Bio-Oriented Synthesis of Novel (S)-Flurbiprofen Clubbed Hydrazone Schiff’s Bases for Diabetic Management: In Vitro and In Silico Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. In Vitro α-Glucosidase Inhibitory Activity
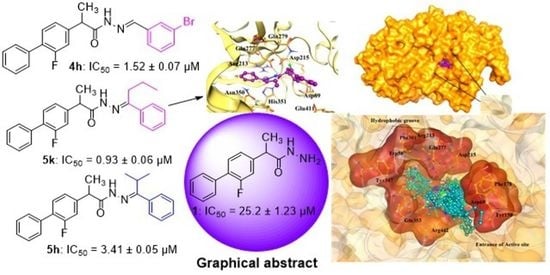
2.3. Molecular Docking Studies
3. Experimental Part
3.1. General Instrumentation
3.2. 2-(2-Fluorobiphenyl-4-yl)propanehydrazide (1)
3.3. General Procedure for the Synthesis of Substituted Schiff’s Bases of Flurbiprofen (4a–p and 5a–n)
3.4. Spectral Interpretation of the Synthesized Compounds
3.4.1. 2-(2-Fluoro-[1,1′-biphenyl]-4-yl)propanehydrazide (1)
3.4.2. (E)-2-(2-Fluoro-[1,1′-biphenyl]-4-yl)-N′-(3,4,5-trimethoxybenzylidene)propanehydrazide (4a)
3.4.3. (E)-2-(2-Fluoro-[1,1′-biphenyl]-4-yl)-N′-(3-nitrobenzylidene)propanehydrazide (4b)
3.4.4. (E)-N′-(2,4-Dihydroxybenzylidene)-2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanehydrazide (4c)
3.4.5. (E)-2-(2-Fluoro-[1,1′-biphenyl]-4-yl)-N′-(2-hydroxy-3-methoxybenzylidene)propanehydrazide (4d)
3.4.6. (E)-N′-(4-(Diethylamino)benzylidene)-2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanehydrazide (4e)
3.4.7. (E)-2-(2-Fluoro-[1, 1′-biphenyl]-4-yl)-N′-(3-hydroxy-4-methoxybenzylidene)propanehydrazide (4f)
3.4.8. (E)-N′-(4-Bromo-2-fluorobenzylidene)-2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanehydrazide (4g)
3.4.9. (E)-N′-(3-Bromobenzylidene)-2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanehydrazide (4h)
3.4.10. (E)-N′-(3,5-Dibromo-4-hydroxybenzylidene)-2-(2-fluoro-[1,1′-biphenyl]-4- yl)propane hydrazide (4i)
3.4.11. (E)-N′-(3-Ethoxy-4-hydroxybenzylidene)-2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanehydrazide (4j)
3.4.12. (E)-N′-Butylidene-2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanehydrazide (4k)
3.4.13. (E)-2-(2-Fluoro-[1, 1′-biphenyl]-4-yl)-N′-hexylidenepropanehydrazide (4l)
3.4.14. (E)-2-(2-Fluoro-[1,1′-biphenyl]-4-yl)-N′-(2-methoxybenzylidene)propanehydrazide (4m)
3.4.15. (E)-2-(2-Fluoro-[1,1′-biphenyl]-4-yl)-N′-(naphthalen-1-ylmethylene)propanehydrazide (4n)
3.4.16. (E)-2-(2-Fluoro-[1,1′-biphenyl]-4-yl)-N′-octylidenepropanehydrazide (4o)
3.4.17. (E)-4-((2-(2-(2-Fluoro-[1,1′-biphenyl]-4-yl)propanoyl)hydrazono)methyl)benzoic acid (4p)
3.4.18. (E)-2-(2-Fluoro-[1,1′-biphenyl]-4-yl)-N′-(1-(4-hydroxyphenyl)ethylidene)propanehydrazide (5a)
3.4.19. (E)-2-(2-Fluoro-[1,1′-biphenyl]-4-yl)-N′-(1-(4-nitrophenyl)ethylidene)propanehydrazide (5b)
3.4.20. (E)-2-(2-Fluoro-[1,1′-biphenyl]-4-yl)-N′-(6-methoxy-3,4-dihydronaphthalen-1(2H) -ylidene)propanehydrazide (5c)
3.4.21. N′-((1Z, 2Z)-1,3-Diphenylallylidene)-2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanehydrazide (5d)
3.4.22. (E)-N′-(Cyclohexyl(phenyl)methylene)-2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanehydrazide (5e)
3.4.23. (E)-2-(2-Fluoro-[1,1′-biphenyl]-4-yl)-N′-(1-phenylethylidene)propanehydrazide (5f)
3.4.24. (E)-2-(2-Fluoro-[1,1′-biphenyl]-4-yl)-N′-(1-phenylpropylidene)propanehydrazide (5g)
