Biflavonoids: Preliminary Reports on Their Role in Prostate and Breast Cancer Therapy
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization—WHO. Available online: https://www.who.int/health-topics/cancer (accessed on 31 August 2023).
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, B.; Geyer, F.C.; Reis-filho, J.S. Histological Types of Breast Cancer: How Special Are They? Mol. Oncol. 2010, 4, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Salles, M.D.A.; Perez, A.A.; Gomes, D.S.; Gobbi, H. Contribuição Da Imuno-Histoquímica Na Avaliação de Fatores Prognósticos e Preditivos Do Câncer de Mama e No Diagnóstico de Lesões Mamárias. J. Bras. Patol. Med. Lab. 2009, 45, 213–222. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, M.; Hristeva, S.; Bielska, M.; Ortega, R.; Kumar, K. Highly Stereoselective Synthesis of a Compound Collection Based on the Bicyclic Scaffolds of Natural Products. Molecules 2017, 22, 827. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, E.; Sankari, L.; Malathi, L.; Krupaa, J. Naturally Occurring Products in Cancer Therapy. J. Pharm. Bioallied Sci. 2015, 7, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.C.; Hsu, C.L.; Lin, H.T.; Yen, G.C. Anticancer Effects of Flavonoid Derivatives Isolated from Millettia Reticulata Benth in Sk-Hep-1 Human Hepatocellular Carcinoma Cells. J. Agric. Food Chem. 2010, 58, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Pesrspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef] [PubMed]
- Tiemy, J.; Siraichi, G.; Felipe, D.F.; Zampar, L.; Brambilla, S.; Terra, A.; Cecchini, A.L.; Elaine, L.; Cortez, R. Antioxidant Capacity of the Leaf Extract Obtained from Arrabidaea Chica Cultivated in Southern Brazil. PLoS ONE 2013, 8, 1–9. [Google Scholar]
- Xiao, J.; Muzashvili, T.S.; Georgiev, M.I. Advances in the Biotechnological Glycosylation of Valuable Flavonoids. Biotechnol. Adv. 2014, 32, 1145–1156. [Google Scholar] [CrossRef]
- Suzart, L.R.; De, J.F.; Daniel, S.; de Carvalho, M.G.; Auxiliadora, M.; Kaplan, C. Flavonoidic biodiversiy and pharmacologic aspects in the species of the Ouratea and Luxemburgia genera (Ochnaceae). Quim. Nova. 2007, 30, 984–987. [Google Scholar] [CrossRef]
- He, X.; Yang, F.; Huang, X. Proceedings of Chemistry, Pharmacology, Pharmacokinetics and Synthesis of Biflavonoids. Molecules 2021, 26, 6088. [Google Scholar] [CrossRef]
- Mercader, A.; Pomilio, A. Naturally-Occurring Dimers of Flavonoids as Anticarcinogens. Anti-Cancer Agents Med. Chem. 2013, 13, 1217–1235. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.P.; Park, H.; Son, K.H.; Chang, H.W.; Kang, S.S. Biochemical Pharmacology of Biflavonoids: Implications for Anti-Inflammatory Action. Arch. Pharmacal Res. 2008, 31, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Gontijo, V.S.; dos Santos, M.H.; Viegas, C., Jr. Biological and Chemical Aspects of Natural Biflavonoids from Plants: A Brief Review. Mini-Rev. Med. Chem. 2016, 17, 834–862. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.F.; Wong, I.L.K.; Kan, J.W.Y.; Yan, C.S.W.; Chow, L.M.C.; Chan, T.H. Amine Linked Flavonoid Dimers as Modulators for P-Glycoprotein-Based Multidrug Resistance: Structure-Activity Relationship and Mechanism of Modulation. J. Med. Chem. 2012, 55, 1999–2014. [Google Scholar] [CrossRef]
