Macrozoobenthic Diversity along an Oxygen Gradient in the Deep Trough of the Gulf of St. Lawrence (Canada)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Statistical Analyses
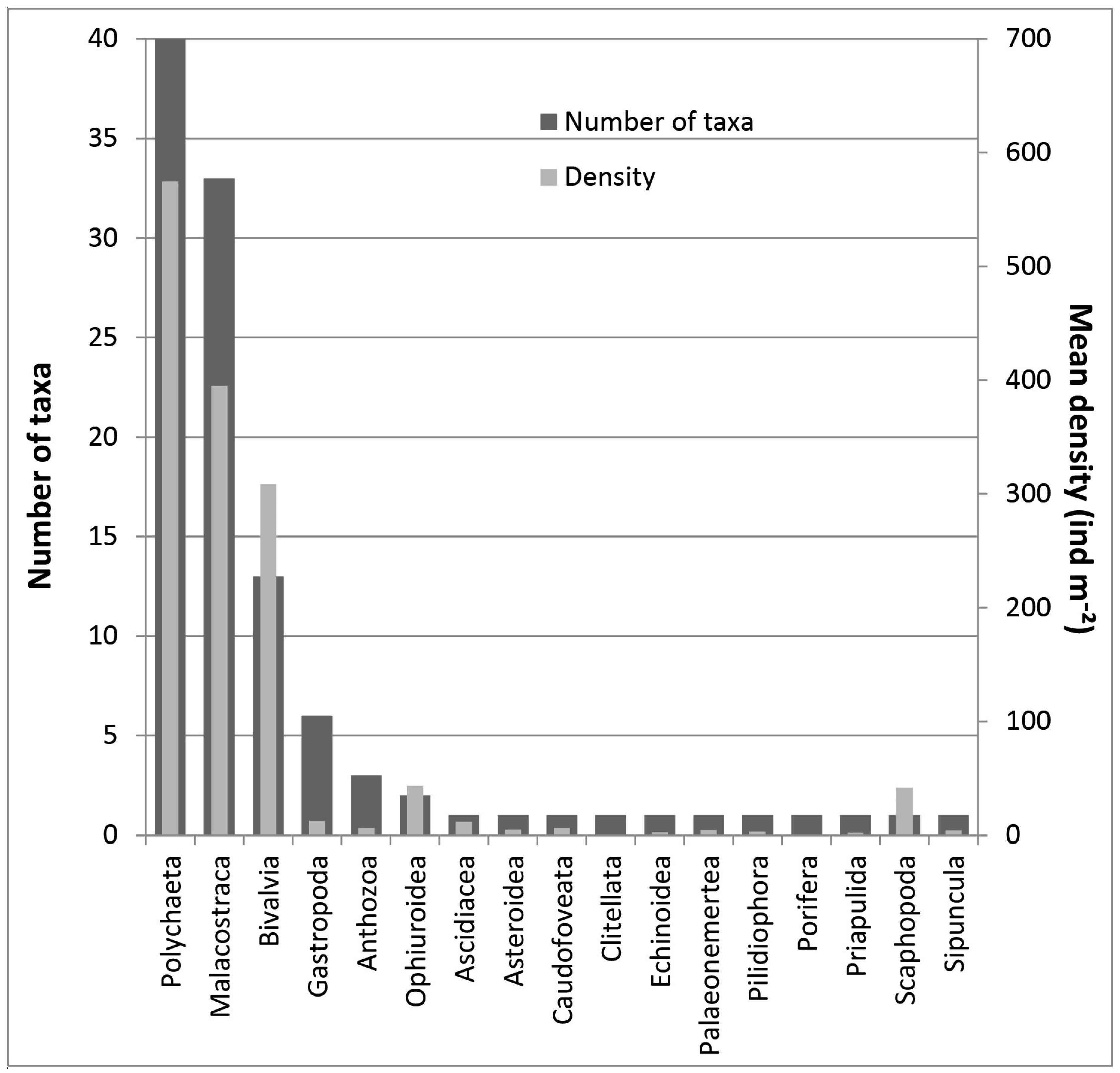
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levin, L.A.; Ekau, W.; Gooday, A.J.; Jorissen, F.; Middelburg, J.J.; Naqvi, S.W.A.; Neira, C.; Rabalais, N.N.; Zhan, J. Effects of natural and human-induced hypoxia on coastal benthos. Biogeosciences 2009, 6, 2063–2098. [Google Scholar] [CrossRef]
- Zhang, J.; Gilbert, D.; Gooday, A.J.; Levin, L.; Naqvi, S.W.A.; Middelburg, J.J.; Scranton, M.; Ekau, W.; Peña, A.; Dewitte, B.; et al. Natural and human-induced hypoxia and consequences for coastal areas: Synthesis and future development. Biogeosciences 2010, 7, 1443–1467. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Cai, W.-J.; Carstensen, J.; Conley, D.J.; Fry, B.; Hu, X.; Quiñones-Rivera, Z.; Rosenberg, R.; Slomp, C.; Turner, E.; et al. Eutrophication-driven deoxygenation in the coastal ocean. Oceanography 2014, 27, 172–183. [Google Scholar] [CrossRef]
- Breitburg, D.; Levin, L.A.; Oschlies, A.; Grégoire, M.; Chavez, F.P.; Conley, D.J.; Garçon, V.; Gilbert, D.; Gutiérrez, D.; Isensee, K.; et al. Declining oxygen in the global ocean and coastal waters. Science 2018, 359, eaam7240. [Google Scholar] [CrossRef]
- Borges, F.O.; Sampaio, E.; Santos, C.P.; Rosa, R. Impacts of low oxygen on marine life: Neglected, but a crucial priority for research. Biol. Bull. 2022, 243, 104–119. [Google Scholar] [CrossRef]
- Levin, L.A. Oxygen minimum zone benthos: Adaptation and community response to hypoxia. In Oceanography and Marine Biology; CRC Press: Boca Raton, FL, USA, 2003; Volume 41, pp. 1–45. [Google Scholar]
