Occurrence of Hepatozoon in Some Reptiles from Brazilian Biomes with Molecular and Morphological Characterization of Hepatozoon caimani
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Morphologic Detection of Parasites
2.3. Molecular Detection of Parasites
2.4. Phylogenetic Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Isolate | Host Species | Parasite Species | #GenBank® | Locality | Biome |
|---|---|---|---|---|---|
| 179 | Caiman yacare | Hepatozoon caimani | OR510629 | Nossa Senhora do Livramento—MT | Pantanal |
| 181 | Paleosuchus palpebrosus | Hepatozoon sp. | OR510639 | Nobre—MT | Brazilian Savanna |
| 285 | Caiman yacare | Hepatozoon sp. | OR510661 | Cáceres—MT | Pantanal |
| 286 | Caiman yacare | Hepatozoon caimani | OR510630 | Cáceres—MT | Pantanal |
| 288 | Caiman yacare | Hepatozoon caimani | OR510631 | Cáceres—MT | Pantanal |
| 289 | Caiman yacare | Hepatozoon sp. | OR510650 | Cáceres—MT | Pantanal |
| 290 | Caiman yacare | Hepatozoon sp. | OR510662 | Cáceres—MT | Pantanal |
| 292 | Caiman yacare | Hepatozoon caimani | OR510632 | Cáceres—MT | Pantanal |
| 293 | Caiman yacare | Hepatozoon sp. | OR510649 | Cáceres—MT | Pantanal |
| 294 | Caiman yacare | Hepatozoon sp. | OR510649 | Cáceres—MT | Pantanal |
| 296 | Caiman yacare | Hepatozoon sp. | OR510642 | Cáceres—MT | Pantanal |
| 297 | Caiman yacare | Hepatozoon sp. | OR510646 | Cáceres—MT | Pantanal |
| 299 | Caiman yacare | Hepatozoon caimani | OR510633 | Cáceres—MT | Pantanal |
| 300 | Caiman yacare | Hepatozoon sp. | OR510663 | Cáceres—MT | Pantanal |
| 302 | Paleosuchus palpebrosus | Hepatozoon sp. | OR510664 | Cáceres—MT | Pantanal |
| 303 | Paleosuchus palpebrosus | Hepatozoon sp. | OR510665 | Cáceres—MT | Pantanal |
| 304 | Paleosuchus palpebrosus | Hepatozoon sp. | OR510666 | Cáceres—MT | Pantanal |
| 306 | Paleosuchus palpebrosus | Hepatozoon sp. | OR510667 | Cáceres—MT | Pantanal |
| 309 | Caiman yacare | Hepatozoon sp. | OR510668 | Barra do Bugres—MT | Amazonia |
| 310 | Caiman yacare | Hepatozoon sp. | OR510654 | Barra do Bugres—MT | Amazonia |
| 312 | Caiman yacare | Hepatozoon sp. | OR510655 | Barra do Bugres—MT | Amazonia |
| 314 | Caiman yacare | Hepatozoon sp. | OR510669 | Barra do Bugres—MT | Amazonia |
| 322 | Paleosuchus palpebrosus | Hepatozoon sp. | OR510641 | Porto Estrela—MT | Brazilian Savanna |
| 323 | Paleosuchus palpebrosus | Hepatozoon sp. | OR510670 | Cuiabá—MT | Brazilian Savanna |
| 325 | Caiman yacare | Hepatozoon caimani | OR510634 | Porto Estrela—MT | Brazilian Savanna |
| 326 | Caiman yacare | Hepatozoon sp. | OR510652 | Porto Estrela—MT | Brazilian Savanna |
| 329 | Caiman yacare | Hepatozoon caimani | OR510635 | Porto Estrela—MT | Brazilian Savanna |
| 330 | Caiman yacare | Hepatozoon sp. | OR510656 | Porto Estrela—MT | Brazilian Savanna |
| 331 | Caiman yacare | Hepatozoon sp. | OR510657 | Porto Estrela—MT | Brazilian Savanna |
| 332 | Caiman yacare | Hepatozoon sp. | OR510644 | Porto Estrela—MT | Brazilian Savanna |
| 333 | Caiman yacare | Hepatozoon sp. | OR510645 | Porto Estrela—MT | Brazilian Savanna |
| 334 | Caiman yacare | Hepatozoon caimani | OR510636 | Porto Estrela—MT | Brazilian Savanna |
| 335 | Caiman yacare | Hepatozoon sp. | OR510647 | Porto Estrela—MT | Brazilian Savanna |
| 337 | Caiman yacare | Hepatozoon sp. | OR510643 | Porto Estrela—MT | Brazilian Savanna |
| 338 | Caiman yacare | Hepatozoon caimani | OR510637 | Porto Estrela—MT | Brazilian Savanna |
| 339 | Caiman yacare | Hepatozoon sp. | OR510671 | Porto Estrela—MT | Brazilian Savanna |
| 340 | Caiman yacare | Hepatozoon caimani | OR510638 | Porto Estrela—MT | Brazilian Savanna |
| 341 | Caiman yacare | Hepatozoon sp. | OR510658 | Porto Estrela—MT | Brazilian Savanna |
| 342 | Caiman yacare | Hepatozoon sp. | OR510659 | Porto Estrela—MT | Brazilian Savanna |
| 343 | Caiman yacare | Hepatozoon sp. | OR510660 | Porto Estrela—MT | Brazilian Savanna |
| 344 | Caiman yacare | Hepatozoon sp. | OR510653 | Porto Estrela—MT | Brazilian Savanna |
| 345 | Caiman yacare | Hepatozoon sp. | OR510648 | Porto Estrela—MT | Brazilian Savanna |
| 346 | Paleosuchus palpebrosus | Hepatozoon sp. | OR510639 | Nobre—MT | Brazilian Savanna |
