Conservation Prioritization of Orthoptera Assemblages on a Mediterranean Island
Abstract
:1. Introduction
2. Materials and Methods
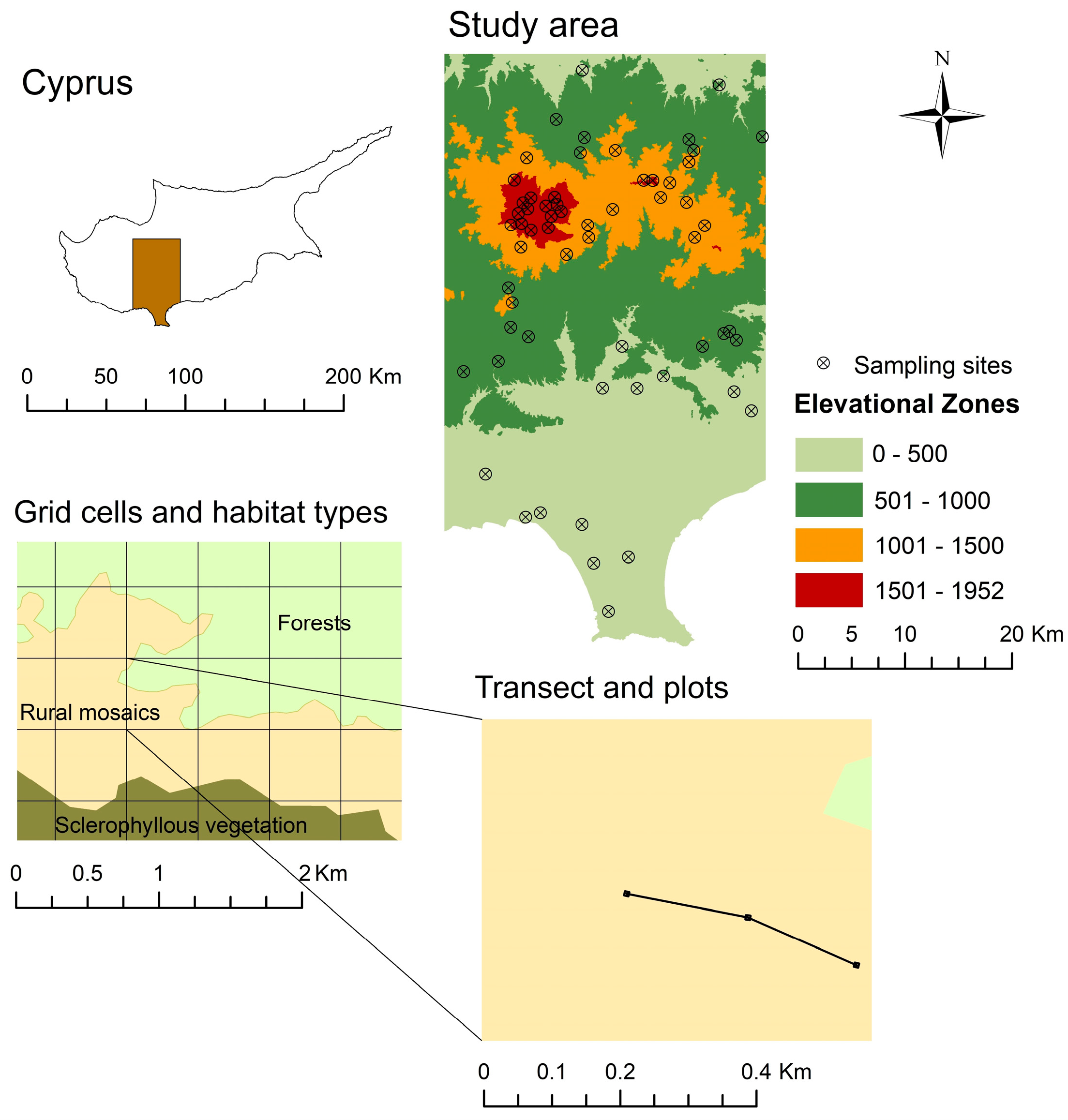
2.1. Study Area
2.2. Site Selection
2.3. Sampling
2.4. Data Analysis
2.4.1. Orthoptera Conservation Value Assessment
2.4.2. Community Composition in Relation to Environmental Variables
2.4.3. Effect of Environmental Variables on Orthoptera Diversity
3. Results
3.1. Diversity Indices
3.2. Standardized Conservation Index (StCI)
3.3. Effects of Environmental Variables on Orthopteran Community Composition
4. Discussion
4.1. Standardized Conservation Index (StCI) and Species Conservation Value (ci)
4.2. Site Conservation Value
4.3. Effects of Environmental Parameters on Orthoptera Community Composition
4.4. Implications for Conservation Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Capmourteres, V.; Anand, M. “Conservation value”: A review of the concept and its quantification. Ecosphere 2016, 7, e01476. [Google Scholar] [CrossRef]
- Thompson, A.C.; Bazelet, C.S.; Naskrecki, P.; Samways, M.J. Adapting the Dragonfly Biotic Index to a katydid (Tettigoniidae) rapid assessment technique: Case study of a biodiversity hotspot, the Cape Floristic Region, South Africa. J. Orth. Res. 2017, 26, 63–71. [Google Scholar] [CrossRef]
- Halmy, M.W.A.; Salem, B.B. Species conservation importance index (SCI) for comparing sites’ conservation value at landscape level. Braz. J. Bot. 2015, 38, 823–835. [Google Scholar] [CrossRef]
- Simaika, J.P.; Samways, M.J. An easy-to-use index of ecological integrity for prioritizing freshwater sites and for assessing habitat quality. Biodivers. Conserv. 2009, 18, 1171–1185. [Google Scholar] [CrossRef]
- Korbaa, M.; Ferreras-Romero, M.; Ruiz-Garcia, A.; Boumaiza, M. TSOI—A new index on Odonata populations to assess the conservation relevance of watercourses in Tunisia. Odonatologica 2018, 47, 43–72. [Google Scholar] [CrossRef]
- Matenaar, D.; Bazelet, C.S.; Hochkirch, A. Simple tools for the evaluation of protected areas for the conservation of grasshoppers. Biol. Conserv. 2015, 192, 192–199. [Google Scholar] [CrossRef]
- Joubert-van der Merwe, L.; Pryke, J.S.; Samways, M.J. Well-managed grassland heterogeneity promotes butterfly conservation in a corridor network. J. Environ. Manag. 2019, 238, 382–395. [Google Scholar] [CrossRef]
- D’Amen, M.; Bombi, P.; Campanaro, A.; Zapponi, L.; Bologna, M.A.; Mason, F. Po ssible directions in the protection of the neglected invertebrate biodiversity. Anim. Conserv. 2013, 16, 383–385. [Google Scholar] [CrossRef]