- Shi, Z.; Assis, J.; Costello, M.J. Vulnerability of marine species to low oxygen under climate change. Imperiled Encycl. Conserv. 2022, 887–894. [Google Scholar] [CrossRef]
- Amorim, K.; Piontkivska, H.; Zettler, M.L.; Sokolov, E.; Hinzke, T.; Nai, A.M.; Sokolova, I.M. Transcriptional response of key metabolic and stress response genes of a nuculanid bivalve, Lembulus bicuspidatus from an oxygen minimum zone exposed to hypoxia-reoxygenation. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2021, 256, 110670. [Google Scholar] [CrossRef] [PubMed]
- Amorim, K.; Loick-Wilde, N.; Yuen, B.; Osvatic, J.T.; Wäge-Recchioni, J.; Hausmann, B.; Petersen, J.M.; Fabian, J.; Wodarg, D.; Zettler, M.L. Chemoautotrophy, symbiosis and sedimented diatoms support high biomass of benthic molluscs in the Namibian shelf. Sci. Rep. 2022, 12, 9731. [Google Scholar] [CrossRef] [PubMed]
- Gogina, M.; Darr, A.; Zettler, M.L. Approach to assess consequences of hypoxia disturbance events for benthic ecosystem functioning. J. Mar. Syst. 2014, 129, 203–213. [Google Scholar] [CrossRef]
- Berger, W.H.; Lange, C.B.; Wefer, G. Upwelling history of the Benguela-Namibia system: A synthesis of Leg 175 results. Proc. Ocean Drill. Progr. Scient. Res. 2002, 175, 1–103. [Google Scholar]
- Amorim, K.; Zettler, M.L. Gradients and instability: Macrozoobenthic communities in the Benguela Upwelling System off Namibia. Estuar. Coast. Shelf. Sci. 2023, 291, 108421. [Google Scholar] [CrossRef]
- Quiroga, E.; Quiñones, R.; Palma, M.; Sellanes, J.; Gallardo, V.A.; Gerdes, D.; Rowe, G. Biomass size-spectra of macrobenthic communities in the oxygen minimum zone off Chile. Estuar Coast. Shelf Sci. 2005, 62, 217–231. [Google Scholar] [CrossRef]
