Phenotypic, Metabolic and Genetic Adaptations of the Ficus Species to Abiotic Stress Response: A Comprehensive Review
Abstract
1. Introduction
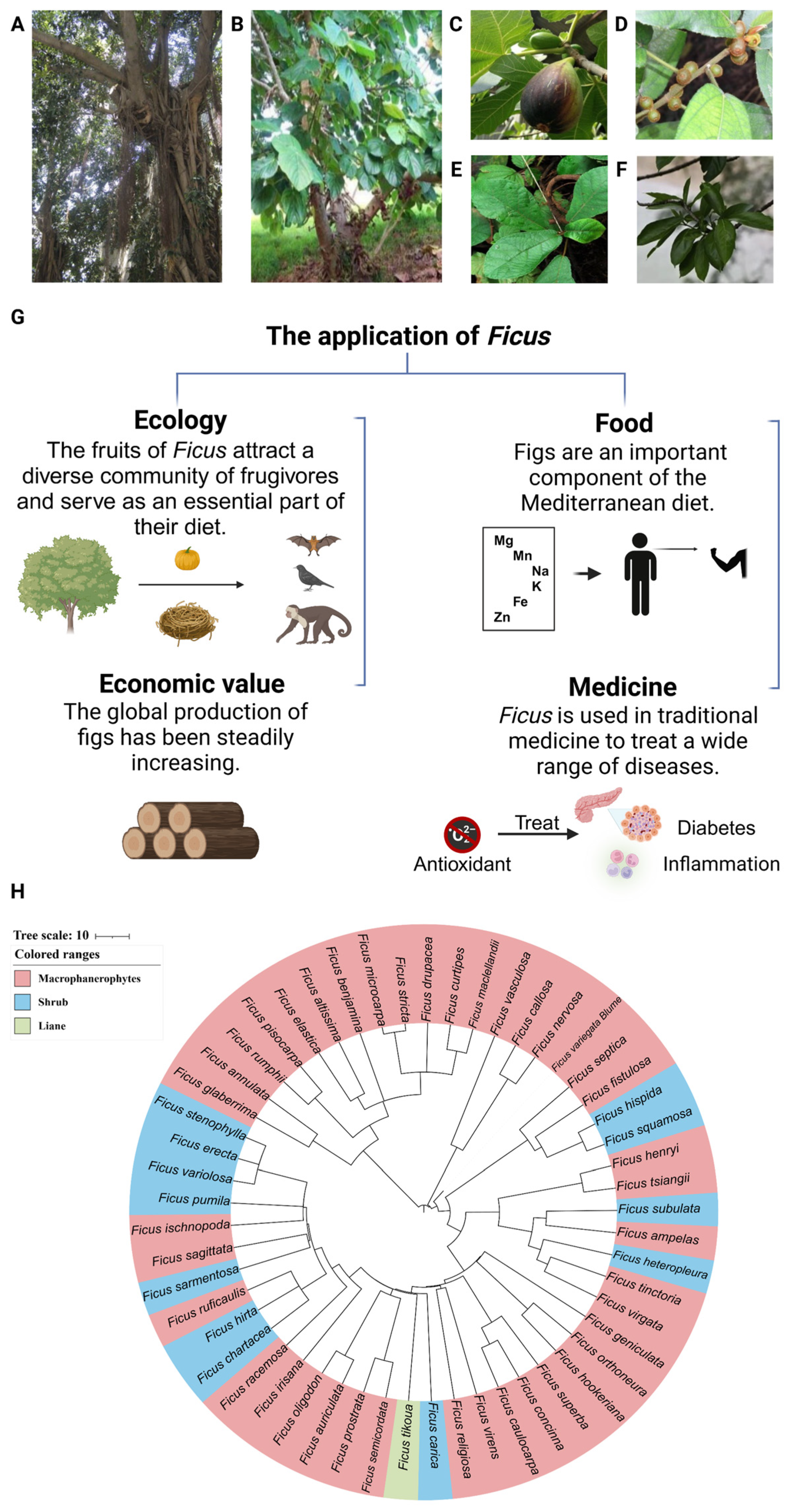
2. Adaption of the Ficus Species to Tropical Environment
3. The Responses of Ficus to Abiotic Stress
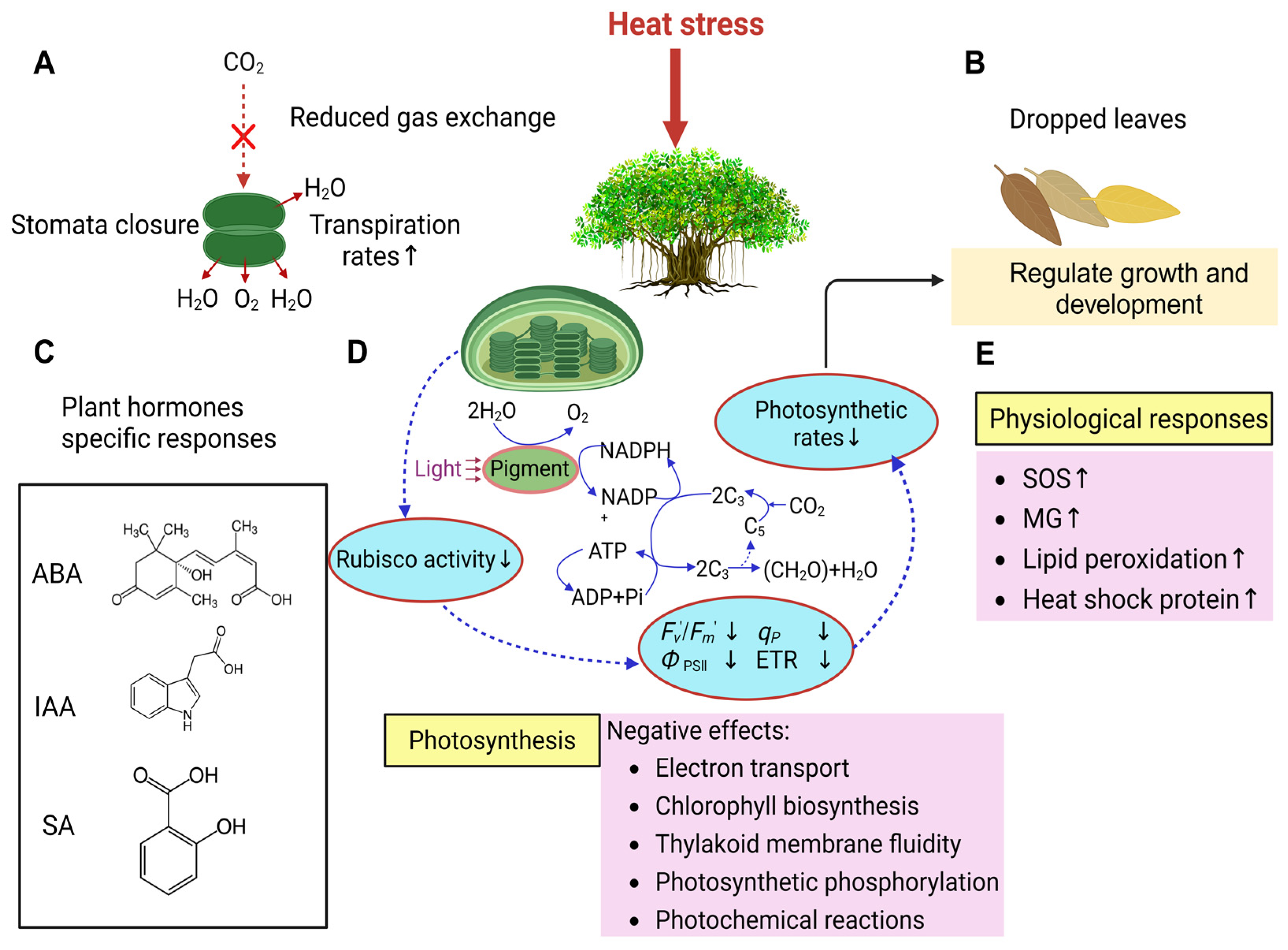
3.1. The Responses of Ficus to Heat Stress
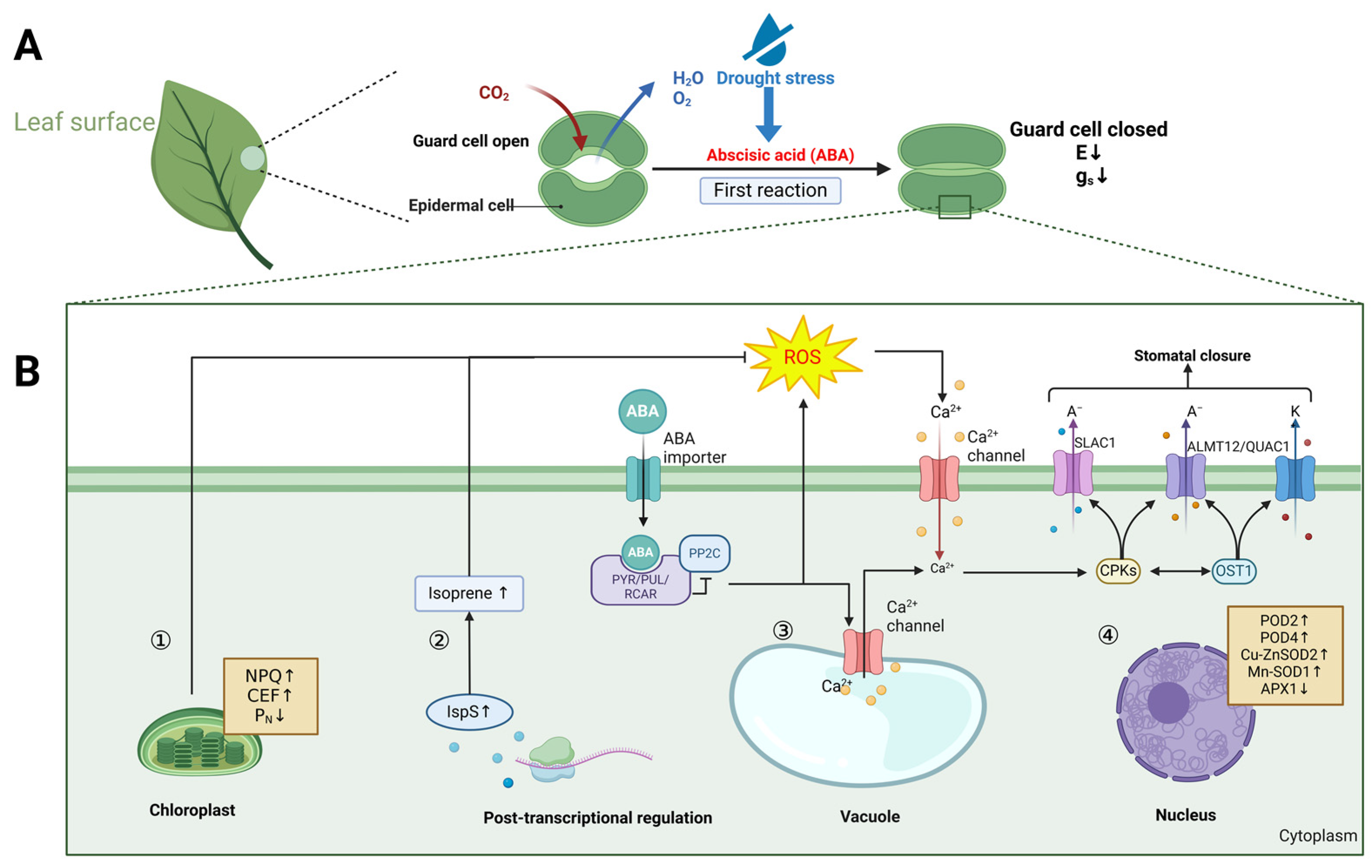
3.2. The Responses of Ficus to Drought Stress
3.3. The Responses of Ficus to Flood Stress
3.4. The Responses of Ficus to Oxidative Stress
3.5. The Responses of Ficus to Saline-Alkali Stress
4. The Adaptive Genes and Molecular Mechanism of Ficus Relating to Abiotic Stress
5. Potential Application of Ficus Genes in Genetic Breeding
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, X.; Wang, G.; Zhang, S.; Cheng, S.; Wang, Y.; Wen, P.; Ma, X.; Shi, Y.; Qi, R.; Yang, Y.; et al. Genomes of the Banyan Tree and Pollinator Wasp Provide Insights into Fig-Wasp Coevolution. Cell 2020, 183, 875–889. [Google Scholar] [CrossRef] [PubMed]
- Jianfeng, H.; Rui, X.; Yanqiong, P. Research progress of interspecific hybridization in genus Ficus. Biodivers. Sci. 2019, 27, 457. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, R.A. Updated review on Indian Ficus species. Arab. J. Chem. 2023, 16, 104976. [Google Scholar] [CrossRef]
- Cheng, J.-X.; Zhang, B.-D.; Zhu, W.-F.; Zhang, C.-F.; Qin, Y.-M.; Abe, M.; Akihisa, T.; Liu, W.-Y.; Feng, F.; Zhang, J. Traditional uses, phytochemistry, and pharmacology of Ficus hispida L.f.: A review. J. Ethnopharmacol. 2020, 248, 112204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-R.; Yang, X.; Li, W.-Y.; Peng, Y.-Q.; Gao, J. Comparative chloroplast genome analysis of Ficus (Moraceae): Insight into adaptive evolution and mutational hotspot regions. Front. Plant Sci. 2022, 13, 965335. [Google Scholar] [CrossRef] [PubMed]
- Debbarma, S.; Banik, B.; Mili, M.; Bora, D.; Das, S.; Datta, B.K.; Baijnath, H. Cryptovivipary: A rare phenomenon in monoecious species of Ficus L. (Moraceae). S. Afr. J. Bot. 2024, 167, 391–398. [Google Scholar] [CrossRef]
- Hao, G.Y.; Goldstein, G.; Sack, L.; Holbrook, N.M.; Liu, Z.H.; Wang, A.Y.; Harrison, R.D.; Su, Z.H.; Cao, K.F. Ecology of hemiepiphytism in fig species is based on evolutionary correlation of hydraulics and carbon economy. Ecology 2011, 92, 2117–2130. [Google Scholar] [CrossRef]
- Mellal, M.K.; Khelifa, R.; Chelli, A.; Djouadi, N.; Madani, K. Combined Effects of Climate and Pests on Fig (Ficus carica L.) Yield in a Mediterranean Region: Implications for Sustainable Agricultural Strategies. Sustainability 2023, 15, 5820. [Google Scholar] [CrossRef]
- Wijaya, I.M.S.; Defiani, M.R. Diversity and distribution of figs (Ficus: Moraceae) in Gianyar District, Bali, Indonesia. Biodiversitas J. Biol. Divers. 2021, 22, 233–246. [Google Scholar] [CrossRef]
- Raji, I.A.; Downs, C.T. Tree visitation and potential seed dispersal of keystone Ficus species by vertebrates in an urban mosaic landscape in eastern South Africa. Acta Oecologica 2022, 117, 103865. [Google Scholar] [CrossRef]
- Raji, I.A.; Downs, C.T. Ficus-frugivore interactions, especially in areas of land-use change, in Africa: A systematic review. Acta Oecologica 2021, 113, 103774. [Google Scholar] [CrossRef]
- Shanahan, M.; So, S.; Compton, S.G.; Corlett, R. Fig-eating by vertebrate frugivores: A global review. Biol. Rev. 2001, 76, 529–572. [Google Scholar] [CrossRef]
- Rønsted, N.; Salvo, G.; Savolainen, V. Biogeographical and phylogenetic origins of African fig species (Ficus section Galoglychia). Mol. Phylogenetics Evol. 2007, 43, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.S. Can fruit pulp meet the calcium needs of tropical frugivorous passerines during reproduction? J. Trop. Ecol. 2014, 30, 79–88. [Google Scholar] [CrossRef]
- Sinu, P.A.; Unni, A.P.; Jose, T. Biotic Seed Dispersal Mechanisms of Tropical Rain Forests—Bats, Fishes, and Migratory Birds. In Reproductive Ecology of Flowering Plants: Patterns and Processes; Tandon, R., Shivanna, K.R., Koul, M., Eds.; Springer: Singapore, 2020; pp. 299–334. [Google Scholar] [CrossRef]