| Hepatozoon (This Study) | Hepatozoon caimani Soares et al. 2017 | Hepatozoon caimani Bouer et al. 2017 [3] | ||||
|---|---|---|---|---|---|---|
| Free stages (N = 50) | ||||||
| SD | (Min–Max) values | Confidence intervals (95%) | SD | (Min–Max) values | SD | |
| Area | 55.319 ± 7.010 | (45.066–77.258) | 55.319 ± 1.586 | 50.689 ± 7.159 | (31.274–77.127) | - |
| Length | 21.277 ± 1.429 | (18.586–25.301) | 21.277 ± 0.323 | 23.891 ± 3.978 | (14.674–34.183) | - |
| Width | 2.974 ± 0.264 | (2.298–3.556) | 2.974 ± 0.0597 | 2.809 ± 0.650 | (1.251–6.292) | - |
| Nucleus area | 14.971 ± 2.832 | (12.079–24.005) | 14.971 ± 0.641 | 18.002 ± 3.917 | (10.254–31.229) | - |
| Nucleus length | 6.040 ± 1.053 | (4.739–9.215) | 6.040 ± 0.238 | 7.027 ± 1.627 | (2.806–13.225) | - |
| Nucleus width | 2.974 ± 0.264 | (2.298–3.556) | 2.974 ± 0.0597 | 2.752 ± 0.721 | (1.073–6.455) | - |
| Intraerythrocytic stages (N = 50) | ||||||
| (Min–Max) values | (Min–Max) values | |||||
| Area | 55.73 ± 10.123 | (33.456–78.023) | 55.731 ± 2.806 | 29.92 ± 4.588 | (22.585–52.082) | 53.20 ± 14.6 |
| Length | 12.97 ± 1.962 | (10.197–21.143) | 12.971 ± 0.544 | 12.45 ± 1.797 | (8.860–22.274) | 12.90 ± 1.6 |
| Width | 5.15 ± 0.925 | (3.355–9.953) | 5.152 ± 0.256 | 4.11 ± 1.001 | (2.200–8.318) | 4.81 ± 1.1 |
| Nucleus area | 13.95 ± 4.291 | (7.641–24.381) | 13.951 ± 1.189 | 16.15 ± 3.445 | (10.518–32.453) | 13.10 ± 4.71 |
| Nucleus length | 5.30 ± 1.222 | (3.456–8.261) | 5.301 ± 0.339 | 5.90 ± 1.674 | (2.410–12.873) | 5.67 ± 1.5 |
| Nucleus width | 3.39 ± 0.797 | (1.989–5.767) | 3.393 ± 0.221 | 3.08 ± 1.038 | (1.403–6.025) | 2.73 ± 0.88 |
References
- Telford, S.R. Hemoparasites of the Reptilia: Color Atlas and Text; CRC Press: Boca Raton, FL, USA; London, UK, 2009; ISBN 978-1-4200-8040-7. [Google Scholar]
- Smith, T.G. The genus Hepatozoon (Apicomplexa: Adeleina). J. Parasitol. 1996, 82, 565–585. [Google Scholar] [CrossRef] [PubMed]
- Bouer, A.; André, M.R.; Gonçalves, L.R.; Luzzi, M.D.C.; Oliveira, J.P.D.; Rodrigues, A.C.; Varani, A.D.M.; Miranda, V.F.O.D.; Perles, L.; Werther, K.; et al. Hepatozoon caimani in Caiman crocodilus yacare (Crocodylia, Alligatoridae) from North Pantanal, Brazil. Rev. Bras. Parasitol. Vet. 2017, 26, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Duszynski, D.W.; McAllister, C.T.; Tellez, M. The Coccidia (Apicomplexa) of the Archosauria (Crocodylia: Eusuchia) of the World. J. Parasitol. 2020, 106, 90. [Google Scholar] [CrossRef] [PubMed]
- Lainson, R.; Paperna, I.; Naiff, R.D. Development of Hepatozoon caimani (Carini, 1909) Pessôa, De Biasi & De Souza, 1972 in the Caiman caiman c. crocodilus, the frog Rana catesbeiana and the mosquito Culex fatigans. Mem. Inst. Oswaldo Cruz 2003, 98, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Sloboda, M.; Kamler, M.; Bulantová, J.; Votýpka, J.; Modrý, D. A new species of Hepatozoon (Apicomplexa: Adeleorina) from Python regius (Serpentes: Pythonidae) and its experimental transmission by a mosquito vector. J. Parasitol. 2007, 93, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Baneth, G. Perspectives on canine and feline hepatozoonosis. Vet. Parasitol. 2011, 181, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Uiterwijk, M.; Vojta, L.; Šprem, N.; Beck, A.; Jurković, D.; Kik, M.; Duscher, G.G.; Hodžić, A.; Reljić, S.; Sprong, H.; et al. Diversity of Hepatozoon species in wild mammals and ticks in Europe. Parasit. Vectors 2023, 16, 27. [Google Scholar] [CrossRef]
- Moço, T.C.; O’Dwyer, L.H.; Vilela, F.C.; Barrella, T.H.; Silva, R.J.D. Morphologic and morphometric analysis of Hepatozoon spp. (Apicomplexa, Hepatozoidae) of snakes. Mem. Inst. Oswaldo Cruz 2002, 97, 1169–1176. [Google Scholar] [CrossRef]
- Viana, L.A.; Paiva, F.; Coutinho, M.E.; Lourenço-de-Oliveira, R. Hepatozoon caimani (Apicomplexa: Hepatozoidae) in wild caiman, Caiman yacare, from the Pantanal Region, Brazil. J. Parasitol. 2010, 96, 83–88. [Google Scholar] [CrossRef]
- Muriel, J.; González-Blázquez, M.; Matta, N.E.; Vargas-León, C.M. Parasitas Sanguíneos de Anfíbios; Teresina, PI, EDUFPI; UFPI: Grand Rapids, MI, USA, 2021; ISBN 978-65-5904-067-4. Available online: https://www.ufpi.br/arquivos_download/arquivos/edufpi/eBook_Parasitas_Sangu%C3%ADneos_de_Anf%C3%ADbios1.pdf (accessed on 10 October 2023).