3.4.25. (E)-2-(2-Fluoro-[1,1′-biphenyl]-4-yl)-N′-(2-methyl-1-phenylpropylidene)propanehydrazide (5h)
3.4.26. (E)-2-(2-Fluoro-[1,1′-biphenyl]-4-yl)-N′-(1-phenylpentylidene)propanehydrazide (5i)
3.4.27. (E)-N′-(1-Cyclopropylethylidene)-2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanehydrazide (5j)
3.4.28. (E)-2-(2-Fluoro-[1,1′-biphenyl]-4-yl)-N′-(1-phenylbutylidene)propanehydrazide (5K)
3.4.29. N′-Cyclohexylidene-2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanehydrazide (5l)
3.4.30. (E)-2-(2-Fluoro-[1,1′-biphenyl]-4-yl)-N′-(1-(2-methoxyphenyl)ethylidene)propanehydrazide (5m)
3.4.31. (E)-2-(2-Fluoro-[1,1′-biphenyl]-4-yl)-N′-(1-(4-methoxyphenyl)ethylidene)propanehydrazide (5n)
3.5. In Vitro α-Glucosidase Inhibition Assay
3.6. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, K.M.; Taha, M.; Rahim, F.; Fakhri, M.I.; Jamil, W.; Khan, M.; Rasheed, S.; Karim, A.; Perveen, S.; IQBAL, M. Acylhydrazide Schiff bases: Synthesis and antiglycation activity. J. Chem. Soc. Pak. 2013, 34, 930–938. [Google Scholar]
- Taha, M.; Ismail, N.H.; Jamil, W.; Yousuf, S.; Jaafar, F.M.; Ali, M.I.; Kashif, S.M.; Hussain, E. Synthesis, evaluation of antioxidant activity and crystal structure of 2, 4-dimethylbenzoylhydrazones. Molecules 2013, 18, 10912–10929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed Khan, K.; Shah, Z.; Uddin Ahmad, V.; Khan, M.; Taha, M.; Rahim, F.; Ali, S.; Ambreen, N.; Perveen, S.; Iqbal Choudhary, M. 2,4,6-Trichlorophenylhydrazine Schiff bases as DPPH radical and super oxide anion scavengers. Med. Chem. 2012, 8, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Anouar, E.H.; Raweh, S.; Bayach, I.; Taha, M.; Baharudin, M.S.; Di Meo, F.; Hasan, M.H.; Adam, A.; Ismail, N.H.; Weber, J.-F.F. Antioxidant properties of phenolic Schiff bases: Structure–activity relationship and mechanism of action. J. Comput.-Aided Mol. Des. 2013, 27, 951–964. [Google Scholar] [CrossRef]
- Cheng, K.; Zheng, Q.-Z.; Qian, Y.; Shi, L.; Zhao, J.; Zhu, H.-L. Synthesis, antibacterial activities and molecular docking studies of peptide and Schiff bases as targeted antibiotics. Bioorg. Med. Chem. 2009, 17, 7861–7871. [Google Scholar] [CrossRef]
- Avupati, V.R.; Yejella, R.P.; Parala, V.R.; Killari, K.N.; Papasani, V.M.R.; Cheepurupalli, P.; Gavalapu, V.R.; Boddeda, B. Synthesis, characterization and in vitro biological evaluation of some novel 1,3,5-triazine–Schiff base conjugates as potential antimycobacterial agents. Bioorg. Med. Chem. Lett. 2013, 23, 5968–5970. [Google Scholar] [CrossRef] [PubMed]
- Jarrahpour, A.; Khalili, D.; De Clercq, E.; Salmi, C.; Brunel, J.M. Synthesis, antibacterial, antifungal and antiviral activity evaluation of some new bis-Schiff bases of isatin and their derivatives. Molecules 2007, 12, 1720–1730. [Google Scholar] [CrossRef] [PubMed]
- Popp, F.D.; Kirsch, W. Synthesis of potential anticancer agents. V. Schiff bases and related Compounds1-2. J. Org. Chem. 1961, 26, 3858–3860. [Google Scholar] [CrossRef]
