Introducing the NUATEI Consortium: A Mexican Research Program for the Identification of Natural and Synthetic Antimicrobial Compounds for Prevalent Infectious Diseases
Abstract
:1. Introduction
2. Consortium Operating Chain
3. Natural Products: Overview
3.1. Antimicrobial Compounds from Plants
3.2. Antimicrobial Compounds of Microbial Origin
4. Antimicrobial Synthetic Compounds
5. Biological Models of Infectious Diseases Targeted by the NUATEI Consortium
5.1. Tuberculosis as a Target of the NUATEI Program
5.1.1. Current Treatment and Drawbacks
5.1.2. Methodological Approach for the Search of Anti-M. tuberculosis Compounds
5.1.3. Compounds Tested against M. tuberculosis
5.2. Chagas Disease (American Trypanosomiasis) as a Target of the NUATEI Program
5.2.1. Current Treatment and Drawbacks
5.2.2. Methodological Approach for the Search of Anti-T. cruzi Compounds
5.2.3. Compounds Tested against T. cruzi
5.3. Amoebiasis by Entamoeba histolytica as a Target of the NUATEI Program
5.3.1. Current Treatment and Drawbacks
5.3.2. Methodological Approach for the Search of Anti-Amoebic Compounds
5.3.3. Compounds Tested against E. histolytica
5.4. HIV-1 as a Target of the NUATEI Program
5.4.1. Methodological Approach for the Search of HIV Inhibitors
5.4.2. New Synthetic Pyridinones against HIV-1 Reverse Transcriptase
6. Concluding Remarks and Future Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Secretaría de Salud. Boletín Epidemiológico Sistema Nacional de Vigilancia Epidemiológica Sistema Único de Información. Available online: https://www.gob.mx/salud/acciones-y-programas/direccion-general-de-epidemiologia-boletin-epidemiologico (accessed on 15 January 2024).
- Segala, F.V.; Bavaro, D.F.; Di Gennaro, F.; Salvati, F.; Marotta, C.; Saracino, A.; Murri, R.; Fantoni, M. Impact of SARS-CoV-2 Epidemic on Antimicrobial Resistance: A Literature Review. Viruses 2021, 13, 2110. [Google Scholar] [CrossRef] [PubMed]
- De Mello, F.C.Q.; Silva, D.R.; Dalcolmo, M.P. Tuberculosis: Where Are We? J. Bras. Pneumol. 2018, 44, 82. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Chagas Disease (American Trypanosomiasis). Available online: https://www.who.int/health-topics/chagas-disease#tab=tab_1 (accessed on 13 April 2024).
- Salazar-Schettino, M.P.; Bucio-Torres, M.I.; Cabrera-Bravo, M.; Citlalli De Alba-Alvarado, M.; Rocío Castillo-Saldaña, D.; Zenteno-Galindo, E.A.; Rojo-Medina, J.; Angélica Fernández-Santos, N.; Perera-Salazar, G. Enfermedad de Chagas En México. Rev. Fac. Méd. 2016, 59, 6–16. [Google Scholar]
- Carrero, J.C.; Reyes-López, M.; Serrano-Luna, J.; Shibayama, M.; Unzueta, J.; León-Sicairos, N.; de la Garza, M. Intestinal Amoebiasis: 160 Years of Its First Detection and Still Remains as a Health Problem in Developing Countries. Int. J. Med. Microbiol. 2020, 310, 151358. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS. UNAIDS Global HIV Statistics; UNAIDS: Geneva, Switzerland, 2023. [Google Scholar]
- Sistema de Vigilancia Epidemiológica de VIH. Informe Histórico de VIH 3er Trimestre 2023; Sistema de Vigilancia Epidemiológica de VIH: Ciudad de Mexico, Mexico, 2023. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Blaskovich, M.A.; Cooper, M.A. Antibiotics in the Clinical Pipeline in 2013. J. Antibiot. 2013, 66, 571–591. [Google Scholar] [CrossRef] [PubMed]
- Baquero, F. Threats of Antibiotic Resistance: An Obliged Reappraisal. Int. Microbiol. 2021, 24, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Wolfender, J.L.; Litaudon, M.; Touboul, D.; Queiroz, E.F. Innovative Omics-Based Approaches for Prioritisation and Targeted Isolation of Natural Products-New Strategies for Drug Discovery. Nat. Prod. Rep. 2019, 36, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tong, Z.; Shi, J.; Li, R.; Upton, M.; Wang, Z. Drug Repurposing for Next-Generation Combination Therapies against Multidrug-Resistant Bacteria. Theranostics 2021, 11, 4910–4928. [Google Scholar] [CrossRef]