- O’Dwyer, L.H.; Moço, T.C.; Paduan, K.D.S.; Spenassatto, C.; Da Silva, R.J.; Ribolla, P.E.M. Description of three new species of Hepatozoon (Apicomplexa, Hepatozoidae) from rattlesnakes (Crotalus durissus terrificus) based on molecular, morphometric and morphologic characters. Exp. Parasitol. 2013, 135, 200–207. [Google Scholar] [CrossRef]
- Ferreira, F.C.; Alves, L.G.M.; Jager, G.B.; Franzini, L.D.; Mesquita, D.O.; Díaz-Delgado, J.; Catão-Dias, J.L.; Braga, É.M. Molecular and pathological investigations of Plasmodium parasites infecting striped forest whiptail lizards (Kentropyx calcarata) in Brazil. Parasitol. Res. 2020, 119, 2631–2640. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.S.; Bahiense, T.C.; Silva, A.A.B.; Onofrio, V.C.; Barral, T.D.; Souza, B.M.P.; Lira-da-Silva, R.M.; Biondi, I.; Meyer, R.; Portela, R.W. Ticks and associated pathogens from rescued wild animals in rainforest fragments of northeastern Brazil. Front. Vet. Sci. 2020, 7, 177. [Google Scholar] [CrossRef] [PubMed]
- Úngari, L.P.; Santos, A.L.Q.; O’Dwyer, L.H.; Da Silva, M.R.L.; De Melo Fava, N.N.; Paiva, G.C.M.; De Melo Costa Pinto, R.; Cury, M.C. Haemogregarina podocnemis sp. nov.: Description of a New Species of Haemogregarina Danilewsky 1885 (Adeleina: Haemogregarinaidae) in Free-Living and Captive Yellow-Spotted River Turtles Podocnemis unifilis (Testudines: Podocnemididae) from Brazil. Parasitol. Res. 2018, 117, 1535–1548. [Google Scholar] [CrossRef] [PubMed]
- Úngari, L.P.; Netherlands, E.C.; Quagliatto Santos, A.L.; Paulino De Alcantara, E.; Emmerich, E.; Da Silva, R.J.; O’Dwyer, L.H. New insights on the diversity of Brazilian anuran blood parasites: With the description of three new species of Hepatozoon (Apicomplexa: Hepatozoidae) from Leptodactylidae anurans. Int. J. Parasitol. Parasites Wildl. 2021, 14, 190–201. [Google Scholar] [CrossRef]
- Jameie, F.; Nasiri, V.; Paykari, H. Morphological detection and molecular characterization of Hepatozoon spp. from venomous terrestrial snakes in Iran. Exp. Parasitol. 2022, 239, 108309. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Lainson, R. Atlas of Protozoan Parasites of the Amazonian Fauna of Brazil; Instituto Evandro Chagas: Ananindeua, Brazil, 2012; ISBN 978-85-60420-07-0. [Google Scholar]
- Ujvari, B.; Madsen, T.; Olsson, M. High prevalence of Hepatozoon spp. (Apicomplexa, Hepatozoidae) infection in water pythons (Liasis fuscus) from tropical Australia. J. Parasitol. 2004, 90, 670–672. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian Inference of Phylogenetic Trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree: Tree Figure Drawing Tool Version 1.4.0; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2010; Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 10 October 2023).
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Soares, P.; Borghesan, T.C.; Tavares, L.E.R.; Ferreira, V.L.; Teixeira, M.M.G.; Paiva, F. Hepatozoon caimani Carini, 1909 (Adeleina: Hepatozoidae) in wild population of Caiman yacare Daudin, 1801 (Crocodylia: Alligatoridae), Pantanal, Brazil. Parasitol. Res. 2017, 116, 1907–1916. [Google Scholar] [CrossRef]
- Lima, I.G.S.; Felix-Nascimento, G.; Picelli, A.M.; Ribeiro, L.B. Contagem diferencial e morfometria de células sanguíneas nos lagartos Ameivula ocellifera (Squamata: Teiidae) e Tropidurus hispidus (Squamata: Tropiduridae) do semiárido brasileiro, com análise dos efeitos por hemoparasitos. Cuad. Herpetol. 2021, 35, 109–119. Available online: https://issuu.com/cuadernosdeherpetologia/docs/v35n1 (accessed on 22 November 2023).