- Palma, M.; Quiroga, E.; Gallardo, V.A.; Arntz, W.; Gerdes, D.; Schneider, W.; Hebbeln, D. Macrobenthic animal assemblages of the continental margin off Chile (22° to 42°S). J. Mar. Biol. Ass. U. K. 2005, 85, 233–245. [Google Scholar] [CrossRef]
- Gilbert, D.; Sundby, B.; Gobeil, C.; Mucci, A.; Tremblay, G.H. A seventy-two-year record of diminishing deep-water oxygen in the St. Lawrence estuary: The northwest Atlantic connection. Limnol. Oceanogr. 2005, 50, 1654–1666. [Google Scholar] [CrossRef]
- Jutras, M.; Mucci, A.; Chaillou, G.; Nesbitt, W.A.; Wallace, D.W.R. Temporal and spatial evolution of bottom-water hypoxia in the Estuary and Gulf of St. Lawrence. Biogeosciences 2023, 20, 839–849. [Google Scholar] [CrossRef]
- Lefort, S.; Gratton, Y.; Mucci, A.; Dadou, I.; Gilbert, D. Hypoxia in the Lower St. Lawrence Estuary: How physics controls spatial patterns. J. Geophys. Res. 2012, 117, C07018. [Google Scholar] [CrossRef]
- Bourgault, D.; Cyr, F. Hypoxia in the St. Lawrence Estuary: How a coding error led to the belief that “Physics Controls Spatial Patterns. PLoS ONE 2015, 10, e0138858. [Google Scholar] [CrossRef] [PubMed]
- Dickie, L.; Trites, R. The Gulf of St. Lawrence. In Ecosystems of the World: Estuaries and Enclosed Seas; Ketchum, B., Ed.; Elsevier: New York, NY, USA, 1983; pp. 403–425. [Google Scholar]
- Saucier, J.-F.; Roy, F.; Gilbert, D.; Pellerin, P.; Ritchie, H. Modelling the formation and circulation processes of water masses and sea ice in the Gulf of St. Lawrence, Canada. J. Geophys. Res. 2003, 108, 3269–3289. [Google Scholar] [CrossRef]
- Belley, R.; Archambault, P.; Sundby, B.; Gilbert, F.; Gagnon, J.-M. Effects of hypoxia on benthic macrofauna and bioturbation in the Estuary and Gulf of St. Lawrence, Canada. Cont. Shelf Res. 2010, 30, 1302–1313. [Google Scholar] [CrossRef]