- Sinha, D.; Tiwari, A.K.; Singh, S.; Shukla, G.; Mishra, P.; Chandra, H.; Mishra, A.K. Synthesis, characterization and biological activity of Schiff base analogues of indole-3-carboxaldehyde. Eur. J. Med. Chem. 2008, 43, 160–165. [Google Scholar] [CrossRef]
- You, Z.-L.; Shi, D.-H.; Xu, C.; Zhang, Q.; Zhu, H.-L. Schiff base transition metal complexes as novel inhibitors of xanthine oxidase. Eur. J. Med. Chem. 2008, 43, 862–871. [Google Scholar] [CrossRef]
- Rafiq, K.; Khan, M.; Muhammed, N.; Khan, A.; Rehman, N.U.; Al-Yahyaei, B.E.M.; Khiat, M.; Halim, S.A.; Shah, Z.; Csuk, R. New amino acid clubbed Schiff bases inhibit carbonic anhydrase II, α-glucosidase, and urease enzymes: In silico and in vitro. Med. Chem. Res. 2021, 30, 712–728. [Google Scholar] [CrossRef]
- Rahim, F.; Zaman, K.; Taha, M.; Ullah, H.; Ghufran, M.; Wadood, A.; Rehman, W.; Uddin, N.; Shah, S.A.A.; Sajid, M. Synthesis, in vitro alpha-glucosidase inhibitory potential of benzimidazole bearing bis-Schiff bases and their molecular docking study. Bioorg. Chem. 2020, 94, 103394. [Google Scholar] [CrossRef]
- Mishra, P.; Gupta, P.; Shakya, A.K.; Shukla, R.; Srimal, R. Anti-inflammatory and diuretic activity of a new class of compounds--Schiff bases of 3-amino-2-methylquinazolin 4 (3H)-ones. Indian J. Physiol. Pharmacol. 1995, 39, 169–172. [Google Scholar]
- Jain, J.S.; Srivastava, R.S.; Aggarwal, N.; Sinha, R. Synthesis and evaluation of Schiff bases for anticonvulsant and behavioral depressant properties. Cent. Nerv. Syst. Agents Med. Chem. 2007, 7, 200–204. [Google Scholar] [CrossRef]
- Khan, M.; Alam, A.; Khan, K.M.; Salar, U.; Chigurupati, S.; Wadood, A.; Ali, F.; Mohammad, J.I.; Riaz, M.; Perveen, S. Flurbiprofen derivatives as novel α-amylase inhibitors: Biology-oriented drug synthesis (BIODS), in vitro, and in silico evaluation. Bioorg. Chem. 2018, 81, 157–167. [Google Scholar] [CrossRef]
- Rao, C.V.; Reddy, B.S. NSAIDs and chemoprevention. Curr. Cancer Drug Targets 2004, 4, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Tian, C.; Qiang, X.; Song, Q.; Cao, Z.; Ye, C.; He, Y.; Deng, Y.; Zhang, L. Flurbiprofen-chalcone hybrid Mannich base derivatives as balanced multifunctional agents against Alzheimer’s disease: Design, synthesis and biological evaluation. Bioorg. Chem. 2020, 94, 103477. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, L.; Zeng, S. Characterizing the effect of UDP-glucuronosyltransferase (UGT) 2B7 and UGT1A9 genetic polymorphisms on enantioselective glucuronidation of flurbiprofen. Biochem. Pharmacol. 2011, 82, 1757–1763. [Google Scholar] [CrossRef]
- Sagdinc, S.; Pir, H. Spectroscopic and DFT studies of flurbiprofen as dimer and its Cu (II) and Hg (II) complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 181–194. [Google Scholar] [CrossRef]
- Musa, K.A.; Eriksson, L.A. Photochemical and photophysical properties, and photodegradation mechanism, of the non-steroid anti-inflammatory drug Flurbiprofen. J. Photochem. Photobiol. A Chem. 2009, 202, 48–56. [Google Scholar] [CrossRef]