- Kostopoulou, E.; Jones, P.D. Assessment of climate extremes in the Eastern Mediterranean. Meteorol. Atmos. Phys. 2005, 89, 69–85. [Google Scholar] [CrossRef]
- Courchamp, F.; Hoffmann, B.D.; Russell, J.C.; Leclerc, C.; Bellard, C. Climate change, sea-level rise, and conservation: Keeping island biodiversity afloat. Trends Ecol. Evol. 2014, 29, 127–130. [Google Scholar] [CrossRef]
- Vogiatzakis, I.N.; Mannion, A.M.; Sarris, D. Mediterranean island biodiversity and climate change: The last 10,000 years and the future. Biodivers. Conserv. 2016, 25, 2597–2627. [Google Scholar] [CrossRef]
- Médail, F. The specific vulnerability of plant biodiversity and vegetation on Mediterranean islands in the face of global change. Reg. Environ. Chang. 2017, 17, 1775–1790. [Google Scholar] [CrossRef]
- Veron, S.; Mouchet, M.; Govaerts, R.; Haevermans, T.; Pellens, R. Vulnerability to climate change of islands worldwide and its impact on the tree of life. Sci. Rep. 2019, 9, 14471. [Google Scholar] [CrossRef] [PubMed]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Trigas, P.; Strid, A.; Dimopoulos, P. Plant Diversity Patterns and Conservation Implications under Climate-Change Scenarios in the Mediterranean: The Case of Crete (Aegean, Greece). Diversity 2020, 12, 270. [Google Scholar] [CrossRef]
- Adu-Acheampong, S.; Samways, M.J.; Landmann, T.; Kyerematen, R.; Minkah, R.; Mukundamago, M.; Moshobane, C.M. Endemic grasshopper species distribution in an agro-natural landscape of the Cape Floristic Region, South Africa. Ecol. Eng. 2017, 105, 133–140. [Google Scholar] [CrossRef]
- Zografou, K.; Adamidis, G.C.; Grill, A.; Kati, V.; Wilson, R.J.; Halley, J.M. Who flies first?—Habitat-specific phenological shifts of butterflies and orthopterans in the light of climate change: A case study from the south-east Mediterranean. Ecol. Entomol. 2015, 40, 562–574. [Google Scholar] [CrossRef]
- Báldi, A.; Kisbenedek, T. Orthopteran assemblages as indicators of grassland naturalness in Hungary. Agric. Ecosyst. Environ. 1997, 66, 121–129. [Google Scholar] [CrossRef]
- Kati, V.; Mani, P.; von Helversen, O.; Willemse, F.; Elsner, N.; Dimopoulos, P. Human Land use Threatens Endemic Wetland Species: The Case of Chorthippus lacustris (La Greca and Messina 1975) (Orthoptera: Acrididae) in Epirus, Greece. J. Insect Conserv. 2006, 10, 65–74. [Google Scholar] [CrossRef]