- Brunel, P.; Bossé, L.; Lamarche, G. Catalogue of the Marine Invertebrates of the Estuary and Gulf of Saint Lawrence; National Research Council of Canada: Ottawa, ON, Canada, 1998; p. 405. [Google Scholar]
- Chabot, D.; Rondeau, A.; Sainte-Marie, B.; Savard, L.; Surette, T.; Archambault, P. Distribution of Benthic Invertebrates in the Estuary and Gulf of St. Lawrence; Research Document; Canadian Science Advisory Secretariat (CSAS): Ottawa, ON, Canada, 2007; Volume 018, pp. 1–108. [Google Scholar]
- Moritz, C.; Lévesque, M.; Gravel, D.; Vaz, S.; Archambault, D.; Archambault, P. Modelling spatial distribution of epibenthic communities in the Gulf of St. Lawrence (Canada). J. Sea Res. 2013, 78, 5–84. [Google Scholar] [CrossRef]
- Nozères, C.; Bourassa, M.-N.; Gendron, M.-H.; Plourde, S.; Savenkoff, C.; Bourdages, H.; Benoît, H.; Bolduc, F. Using annual ecosystemic multispecies surveys to assess biodiversity in the Gulf of St. Lawrence. Can. Tech. Rep. Fish. Aquat. Sci. 2015, 3149, 126. [Google Scholar]
- Stortini, C.H.; Chabot, D.; Shackell, N.L. Marine species in ambient low-oxygen regions subject to double jeopardy impacts of climate change. Glob. Chang. Biol. 2016, 23, 2284–2296. [Google Scholar] [CrossRef]
- Bourdages, H.; Brassard, C.; Desgagnés, M.; Galbraith, P.; Gauthier, J.; Nozères, C.; Scallon-Chouinard, P.-M.; Senay, C. Preliminary Results from the Ecosystemic Survey in August 2019 in the Estuary and Northern Gulf of St. Lawrence; Research Document; Canadian Science Advisory Secretariat (CSAS): Ottawa, ON, Canada, 2020; Volume 009, pp. 1–93. [Google Scholar]
- Miatta, M.; Snelgrove, P.V.R. Benthic nutrient fluxes in deep-sea sediments within the Laurentian Channel MPA (eastern Canada): The relative roles of macrofauna, environment and sea pen octocorals. Deep-Sea Res. Part I 2021, 178, 103655. [Google Scholar] [CrossRef]