- Silva, L.N.; Zimmer, K.R.; Macedo, A.J.; Trentin, D.S. Plant Natural Products Targeting Bacterial Virulence Factors. Chem. Rev. 2016, 116, 9162–9236. [Google Scholar] [CrossRef]
- Frankenberg, R. Allopathic Medicine, Profession, and Capitalist Ideology in India. Soc. Sci. Med. Part A Med. Psychol. Med. Sociol. 1981, 15, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Tainter, M.L.; Marcelli, G.M.A. The Rise of Synthetic Drugs in the American Pharmaceutical Industry. Bull. N. Y. Acad. Med. 1959, 35, 387–405. [Google Scholar] [PubMed]
- Villaseñor, J.L. Los Géneros de Plantas Vasculares de La Flora de México. Bot. Sci. 2004, 75, 105–135. [Google Scholar] [CrossRef]
- Aguilar, A.; Camacho, J.R.; Chino, S.; Jacquez, P.; López, M.E. Herbario Medicinal Del Instituto Mexicano Del Seguro Social. Inf. Etnobotán. 1994, 1, 253. [Google Scholar]
- Reyes-Chilpa, R.; Guzmán-Gutiérrez, S.L.; Campos-Lara, M.; Bejar, E.; Osuna-Fernández, H.R.; Hernández-Pasteur, G. On the First Book of Medicinal Plants Written in the American Continent: The Libellus Medicinalibus Indorum Herbis from Mexico, 1552. A Review. Bol. Latinoam. Caribe Plantas Med. Aromát. 2021, 20, 1–27. [Google Scholar] [CrossRef]
- Cilia-López, V.G.; Cariño-Cortés, R.; Zurita-Salinas, L.R. Ethnopharmacology of the Asteraceae Family in Mexico. Bot. Sci. 2021, 99, 455–486. [Google Scholar] [CrossRef]
- Gomez-Cansino, R.; Guzman-Gutierrez, S.L.; Campos-Lara, M.G.; Espitia-Pinzon, C.I.; Reyes-Chilpa, R. Natural Compounds from Mexican Medicinal Plants as Potential Drug Leads for Anti-Tuberculosis Drugs. An. Acad. Bras. Ciências 2017, 89, 31–43. [Google Scholar] [CrossRef]
- Romo de Vivar, A. Productos Naturales de La Flora Mexicana; Editorial Limusa: Mexico City, Mexico, 1985; 220p. [Google Scholar]
- Drago-Serrano, M.E.; Campos-Rodríguez, R.; Carrero, J.C.; Delagarza, M. Lactoferrin: Balancing Ups and Downs of Inflammation Due to Microbial Infections. Int. J. Mol. Sci. 2017, 18, 501. [Google Scholar] [CrossRef] [PubMed]
- Katsuno, K.; Burrows, J.N.; Duncan, K.; Van Huijsduijnen, R.H.; Kaneko, T.; Kita, K.; Mowbray, C.E.; Schmatz, D.; Warner, P.; Slingsby, B.T. Hit and Lead Criteria in Drug Discovery for Infectious Diseases of the Developing World. Nat. Rev. Drug Discov. 2015, 14, 751–758. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; Wiley: Chichester, UK, 2009. [Google Scholar]
- Dias, D.A.; Urban, S.; Roessner, U. A Historical Overview of Natural Products in Drug Discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Chen, Y.; Garcia de Lomana, M.; Friedrich, N.-O.; Kirchmair, J. Characterization of the Chemical Space of Known and Readily Obtainable Natural Products. J. Chem. Inf. Model. 2018, 58, 1518–1532. [Google Scholar] [CrossRef]
- Ismail, F.M.D.; Nahar, L.; Zhang, K.Y.; Sarker, S.D. Antiparasitic Natural Products. In Annual Reports in Medicinal Chemistry; Sarker, S.D., Nahar, L., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 55, pp. 115–151. [Google Scholar]
- World Health Organization. Malaria. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 1 February 2024).
- Yearsley, G.K.; Last, P.R. A New Genus of Stingarees (Myliobatiformes: Urolophidae) with Comments on Other Urolophid Genera and an Annotated Checklist of Species. In Rays of the World: Supplementary Information; Last, P.R., Yearsley, G.K., Eds.; CSIRO Special Publication: Clayton, Australia, 2016; pp. 35–40. [Google Scholar]
- Miller, L.H.; Su, X. Artemisinin: Discovery from the Chinese Herbal Garden. Cell 2011, 146, 855–858. [Google Scholar] [CrossRef]
- World Health Organization. The First WHO Traditional Medicine Global Summit. Available online: https://www.who.int/news-room/events/detail/2023/08/17/default-calendar/the-first-who-traditional-medicine-global-summit (accessed on 16 December 2023).