- Schirmel, J.R.; Gerlach, R.; Buhk, C. Disentangling the role of management, vegetation structure, and plant quality for Orthoptera in lowland meadows. Insect Sci. 2019, 26, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Hochkirch, A.; Nieto, A.; García Criado, M.; Cálix, M.; Braud, Y.; Buzzetti, F.M.; Chobanov, D.; Odé, B.; Presa Asensio, J.J.; Willemse, L.; et al. European Red List of Grasshoppers, Crickets and Bush-Crickets; Publications Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Médail, F.; Diadema, K. Glacial refugia influence plant diversity patterns in the Mediterranean Basin. J. Biogeogr. 2009, 36, 1333–1345. [Google Scholar] [CrossRef]
- Christodoulou, C.S.; Griffiths, G.H.; Vogiatzakis, I.N. Using threatened plant species to identify conservation gaps and opportunities on the island of Cyprus. Biodivers. Conserv. 2018, 27, 2837–2858. [Google Scholar] [CrossRef]
- Kadis, K.; et al. Strategy for Biodiversity in Cyprus; Frederick University: Nicosia, Cyprus, 2012. [Google Scholar]
- Hewitt, G. Mediterranean Peninsulas: The Evolution of Hotspots. In Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas; Zachos, F.E., Habel, J.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 123–147. [Google Scholar]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial Ecoregions of the World: A New Map of Life on Earth: A new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. Bioscience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- Tumbrinck, J. An annotated checklist of the Orthoptera (Saltatoria) of Cyprus. Articulata 2006, 21, 121–159. [Google Scholar]
- Siedle, K.; Tumbrinck, J.; Tzirkalli, E. Orthoptera. In An Introduction to the Wildlife of Cyprus; Sparrow, D.J., John, E., Eds.; Terra Cypria: Nicosia, Cyprus, 2016; pp. 132–173. [Google Scholar]
- Sevgili, H.; Ciplak, B.; Heller, G.K.; Demirsoy, A. Morphology, bioacoustics and phylogeography of the Isophya major group (Orthoptera: Tettigoniidae: Phaneropterinae): A species complex occurring in Anatolia and Cyprus. Eur. J. Entomol. 2006, 103, 657–671. [Google Scholar] [CrossRef]
- Ünal, M. Tettigoniidae (Orthoptera) from Turkey and the Middle East II. Trans. Am. Entomol. 2012, 138, 21–54. [Google Scholar]
- Tsintides, T.; Hadjikyriakos, G.; Christodoulou, C. Trees and Shrubs in Cyprus; Foundation, A.G. Leventis, Cyprus Forest Association: Nicosia, Cyprus, 2002. [Google Scholar]
- Tsintides, T.; Christodoulou, C.S.; Delipetrou, P.; Georghiou, K. The Red Data Book of the Flora of Cyprus; Cyprus Forestry Association: Nicosia, Cyprus, 2007. [Google Scholar]
- ESRI ArcGIS 10; Environmental Systems Research Institute: Redlands, CA, USA, 2010.
- EEA. Corine Land Cover 2006. Available online: https://www.eea.europa.eu/data-and-maps/data/clc-2006-vector-data-version (accessed on 10 January 2011).