- Miatta, M.; Snelgrove, P.V.R. Sea pens as indicators of macrofaunal communities in deep-sea sediments: Evidence from the Laurentian Channel Marine Protected Area. Deep-Sea Res. Part I 2022, 182, 103702. [Google Scholar] [CrossRef]
- Loring, D.H.; Nota, D.J.G. Morphology and sediment of the Gulf of St. Lawrence; Bulletin of the Fisheries Research Board of Canada; Fisheries and Marine Service: Ottawa, ON, Canada, 1973; Volume 182, pp. 1–147. [Google Scholar]
- Clarke, K.R.; Corley, R.N. PRIMER v6: User Manual/Tutorial; Primer-E Primer: Plymouth, UK, 2006. [Google Scholar]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA). In Wiley StatsRef: Statistics Reference Online; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation; Plymouth Marine Laboratory: Plymouth, UK, 1994. [Google Scholar]
- Alkhatib, M.; Lehmann, M.F.; del Giorgio, P.A. The nitrogen isotope effect of benthic remineralization-nitrification-denitrification coupling in an estuarine environment. Biogeosciences 2012, 9, 1633–1646. [Google Scholar] [CrossRef]
- Galbraith, P.S.; Chassé, J.; Nicot, P.; Caverhill, C.; Gilbert, D.; Pettigrew, B.; Lefaivre, D.; Brickman, D.; Devine, L.; Lafleur, C. Physical Oceanographic Conditions in the Gulf of St. Lawrence in 2014; Research Document; Canadian Science Advisory Secretariat (CSAS): Ottawa, ON, Canada, 2015; Volume 032, pp. 1–87. [Google Scholar]
- Vaquer-Sunyer, R.; Duarte, C.M. Thresholds of hypoxia for marine biodiversity. Proc. Natl. Acad. Sci. USA 2008, 107, 15452–15457. [Google Scholar] [CrossRef]
- Anderson, S.J.; Taylor, A.C.; Atkinson, R.J.A. Anaerobic metabolism during anoxia in the burrowing shrimp Calocaris macandreae Bell (Crustacea, Thalassinidea). Comp. Biochem. Physiol. Part A 1994, 108, 515–522. [Google Scholar] [CrossRef]