- Isa, M.; Jaafar, M.; Kasim, K.; Mutalib, M. Cultivation of fig (Ficus carica L.) as an alternative high value crop in Malaysia: A brief review. IOP Conf. Ser. Mater. Sci. Eng. 2020, 864, 012134. [Google Scholar] [CrossRef]
- Devi, R.; Manjula, B.; Kumar, M.; Manjula, B. Food and medicinal values of some Ficus species. Med. Bio-Wealth India 2022, 6, 21–26. [Google Scholar] [CrossRef]
- Mascellani, A.; Natali, L.; Cavallini, A.; Mascagni, F.; Caruso, G.; Gucci, R.; Havlik, J.; Bernardi, R. Moderate Salinity Stress Affects Expression of Main Sugar Metabolism and Transport Genes and Soluble Carbohydrate Content in Ripe Fig Fruits (Ficus carica L. cv. Dottato). Plants 2021, 10, 1861. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, A.; Magarelli, A.; Ferrara, G. The fig (Ficus carica L.): Varietal evolution from Asia to Puglia region, southeastern Italy. CABI Agric. Biosci. 2024, 5, 57. [Google Scholar] [CrossRef]
- Ammar, A.; Aissa, I.B.E.N.; Mars, M.; Gouiaa, M. Seasonal variation of fig tree (Ficus carica L.) physiological characteristics reveals its adaptation performance. S. Afr. J. Bot. 2020, 132, 30–37. [Google Scholar] [CrossRef]
- Ashraf, K.; Haque, M.R.; Amir, M.; Amir, M.; Ahmad, N.; Ahmad, W.; Sultan, S.; Ali Shah, S.A.; Alafeefy, A.M.; Mujeeb, M.; et al. An Overview of Phytochemical and Biological Activities: Ficus deltoidea Jack and Other Ficus spp. J. Pharm. Bioallied Sci. 2021, 13, 11–25. [Google Scholar] [CrossRef]
- Mohammad, N.; Wei, Y.K.; Bakar, N.F.A. Determination of Mineral Content in The Ficus Deltoidea Leaves. J. Sains Kesihat. Malays. 2012, 10, 25–29. [Google Scholar] [CrossRef]
- Lansky, E.P.; Paavilainen, H.M.; Pawlus, A.D.; Newman, R.A. Ficus spp. (fig): Ethnobotany and potential as anticancer and anti-inflammatory agents. J. Ethnopharmacol. 2008, 119, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Qian, J.; Yuan, W.; Yang, X.; Di, B.; Meng, Y.; Shao, J. Effects of Interval Flooding Stress on Physiological Characteristics of Apple Leaves. Horticulturae 2021, 7, 331. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, W.; Liu, J.; Song, S.; Hou, X.; Jia, C.; Li, J.; Miao, H.; Wang, Z.; Tie, W.; et al. An aquaporin gene MaPIP2-7 is involved in tolerance to drought, cold and salt stresses in transgenic banana (Musa acuminata L.). Plant Physiol. Biochem. 2020, 147, 66–76. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Wang, Q.; Chen, M.; Ye, X.; Li, D.; Chen, X.; Li, L.; Gao, D. 24-Epibrassinolide-alleviated drought stress damage influences antioxidant enzymes and autophagy changes in peach (Prunus persicae L.) leaves. Plant Physiol. Biochem. 2019, 135, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, L.M.; Shalan, A.M.; El-Boray, M.S.; Vincent, C.I.; El-Kady, M.E.; Grosser, J.W.; Dutt, M. Application of silicon nanoparticles enhances oxidative stress tolerance in salt stressed ‘Valencia’ sweet orange plants. Sci. Hortic. 2022, 295, 110856. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Xu, J.; Lv, Z.; Manzoor, M.A.; Mao, J.; Zhang, X.; Liu, R.; Wang, S.; Whiting, M.D.; et al. Strigolactone and salicylic acid regulate the expression of multiple stress-related genes and enhance the drought resistance of cherry rootstocks. Sci. Hortic. 2023, 313, 111827. [Google Scholar] [CrossRef]
- Ahmad, M.; Alabd, A.; Gao, Y.; Yu, W.; Jamil, W.; Wang, X.; Wei, J.; Ni, J.; Teng, Y.; Bai, S. Three stress-responsive NAC transcription factors, Pp-SNACs, differentially and synergistically regulate abiotic stress in pear. Sci. Hortic. 2022, 305, 111393. [Google Scholar] [CrossRef]
- Samarasinghe, C.R.K.; Meegahakumbura, M.K.; Dissanayaka, H.D.M.A.C.; Kumarathunge, D.; Perera, L. Variation in yield and yield components of different coconut cultivars in response to within year rainfall and temperature variation. Sci. Hortic. 2018, 238, 51–57. [Google Scholar] [CrossRef]
- Liang, C. Research Progress of Fig Cultivation and Postharvest Technology. Chin. Fruits Veg. 2022, 42, 78–84. [Google Scholar] [CrossRef]
- Krause, G.H.; Winter, K.; Krause, B.; Jahns, P.; García, M.; Aranda, J.; Virgo, A. High-temperature tolerance of a tropical tree, Ficus insipida: Methodological reassessment and climate change considerations. Funct. Plant Biol. 2010, 37, 890–900. [Google Scholar] [CrossRef]