- Kati, V.; Zografou, K.; Tzirkalli, E.; Chitos, T.; Willemse, L. Butterfly and grasshopper diversity patterns in humid Mediterranean grasslands: The roles of disturbance and environmental factors. J. Insect Conserv. 2012, 16, 807–818. [Google Scholar] [CrossRef]
- Willemse, F. A Key to the Orthoptera Species of Greece; Hellenic Zoological Society: Athens, Greece, 1985. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. vegan: Community Ecology Package. R package version 2.5–4. 2020. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 3 February 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org/ (accessed on 3 February 2020).
- Zuur, A.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 2nd ed.; Sage: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- Nagy, A.; Rácz, I.A.; Arnóczkyné Jakab, D.; Szanyi, S. Setting priorities and evaluation of habitats for the conservation of orthopterans: Case study in the Aggtelek National Park (N Hungary). Biol. Futura 2023, 74, 401–412. [Google Scholar] [CrossRef]
- Çiplak, B. Distribution of Tettigoniinae (Orthoptera, Tettigoniidae) bush-crickets in Turkey: The importance of the Anatolian Taurus Mountains in biodiversity and implications for conservation. Biodivers. Conserv. 2003, 12, 47–64. [Google Scholar] [CrossRef]
- Kruess, A.; Tscharntke, T. Grazing Intensity and the Diversity of Grasshoppers, Butterflies, and Trap-Nesting Bees and Wasps. Conserv. Biol. 2002, 16, 1570–1580. [Google Scholar] [CrossRef]
- Kati, V.; Dufrêne, M.; Legakis, A.; Grill, A.; Lebrun, P. Conservation management for Orthoptera in the Dadia reserve, Greece. Biol. Conserv. 2004, 115, 33–44. [Google Scholar] [CrossRef]
- Zografou, K.; Sfenthourakis, S.; Pullin, A.; Kati, V. On the surrogate value of red-listed butterflies for butterflies and grasshoppers: A case study in Grammos site of Natura 2000, Greece. J. Insect Conserv. 2009, 13, 505–514. [Google Scholar] [CrossRef]
- Department of Forest; Food and Agricultural Organization. Forestry Outlook Study for Cyprus, Opportunities and Challenges towards 2020; Department of Forest, Ministry of Agriculture, Rural Development and Environment: Nicosia, Cyprus, 2005. [Google Scholar]
- Zakkak, S.; Radovic, A.; Nikolov, S.C.; Shumka, S.; Kakalis, L.; Kati, V. Assessing the effect of agricultural land abandonment on bird communities in southern-eastern Europe. J. Environ. Manag. 2015, 164, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, J.; Riesen, M. Bracken fern (Pteridium aquilinum (L.) Kuhn) overgrowth on dry Alpine grassland impedes Red List Orthoptera but supports local orthopteran beta diversity. J. Insect Conserv. 2021, 25, 657–669. [Google Scholar] [CrossRef]
- Gebeyehu, S.; Samways, M.J. Responses of grasshopper assemblages to long-term grazing management in a semi-arid African savanna. Agric. Ecosyst. Environ. 2003, 95, 613–622. [Google Scholar] [CrossRef]
- Wettstein, W.; Schmid, B. Conservation of arthropod diversity in montane wetlands: Effect of altitude, habitat quality and habitat fragmentation on butterflies and grasshoppers. J. Appl. Ecol. 1999, 36, 363–373. [Google Scholar] [CrossRef]
- Ingrisch, S. The plurennial life cycles of the European Tettigoniidae (Insecta: Orthoptera). Oecologia 1986, 70, 624–630. [Google Scholar] [CrossRef]