- Tarafder, M.; Kasbollah, A.; Saravanan, N.; Crouse, K.A.; Ali, A.M. S-methyldithiocarbazate and its Schiff bases: Evaluation of bondings and biological properties. J. Biochem. Mol. Biol. Biophys. JBMBB Off. J. Fed. Asian Ocean. Biochem. Mol. Biol. (FAOBMB) 2002, 6, 85–91. [Google Scholar] [CrossRef]
- Mohammed Khan, K.; Rahim, F.; Ambreen, N.; Taha, M.; Khan, M.; Jahan, H.; Shaikh, A.; Iqbal, S.; Perveen, S.; Iqbal Choudhary, M. Synthesis of benzophenonehydrazone Schiff bases and their in vitro antiglycating activities. Med. Chem. 2013, 9, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Rahim, F.; Malik, F.; Ullah, H.; Wadood, A.; Khan, F.; Javid, M.T.; Taha, M.; Rehman, W.; Rehman, A.U.; Khan, K.M. Isatin based Schiff bases as inhibitors of α-glucosidase: Synthesis, characterization, in vitro evaluation and molecular docking studies. Bioorg. Chem. 2015, 60, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Naz, H.; Rasheed, S.; Ismail, N.H.; Rahman, A.A.; Yousuf, S.; Choudhary, M.I. Synthesis of 4-methoxybenzoylhydrazones and evaluation of their antiglycation activity. Molecules 2014, 19, 1286–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Küçükgüzel, S.G.; Mazi, A.; Sahin, F.; Öztürk, S.; Stables, J. Synthesis and biological activities of diflunisal hydrazide–hydrazones. Eur. J. Med. Chem. 2003, 38, 1005–1013. [Google Scholar] [CrossRef]
- Küçükgüzel, G.; Kocatepe, A.; De Clercq, E.; Şahin, F.; Güllüce, M. Synthesis and biological activity of 4-thiazolidinones, thiosemicarbazides derived from diflunisal hydrazide. Eur. J. Med. Chem. 2006, 41, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.S.; Kavrekar, V.; Mishra, A. In vitro studies on alpha amylase and alpha glucosidase inhibitory activities of selected plant extracts. Eur. J. Exp. Biol. 2013, 3, 128–132. [Google Scholar]
- Kawde, A.-N.; Taha, M.; Alansari, R.S.; Almandil, N.B.; Uddin, N.; Rahim, F.; Chigurupati, S.; Nawaz, M.; Hayat, S.; Ibrahim, M. Exploring efficacy of indole-based dual inhibitors for α-glucosidase and α-amylase enzymes: In silico, biochemical and kinetic studies. Int. J. Biol. Macromol. 2020, 154, 217–232. [Google Scholar] [CrossRef]
- Taha, M.; Noreen, T.; Imran, S.; Nawaz, F.; Chigurupati, S.; Selvaraj, M.; Rahim, F.; Ismail, N.H.; Kumar, A.; Mosaddik, A. Synthesis, α-amylase inhibition and molecular docking study of bisindolylmethane sulfonamide derivatives. Med. Chem. Res. 2019, 28, 2010–2022. [Google Scholar] [CrossRef]
- Chigurupati, S.; Yiik, E.W.K.; Vijayabalan, S.; Selvarajan, K.K.; Alhowail, A.; Nanda, S.S.; Das, S. Antioxidant and antidiabetic properties of Tamarindus indica leaf ethanolic extract from Malaysia. Southeast Asian J. Trop. Med. Public Health 2020, 51, 559–569. [Google Scholar]