- Harris, D.J.; Borges-Nojosa, D.M.; Maia, J.P. Prevalence and diversity of Hepatozoon in native and exotic geckos from Brazil. J. Parasitol. 2015, 101, 80–85. [Google Scholar] [CrossRef]
- Viana, L.A.; Marques, E.J. Haemogregarine parasites (Apicomplexa: Hepatozoidae) in Caiman crocodilus yacare (Crocodilia: Alligatoridae) from Pantanal, Corumbá, MS, Brazil. Rev. Bras. Parasitol. Vet. 2005, 14, 173–175. [Google Scholar]
- Carini, A. Sur une hémogrégarine du Caiman latirostris Daud. Bull. Soc. Pathol. Exot. 1909, 2, 471–472. [Google Scholar]
- Zechmeisterová, K.; Javanbakht, H.; Kvičerová, J.; Široký, P. Against growing synonymy: Identification pitfalls of Hepatozoon and Schellackia demonstrated on North Iranian reptiles. Eur. J. Protistol. 2021, 79, 125780. [Google Scholar] [CrossRef] [PubMed]
- Lainson, R. Trypanosoma cecili n. sp., a parasite of the south american cayman Caiman crocodilus crocodilus (Linnaeus, 1758) (Crocodilia: Alligatoridae). In Protozoology; Clunbury Cottrell Press: Berkhampstead, UK, 1977; Volume III, pp. 87–93. [Google Scholar]
- Campos, Z. Caiman crocodilus yacare. Food-related movement. Herp. Rev. 2003, 34, 141. [Google Scholar]
- Campos, Z.; Coutinho, M.; Magnusson, W. Terrestrial activity of caiman in the Pantanal, Brazil. Copeia 2003, 2003, 628–634. [Google Scholar] [CrossRef]
- Anderson, R.M.; May, R.M. Population biology of infectious diseases: Part I. Nature 1979, 280, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; May, R.M. The population dynamics of microparasites and their invertebrate hosts. Philos. Trans. R. Soc. Lond. B 1981, 291, 451–524. [Google Scholar]
- McCallum, H.; Barlow, N.; Hone, J. How should pathogen transmission be modelled? Trends Ecol. Evol. 2001, 16, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Landau, I.; Michel, J.C.; Chabaud, A.G. Cycle biologique d’Hepatozoon domerguei; discussion sur les caractères fondamentaux d’un cycle de Coccidie. Parasitol. Res. 1972, 38, 250–270. [Google Scholar]
- Projeto MapBiomas—Coleção [v. 7.1] da Série Anual de Mapas de Cobertura e Uso da Terra do Brasil. Available online: https://plataforma.brasil.mapbiomas.org/ (accessed on 7 August 2023).
- Harris, D.J.; Maia, J.P.; Perera, A. Molecular characterization of Hepatozoon species in reptiles from the Seychelles. J. Parasitol. 2011, 97, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Viana, L.A.; Soares, P.; Silva, J.E.; Paiva, F.; Coutinho, M.E. Anurans as paratenic hosts in the transmission of Hepatozoon caimani to caimans Caiman yacare and Caiman latirostris. Parasitol. Res. 2012, 110, 883–886. [Google Scholar] [CrossRef]
- Maia, J.P.; Harris, D.J.; Perera, A. Molecular survey of Hepatozoon species in lizards from North Africa. J. Parasitol. 2011, 97, 513–517. [Google Scholar] [CrossRef]
- Cardoso, W.A.; Perles, L.; Picelli, A.M.; Correa, J.K.C.; André, M.R.; Viana, L.A. Hepatozoon parasites (Apicomplexa: Hepatozoidae) in fish Hoplias aimara (Characiformes, Erythrinidae) from the Eastern Amazon, Brazil. Parasitol. Res. 2022, 121, 1041–1046. [Google Scholar] [CrossRef]
- Magnusson, W.E.; da Silva, E.V.; Lima, A.P. Diets of Amazonian Crocodilians. J. Herpetol. 1987, 21, 85–95. [Google Scholar] [CrossRef]
- Mudrek, J.R. Ecologia Populacional e Alimentar do Jacaré-Paguá Paleosuchus palpebrosus (Crocodylia: Alligatoridae) em Córregos Urbanos. 2016. 70 f. Dissertação (Mestrado em Ecologia e Conservação da Biodiversidade)—Universidade Federal de Mato Grosso, Instituto de Biociências, Cuiabá. 2016. Available online: https://ri.ufmt.br/handle/1/1733 (accessed on 10 October 2023).
