, D. Inflammation, Autoinflammation and Autoimmunity in Inflammatory Bowel Diseases. Curr. Issues Mol. Biol. 2023, 45, 5534–5557. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, J.; Liu, H.; Li, S.; Wang, Q. The Role of Tissue-Resident Macrophages in the Development and Treatment of Inflammatory Bowel Disease. Front. Cell Dev. Biol. 2022, 10, 896591. [Google Scholar] [CrossRef]
- Wang, E.J.; Wu, M.Y.; Ren, Z.Y.; Zheng, Y.; Ye, R.D.; Tan, C.S.H.; Wang, Y.; Lu, J.H. Targeting macrophage autophagy for inflammation resolution and tissue repair in inflammatory bowel disease. Burns Trauma 2023, 11, tkad004. [Google Scholar] [CrossRef]
- Laha, D.; Grant, R.; Mishra, P.; Nilubol, N. The Role of Tumor Necrosis Factor in Manipulating the Immunological Response of Tumor Microenvironment. Front. Immunol. 2021, 12, 656908. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Alexander, M.; Misharin, A.V.; Budinger, G.R.S. The role of macrophages in the resolution of inflammation. J. Clin. Investig. 2019, 129, 2619–2628. [Google Scholar] [CrossRef]
- Kataru, R.P.; Jung, K.; Jang, C.; Yang, H.; Schwendener, R.A.; Baik, J.E.; Han, S.H.; Alitalo, K.; Koh, G.Y. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood 2009, 113, 5650–5659. [Google Scholar] [CrossRef] [PubMed]
- Smythies, L.E.; Shen, R.; Bimczok, D.; Novak, L.; Clements, R.H.; Eckhoff, D.E.; Bouchard, P.; George, M.D.; Hu, W.K.; Dandekar, S.; et al. Inflammation anergy in human intestinal macrophages is due to Smad-induced IkappaBalpha expression and NF-kappaB inactivation. J. Biol. Chem. 2010, 285, 19593–19604. [Google Scholar] [CrossRef]
- Lai, J.L.; Liu, Y.H.; Liu, C.; Qi, M.P.; Liu, R.N.; Zhu, X.F.; Zhou, Q.G.; Chen, Y.Y.; Guo, A.Z.; Hu, C.M. Indirubin Inhibits LPS-Induced Inflammation via TLR4 Abrogation Mediated by the NF-kB and MAPK Signaling Pathways. Inflammation 2017, 40, 1–12. [Google Scholar] [CrossRef]
- Pua, L.J.W.; Mai, C.W.; Chung, F.F.; Khoo, A.S.; Leong, C.O.; Lim, W.M.; Hii, L.W. Functional Roles of JNK and p38 MAPK Signaling in Nasopharyngeal Carcinoma. Int. J. Mol. Sci. 2022, 23, 1108. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Bian, Q.; Rong, D.; Wang, L.; Song, J.; Huang, H.S.; Zeng, J.; Mei, J.; Wang, P.Y. JAK/STAT pathway: Extracellular signals, diseases, immunity, and therapeutic regimens. Front. Bioeng. Biotechnol. 2023, 11, 1110765. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- De-Leon-Lopez, Y.S.; Thompson, M.E.; Kean, J.J.; Flaherty, R.A. The PI3K-Akt pathway is a multifaceted regulator of the macrophage response to diverse group B Streptococcus isolates. Front. Cell Infect. Microbiol. 2023, 13, 1258275. [Google Scholar] [CrossRef]
- Hug, H.; Mohajeri, M.H.; La Fata, G. Toll-Like Receptors: Regulators of the Immune Response in the Human Gut. Nutrients 2018, 10, 203. [Google Scholar] [CrossRef]
- Dorrington, M.G.; Fraser, I.D.C. NF-kappaB Signaling in Macrophages: Dynamics, Crosstalk, and Signal Integration. Front. Immunol. 2019, 10, 705. [Google Scholar] [CrossRef] [PubMed]