- Antonatos, S.; Emmanouel, N.; Fantinou, A.; Tsagkarakis, A.; Anagnostopoulos, A.; Ntampakis, D. Seasonal population fluctuation and spatial distribution of Orthoptera in two grassland areas of Attica—Greece. J. Nat. Hist. 2014, 48, 661–674. [Google Scholar] [CrossRef]
- Gavlas, V.; Bednár, J.; Krištín, A. A comparative study on orthopteroid assemblages along a moisture gradient in the Western Carpathians. Biologia 2007, 62, 95–102. [Google Scholar] [CrossRef]
- Sliacka, A.; Krištín, A.; Naďo, L. Response of Orthoptera to clear-cuts in beech forests. Eur. J. Entomol. 2013, 110, 319–326. [Google Scholar] [CrossRef]
- Helbing, F.; Blaeser, T.P.; Löffler, F.; Fartmann, T. Response of Orthoptera communities to succession in alluvial pine woodlands. J. Insect Conserv. 2014, 18, 215–224. [Google Scholar] [CrossRef]
- Pina, S.; Vasconcelos, S.; Reino, L.; Santana, J.; Beja, P.; Sánchez-Oliver, J.S.; Catry, I.; Moreira, F.; Ferreira, S. The Orthoptera of Castro Verde Special Protection Area (Southern Portugal): New data and conservation value. ZooKeys 2017, 691, 19–48. [Google Scholar] [CrossRef]
- Crous, C.J.; Samways, M.J.; Pryke, J.S. Grasshopper assemblage response to surface rockiness in Afro-montane grasslands. Insect Conserv. Divers. 2014, 7, 185–194. [Google Scholar] [CrossRef]
- Zografou, K.; Adamidis, G.C.; Komnenov, M.; Kati, V.; Sotirakopoulos, P.; Pitta, E.; Chatzaki, M. Diversity of spiders and orthopterans respond to intra-seasonal and spatial environmental changes. J. Insect Conserv. 2017, 21, 531–543. [Google Scholar] [CrossRef]
- Joubert-Van der Merwe, L.; Pryke, J.S. Is cattle grazing more important than landscape heterogeneity for grasshoppers in Afromontane grassland? J. Orthoptera Res. 2018, 27, 13–21. [Google Scholar] [CrossRef]
- Krämer, B.; Poniatowski, D.; Villar, L.; Fartmann, T. The Orthoptera communities of sub-Mediterranean dry grasslands (Aphyllanthion alliance) in the western Spanish Pyrenees. Articulata 2010, 25, 59–72. [Google Scholar]
- Löffler, F.; Fartmann, T. Effects of landscape and habitat quality on Orthoptera assemblages of pre-alpine calcareous grasslands. Agric. Ecosyst. Environ. 2017, 248, 71–81. [Google Scholar] [CrossRef]
- Pe’er, G.; Dicks, L.V.; Visconti, P.; Arlettaz, R.; Báldi, A.; Benton, T.G.; Collins, S.; Dieterich, M.; Gregory, R.D.; Hartig, F.; et al. EU agricultural reform fails on biodiversity. Science 2014, 344, 1090–1092. [Google Scholar] [CrossRef]
- Zakkak, S.; Chatzaki, M.; Karamalis, N.; Kati, V. Spiders in the context of agricultural land abandonment in Greek Mountains: Species responses, community structure and the need to preserve traditional agricultural landscapes. J. Insect Conserv. 2014, 18, 599–611. [Google Scholar] [CrossRef]
- Nogués-Bravo, D.; Araújo, M.B.; Lasanta, T.; Moreno, J.I.L. Climate Change in Mediterranean Mountains during the 21st Century. AMBIO 2008, 37, 280–285. [Google Scholar] [CrossRef]
- Hadjinicolaou, P.; Giannakopoulos, C.; Zerefos, C.; Lange, M.A.; Pashiardis, S.; Lelieveld, J. Mid-21st century climate and weather extremes in Cyprus as projected by six regional climate models. Reg. Environ. Chang. 2011, 11, 441–457. [Google Scholar] [CrossRef]
- Balleto, E.; Casale, A. Mediterranean Insect Conservation. In The Conservation of Insects and Their Habitats; Collins, N., Thomas, J., Eds.; Academic Press: London, UK, 1991; pp. 121–142. [Google Scholar]