- Sadder, M.T.; Alshomali, I.; Ateyyeh, A.; Musallam, A. Physiological and molecular responses for long term salinity stress in common fig (Ficus carica L.). Physiol. Mol. Biol. Plants 2021, 27, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.; Aissa, I.B.; Gouiaa, M.; Mars, M. Fig (Ficus carica L.) vulnerability to climate change: Combined effects of water stress and high temperature on ecophysiological behaviour of different cultivars. S. Afr. J. Bot. 2022, 147, 482–492. [Google Scholar] [CrossRef]
- Ammar, A.; Ben Aissa, I.; Zaouay, F.; Gouiaa, M.; Mars, M. Physiological behaviour of fig tree (Ficus carica L.) under different climatic conditions. In Fig (Ficus carica): Production, Processing, and Properties; Springer International Publishing: Cham, Switzerland, 2023; pp. 247–257. [Google Scholar] [CrossRef]
- Richter, S.; Anders, N.; Wolters, H.; Beckmann, H.; Thomann, A.; Heinrich, R.; Schrader, J.; Singh, M.K.; Geldner, N.; Mayer, U.; et al. Role of the GNOM gene in Arabidopsis apical-basal patterning—From mutant phenotype to cellular mechanism of protein action. Eur. J. Cell Biol. 2010, 89, 138–144. [Google Scholar] [CrossRef]
- Jia, W.; Ma, M.; Chen, J.; Wu, S. Plant Morphological, Physiological and Anatomical Adaption to Flooding Stress and the Underlying Molecular Mechanisms. Int. J. Mol. Sci. 2021, 22, 1088. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Lin, Z. Reactive Oxygen Species and Relative Enzyme Activities in the Development of Aerial Roots of Chinese Banyan (Ficus microcarpa). J. Plant Growth Regul. 2014, 33, 160–168. [Google Scholar] [CrossRef]
- Gautam, R.; Meena, R.K.; Rampuria, S.; Shukla, P.; Kirti, P.B. Ectopic expression of DnaJ type-I protein homolog of Vigna aconitifolia (VaDJI) confers ABA insensitivity and multiple stress tolerance in transgenic tobacco plants. Front. Plant Sci. 2023, 14, 1135552. [Google Scholar] [CrossRef]
- He, J.; Li, P.; Huo, H.; Liu, L.; Tang, T.; He, M.; Huang, J.; Liu, L. Heterologous expression of HpBHY and CrBKT increases heat tolerance in Physcomitrella patens. Plant Divers. 2019, 41, 266–274. [Google Scholar] [CrossRef]
- Ramkumar, T.R.; Lenka, S.K.; Arya, S.S.; Bansal, K.C. A short history and perspectives on plant genetic transformation. In Biolistic DNA Delivery in Plants: Methods and Protocols; Springer: New York, NY, USA, 2020; pp. 39–68. [Google Scholar] [CrossRef]
- Kumar, S.; Suleski, M.; Craig, J.M.; Kasprowicz, A.E.; Sanderford, M.; Li, M.; Stecher, G.; Hedges, S.B. TimeTree 5: An Expanded Resource for Species Divergence Times. Mol. Biol. Evol. 2022, 39, msac174. [Google Scholar] [CrossRef]
- Harrison, R.D. Figs and the diversity of tropical rainforests. Bioscience 2005, 55, 1053–1064. [Google Scholar] [CrossRef]
- Harrison, R.D.; Shanahan, M. Seventy-seven ways to be a fig: Overview of a diverse plant assemblage. In Pollination Ecology and the Rain Forest: Sarawak Studies; Springer: New York, NY, USA, 2005; pp. 111–127. [Google Scholar] [CrossRef]
- Bao, Y.; He, M.; Zhang, C.; Jiang, S.; Zhao, L.; Ye, Z.; Sun, Q.; Xia, Z.; Zou, M. Advancing understanding of Ficus carica: A comprehensive genomic analysis reveals evolutionary patterns and metabolic pathway insights. Front. Plant Sci. 2023, 14, 1298417. [Google Scholar] [CrossRef] [PubMed]
- Usai, G.; Mascagni, F.; Giordani, T.; Vangelisti, A.; Bosi, E.; Zuccolo, A.; Ceccarelli, M.; King, R.; Hassani-Pak, K.; Zambrano, L.S.; et al. Epigenetic patterns within the haplotype phased fig (Ficus carica L.) genome. Plant J. 2020, 102, 600–614. [Google Scholar] [CrossRef]
- Ashalatha, K.L.; Arunkumar, K.P.; Gowda, M. Genomic and transcriptomic analysis of sacred fig (Ficus religiosa). BMC Genom. 2023, 24, 197. [Google Scholar] [CrossRef] [PubMed]
- Shirasawa, K.; Yakushiji, H.; Nishimura, R.; Morita, T.; Jikumaru, S.; Ikegami, H.; Toyoda, A.; Hirakawa, H.; Isobe, S. The Ficus erecta genome aids Ceratocystis canker resistance breeding in common fig (F. carica). Plant J. 2020, 102, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Brodersen, C.R. Finding support for theoretical tradeoffs in xylem structure and function. New Phytol. 2015, 209, 8–10. [Google Scholar] [CrossRef]