- Wu, B.; Song, H.-P.; Zhou, X.; Liu, X.-G.; Gao, W.; Dong, X.; Li, H.-J.; Li, P.; Yang, H. Screening of minor bioactive compounds from herbal medicines by in silico docking and the trace peak exposure methods. J. Chromatogr. A 2016, 1436, 91–99. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.; Menichini, F. Natural products as α-amylase and α-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: An update. Mini Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef]
- Chougale, A.D.; Ghadyale, V.A.; Panaskar, S.N.; Arvindekar, A.U. Alpha glucosidase inhibition by stem extract of Tinospora cordifolia. J. Enzym. Inhib. Med. Chem. 2009, 24, 998–1001. [Google Scholar] [CrossRef]
- Raju, B.C.; Tiwari, A.K.; Kumar, J.A.; Ali, A.Z.; Agawane, S.B.; Saidachary, G.; Madhusudana, K. α-Glucosidase inhibitory antihyperglycemic activity of substituted chromenone derivatives. Bioorg. Med. Chem. 2010, 18, 358–365. [Google Scholar] [CrossRef]
- Rahim, F.; Ullah, H.; Javid, M.T.; Wadood, A.; Taha, M.; Ashraf, M.; Shaukat, A.; Junaid, M.; Hussain, S.; Rehman, W. Synthesis, in vitro evaluation and molecular docking studies of thiazole derivatives as new inhibitors of α-glucosidase. Bioorg. Chem. 2015, 62, 15–21. [Google Scholar] [CrossRef]
- Ding, Y.; Mao, L.; Xu, D.; Xie, H.; Yang, L.; Xu, H.; Geng, W.; Gao, Y.; Xia, C.; Zhang, X. C-Aryl glucoside SGLT2 inhibitors containing a biphenyl motif as potential anti-diabetic agents. Bioorg. Med. Chem. Lett. 2015, 25, 2744–2748. [Google Scholar] [CrossRef]
- Taha, M.; Ismail, N.H.; Imran, S.; Wadood, A.; Rahim, F.; Saad, S.M.; Khan, K.M.; Nasir, A. Synthesis, molecular docking and α-glucosidase inhibition of 5-aryl-2-(6′-nitrobenzofuran-2′-yl)-1,3,4-oxadiazoles. Bioorg. Chem. 2016, 66, 117–123. [Google Scholar] [CrossRef]
- Taha, M.; Ismail, N.H.; Imran, S.; Wadood, A.; Ali, M.; Rahim, F.; Khan, A.A.; Riaz, M. Novel thiosemicarbazide–oxadiazole hybrids as unprecedented inhibitors of yeast α-glucosidase and in silico binding analysis. RSC Adv. 2016, 6, 33733–33742. [Google Scholar] [CrossRef]
- Rehman, N.U.; Khan, A.; Al-Harrasi, A.; Hussain, H.; Wadood, A.; Riaz, M.; Al-Abri, Z. New α-glucosidase inhibitors from the resins of Boswellia species with structure–glucosidase activity and molecular docking studies. Bioorg. Chem. 2018, 79, 27–33. [Google Scholar] [CrossRef]
- Ur Rehman, N.; Rafiq, K.; Khan, A.; Ahsan Halim, S.; Ali, L.; Al-Saady, N.; Hilal Al-Balushi, A.; Al-Busaidi, H.K.; Al-Harrasi, A. α-Glucosidase inhibition and molecular docking studies of natural brominated metabolites from marine macro brown alga Dictyopteris hoytii. Mar. Drugs 2019, 17, 666. [Google Scholar] [CrossRef] [Green Version]
- Ur Rehman, N.; Halim, S.A.; Al-Azri, M.; Khan, M.; Khan, A.; Rafiq, K.; Al-Rawahi, A.; Csuk, R.; Al-Harrasi, A. Triterpenic acids as non-competitive α-glucosidase inhibitors from Boswellia elongata with structure-activity relationship: In vitro and in silico studies. Biomolecules 2020, 10, 751. [Google Scholar] [CrossRef] [PubMed]
- Molecular Operating Environment (MOE); 2020.09; Chemical Computing Group ULC: Montreal, QC, Canada, 2022.