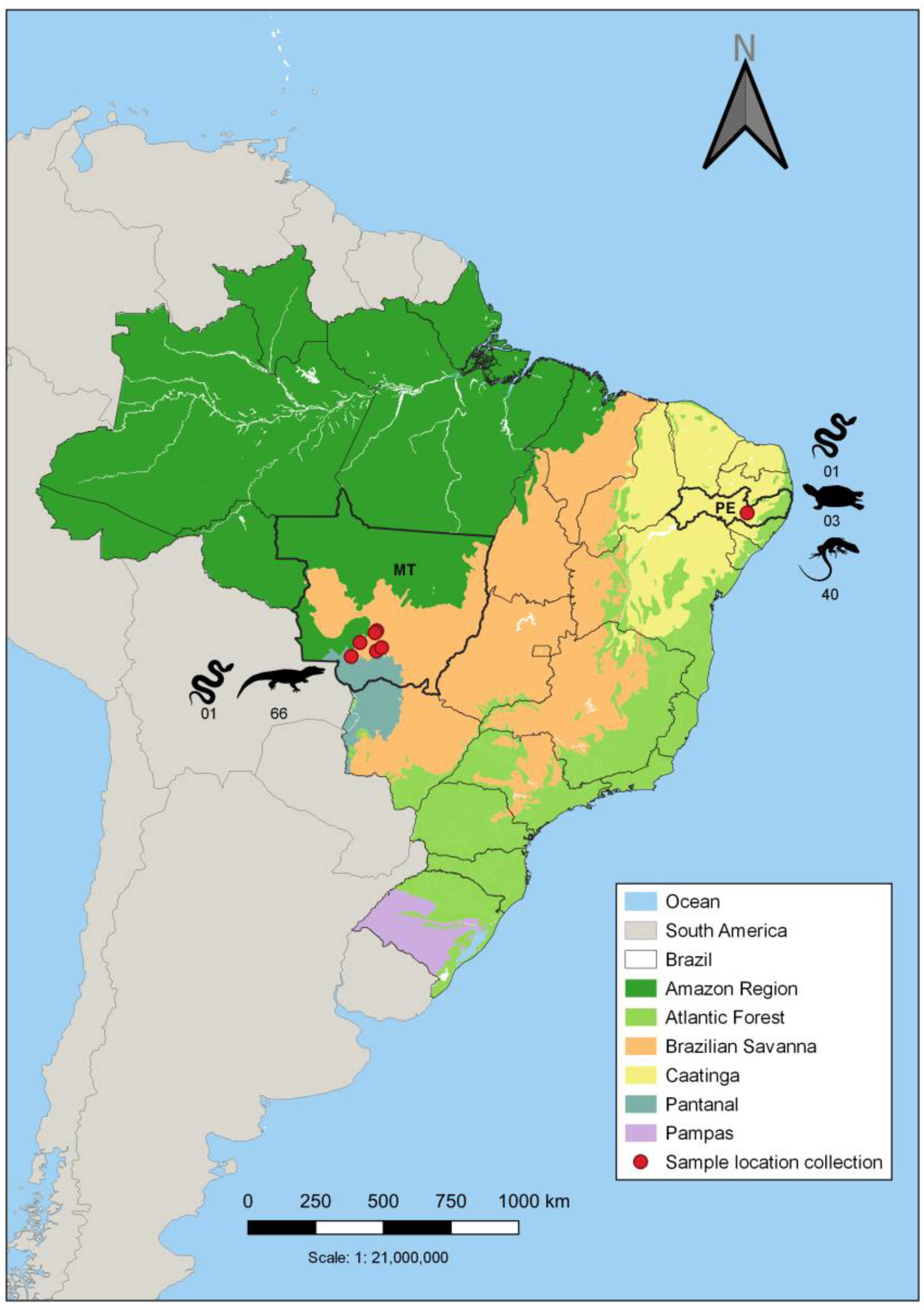

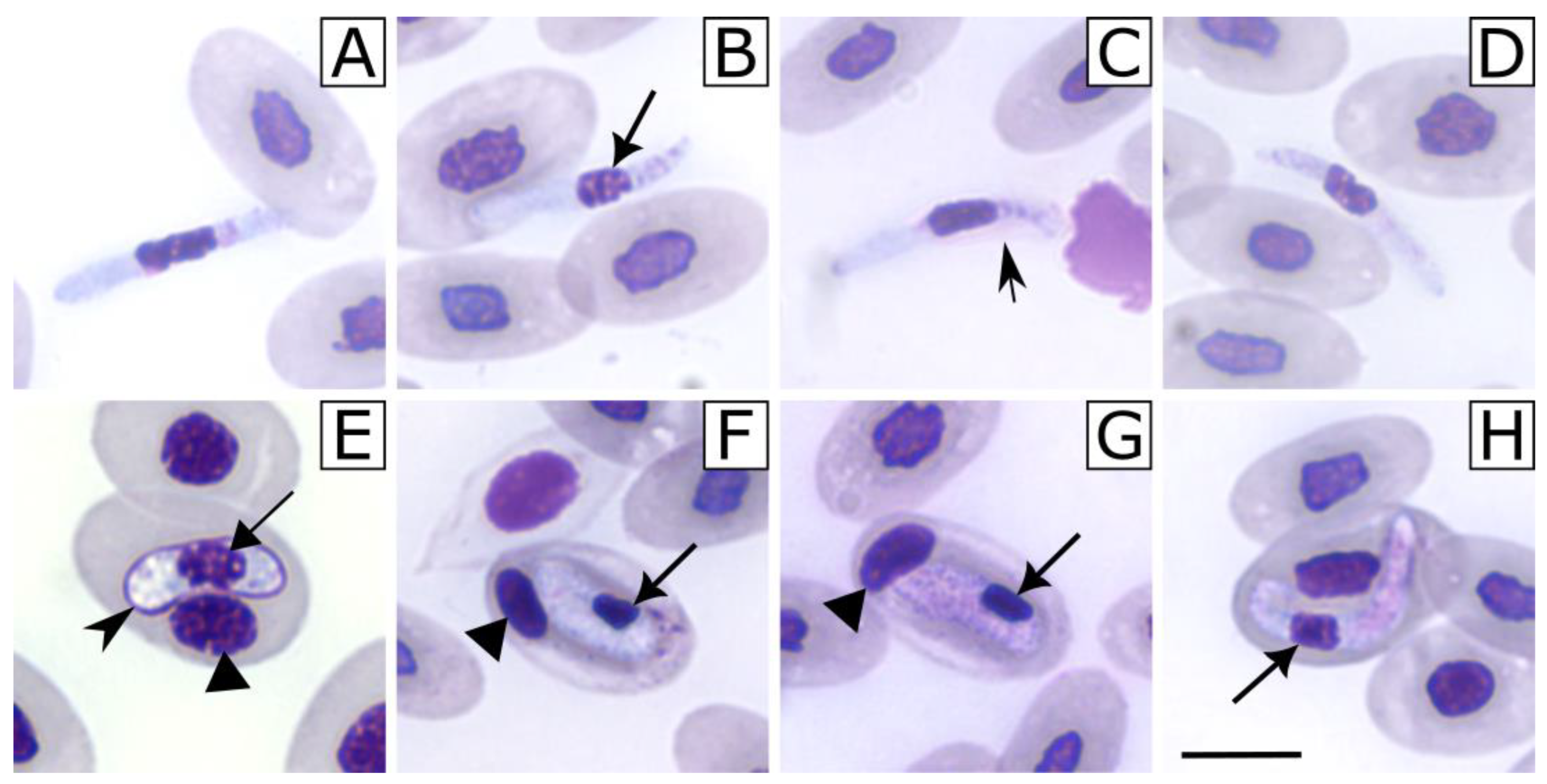
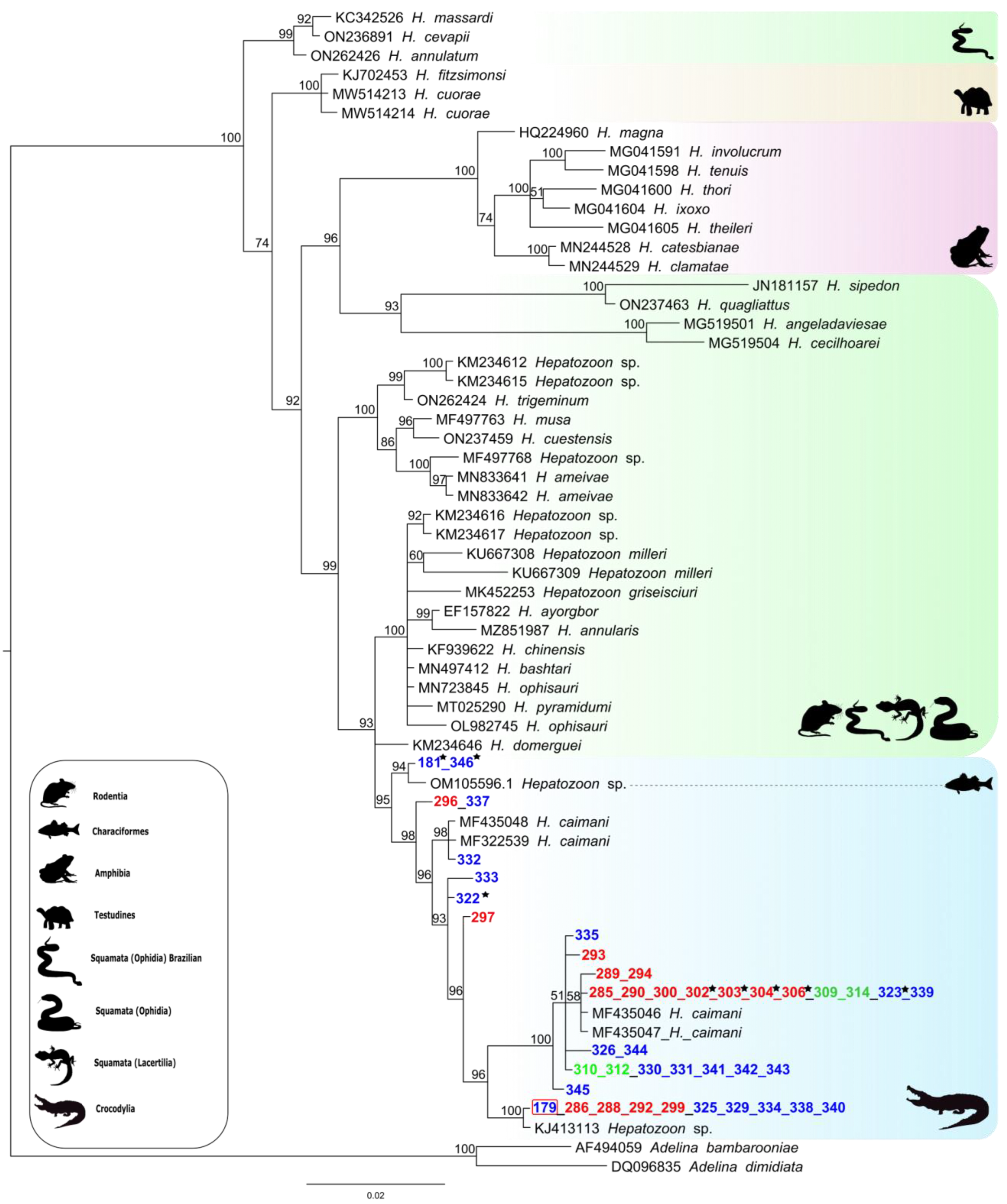
| Order Family | Host Species | PCR Samples (Infected) | Microscopy Samples (Infected) | Biome * (Number of Hepatozoon Occurrences) |
|---|---|---|---|---|
| Crocodylia | ||||
| Alligatoridae | Caiman crocodilus | 2 | 2 | BS (0) |
| Caiman yacare | 47 (43) | 21 (19) | AM (5), BS (20), PA (18) | |
| Paleosuchus palpebrosus | 17 (9) | 10 (3) | AM (1), BS (4), PA (4) | |
| Chelonia | ||||
| Kinosternidae | Kinosternon scorpioides | 3 | 3 | CA (0) |
| Squamata | ||||
| Dipsadidae | Erythrolamprus poecilogyrus | 1 | 0 | BS (0) |
| Oxyrhopus trigeminus | 1 | 1 | CA (0) | |
| Polychrotidae | Polychrus acutirostris | 1 | 1 | CA (0) |
| Teiidae | Ameivula ocellifera | 16 | 6 | CA (0) |
| Tropiduridae | Tropidurus cocorobensis | 23 | 9 | CA (0) |
| Total | 111 (52) | 53 (22) |
| Hepatozoon (This Study) | H. caimani | ||||
|---|---|---|---|---|---|
| Soares et al. 2017 [25] | Bouer et al. 2017 [3] | ||||
| Free stages (N = 50) | |||||
| (Min–Max) values | (Min–Max) values | ||||
| Area | 55.319 ± 7.010 (µm2) | (45.066–77.258) | 50.689 ± 7.159 (µm2) | (31.274–77.127) | |
| Length | 21.277 ± 1.429 | (18.586–25.301) | 23.891 ± 3.978 | (14.674–34.183) | |
| Width | 2.974 ± 0.264 | (2.298–3.556) | 2.809 ± 0.650 | (1.251–6.292) | |
| Nucleus area | 14.971 ± 2.832 (µm2) | (12.079–24.005) | 18.002 ± 3.917 (µm2) | (10.254–31.229) | |
| Nucleus length | 6.040 ± 1.053 | (4.739–9.215) | 7.027 ± 1.627 | (2.806–13.225) | |
| Nucleus width | 2.974 ± 0.264 | (2.298–3.556) | 2.752 ± 0.721 | (1.073–6.455) | |
| Intraerythrocytic gamonts (N = 50) | |||||
| (Min–Max) values | (Min–Max) values | ||||