- Dando, P.R.; Southward, A.J. Chemoautotrophy in bivalve molluscs of the genus Thyasira. J. Mar. Biol. Ass. U. K. 1986, 66, 915–929. [Google Scholar] [CrossRef]
- Holmes, A.; Graham, P.G.; Sellanes, J. A new species of Lucinoma (Bivalvia: Lucinoidea) from a methane gas seep off the southwest coast of Chile. J. Conch. 2005, 38, 673–682. [Google Scholar]
- Oliver, P.G.; Levin, L. A new species of the family Thyasiridae (Mollusca: Bivalvia) from the oxygen minimum zone of the Pakistan margin. J. Mar. Biol. Assoc. U. K. 2006, 86, 411–416. [Google Scholar] [CrossRef]
- Åström, E.K.L.; Oliver, P.G.; Carroll, M.L. A new genus and two new species of Thyasiridae associated with methane seeps off Svalbard, Arctic Ocean. Mar. Biol. Res. 2017, 13, 402–416. [Google Scholar] [CrossRef]
- Vistisen, B.; Vismann, B. Tolerance to low oxygen and sulfide in Amphiura filiformis and Ophiura albida (Echinodermata: Ophiuroidea). Mar. Biol. 1997, 128, 241–246. [Google Scholar] [CrossRef]
- Zettler, M.L.; Friedland, R.; Gogina, M.; Darr, A. Variation in benthic long-term data of transitional waters: Is interpretation more than speculation? PLoS ONE 2017, 12, e0175746. [Google Scholar] [CrossRef] [PubMed]
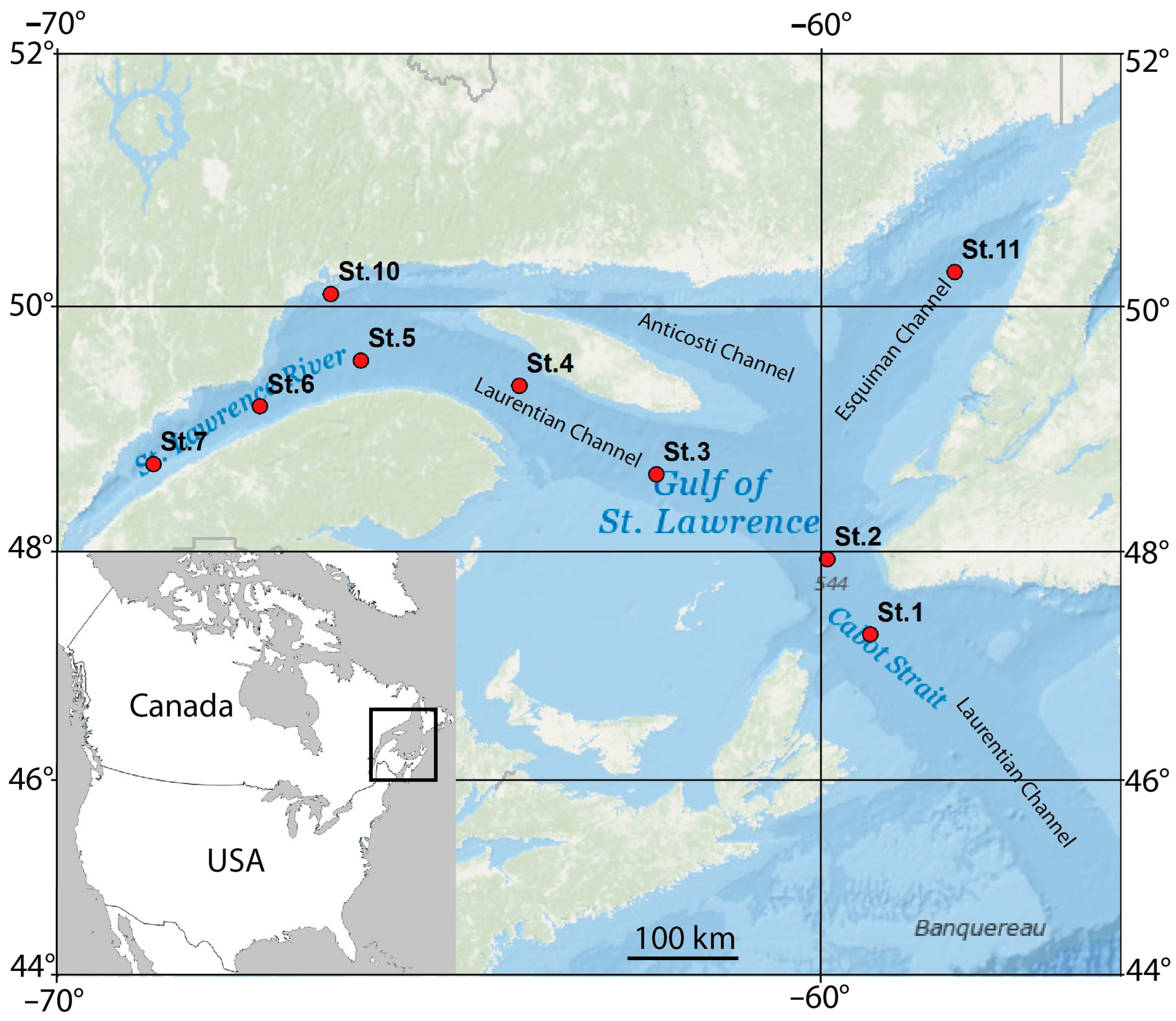
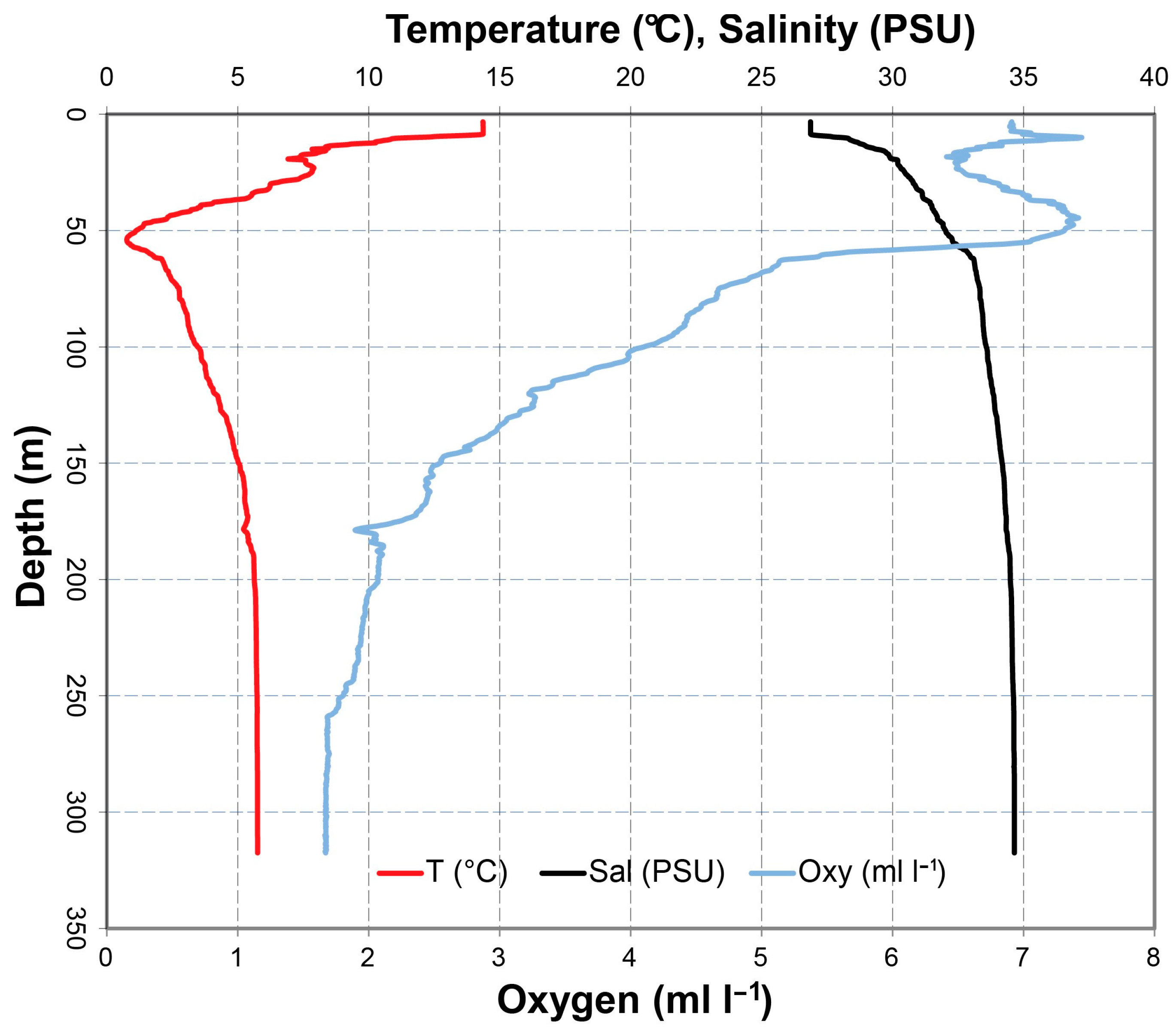

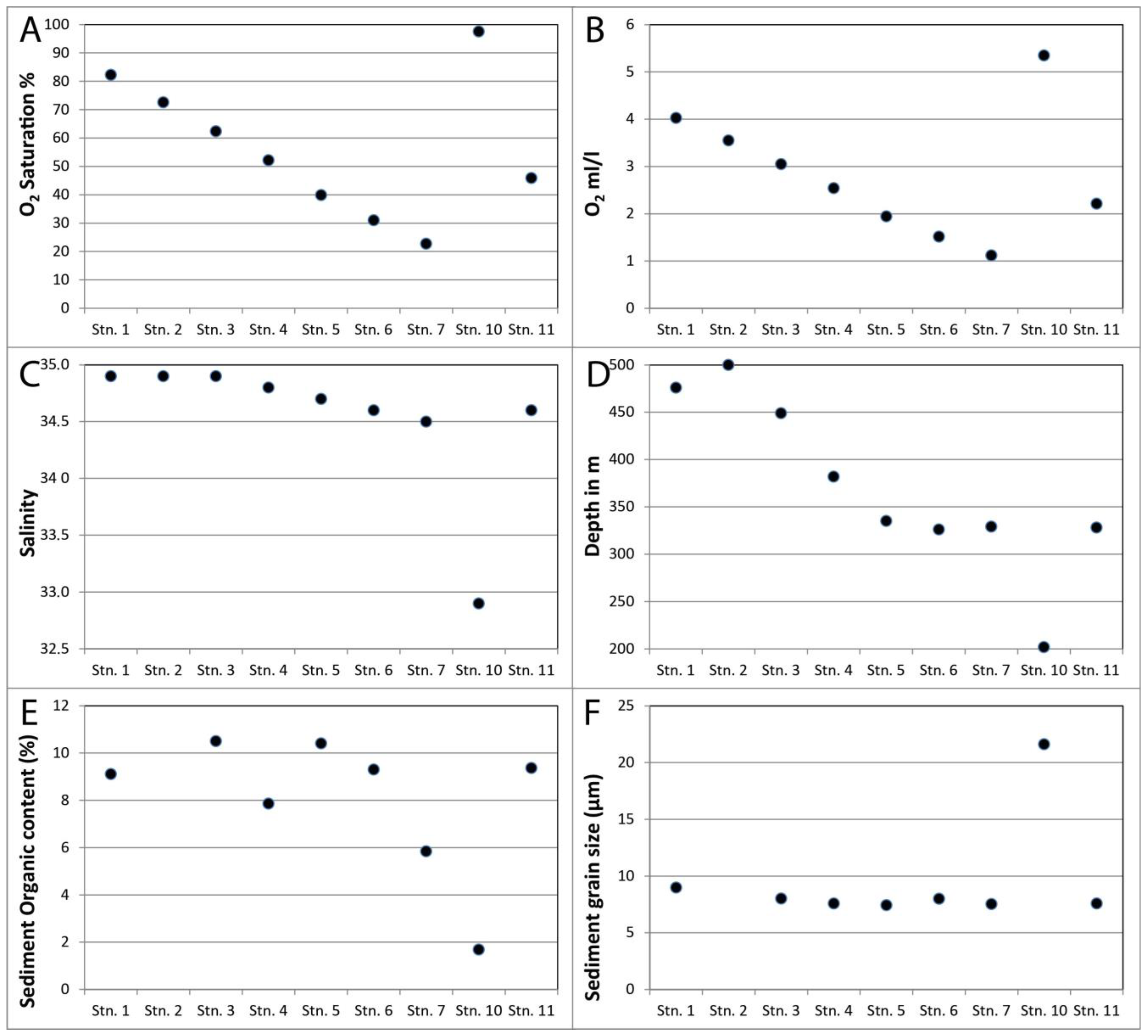

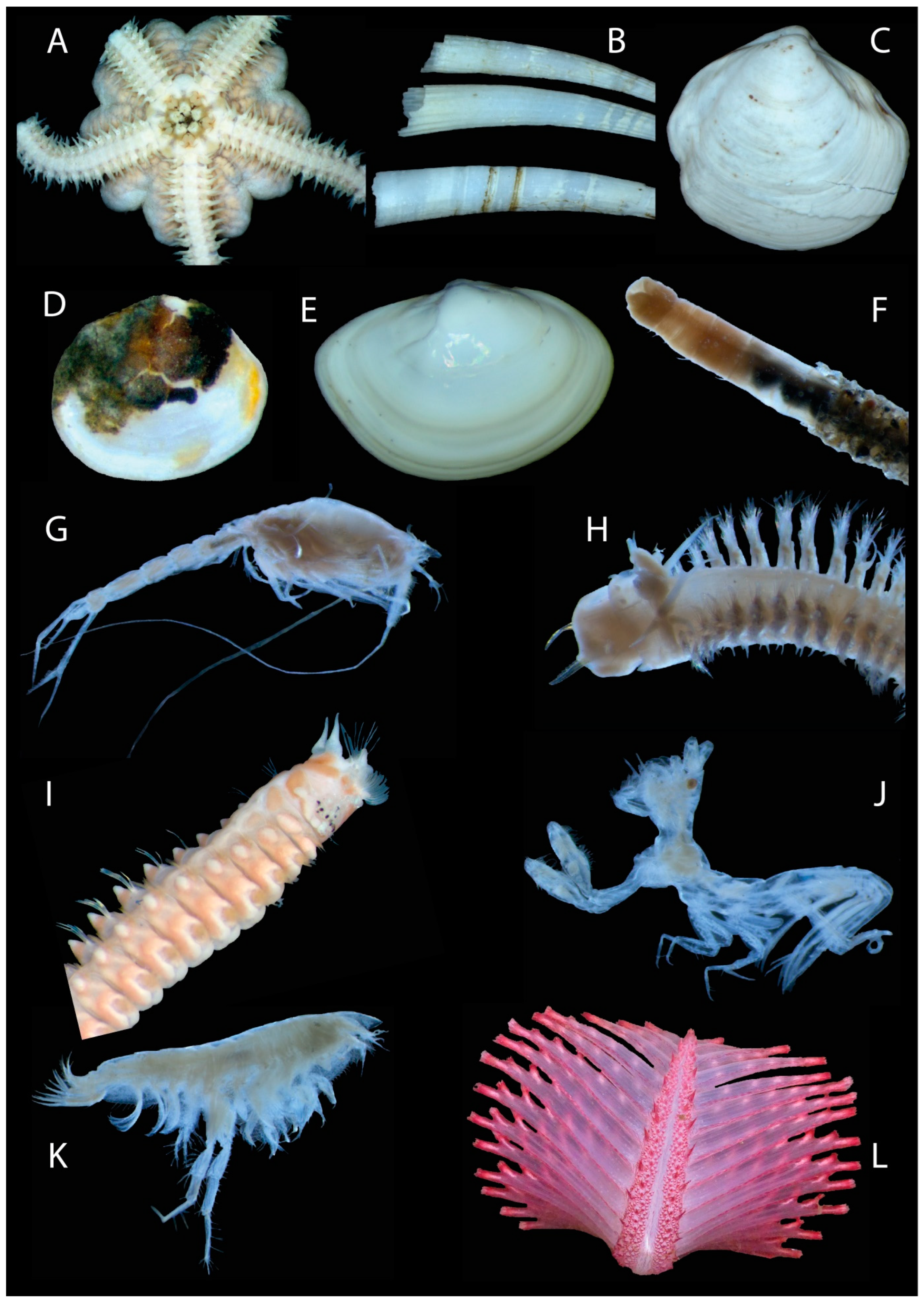
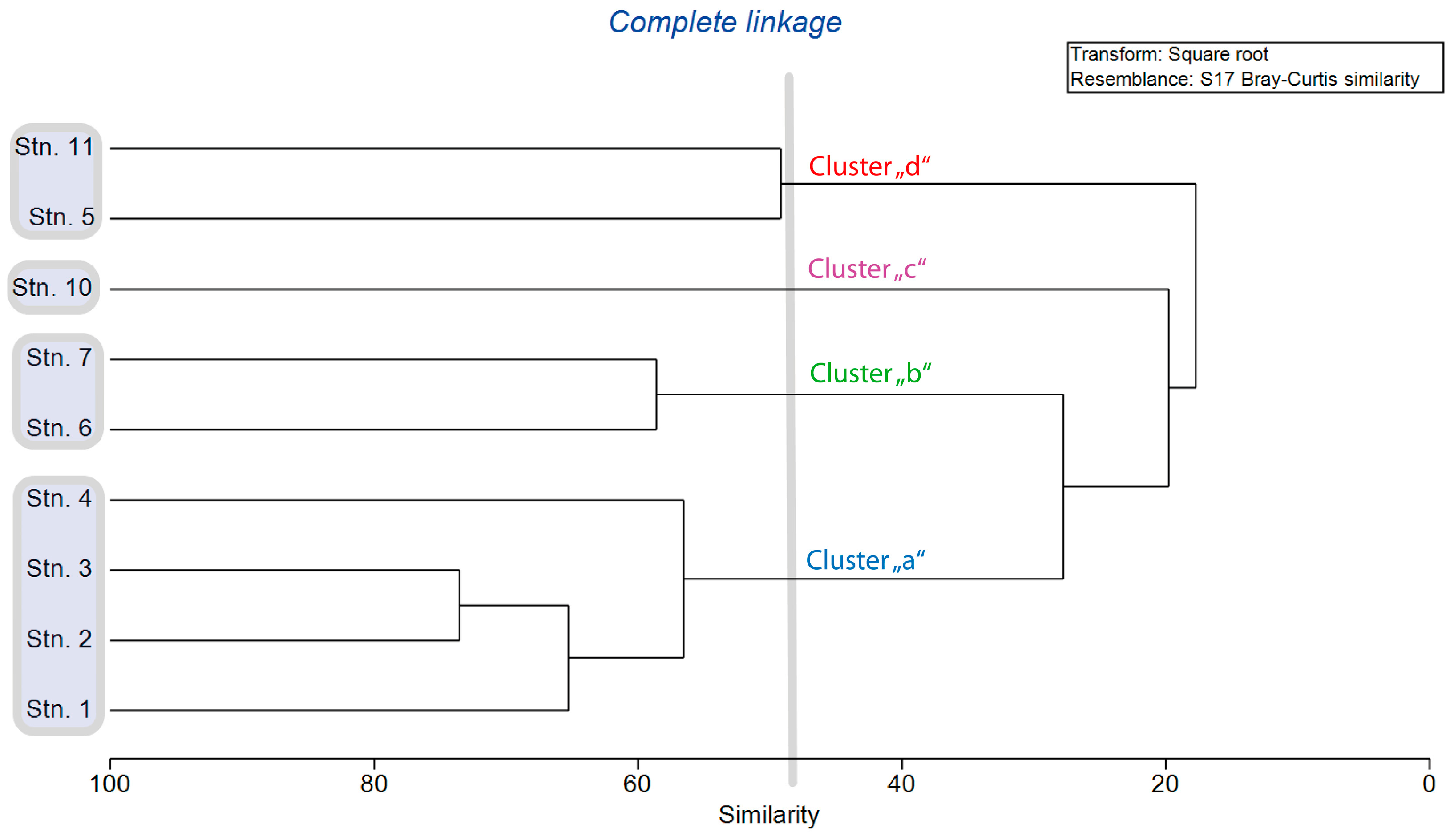

| Station | Latitude | Longitude | Depth (m) | B T (°C) | B O2 (mL/L) | B O2 Sat (%) | B S (PSU) | Sed Org (%) | Sed GS (µm) |
|---|---|---|---|---|---|---|---|---|---|
| Stn. 1 | 47.1859 | −59.5380 | 476 | 5.6 | 4.03 | 82.23 | 34.9 | 9.11 | 9 |
| Stn. 2 | 47.8333 | −60.0835 | 500 | 5.6 | 3.55 | 72.57 | 34.9 | no data | no data |
| Stn. 3 | 48.5503 | −62.2495 | 449 | 5.7 | 3.05 | 62.40 | 34.9 | 10.50 | 8 |