- Yamamoto, K.; Miyake, H.; Kusunoki, M.; Osaki, S. Crystal structures of isomaltase from Saccharomyces cerevisiae and in complex with its competitive inhibitor maltose. FEBS J. 2010, 277, 4205–4214. [Google Scholar] [CrossRef] [PubMed]
- Halim, S.A.; Jabeen, S.; Khan, A.; Al-Harrasi, A. Rational Design of Novel Inhibitors of α-Glucosidase: An Application of Quantitative Structure Activity Relationship and Structure-Based Virtual Screening. Pharmaceuticals 2021, 14, 482. [Google Scholar] [CrossRef] [PubMed]




 1 | |||||
|---|---|---|---|---|---|
| Compounds | R | IC50 ± µM (S.E.M) | Compounds | R | IC50 ± µM (S.E.M) |
| 4a |  | N/A | 5a |  | N/A |
| 4b |  | 7.16 ± 3.25 | 5b |  | N/A |
| 4c |  | 48.39 ± 3.75 | 5c |  | 35.52 ± 2.04 |
| 4d |  | 4.77 ± 2.03 | 5d |  | 42.03 ± 3.51 |
| 4e |  | N/A | 5e |  | N/A |
| 4f |  | 426.82 ± 4.63 | 5f |  | 136.36 ± 2.57 |
| 4g |  | 11.42 ± 1.09 | 5g |  | 68.75 ± 1.42 |
| 4h |  | 1.52 ± 0.07 | 5h |  | 3.41 ± 0.05 |
| 4i |  | 13.20 ± 2.47 | 5i |  | 10.26 ± 0.13 |
| 4j |  | 18.71 ± 0.25 | 5j |  | 29.31 ± 0.60 |
| 4k |  | 146.78 ± 0.86 | 5k |  | 0.93 ± 0.06 |
| 4l |  | 17.36 ± 1.68 | 5l |  | 14.73 ± 0.27 |
| 4m |  | 19.84 ± 1.89 | 5m |  | 11.83 ± 0.18 |
| 4n |  | 147.26 ± 2.78 | 5n |  | 14.24 ± 0.16 |
| 4o |  | 22.72 ± 1.46 | Acarbose | 875.75 ± 1.24 | |
| 4p |  | 18.61 ± 0.14 | |||
| Compounds | Docking Score (kcal/mol) | Protein-Ligand Interaction | |||
|---|---|---|---|---|---|
| Ligand Atom | Receptor Atom | Interaction | Distance (Å) | ||
| 5k | −7.51 | N9 O8 6-ring | OE2-GLU277 NE2-HIS351 CE1-PHE178 | HBD HBA π-H | 1.97 1.74 3.21 |
| 4h | −6.98 | N9 O8 6-ring | OE2-GLU277 NE2-HIS351 CD2-TYR158 | HBD HBA π-H | 1.84 1.69 2.72 |
| 5h | −6.71 | O8 6-ring | NH2-ARG213 6-ring-PHE303 | HBA π-π | 2.65 2.49 |
| 4d | −6.41 | N9 N11 |
OD1-ASP215 NE2-HIS351 |
HBD HBA |
1.80 1.95 |
| 4b | −6.04 |
O26 6-ring |
NE-ARG213 6-ring-PHE303 | HBA π-π |
3.09 3.75 |
| 5i | −5.70 | N9 O8 6-ring | OE1-GLU277 NE2-HIS351 6-ring-PHE303 | HBD HBA π-π | 2.53 2.26 2.52 |
| 4g | −5.36 |
N9 O8 |
OE2-GLU277 NE2-HIS351 |
HBD HBA |
2.62 2.67 |
| 5m | −5.70 | N9 O8 6-ring 6-ring 6-ring | OE2-GLU277 ND2-ASN350 6-ring-TYR347 6-ring-PHE301 6-ring-PHE178 | HBD HBA π-π π-π π-π | 2.82 2.98 3.75 3.27 3.59 |
| 4i | −5.69 | O8 | NH2-ARG213 | HBA | 2.67 |
| 5n | −5.24 | N9 O8 | OE2-GLU277 NE2-HIS351 | HBD HBA | 1.84 1.70 |
| 5l | −5.70 | N9 O8 | OE2-GLU277 NE2-HIS351 | HBD HBA | 1.82 1.71 |