| Area | 55.731 ± 10.123 (µm2) | (33.456–78.023) | 29.922 ± 4.588 (µm2) | (22.585–52.082) | 53.2 ± 14.6 (µm2) |
| Length | 12.971 ± 1.962 | (10.197–21.143) | 12.449 ± 1.797 | (8.860–22.274) | 12.9 ± 1.6 |
| Width | 5.152 ± 0.925 | (3.355–9.953) | 4.111 ± 1.001 | (2.200–8.318) | 4.81 ± 1.1 |
| Nucleus area | 13.951 ± 4.291 (µm2) | (7.641–24.381) | 16.150 ± 3.445 (µm2) | (10.518–32.453) | 13.1 ± 4.71 (µm2) |
| Nucleus length | 5.301 ± 1.222 | (3.456–8.261) | 5.895 ± 1.674 | (2.410–12.873) | 5.67 ± 1.5 |
| Nucleus width | 3.393 ± 0.797 | (1.989–5.767) | 3.080 ± 1.038 | (1.403–6.025) | 2.73 ± 0.88 |
| Single Nucleotide Polymorphisms (SNPs) | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 4 | 4 | 5 | 5 | 5 | |||
| 9 | 7 | 1 | 1 | 4 | 5 | 5 | 5 | 6 | 6 | 7 | 8 | 8 | 8 | 8 | 8 | 3 | 3 | 1 | 1 | 2 | ||
| Haplotype | 8 | 9 | 5 | 9 | 0 | 3 | 4 | 7 | 4 | 5 | 6 | 0 | 1 | 4 | 5 | 8 | 3 | 8 | 4 | 8 | 4 | |
| 1 | 179/286/288/292/299/325/329/334/338/340 | T | G | A | G | T | C | C | T | T | A | T | A | A | T | A | A | G | T | G | G | A |
| 2 | 181/346 | . | . | . | A | . | . | . | . | . | T | C | . | . | A | G | . | . | C | . | . | G |
| 3 | 285/290/300/302/303/304/306/323/309/314/339 | C | A | . | A | C | . | . | G | . | C | . | . | G | . | . | T | . | C | A | A | . |
| 4 | 289/294 | C | A | . | A | C | . | . | G | . | C | . | . | . | . | . | T | . | C | A | A | . |
| 5 | 293 | C | A | . | A | C | . | . | G | . | T | . | . | G | . | . | T | . | C | . | A | . |
| 6 | 296/337 | . | . | . | A | C | T | . | . | C | T | . | . | . | A | G | . | . | C | . | . | G |
| 7 | 297 | . | . | . | A | C | . | . | . | C | . | . | . | . | . | . | . | . | C | . | . | G |
| 8 | 310/312/330/331/341/342/343 | C | A | . | A | C | . | . | G | . | C | . | . | G | . | . | T | . | C | . | A | . |