- Mussbacher, M.; Derler, M.; Basilio, J.; Schmid, J.A. NF-kappaB in monocytes and macrophages—An inflammatory master regulator in multitalented immune cells. Front. Immunol. 2023, 14, 1134661. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-kappaB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, M.; Wen, Y.; Zhang, H.; Zhao, G.N.; Gao, Q. Signaling pathways in macrophages: Molecular mechanisms and therapeutic targets. MedComm 2023, 4, e349. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Costinean, S.; Croce, C.M.; Brasier, A.R.; Merwat, S.; Larson, S.A.; Basra, S.; Verne, G.N. MicroRNA 29 targets nuclear factor-kappaB-repressing factor and Claudin 1 to increase intestinal permeability. Gastroenterology 2015, 148, 158–169.e8. [Google Scholar] [CrossRef]
- Fan, W.; Liang, C.; Ou, M.; Zou, T.; Sun, F.; Zhou, H.; Cui, L. MicroRNA-146a Is a Wide-Reaching Neuroinflammatory Regulator and Potential Treatment Target in Neurological Diseases. Front. Mol. Neurosci. 2020, 13, 90. [Google Scholar] [CrossRef]
- Sprenkle, N.T.; Serezani, C.H.; Pua, H.H. MicroRNAs in Macrophages: Regulators of Activation and Function. J. Immunol. 2023, 210, 359–368. [Google Scholar] [CrossRef]
- Chacon-Millan, P.; Lama, S.; Del Gaudio, N.; Gravina, A.G.; Federico, A.; Pellegrino, R.; Luce, A.; Altucci, L.; Facchiano, A.; Caraglia, M.; et al. A Combination of Microarray-Based Profiling and Biocomputational Analysis Identified miR331-3p and hsa-let-7d-5p as Potential Biomarkers of Ulcerative Colitis Progression to Colorectal Cancer. Int. J. Mol. Sci. 2024, 25, 5699. [Google Scholar] [CrossRef]
- Rao, J.; Shao, L.; Lin, M.; Huang, J.; Fan, L. LncRNA UCA1 Accelerates the Progression of Ulcerative Colitis via Mediating the miR-331-3p/BRD4 Axis. Int. J. Gen. Med. 2021, 14, 2427–2435. [Google Scholar] [CrossRef]
- Lightbody, R.J.; Taylor, J.M.W.; Dempsie, Y.; Graham, A. Induction of microRNA hsa-let-7d-5p, and repression of HMGA2, contribute protection against lipid accumulation in macrophage ‘foam’ cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 159005. [Google Scholar] [CrossRef]
- Altaf-Ul-Amin, M.; Karim, M.B.; Hu, P.; Ono, N.; Kanaya, S. Discovery of inflammatory bowel disease-associated miRNAs using a novel bipartite clustering approach. BMC Med. Genom. 2020, 13 (Suppl. S3), 10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yang, L.; Larson, S.; Basra, S.; Merwat, S.; Tan, A.; Croce, C.; Verne, G.N. Decreased miR-199 augments visceral pain in patients with IBS through translational upregulation of TRPV1. Gut 2016, 65, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X.; Zhang, D.; Sun, X.; Wu, Y.; Wang, J.; Li, Q.; Jiang, G. The macrophage polarization by miRNAs and its potential role in the treatment of tumor and inflammation (Review). Oncol. Rep. 2023, 50, 190. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.T.; Wang, J.; Lu, W.; Cao, Y.; Cai, W. Downregulated expression of microRNA-124 in pediatric intestinal failure patients modulates macrophages activation by inhibiting STAT3 and AChE. Cell Death Dis. 2016, 7, e2521. [Google Scholar] [CrossRef]
- Koukos, G.; Polytarchou, C.; Kaplan, J.L.; Morley-Fletcher, A.; Gras-Miralles, B.; Kokkotou, E.; Baril-Dore, M.; Pothoulakis, C.; Winter, H.S.; Iliopoulos, D. MicroRNA-124 regulates STAT3 expression and is down-regulated in colon tissues of pediatric patients with ulcerative colitis. Gastroenterology 2013, 145, 842–852.e2. [Google Scholar] [CrossRef]