- Dury, L.; Nasr, R.; Lorendeau, D.; Comsa, E.; Falson, P.; di Pietro, A.; Baubichon-Cortay, H.; Wong, I.; Zhu, X.; Chan, K.F.; et al. Flavonoid Dimers Are Highly Potent Killers of Multidrug Resistant Cancer Cells Overexpressing MRP1. Biochem. Pharmacol. 2017, 124, 10–18. [Google Scholar] [CrossRef]
- Liu, Y.; Kelsang, N.; Lu, J.; Zhang, Y.; Liang, H.; Tu, P.; Kong, D.; Zhang, Q. Oxytrodiflavanone A and Oxytrochalcoflavanones A, B: New Biflavonoids from Oxytropis chiliophylla. Molecules 2019, 24, 1468. [Google Scholar] [CrossRef]
- Groshi, A.A.; Jasim, H.A.; Evans, A.R.; Ismail, F.M.D.; Dempster, N.M.; Nahar, L.; Sarker, S.D. Growth inhibitory activity of biflavonoids and diterpenoids from the leaves of the Libyan Juniperus phoenicea against human cancer cells. Phytother. Res. 2019, 33, 2075–2082. [Google Scholar] [CrossRef]
- Banzato, T.; Gubiani, J.; Bernardi, D.; Nogueira, C.; Monteiro, A.; Juliano, F.; Alencar, S.; Pilli, R.; Lima, C.; Longato, G.; et al. Antiproliferative Flavanoid Dimers Isolated from Brazilian Red Propolos. J. Nat. Prod. 2020, 83, 1784–1793. [Google Scholar] [CrossRef]
- Liu, Q.; Cheung, F.W.K.; Liu, B.P.L.; Li, C.; Ye, W.; Che, C. Involvement of p21 and FasL in Induction of Cell Cycle Arrest and Apoptosis by Neochamaejasmin A in Human Prostate LNCaP Cancer Cells. J. Nat. Prod. 2008, 71, 842–846. [Google Scholar] [CrossRef] [PubMed]
- You, O.H.; Kim, S.; Kim, B.; Sohn, E.J.; Lee, H.; Shim, B.; Yun, M.; Kwon, B.; Kim, S. Ginkgetin induces apoptosis via activation of caspase and inhibition of survival genes in PC-3 prostate cancer cells. Bioorganic Med. Chem. Lett. 2013, 23, 2692–2695. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.J.; Jung, S.; Yun, J.; Lee, C.W.; Choi, J.; Lee, Y.; Han, D.C.; Kwon, B. Ginkgetin inhibits the growth of DU 145 prostate cancer cells through inhibition of signal transducer and activator of transcription 3 activity. Cancer Sci. 2015, 106, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.; Cubero, M.; Rodrigues, C.; Franco, Y.; Nascimento, J.; Vendramini-Costa, D.; da Rocha, C.; Longato, G. Antiproliferative Activity of Two Unusual Dimeric Flavonoids, Brachydin E and Brachydin F, Isolated from Fridericia platyphylla (Cham.) L.G.Lohmann: In Vitro and Molecular Docking Evaluation. BioMed Res. Int. 2022, 2022, 3319203. [Google Scholar] [CrossRef] [PubMed]