| 4l | −5.59 |
N9 O8 |
OE2-GLU277 NE2-HIS351 |
HBD HBA |
2.09 2.59 |
| 4p | −5.59 | N18 N17 | OD1-ASP215 NE2-HIS351 | HBD HBA | 1.71 1.95 |
| 4j | −5.36 | O8 O46 | NE2-HIS351 OH-TYR347 | HBA HBA | 2.28 2.09 |
| 4m | −5.44 |
N9 O8 |
OE2-GLU277 NE2-HIS351 |
HBD HBA |
1.95 1.73 |
| 4o | −2.12 | O8 | OH-TYR347 | HBA | 2.05 |
| 1 | −5.11 | N11 O8 6-ring | OD2-ASP69 NH1-ARG442 CD2-TYR158 | HBD HBA π-H | 1.96 1.93 3.64 |
| 5j | −4.77 | N9 O8 | OE2-GLU277 NE2-HIS351 | HBD HBA | 1.82 1.69 |
| 5c | −4.27 |
N9 O8 |
OE2-GLU277 NE2-HIS351 |
HBD HBA |
1.85 1.70 |
| 5d | −4.24 |
6-ring 6-ring |
CG-GLU277 6-ring-PHE303 |
π-H π-π |
3.03 2.58 |
| 4c | −4.21 |
N9 O46 O8 |
OD1-ASP215 OD2-ASP352 NE2-HIS351 |
HBD HBD HBA |
1.80 1.83 2.62 |
| 5g | −3.48 | 6-ring | 6-ring TYR72 | π-π | 2.80 |
| 5f | −3.12 |
N9 O8 |
OE2-GLU277 NE2-HIS351 |
HBD HBA |
2.08 2.44 |
| 4k | −3.50 | O8 | NH1-ARG442 | HBA | 2.46 |
| 4n | −3.64 | N9 | OD1-ASP215 | HBD | 2.73 |
| 4f | −3.36 | 6-ring | 6-ring-PHE303 | π-π | 3.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, A.; Ali, M.; Rehman, N.U.; Ullah, S.; Halim, S.A.; Latif, A.; Zainab; Khan, A.; Ullah, O.; Ahmad, S.; et al. Bio-Oriented Synthesis of Novel (S)-Flurbiprofen Clubbed Hydrazone Schiff’s Bases for Diabetic Management: In Vitro and In Silico Studies. Pharmaceuticals 2022, 15, 672. https://doi.org/10.3390/ph15060672
Alam A, Ali M, Rehman NU, Ullah S, Halim SA, Latif A, Zainab, Khan A, Ullah O, Ahmad S, et al. Bio-Oriented Synthesis of Novel (S)-Flurbiprofen Clubbed Hydrazone Schiff’s Bases for Diabetic Management: In Vitro and In Silico Studies. Pharmaceuticals. 2022; 15(6):672. https://doi.org/10.3390/ph15060672
Chicago/Turabian StyleAlam, Aftab, Mumtaz Ali, Najeeb Ur Rehman, Saeed Ullah, Sobia Ahsan Halim, Abdul Latif, Zainab, Ajmal Khan, Obaid Ullah, Shujaat Ahmad, and et al. 2022. "Bio-Oriented Synthesis of Novel (S)-Flurbiprofen Clubbed Hydrazone Schiff’s Bases for Diabetic Management: In Vitro and In Silico Studies" Pharmaceuticals 15, no. 6: 672. https://doi.org/10.3390/ph15060672
APA StyleAlam, A., Ali, M., Rehman, N. U., Ullah, S., Halim, S. A., Latif, A., Zainab, Khan, A., Ullah, O., Ahmad, S., Al-Harrasi, A., & Ahmad, M. (2022). Bio-Oriented Synthesis of Novel (S)-Flurbiprofen Clubbed Hydrazone Schiff’s Bases for Diabetic Management: In Vitro and In Silico Studies. Pharmaceuticals, 15(6), 672. https://doi.org/10.3390/ph15060672