- Fan, Z.-X.; Sterck, F.; Zhang, S.-B.; Fu, P.-L.; Hao, G.-Y. Tradeoff between Stem Hydraulic Efficiency and Mechanical Strength Affects Leaf–Stem Allometry in 28 Ficus Tree Species. Front. Plant Sci. 2017, 8, 1619. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Fu, P.-L.; Wang, Y.-J.; Cao, K.-F. Different Drought-adaptation Strategies as Characterized by Hydraulic and Water-relations Traits of Evergreen and Deciduous Figs in a Tropical Karst Forest. Plant Sci. J. 2012, 30, 484. [Google Scholar] [CrossRef]
- Wisniewska, J.; Xu, J.; Seifertová, D.; Brewer, P.B.; Ruzicka, K.; Blilou, I.; Rouquié, D.; Benková, E.; Scheres, B.; Friml, J. Polar PIN localization directs auxin flow in plants. Science 2006, 312, 883. [Google Scholar] [CrossRef]
- Marhava, P. Recent developments in the understanding of PIN polarity. New Phytol. 2022, 233, 624–630. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, S.; Ren, T.; Niu, M.; Liu, X.; Liu, C.; Wang, H.; Yin, W.; Xia, X. Crucial Abiotic Stress Regulatory Network of NF-Y Transcription Factor in Plants. Int. J. Mol. Sci. 2023, 24, 4426. [Google Scholar] [CrossRef] [PubMed]
- Junaid, M.; Gokce, A. Global agricultural losses and their causes. Bull. Biol. Allied Sci. Res. 2024, 2024, 66. [Google Scholar] [CrossRef]
- Fang, C.; Chen, H.; Castillo-Díaz, D.; Wen, B.; Cao, K.; Goodale, U. Regeneration and Endogenous Phytohormone Responses to High-Temperature Stress Drive Recruitment Success in Hemiepiphytic Fig Species. Front. Plant Sci. 2021, 12, 754207. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, X.; Shen, J.; Li, Z.; Sang, Y.; Wu, X.; Zha, Y.; Liang, W.; Wang, C.; Wang, K.; et al. Clinically Applicable AI System for Accurate Diagnosis, Quantitative Measurements, and Prognosis of COVID-19 Pneumonia Using Computed Tomography. Cell 2020, 182, 1360. [Google Scholar] [CrossRef]
- Hao, G.-Y.; Wang, A.-Y.; Liu, Z.-H.; Franco, A.C.; Goldstein, G.; Cao, K.-F. Differentiation in light energy dissipation between hemiepiphytic and non-hemiepiphytic Ficus species with contrasting xylem hydraulic conductivity. Tree Physiol. 2011, 31, 626–636. [Google Scholar] [CrossRef]
- Ammar, A.; Ben Aissa, I.; Mars, M.; Gouiaa, M. Comparative physiological behavior of fig (Ficus carica L.) cultivars in response to water stress and recovery. Sci. Hortic. 2020, 260, 108881. [Google Scholar] [CrossRef]
- Zafer Can, H.; Aksoy, U. Seasonal and diurnal photosynthetic behaviour of fig (Ficus carica L.) under semi-arid climatic conditions. Acta Agric. Scand. Sect. B Soil Plant Sci. 2007, 57, 297–306. [Google Scholar] [CrossRef]
- González-Rodríguez, A.M.; Peters, J. Strategies of leaf expansion in Ficus carica under semiarid con ditions. Plant Biol. 2010, 12, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Vangelisti, A.; Zambrano, L.S.; Caruso, G.; Macheda, D.; Bernardi, R.; Usai, G.; Mascagni, F.; Giordani, T.; Gucci, R.; Cavallini, A.; et al. How an ancient, salt-tolerant fruit crop, Ficus carica L., copes with salinity: A transcriptome analysis. Sci. Rep. 2019, 9, 2561. [Google Scholar] [CrossRef]
- Jin, S.H.; Li, X.Q.; Wang, J.G. Effects of high temperature stress on photosynthesis in Ficus concinna var. subsessilis. Chin. Agric. Sci. Bull. 2009, 25, 83–87. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, W.; Zhang, J.; Cao, K. Differential responses of photosystems I and II to seasonal drought in two Ficus species. Acta Oecologica-Int. J. Ecol. 2016, 73, 53–60. [Google Scholar] [CrossRef]
- Khandaker, M.M.; Jamaludin, R.; Majrashi, A.; Rashid, Z.M.; Karim, S.M.R.; Al-Yasi, H.M.; Badaluddin, N.A.; Alenazi, M.M.; Mohd, K.S. Enhancing Rubisco gene expression and metabolites accumulation for better plant growth in Ficus deltoidea under drought stress using hydrogen peroxide. Front. Plant Sci. 2022, 13, 965765. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Rashid, M.H.-U.; Inafuku, M.; Oku, H. Molecular regulatory mechanism of isoprene emission under short-term drought stress in the tropical tree Ficus septica. Tree Physiol. 2018, 39, 440–453. [Google Scholar] [CrossRef]