- Nunes, H.; Tuttisa, K.; Serpelonia, J.; Nascimento, J.; da Rocha, C.; Silva, V.; Lengert, A.; Reis, R.; Colus, I. Characterization of the in vitro cytotoxic effects of brachydins isolated from Fridericia platyphylla in a prostate cancer cell line. J. Toxicol. Environ. Health 2020, 83, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.; Tuttis, K.; Gomes, I.; Oliveira, L.; Serpeloni, J.; Lengert, A.; Reis, R.; Colus, I.; da Rocha, C.; Antunes, L. The Antitumoral/Antimetastatic Action of the Flavonoid Brachydin A in Metastatic Prostate Tumor Spheroids In Vitro Is Mediated by (Parthanatos) PARP-Related Cell Death. Pharmaceutics 2022, 14, 963. [Google Scholar] [CrossRef]
- Serpeloni, J.; Ribeiro, D.; Weiss, G.; Oliveira, L.; Fujiike, A.; Nunes, H.; da Rocha, C.; Guembarovski, R.; Colus, I. Flavonoid brachydin B decreases viability, proliferation, and migration in human metastatic prostate (DU145) cells grown in 2D and 3D culture models. Toxicol. Res. 2023, 12, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.; Ribeiro, D.; Nascimento, J.; da Rocha, C.; Colus, I.; Serpeloni, J. Anticancer activities of Brachydin C in human prostate tumor cells (DU145) grown in 2D and 3D models: Stimulation of cell death and downregulation of metalloproteinases in spheroids. Chem. Biol. Drug Des. 2022, 100, 747–762. [Google Scholar] [CrossRef] [PubMed]
- Maciel-Silva, V.L.; Da Rocha, C.Q.; Alencar, L.M.R.; Castelo-Branco, P.V.; Sousa, I.H.; Azevedo-Santos, A.P.; Vale, A.A.M.; Monteiro, S.G.; Soares, R.P.; Guimarães, S.J.A.; et al. Unusual dimeric flavonoids (brachydins) induce ultrastructural membrane alterations associated with antitumor activity in cancer cell lines. Drug Chem. Toxicol. 2022, 46, 665–676. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, X.; Li, J.; Yao, X.; Liu, W.; Kang, F.; Zou, Z.; Xu, K.; Xu, P.; Tan, G. Identification of a new natural biflavonoids against breast cancer cells induced ferroptosis via the mitochondrial pathway. Bioorganic Chem. 2021, 109, 104744. [Google Scholar] [CrossRef]
- Chen, T.; Yang, P.; Chen, H.; Huang, B. A new biflavonoids from Aster tataricus induced non-apoptotic cell death in A549 cells. Nat. Prod. Res. 2021, 36, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Kalenga, T.M.; Ndoile, M.M.; Atilaw, Y.; Munissi, J.J.E.; Gilissen, P.J.; Rudenko, A.; Bourgard, C.; Sunnerhagen, P.; Nyandoro, S.S.; Erdelyi, M. Antibacterial and cytotoxic biflavonoids from the root bark of Ochna kirkii. Fitoterapia 2021, 151, 104857. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Zhang, S.; Tan, J.; Chen, D.; Xu, Y.; Xu, K.; Tan, G.S. Two new biflavonoids from Selaginella doederleinii. Phytochem. Lett. 2020, 40, 126–129. [Google Scholar] [CrossRef]
- Augusta, D.D.; Dianhar, H.; Rahayu, D.U.C.; Suparto, I.H.; Sugita, P. Anticancer and Antivirus Activities of two Biflavonoids from Indonesian Araucaria hunsteinii K Schum Leaves. J. Hunan Univ. Nat. Sci. 2022, 49, 168–177. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Z.; He, Z.; Xu, J.; Xu, W.; Yang, X. Flavonoids dimers from the fruits of Psoralea corylifolia and their cytotoxicity against MCF-7 cells. Bioorg. Chem. 2023, 130, 1–14. [Google Scholar]