| Stn. 4 | 49.2903 | −63.9893 | 382 | 5.9 | 2.54 | 52.19 | 34.8 | 7.86 | 8 |
| Stn. 5 | 49.5005 | −65.9982 | 335 | 5.8 | 1.94 | 39.89 | 34.7 | 10.40 | 7 |
| Stn. 6 | 49.1196 | −67.2796 | 326 | 5.8 | 1.52 | 31.03 | 34.6 | 9.30 | 8 |
| Stn. 7 | 48.6390 | −68.6319 | 329 | 5.5 | 1.12 | 22.77 | 34.5 | 5.84 | 8 |
| Stn. 10 | 50.0441 | −66.3791 | 202 | 1.6 | 5.35 | 97.53 | 32.9 | 1.69 | 22 |
| Stn. 11 | 50.2230 | −58.4656 | 328 | 6.3 | 2.21 | 45.91 | 34.6 | 9.37 | 8 |
| Stations | Stn. 1 | Stn. 2 | Stn. 3 | Stn. 4 | Stn. 5 | Stn. 6 | Stn. 7 | Stn. 10 | Stn. 11 |
|---|---|---|---|---|---|---|---|---|---|
| Depth (m) | 476 m | 500 m | 449 m | 382 m | 335 m | 326 m | 329 m | 202 m | 328 m |
| Oxygen Saturation (%) | 82 | 73 | 62 | 52 | 40 | 31 | 23 | 98 | 46 |
| Anthozoa | |||||||||
| Pennatula aculeata | 0 | 0 | 0 | 10 | 20 | 3 | 13 | 0 | 3 |
| Scaphopoda | |||||||||
| Antalis sp. | 103 | 110 | 113 | 50 | 0 | 0 | 0 | 0 | 0 |
| Bivalvia | |||||||||
| Mendicula ferruginosa | 303 | 293 | 220 | 93 | 0 | 87 | 77 | 0 | 0 |
| Thyasira sp. | 70 | 87 | 43 | 47 | 27 | 167 | 700 | 83 | 20 |
| Yoldiella sp. | 17 | 30 | 53 | 7 | 3 | 7 | 0 | 0 | 3 |
| Malacostraca | |||||||||
| Diastylis abbreviata | 0 | 0 | 0 | 0 | 0 | 0 | 1600 | 0 | 0 |
| Diastylis goodsiri | 0 | 0 | 0 | 0 | 0 | 20 | 107 | 0 | 0 |
| Dyopedos monacanthus | 3 | 30 | 0 | 0 | 0 | 3 | 70 | 0 | 0 |
| Harpinia sp. | 247 | 160 | 153 | 200 | 37 | 103 | 97 | 0 | 13 |
| Ischyrocerus sp. | 0 | 0 | 0 | 0 | 0 | 33 | 73 | 0 | 0 |
| Leptostylis sp. | 0 | 0 | 0 | 7 | 0 | 110 | 0 | 0 | 0 |
| Polychaeta | |||||||||
| Ampharete sp. | 0 | 3 | 7 | 23 | 13 | 53 | 227 | 3 | 0 |
| Capitellidae | 30 | 23 | 17 | 60 | 27 | 120 | 10 | 23 | 27 |
| Ceratocephale loveni | 0 | 0 | 0 | 13 | 23 | 143 | 197 | 0 | 103 |