- Suggitt, A.J.; Stefanescu, C.; Páramo, F.; Oliver, T.; Anderson, B.J.; Hill, J.K.; Roy, D.B.; Brereton, T.; Thomas, C.D. Habitat associations of species show consistent but weak responses to climate. Biol. Lett. 2012, 8, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Sergeev, M.G. Distribution Patterns of Grasshoppers and Their Kin over the Eurasian Steppes. Insects 2021, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Blondel, J.; Aronson, J.; Bodiou, J.-Y.; Boeuf, G. The Mediterranean Region: Biological Diversity in Space and Time, 2nd ed.; Oxford University Press: New York, NY, USA, 2010. [Google Scholar]

| Habitat Types | Overall Diversity | Average Site Diversity | N Sites | |||||
|---|---|---|---|---|---|---|---|---|
| S | SE | Mean S | Mean SE | Mean H | Mean ci | Mean StCI | ||
| Rural mosaics | 22 | 5 (1) | 6.25 (±0.75) | 1.13 (±0.30) | 1.62 (±0.13) | 0.42 (±0.02) | 72.41 (±3.97) | 8 (24) |
| Forests | 15 | 5 | 2.62 (±0.23) | 0.71 (±0.23) | 0.79 (±0.09) | 0.40 (±0.02) | 68.83 (±3.24) | 21 (63) |
| Sclerophyllous vegetation | 15 | 4 | 2.89 (±0.35) | 0.56 (±0.18) | 0.89 (±0.10) | 0.39 (±0.02) | 67.12 (±3.25) | 9 (27) |
| Grasslands | 11 | 3 | 3.50 (±1.19) | 0.75 (±0.25) | 0.78 (±0.32) | 0.39 (±0.03) | 66.76 (±5.54) | 4 (12) |
| Transitional woodland/shrubland | 12 | 2 | 4.25 (±1.38) | 0.75 (±0.48) | 1.07 (±0.39) | 0.38 (±0.03) | 64.54 (±4.53) | 4 (12) |
| Agricultural areas | 14 | 3 | 4.17 (±0.60) | 0.5 (±0.34) | 1.28 (±0.17) | 0.37 (±0.02) | 62.81 (±3.15) | 6 (18) |
| Riverine vegetation | 16 | 3 | 3.88 (±0.81) | 0.25 (±0.16) | 1.07 (±0.23) | 0.36 (±0.02) | 61.83 (±2.95) | 8 (24) |
| Total | 34 | 11 | 3.63 (±0.26) | 0.67 (±0.11) | 1.02 (±0.07) | 0.39 (±0.01) | 67.09 (±1.51) | 60 |
| Variable | Estimate | SE | z Value | p |
|---|---|---|---|---|
| S (D2 = 51) | ||||
| (Intercept) | 1.22 | 0.07 | 16.77 | <0.001 |
| Altitude | −0.25 | 0.07 | −3.46 | <0.001 |
| Flower heads | 0.20 | 0.06 | 3.16 | <0.05 |
| Slope | −0.12 | 0.07 | −1.66 | 0.10 |
| H (D2 = 48) | ||||
| Intercept | 1.02 | 0.05 | 19.57 | <0.001 |
| Low shrubs | −0.13 | 0.08 | −1.69 | 0.10 |
| Herbs | −0.15 | 0.08 | −1.94 | 0.06 |
| High shrubs | −0.17 | 0.09 | −1.95 | 0.06 |
| Flower heads | 0.19 | 0.07 | 2.71 | <0.05 |
| Tree cover | −0.23 | 0.08 | −2.78 | <0.05 |
| Altitude | −0.28 | 0.06 | −4.97 | <0.001 |
| ci (R2 = 0.23) | ||||
| Intercept | 0.33 | 0.01 | 59.97 | <0.001 |
| Slope | −0.01 | 0.01 | −1.56 | 0.12 |
| High shrubs | −0.02 | 0.01 | −2.47 | <0.05 |
| Rocks | 0.01 | 0.01 | 2.61 | <0.05 |
| Tree cover | −0.02 | 0.01 | −3.51 | <0.001 |
| StCI (R2 = 0.24) | ||||
| Intercept | 4.21 | 0.02 | 228.88 | <0.001 |
| Slope | −0.03 | 0.02 | −1.70 | 0.10 |
| High shrubs | 0.05 | 0.02 | −2.52 | <0.05 |
| Rocks | −0.05 | 0.02 | 2.52 | <0.05 |
| Tree cover | −0.08 | 0.02 | −3.67 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzirkalli, E.; Zografou, K.; Willemse, L.; Vogiatzakis, I.N.; Kati, V. Conservation Prioritization of Orthoptera Assemblages on a Mediterranean Island. Diversity 2024, 16, 347. https://doi.org/10.3390/d16060347
Tzirkalli E, Zografou K, Willemse L, Vogiatzakis IN, Kati V. Conservation Prioritization of Orthoptera Assemblages on a Mediterranean Island. Diversity. 2024; 16(6):347. https://doi.org/10.3390/d16060347
Chicago/Turabian StyleTzirkalli, Elli, Konstantina Zografou, Luc Willemse, Ioannis N. Vogiatzakis, and Vassiliki Kati. 2024. "Conservation Prioritization of Orthoptera Assemblages on a Mediterranean Island" Diversity 16, no. 6: 347. https://doi.org/10.3390/d16060347