- Zhang, G.; Jing, Y.; Zhang, H.; Ma, E.; Guan, J.; Xue, F.; Liu, H.; Sun, X. Isolation and cytotoxic activity of selaginellin derivatives and biflavonoids from Selaginella tamariscina. Planta Medica 2012, 78, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Liu, C.; Liu, F.; Liu, Z.; Lai, G.; Yi, J. Hinokiflavone induces apoptosis and inhibits migration of breast cancer cells via EMT signalling pathway. Cell Biochem. Funct. 2020, 38, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Shin, S.; Lee, H.; Chun, H.; Chung, A. Isoginkgetin inhibits tumor cell invasion by regulating phosphatidylinositol 3-kinase/Akt–dependent matrix metalloproteinase-9 expression. Mol. Cancer Ther. 2006, 5, 2666–2675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yu, H.; Dong, G.; Cai, L.; Bai, Y. Chamaejasmine Arrests Cell Cycle, Induces Apoptosis and Inhibits Nuclear NF-κB Translocation in the Human Breast Cancer Cell Line MDA-MB-231. Molecules 2013, 18, 845–858. [Google Scholar] [CrossRef]
- Adem, A.; Mbaveng, A.T.; Kuete, V.; Heydenreich, M.; Ndakala, A.; Irungu, B.; Yenesew, A.; Efferth, T. Cytotoxicity of isoflavones and biflavonoids from Ormocarpum kirkii towards multi-factorial drug resistant cancer. Phytomedicine 2019, 58, 152853. [Google Scholar] [CrossRef]
- Yeh, P.; Shieh, Y.; Hsu, L.; Kuo, L.Y.; Lin, J.; Liaw, C.; Kuo, Y. Naturally Occurring Cytotoxic [3′ → 8″]-Biflavonoids from Podocarpus nakaii. J. Tradit. Complement. Med. 2012, 2, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Liu, C.; Hsu, Y.; Lin, L.; Wang, S.; Chun, J.; Bau, D.; Lin, S. Amentoflavone Induces Cell-cycle Arrest and Apoptosis in MCF-7 Human Breast Cancer Cells via Mitochondria-dependent Pathway. In Vivo 2012, 26, 963–970. [Google Scholar] [PubMed]
- Park, Y.; Woo, S.H.; Seo, S.; Kim, H.; Noh, W.C.; Lee, J.K.; Kwon, B.; Min, K.N.; Choes, T.; Park, I. Ginkgetin induces cell death in breast cancer cells via downregulation of the estrogen receptor. Oncol. Lett. 2017, 14, 5027–5033. [Google Scholar] [CrossRef] [PubMed]
- Wong, R. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [PubMed]
- Chaabane, W.; User, S.; El-Gazzah, M.; Jaksik, R.; Sajjadi, E.; Rzeszowska-Wolny, J.; Los, M. Autophagy, Apoptosis, Mitoptosis and Necrosis: Interdependence Between Those Pathways and Effects on Cancer. Arch. Immunol. Ther. Exp 2013, 61, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, Z.; Pan, K.; Li, J.; Chen, Q. The function and mechanism of ferroptosis in cancer. Apoptosis 2020, 25, 786–798. [Google Scholar] [CrossRef]
- Radha, G.; Raghavan, S. BCL2: A promising cancer therapeutic target. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2017, 1868, 309–314. [Google Scholar] [CrossRef]