| 9 | 322 | . | . | . | A | C | . | . | . | C | . | . | . | . | A | . | . | . | C | . | . | G |
| 10 | 326/344 | C | A | T | A | C | . | T | G | . | C | . | . | G | . | . | T | . | C | . | A | . |
| 11 | 332 | . | . | . | A | C | . | . | . | C | . | . | T | . | A | G | . | . | C | . | . | G |
| 12 | 333 | . | . | . | A | C | . | . | . | C | . | . | . | . | A | . | . | . | C | A | A | G |
| 13 | 335 | C | A | . | A | C | . | . | G | . | C | . | . | . | . | . | T | . | C | . | A | . |
| 14 | 345 | C | A | . | A | C | . | . | G | . | T | . | . | . | . | . | T | . | C | . | A | . |
| 1 | KJ413113 Hepatozoon sp. | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| 3 | MF435046/MF435047 H. caimani | C | A | . | A | C | . | . | G | . | C | . | . | G | . | . | T | . | C | A | A | . |
| 11 | MF322538/MF322539/MF435048 H. caimani | . | . | . | A | C | . | . | . | C | . | . | T | . | A | G | . | . | C | . | . | G |
| 15 | OM105596 Hepatozoon sp. | . | . | . | A | . | . | . | . | . | T | C | . | . | A | G | . | A | C | - | - | - |
| Amphibia | Crocodylia 1 * | Crocodylia 2A * | Crocodylia 2B * | Crocodylia 2C * | Squamata | Squamata (Ophidia) Brazilian | Testudines | Outgroup Adelina spp. | |
|---|---|---|---|---|---|---|---|---|---|
| Amphibia | 0.02 | ||||||||
| Crocodylia 1 * | 0.06 | 0.00 | |||||||
| Crocodylia 2A * | 0.06 | 0.01 | 0.00 | ||||||
| Crocodylia 2B * | 0.07 | 0.02 | 0.02 | 0.00 | |||||
| Crocodylia 2C * | 0.06 | 0.01 | 0.01 | 0.02 | 0.00 | ||||
| Squamata | 0.06 | 0.03 | 0.04 | 0.04 | 0.04 | 0.04 | |||
| Squamata (Ophidia) Brazilian | 0.05 | 0.03 | 0.03 | 0.04 | 0.03 | 0.04 | 0.00 | ||
| Testudines | 0.05 | 0.03 | 0.03 | 0.04 | 0.03 | 0.04 | 0.02 | 0.00 | |