- Johnson, C.R.; Nell, T.A.; Rosenbaum, S.E.; Lauritis, J.A. Influence of light intensity and drought stress on Ficus benjamina L. J. Am. Soc. Hortic. Sci. 1982, 107, 252–255. [Google Scholar] [CrossRef]
- Yamasaki, H.; Takahashi, S.; Heshiki, R. The tropical fig Ficus microcarpa L. f. cv. golden leaves lacks heat-stable dehydroascorbate reductase activity. Plant Cell Physiol. 1999, 40, 640–646. [Google Scholar] [CrossRef]
- Liu, N.; Cao, C.; Sun, Z.; Lin, Z.; Deng, R. Pollutant-induced cell death and reactive oxygen species accumulation in the aerial roots of Chinese banyan (Ficus microcarpa). Sci. Rep. 2016, 6, 36276. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-h.; Li, X.-q.; Hu, J.-y.; Wang, J.-g. Cyclic electron flow around photosystem I is required for adaptation to high temperature in a subtropical forest tree, Ficus concinna. J. Zhejiang Univ. Sci. B 2009, 10, 784–790. [Google Scholar] [CrossRef]
- Voesenek, L.A.; Bailey-Serres, J. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Y.; Chan, Z. Perspectives on screening winter-flood-tolerant woody species in the riparian protection forests of the Three Gorges Reservoir. PLoS ONE 2014, 9, e108725. [Google Scholar] [CrossRef]
- Pothasin, P.; Compton, S.G.; Wangpakapattanawong, P. Riparian Ficus tree communities: The distribution and abundance of riparian fig trees in northern Thailand. PLoS ONE 2014, 9, e108945. [Google Scholar] [CrossRef]
- Pothasin, P.; Compton, S.G.; Wangpakapattanawong, P. Seasonality of leaf and fig production in Ficus squamosa, a fig tree with seeds dispersed by water. PLoS ONE 2016, 11, e0152380. [Google Scholar] [CrossRef]
- Smitha, R.B.; Bennans, T.; Mohankumar, C.; Benjamin, S. Oxidative stress enzymes in Ficus religiosa L.: Biochemical, histochemical and anatomical evidences. J. Photochem. Photobiol. B Biol. 2009, 95, 17–25. [Google Scholar] [CrossRef]
- Sato, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Complex plant responses to drought and heat stress under climate change. Plant J. 2024, 117, 1873–1892. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.E.; Meacham-Hensold, K.; Lemonnier, P.; Slattery, R.A.; Benjamin, C.; Bernacchi, C.J.; Lawson, T.; Cavanagh, A.P. The effect of increasing temperature on crop photosynthesis: From enzymes to ecosystems. J. Exp. Bot. 2021, 72, 2822–2844. [Google Scholar] [CrossRef] [PubMed]
- Guilioni, L.; Wery, J.; Tardieu, F. Heat Stress-induced Abortion of Buds and Flowers in Pea: Is Sensitivity Linked to Organ Age or to Relations between Reproductive Organs? Ann. Bot. 1997, 80, 159–168. [Google Scholar] [CrossRef]
- Srivalli, B.; Khanna-Chopra, R. The developing reproductive ‘sink’ induces oxidative stress to mediate nitrogen mobilization during monocarpic senescence in wheat. Biochem. Biophys. Res. Commun. 2004, 325, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Singh, S. Plant Adaptation and Tolerance to Heat Stress: Advance Approaches and Future Aspects. CCHTS 2024, 27, 1701–1715. [Google Scholar] [CrossRef]
- Hu, S.; Ding, Y.; Zhu, C. Sensitivity and Responses of Chloroplasts to Heat Stress in Plants. Front. Plant Sci. 2020, 11, 375. [Google Scholar] [CrossRef]
- Wu, X.; Yao, X.; Chen, J.; Zhu, Z.; Zhang, H.; Zha, D. Brassinosteroids protect photosynthesis and antioxidant system of eggplant seedlings from high-temperature stress. Acta Physiol. Plant. 2013, 36, 251–261. [Google Scholar] [CrossRef]
- Guo, M.; Liu, J.-H.; Ma, X.; De-Xu Luo, D.-X.; Gong, Z.-H.; Lu, M.-H. The Plant Heat Stress Transcription Factors (HSFs): Structure, Regulation, and Function in Response to Abiotic Stresses. Front. Plant Sci. 2016, 7, 114. [Google Scholar] [CrossRef]
- Goyal, M.; Banerjee, C.; Nag, S.; Bandyopadhyay, U. The Alba protein family: Structure and function. Biochim. Et Biophys. Acta (BBA)-Proteins Proteom. 2016, 1864, 570–583. [Google Scholar] [CrossRef]
- Tong, J.; Ren, Z.; Sun, L.; Zhou, S.; Yuan, W.; Hui, Y.; Ci, D.; Wang, W.; Fan, L.-M.; Wu, Z.; et al. ALBA proteins confer thermotolerance through stabilizing HSF messenger RNAs in cytoplasmic granules. Nat. Plants 2022, 8, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Mahfouz, M.M.; Zhou, S. Pre-mRNA alternative splicing as a modulator for heat stress response in plants. Trends Plant Sci. 2021, 26, 1153–1170. [Google Scholar] [CrossRef]