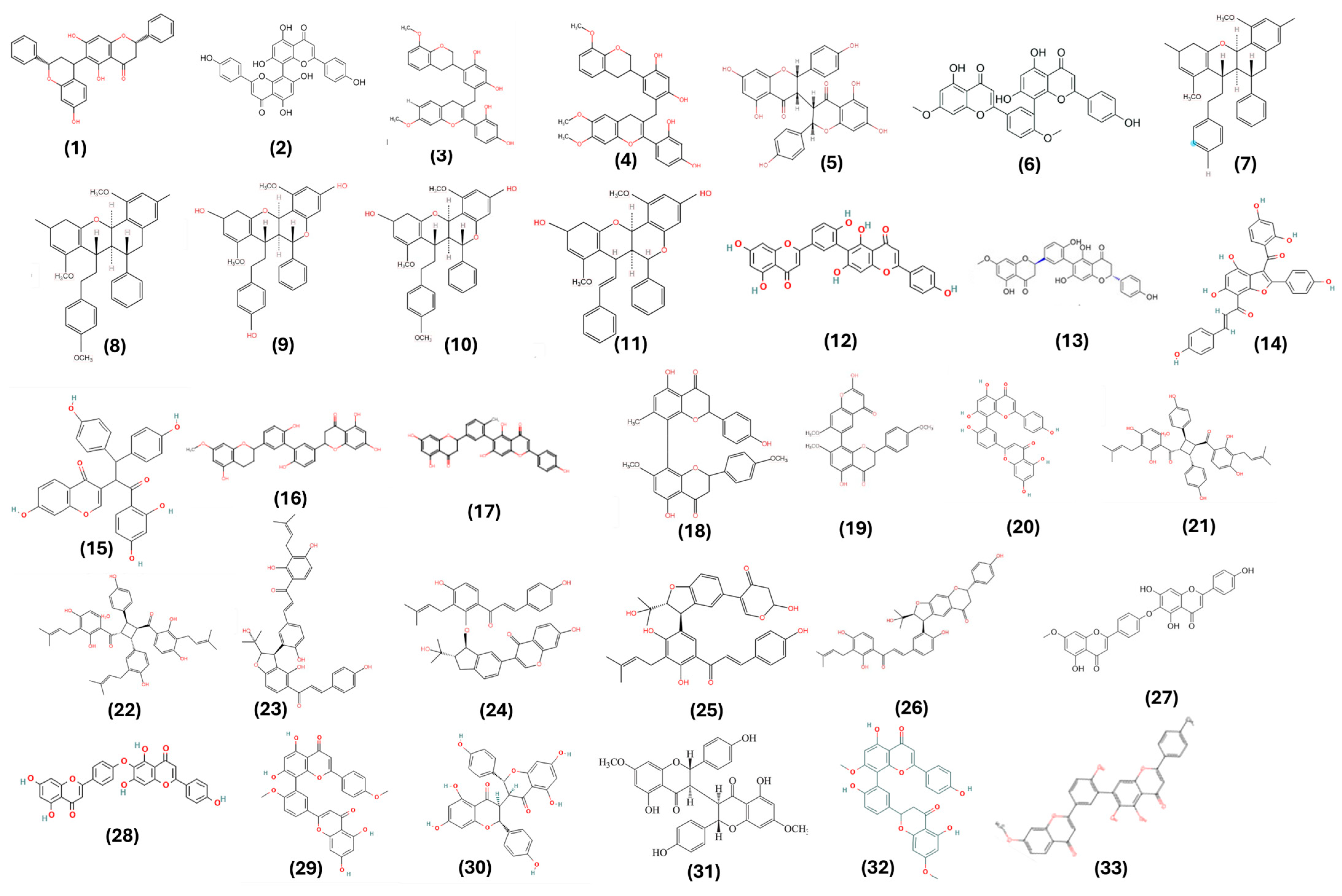
| Biflavonoids | Subtype | Monomer Type | Cell Lines | Assays | *IC50/#EC50/@TGI | Authors |
|---|---|---|---|---|---|---|
| Oxitrodiflavanone A (1) | C-C | BB | PC-3 | MTT (cell viability) | 6.64 μΜ (1) | [19] |
| Cupressuflavone (2) | C-C | AA | PC-3 | MTT (cell viability) | 19.9 μΜ (2) | [20] |
|
Propolone B (3) Propolone A (4) | C-linear fragment-C | EE | PC-3 | MTT (cell viability) | 19.1 μM (3) 21.9 μM (4) | [21] |
| Neochamaejasmin A (5) | C-C | AA | LNCaP | MTT (cell viability) Western blot analysis Cell cycle analysis | 12.5 μg/mL (5) | [22] |
| Ginkgetin (6) | C-C | BB | PC-3 | MTT (cell viability) Western blot analysis Cell cycle analysis | 15–30 μM (6) | [23] |
| Ginkgetin (6) | C-C | BB | DU-145 | MTT (cell viability) Western blot analysis Cell cycle analysis Mouse xenograft in vivo | 5 μM (6) | [24] |
|
Brachydin E (7) Brachydin F (8) | Complex | GG | PC-3 | MTT (cell viability), Molecular docking, Wound healing assay, Clonogenic assay, Phosphatidylserine (PS) Externalization assay, In silico pharmacodynamics | 6.9 μM (7) 37.1 μM (8) | [25] |
|
Brachydin A (9) Brachydin B (10) Brachydin C (11) | Complex | GG | PC-3 | MTT (cell viability) Neutral red assay, LDH activity release assay, Cell death assay, Comet assay, Western blot analysis | 23.41 μM (9) 4.28 μM (10) 4.44 μM (11) | [26] |
| Brachydin A (9) | Complex | GG | DU145 | Cytotoxicity assay, Tumor spheroids, Clonogenicity, Cell migration, Cell death assay, Protein Expression | 60.0–100.0 μM (9) | [27] |
| Brachydin B (10) | Complex | GG | DU145 | MTT (cell viability), Clonogenicity, Cell death assay, LDH, Cell migration | 7.45 μM (10) | [28] |
| Brachydin C (11) | Complex | GG | DU145 | Cytotoxicity assay, Cell migration, Clonogenicity, Protein expression | 47.31 μM (11) | [29] |
|
DCMF containing Brachydin A (9) Brachydin B (10) Brachydin C (11) | Complex | GG | DU145 | Sulforhodamine B (cell viability), Comet (genotoxicity), Clonogenicity (reproductive capacity) and Wound healing (cell migration) assays, and Atomic force microscopy (AFM) for ultrastructural cell membrane alterations | 2.51 μg/mL (9–11) | [30] |
| Biflavonoids | Subtype | Monomer Type | Cell Lines | Assays | *IC50/#EC50/@TGI | Authors |
|---|---|---|---|---|---|---|
|
DCMF containing Brachydin A (9) Brachydin B (10) Brachydin C (11) | Complex | GG | MCF7 | Sulforhodamine B (cell viability), Comet (genotoxicity), Clonogenicity (reproductive capacity), Wound healing (cell migration) assays, and Atomic force microscopy (AFM) for ultrastructural cell membrane alterations | 2.77 μg/mL (9–11) | [30] |
| Robustaflavone (12) | C-C | BB | MCF7 | MTT (cell viability), Detection of apoptotic cells, RNA extraction and sequencing, ROS, Molecular docking, Western blot | 11.89 μΜ (12) | [31] |
| (2R,2′R′)-7-O-methyl-2,3,2″,3″-tetrahydrorobustaflavone (13) | C-C | AA | MCF7 | MTT (cell viability) | 5.4 μΜ (13) | [32] |