| Chaetopteridae | 7 | 3 | 0 | 7 | 0 | 3 | 0 | 130 | 3 |
| Cirratulidae | 0 | 3 | 7 | 70 | 20 | 67 | 70 | 17 | 7 |
| Diplocirrus sp. | 53 | 13 | 27 | 43 | 0 | 7 | 10 | 0 | 0 |
| Galathowenia oculata | 20 | 13 | 37 | 177 | 0 | 177 | 617 | 0 | 0 |
| Lumbrineris sp. | 27 | 10 | 13 | 73 | 20 | 87 | 13 | 33 | 60 |
| Prionospio steenstrupi | 10 | 23 | 33 | 10 | 0 | 3 | 3 | 890 | 0 |
| Trochochaeta multisetosa | 0 | 0 | 0 | 0 | 0 | 43 | 170 | 0 | 13 |
| Ophiuroidea | |||||||||
| Amphiura sp. | 13 | 30 | 47 | 33 | 70 | 70 | 27 | 0 | 0 |
| Ophiura sp. | 90 | 0 | 3 | 7 | 0 | 0 | 0 | 0 | 0 |
| Ascidiacea | |||||||||
| Molgula sp. | 0 | 0 | 0 | 0 | 0 | 10 | 97 | 0 | 0 |
| SoD (above) | 993 | 833 | 773 | 930 | 260 | 1317 | 4177 | 1180 | 253 |
| TD density (all taxa) ind m−2 | 1180 | 997 | 980 | 1140 | 323 | 1587 | 4554 | 1757 | 287 |
| TSN (all taxa) | 37 | 37 | 37 | 44 | 21 | 51 | 47 | 36 | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zettler, M.L.; Pollehne, F. Macrozoobenthic Diversity along an Oxygen Gradient in the Deep Trough of the Gulf of St. Lawrence (Canada). Diversity 2023, 15, 854. https://doi.org/10.3390/d15070854
Zettler ML, Pollehne F. Macrozoobenthic Diversity along an Oxygen Gradient in the Deep Trough of the Gulf of St. Lawrence (Canada). Diversity. 2023; 15(7):854. https://doi.org/10.3390/d15070854
Chicago/Turabian StyleZettler, Michael L., and Falk Pollehne. 2023. "Macrozoobenthic Diversity along an Oxygen Gradient in the Deep Trough of the Gulf of St. Lawrence (Canada)" Diversity 15, no. 7: 854. https://doi.org/10.3390/d15070854
APA StyleZettler, M. L., & Pollehne, F. (2023). Macrozoobenthic Diversity along an Oxygen Gradient in the Deep Trough of the Gulf of St. Lawrence (Canada). Diversity, 15(7), 854. https://doi.org/10.3390/d15070854