- Diatta, A.A.; Fike, J.H.; Battaglia, M.L.; Galbraith, J.M.; Baig, M.B. Effects of biochar on soil fertility and crop productivity in arid regions: A review. Arab. J. Geosci. 2020, 13, 595. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, E. Towards adaptation of water resource systems to climatic and socio-economic change. Water Resour. Manag. 2017, 31, 2965–2984. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, S.N. The global governance of water, energy, and food nexus: Allocation and access for competing demands. Int. Environ. Agreem. Politics Law Econ. 2020, 20, 377–391. [Google Scholar] [CrossRef]
- Ahmad, H.M.; Fiaz, S.; Hafeez, S.; Zahra, S.; Shah, A.N.; Gul, B.; Aziz, O.; Fakhar, A.; Rafique, M.; Chen, Y.; et al. Plant Growth-Promoting Rhizobacteria Eliminate the Effect of Drought Stress in Plants: A Review. Front. Plant Sci. 2022, 13, 875774. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, L.; Gargouri, K.; Ben Hassena, A.; Mbadra, C.; Ghrab, M.; Ncube, B.; Van Staden, J.; Gargouri, R. Impact of drought and salinity on olive water status and physiological performance in an arid climate. Agric. Water Manag. 2019, 213, 749–759. [Google Scholar] [CrossRef]
- Pirasteh-Anosheh, H.; Saed-Moucheshi, A.; Pakniyat, H.; Pessarakli, M. Stomatal responses to drought stress. In Water Stress and Crop Plants: A Sustainable Approach; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 24–40. [Google Scholar] [CrossRef]
- Abdolinejad, R.; Shekafandeh, A. Tetraploidy Confers Superior in vitro Water-Stress Tolerance to the Fig Tree (Ficus carica) by Reinforcing Hormonal, Physiological, and Bioc hemical Defensive Systems. Front. Plant Sci. 2022, 12, 796215. [Google Scholar] [CrossRef]
- Knipfer, T.; Bambach, N.; Hernandez, M.I.; Bartlett, M.K.; Sinclair, G.; Duong, F.; Kluepfel, D.A.; McElrone, A.J. Predicting stomatal closure and turgor loss in woody plants using predawn and midday water potential. Plant Physiol. 2020, 184, 881–894. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.H.; Fang, X.W.; Su, P.X.; Wang, G. Ecophysiological responses of Caragana korshinskii Kom. under extreme drought stress: Leaf abscission and stem survives. Photosynthetica 2012, 50, 541–548. [Google Scholar] [CrossRef]
- Jackson, M.B.; Colmer, T.D. Response and adaptation by plants to flooding stress. Ann. Bot. 2005, 96, 501–505. [Google Scholar] [CrossRef]
- Fukao, T.; Barrera-Figueroa, B.E.; Juntawong, P.; Peña-Castro, J.M. Submergence and Waterlogging Stress in Plants: A Review Highlighting Research Opportunities and Understudied Aspects. Front. Plant Sci. 2019, 10, 340. [Google Scholar] [CrossRef]
- Lee, S.C.; Mustroph, A.; Sasidharan, R.; Vashisht, D.; Pedersen, O.; Oosumi, T.; Voesenek, L.A.C.J.; Bailey-Serres, J. Molecular characterization of the submergence response of the Arabidopsis thaliana ecotype Columbia. New Phytol. 2011, 190, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.-C.; Chou, M.-Y.; Chou, S.-J.; Li, Y.R.; Peng, H.-P.; Shih, M.-C. Submergence confers immunity mediated by the WRKY22 transcription factor in Arabidopsis. Plant Cell 2013, 25, 2699–2713. [Google Scholar] [CrossRef]
- Rivera-Contreras, I.K.; Zamora-Hernández, T.; Huerta-Heredia, A.A.; Capataz-Tafur, J.; Barrera-Figueroa, B.E.; Juntawong, P.; Peña-Castro, J.M. Transcriptomic analysis of submergence-tolerant and sensitive Brachypodium distachyon ecotypes reveals oxidative stress as a major tolerance factor. Sci. Rep. 2016, 6, 27686. [Google Scholar] [CrossRef]
- Furness, H.; Breen, C. The vegetation of seasonally flooded areas of the Pongolo River Floodplain. Bothalia 1980, 13, 217–231. [Google Scholar] [CrossRef][Green Version]
- Hasanuzzaman, M.; Al Mahmud, J.; Nahar, K.; Anee, T.I.; Inafuku, M.; Oku, H.; Fujita, M. Adaptation, and ROS Metabolism in Plants Exposed to Waterlogging Stress. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress; Khan, M.I.R., Khan, N.A., Eds.; Springer: Singapore, 2017; pp. 257–281. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; da Silva, J.A.T.; Fujita, M. Plant Response and Tolerance to Abiotic Oxidative Stress: Antioxidant Defense Is a Key Factor. In Crop Stress and Its Management: Perspectives and Strategies; Venkateswarlu, B., Shanker, A., Shanker, C., Maheswari, M., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 261–315. [Google Scholar] [CrossRef]