| Outgroup Adelina spp. | 0.15 | 0.12 | 0.13 | 0.13 | 0.12 | 0.13 | 0.11 | 0.11 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clemente, G.R.C.; Gutierrez-Liberato, G.A.; Anjos, C.C.; Simões, P.I.; Mudrek, J.R.; Fecchio, A.; Lima, J.H.A.; Oliveira, P.M.A.; Pinho, J.B.; Mathias, B.S.; et al. Occurrence of Hepatozoon in Some Reptiles from Brazilian Biomes with Molecular and Morphological Characterization of Hepatozoon caimani. Diversity 2023, 15, 1192. https://doi.org/10.3390/d15121192
Clemente GRC, Gutierrez-Liberato GA, Anjos CC, Simões PI, Mudrek JR, Fecchio A, Lima JHA, Oliveira PMA, Pinho JB, Mathias BS, et al. Occurrence of Hepatozoon in Some Reptiles from Brazilian Biomes with Molecular and Morphological Characterization of Hepatozoon caimani. Diversity. 2023; 15(12):1192. https://doi.org/10.3390/d15121192
Chicago/Turabian StyleClemente, Gabriella R. C., Germán A. Gutierrez-Liberato, Carolina C. Anjos, Pedro I. Simões, Jessica R. Mudrek, Alan Fecchio, José H. A. Lima, Patricia M. A. Oliveira, João B. Pinho, Bruno S. Mathias, and et al. 2023. "Occurrence of Hepatozoon in Some Reptiles from Brazilian Biomes with Molecular and Morphological Characterization of Hepatozoon caimani" Diversity 15, no. 12: 1192. https://doi.org/10.3390/d15121192
APA StyleClemente, G. R. C., Gutierrez-Liberato, G. A., Anjos, C. C., Simões, P. I., Mudrek, J. R., Fecchio, A., Lima, J. H. A., Oliveira, P. M. A., Pinho, J. B., Mathias, B. S., Guimarães, L. O., & Kirchgatter, K. (2023). Occurrence of Hepatozoon in Some Reptiles from Brazilian Biomes with Molecular and Morphological Characterization of Hepatozoon caimani. Diversity, 15(12), 1192. https://doi.org/10.3390/d15121192