- Jiao, P.; Wang, X.P.; Luoreng, Z.M.; Yang, J.; Jia, L.; Ma, Y.; Wei, D.W. miR-223: An Effective Regulator of Immune Cell Differentiation and Inflammation. Int. J. Biol. Sci. 2021, 17, 2308–2322. [Google Scholar] [CrossRef]
- Sun, L.; Sun, M.; Ma, K.; Liu, J. Let-7d-5p suppresses inflammatory response in neonatal rats with necrotizing enterocolitis via LGALS3-mediated TLR4/NF-kappaB signaling pathway. Am. J. Physiol. Cell Physiol. 2020, 319, C967–C979. [Google Scholar] [CrossRef]
- Bai, J.; Wang, Y.; Li, F.; Wu, Y.; Chen, J.; Li, M.; Wang, X.; Lv, B. Research advancements and perspectives of inflammatory bowel disease: A comprehensive review. Sci. Prog. 2024, 107, 368504241253709. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, C.; Patil, S.; Dong, S. Unveiling the therapeutic symphony of probiotics, prebiotics, and postbiotics in gut-immune harmony. Front. Nutr. 2024, 11, 1355542. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Ma, Y.; Yan, B.; Pei, W.; Wu, Q.; Ding, C.; Huang, C. The promotion mechanism of prebiotics for probiotics: A review. Front. Nutr. 2022, 9, 1000517. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, N.; Su, X.; Gao, Y.; Yang, R. Gut-Microbiota-Derived Metabolites Maintain Gut and Systemic Immune Homeostasis. Cells 2023, 12, 793. [Google Scholar] [CrossRef]
- Shin, A.; Kashyap, P.C. Multi-omics for biomarker approaches in the diagnostic evaluation and management of abdominal pain and irritable bowel syndrome: What lies ahead. Gut Microbes 2023, 15, 2195792. [Google Scholar] [CrossRef]
- Goetz, L.H.; Schork, N.J. Personalized medicine: Motivation, challenges, and progress. Fertil. Steril. 2018, 109, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Cai, Y.; Yao, H.; Lin, C.; Xie, Y.; Tang, S.; Zhang, A. Small molecule metabolites: Discovery of biomarkers and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 132. [Google Scholar] [CrossRef]
- He, X.; Tan, S.; Shao, Z.; Wang, X. Latitudinal and longitudinal regulation of tissue macrophages in inflammatory diseases. Genes Dis. 2022, 9, 1194–1207. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, E.X.; Verne, G.N.; Zhou, Q. Macrophages and Gut Barrier Function: Guardians of Gastrointestinal Health in Post-Inflammatory and Post-Infection Responses. Int. J. Mol. Sci. 2024, 25, 9422. https://doi.org/10.3390/ijms25179422
Meng EX, Verne GN, Zhou Q. Macrophages and Gut Barrier Function: Guardians of Gastrointestinal Health in Post-Inflammatory and Post-Infection Responses. International Journal of Molecular Sciences. 2024; 25(17):9422. https://doi.org/10.3390/ijms25179422
Chicago/Turabian StyleMeng, Edward Xiangtai, George Nicholas Verne, and Qiqi Zhou. 2024. "Macrophages and Gut Barrier Function: Guardians of Gastrointestinal Health in Post-Inflammatory and Post-Infection Responses" International Journal of Molecular Sciences 25, no. 17: 9422. https://doi.org/10.3390/ijms25179422
APA StyleMeng, E. X., Verne, G. N., & Zhou, Q. (2024). Macrophages and Gut Barrier Function: Guardians of Gastrointestinal Health in Post-Inflammatory and Post-Infection Responses. International Journal of Molecular Sciences, 25(17), 9422. https://doi.org/10.3390/ijms25179422