- Liu, N.; Lin, Z.; Mo, H. Metal (Pb, Cd, and Cu)-induced reactive oxygen species accumulations in aerial root cells of the Chinese banyan (Ficus microcarpa). Ecotoxicology 2012, 21, 2004–2011. [Google Scholar] [CrossRef]
- Prăvălie, R.; Patriche, C.; Borrelli, P.; Panagos, P.; Roșca, B.; Dumitraşcu, M.; Nita, I.-A.; Săvulescu, I.; Birsan, M.-V.; Bandoc, G. Arable lands under the pressure of multiple land degradation processes. A global perspective. Environ. Res. 2021, 194, 110697. [Google Scholar] [CrossRef]
- Negacz, K.; Malek, Ž.; de Vos, A.; Vellinga, P. Saline soils worldwide: Identifying the most promising areas for saline agriculture. J. Arid Environ. 2022, 203, 104775. [Google Scholar] [CrossRef]
- Tarolli, P.; Luo, J.; Park, E.; Barcaccia, G.; Masin, R. Soil salinization in agriculture: Mitigation and adaptation strategies combining nature-based solutions and bioengineering. iScience 2024, 27, 108830. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, G.; Akhtar, M.S.; Abdullah, R. Global concern for salinity on various agro-ecosystems. In Salt Stress, Microbes, and Plant Interactions: Causes and Solution: Volume 1; Akhtar, M.S., Ed.; Springer: Singapore, 2019; pp. 1–19. [Google Scholar] [CrossRef]
- Hassani, A.; Azapagic, A.; Shokri, N. Global predictions of primary soil salinization under changing climate in the 21st century. Nat. Commun. 2021, 12, 6663. [Google Scholar] [CrossRef]
- Pradhan, C.; Mohanty, M. Submergence Stress: Responses and adaptations in crop plants. In Molecular Stress Physiology of Plants; Rout, G.R., Das, A.B., Eds.; Springer: New Delhi, India, 2013; pp. 331–357. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, S.; Yang, D.; Zheng, J. Genome-wide in silico identification of glutathione S-transferase (GST) gene family members in fig (Ficus carica L.) and expression characteristics during fruit color development. Peerj 2023, 11, e14406. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Cui, Y.; Song, M.; Vainstein, A.; Chen, S.; Ma, H. Papain-Like Cysteine Protease Gene Family in Fig (Ficus carica L.): Genome-Wide Analysis and Expression Patterns. Front. Plant Sci. 2021, 12, 681801. [Google Scholar] [CrossRef]
- Song, M.; Wang, H.; Wang, Z.; Huang, H.; Chen, S.; Ma, H. Genome-Wide Characterization and Analysis of bHLH Transcription Factors Related to Anthocyanin Biosynthesis in Fig (Ficus carica L.). Front. Plant Sci. 2021, 12, 730692. [Google Scholar] [CrossRef]
- Kim, A.S.; Kim, Y.O.; Ryu, H.J.; Kwak, Y.S.; Lee, J.Y.; Kang, H. Isolation of stress-related genes of rubber particles and latex in fig tree (Ficus carica) and their expressions by abiotic stress or plant hormone treatments. Plant Cell Physiol. 2003, 44, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X.; Ding, Y.L.; Yang, Y.Q.; Song, C.P.; Wang, B.S.; Yang, S.H.; Guo, Y.; Gong, Z.Z. Protein kinases in plant responses to drought, salt, and cold stress. J. Integr. Plant Biol. 2021, 63, 53–78. [Google Scholar] [CrossRef]
- Gong, Z. Plant abiotic stress: New insights into the factors that activate and modulate plant responses. J. Integr. Plant Biol. 2021, 63, 429–430. [Google Scholar] [CrossRef]
| Latin Name | Level | GenBank | Release Date | Genome Size | References |
|---|---|---|---|---|---|
| Ficus carica | Chromosome | GCA_009761775.1 | December 2019 | 333.4 Mb | [46] |
| Ficus microcarpa | Chromosome | GCA_025413485.1 | September 2022 | 426.6 Mb | [1] |
| Ficus hispida | Chromosome | GCA_025413025.1 | September 2022 | 369.8 Mb | [1] |
| Ficus religiosa | Scaffold | GCA_024759925.1 | August 2022 | 406.1 Mb | [47] |
| Ficus erecta | Contig | GCA_008635985.1 | September 2019 | 595.8 Mb | [48] |
| Species | Response | Stress | References |
|---|---|---|---|
| Hemiepiphytic Ficus | Morphology, behavior, physiology | Heat, drought | [57,58,59] |
| Ficus carica L. | Morphology, behavior, physiology | Heat, drought, salt | [18,33,60,61,62,63] |
| Ficus chartacea var. torulosa | Physiology | Heat | [64] |