|
Calodenin B (14) Lophirone A (15) | C-C (14) Complex (15) | AA (14) EG (15) | MCF7 | Cytotoxicity assay | 219.3 μM (14) 19.2 μM (15) | [33] |
|
7-O-methyl-2,3,2″,3″-tetrahydro-3′,3‴-biapigenin (16) 4′-O-methylrobustaflavone (17) | C-C | BB | MCF7 | MTT (cell viability) | 41.44 μM (16) 16.68 μM (17) | [34] |
|
4′,7,7″-tri-O-methylcupressuflavone (18) 4‴,7,7″-tri-O-methylagathisflavone (19) | C-C | BB | MCF7 | MTT (cell viability) | 91.74 μg/mL (18) 314.44 μg/mL (19) | [35] |
|
Amentoflavone (20) Cupressuflavone (2) | C-C | AA | MDA-MB-231 | MTT (cell viability) | 16.1 μM (20) 12.7 μM (2) | [20] |
|
Psocorylin R (21) Psocorylin S (22) Psocorylin U (23) Psocorylin V (24) Psocorylin W (25) Psocorylin Y (26) | C-C or C-linear fragment-C | GG | MCF7 | Cytotoxicity assay and Apoptosis assay | 7.35 μM (21) 17.40 μM (22) 10.01μM (23) 21.98 μM (24) 8.42 μM (25) 9.01 μM (26) | [36] |
| Neocryptomerin (27) Hinokiflavone (28) | C-linear fragment | BB | MCF7 | MTT (cell viability) | 30.09 µg/mL (27) 39.32 µg/mL (28) | [37] |
| Hinokiflavone (28) | C-linear fragment | BB | MDA-MB-231 | MTT (cell viability), Clonogenicity (reproductive capacity), Western blot, cell migration, In vivo tumor model in mice | 40 μM (28) | [38] |
| Isoginkgetin (29) | C-C | BB | MDA-MB-231 | Cytotoxicity assay Western blot | 20 μM (29) | [39] |
| Chamaejasmin (30) | C-C | AA | MDA-MB-231 | MTT (cell viability), Western blot, Cell cycle analysis | 5.11 μM (30) | [40] |
| 7,7″-di-O-methylchamaejasmin (31) | C-C | AA | MDA-MB-231 | Cytotoxicity assay, Western blot, Apoptosis assay | 7.76 μM (31) | [41] |
|
Podocarpusflavone-A (32) II-4″,I-7-Dimethoxyamentoflavone (33) | C-C | BB | MCF7 | MTT (cell viability), Cell cycle Topoisomerase I assay | 16.24 µg/mL (32) 15.17 µg/mL (33) | [42] |
| Amentoflavone (20) | C-C | AA | MCF7 | MTT (cell viability), Cell cycle Western blot Cometa assay | 150 μM (20) | [43] |
| Ginkgetin (6) | C-C | BB | MCF7 | MTT (cell viability), Cell cycle Western blot Apoptosis assay ER-α expression | 10 µM (6) | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, C.A.d.; Maquedano, L.K.; Jaalouk, L.S.; Santos, D.C.d.; Longato, G.B. Biflavonoids: Preliminary Reports on Their Role in Prostate and Breast Cancer Therapy. Pharmaceuticals 2024, 17, 874. https://doi.org/10.3390/ph17070874
Lima CAd, Maquedano LK, Jaalouk LS, Santos DCd, Longato GB. Biflavonoids: Preliminary Reports on Their Role in Prostate and Breast Cancer Therapy. Pharmaceuticals. 2024; 17(7):874. https://doi.org/10.3390/ph17070874
Chicago/Turabian StyleLima, Carolina Afonso de, Larissa Kaori Maquedano, Luiza Sertek Jaalouk, Dina Cardoso dos Santos, and Giovanna Barbarini Longato. 2024. "Biflavonoids: Preliminary Reports on Their Role in Prostate and Breast Cancer Therapy" Pharmaceuticals 17, no. 7: 874. https://doi.org/10.3390/ph17070874