| Ficus tinctoria | Physiology | Drought | [65] |
| Ficus racemosa | Physiology | Drought | [65] |
| Ficus deltoidea | Physiology | Drought | [66] |
| Ficus septica | Physiology | Drought | [67] |
| Ficus benjamina L. | Physiology, cytology | Drought | [68] |
| Ficus orthoneura | Morphology | Drought | [51] |
| Ficus microcarpa | Physiology, morphology | Drought, oxidative | [69,70] |
| Ficus concinna | Molecular mechanisms | Drought | [71] |
| Ficus ssp. | Morphology | Flood | [72] |
| Ficus tikoua | Viability | Flood | [73] |
| Ficus crytophylla | Behavior | Flood | [74] |
| Ficus squamosa | Behavior | Flood | [75] |
| Ficus religiosa L. | Physiology | oxidative | [76] |
| Hemiepiphytic Ficus | Morphology, behavior, physiology | Heat, drought | [57,58,59] |
| Stress | Responses | |
|---|---|---|
| Heat stress | Morphology | Stomatal closure and leaf abscission. |
| Physiology | Decreased photosystem activity; reduced photosynthetic rate; increased transpiration rate; elevated levels of IAA, ROS, MG, and lipid peroxidation. | |
| Cytology | Reduced chlorophyll synthesis. | |
| Molecular | Inactivation of heat-sensitive proteins; synthesis of heat shock proteins. | |
| Drought stress | Morphology | Regulated leaf temperature, increased leaf abscission, reduced stomatal conductance; decreased root hydraulic conductance (Lp); and unchanged leaf turgor pressure. |
| Physiology | Decreased photosynthetic and transpiration rates; accumulation of dry matter; reduced glutamine; enhanced non-photochemical quenching (NPQ); activation of cyclic electron flow (CEF) and increased isoprene emission rate. | |
| Cytology | Reduced chlorophyll synthesis and damaged thylakoid structure. | |
| Molecular | Increased transcription of POD2, POD4, Cn-ZnSOD2, and Mn-SOD1; decreased transcription of APX1. | |
| Flood stress | Morphology | Seed dispersal via water; reduced stomatal conductance; formation of aerial prop roots. |
| Physiology | Nutrient imbalance; accumulation of ROS; decreased photosynthetic rate; increased ethylene production. | |
| Cytology | Damaged membrane integrity. | |
| Molecular | Increased ADH activity and proline content. | |
| Oxidative stress | Morphology | Decreased antioxidant capacity during senescence. |
| Physiology | Increased hydrogen peroxide and malondialdehyde levels, increased POX activity; and lipid peroxidation. | |
| Cytology | Decreased cell viability in adventitious roots; damage to the cell wall and plasma membrane. | |
| Molecular | Ascorbate-glutathione (AsA-GSH) pathway. | |
| Salt stress | Morphology | Reduced stomatal conductance. |
| Physiology | Decreased photosynthetic rate; increased sucrose and d-sorbitol; downregulated glycolytic metabolism. | |
| Cytology | Decreased chlorophyll content; altered cell wall composition. | |
| Molecular | Increased transcription of carbohydrate transport genes; overexpression of ROS signaling proteins and proline synthesis coding genes. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, S.; Yin, T.; He, H.; Liu, X.; Long, X.; Dong, P.; Zhu, Z. Phenotypic, Metabolic and Genetic Adaptations of the Ficus Species to Abiotic Stress Response: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 9520. https://doi.org/10.3390/ijms25179520
Yuan S, Yin T, He H, Liu X, Long X, Dong P, Zhu Z. Phenotypic, Metabolic and Genetic Adaptations of the Ficus Species to Abiotic Stress Response: A Comprehensive Review. International Journal of Molecular Sciences. 2024; 25(17):9520. https://doi.org/10.3390/ijms25179520
Chicago/Turabian StyleYuan, Shengyun, Tianxiang Yin, Hourong He, Xinyi Liu, Xueyan Long, Pan Dong, and Zhenglin Zhu. 2024. "Phenotypic, Metabolic and Genetic Adaptations of the Ficus Species to Abiotic Stress Response: A Comprehensive Review" International Journal of Molecular Sciences 25, no. 17: 9520. https://doi.org/10.3390/ijms25179520
APA StyleYuan, S., Yin, T., He, H., Liu, X., Long, X., Dong, P., & Zhu, Z. (2024). Phenotypic, Metabolic and Genetic Adaptations of the Ficus Species to Abiotic Stress Response: A Comprehensive Review. International Journal of Molecular Sciences, 25(17), 9520. https://doi.org/10.3390/ijms25179520