- Gómez-Cansino, R.; Espitia-Pinzon, C.I.; Campos-Lara, M.G.; Guzman-Gutierrez, S.L.; Segura-Salinas, E.; Echeverria-Valencia, G.; Torras-Claveria, L.; Cuevas-Figueroa, X.M.; Reyes-Chilpa, R. Antimycobacterial and HIV-1 Reverse Transcriptase Activity of Julianaceae and Clusiaceae Plant Species from Mexico. Evid.-Based Complement. Altern. Med. 2015, 2015, 183036. [Google Scholar] [CrossRef]
- Huerta Reyes, M.E. Evaluacion de La Actividad Inhibidora de Metabolitos Secundarios de Clusiaceae Mexicanas Sobre El VIH-1. Ph.D. Thesis, Universidad Nacional Autónoma de México, Ciudad de México, Mexico, 2004. [Google Scholar]
- Wright, G.D. Something Old, Something New: Revisiting Natural Products in Antibiotic Drug Discovery. Can. J. Microbiol. 2014, 60, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Bérdy, J. Microorganisms Producing Antibiotics. In Antibiotics: Current Innovations and Future Trends; Sanchez, S., Demain, A.L., Eds.; Caister Academic Press: Wymondham, UK, 2015; pp. 49–64. ISBN 9781908230546. [Google Scholar]
- Mella, S.; Zemelman, C.; Bello, H.; Dominguez, M.; Gonzalez, G.; Zemelman, R. Propiedades Microbiológicas, Clasificación y Relación Estructura-Actividad de Cefalosporinas e Importancia de Las Cefalosporinas de Cuarta Generación. Rev. Chil. Infectol. 2001, 18, 7–19. [Google Scholar] [CrossRef]
- Bérdy, J. Thoughts and Facts about Antibiotics: Where We Are Now and Where We Are Heading. J. Antibiot. 2012, 65, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Challis, G.L. Exploitation of the Streptomyces coelicolor A3(2) Genome Sequence for Discovery of New Natural Products and Biosynthetic Pathways. J. Ind. Microbiol. Biotechnol. 2014, 41, 219–232. [Google Scholar] [CrossRef]
- Gosse, J.T.; Hill, P.; Dowd, S.E.; Boddy, C.N. Draft Genome Sequence of Streptomyces Sp. Strain PBH53, Isolated from an Urban Environment. Genome Announc. 2015, 3, e00859-15. [Google Scholar] [CrossRef]
- Mukherjee, S.; Stamatis, D.; Bertsch, J.; Ovchinnikova, G.; Sundaramurthi, J.C.; Lee, J.; Kandimalla, M.; Chen, I.M.A.; Kyrpides, N.C.; Reddy, T.B.K. Genomes OnLine Database (GOLD) v.8: Overview and Updates. Nucleic Acids Res. 2021, 49, D723–D733. [Google Scholar] [CrossRef]
- Ramadhar, T.R.; Beemelmanns, C.; Currie, C.R.; Clardy, J. Bacterial Symbionts in Agricultural Systems Provide a Strategic Source for Antibiotic Discovery. J. Antibiot. 2014, 67, 53–58. [Google Scholar] [CrossRef]
- Piel, J. Approaches to Capturing and Designing Biologically Active Small Molecules Produced by Uncultured Microbes. Annu. Rev. Microbiol. 2011, 65, 431–453. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.A.; De Mecca, M.M.; Bartel, L.C. Toxic Side Effects of Drugs Used to Treat Chagas’ Disease (American Trypanosomiasis). Hum. Exp. Toxicol. 2006, 25, 471–479. [Google Scholar] [CrossRef]
- Bhalani, D.V.; Nutan, B.; Kumar, A.; Singh Chandel, A.K. Bioavailability Enhancement Techniques for Poorly Aqueous Soluble Drugs and Therapeutics. Biomedicines 2022, 10, 2055. [Google Scholar] [CrossRef] [PubMed]
- Ong, Y.C.; Roy, S.; Andrews, P.C.; Gasser, G. Metal Compounds against Neglected Tropical Diseases. Chem. Rev. 2019, 119, 730–796. [Google Scholar] [CrossRef] [PubMed]
- Maya, J.D.; Orellana, M.; Ferreira, J.; Kemmerling, U.; López-Muñoz, R.; Morello, A. Chagas Disease: Present Status of Pathogenic Mechanisms and Chemotherapy. Biol. Res. 2010, 43, 323–331. [Google Scholar] [CrossRef]
- Bajorath, J.; Peltason, L.; Wawer, M.; Guha, R.; Lajiness, M.S.; Van Drie, J.H. Navigating Structure-Activity Landscapes. Drug Discov. Today 2009, 14, 698–705. [Google Scholar] [CrossRef]
- Medina-Franco, J.L.; Méndez-Lucio, O.; Martinez-Mayorga, K. The Interplay between Molecular Modeling and Chemoinformatics to Characterize Protein-Ligand and Protein-Protein Interactions Landscapes for Drug Discovery. In Advances in Protein Chemistry and Structural Biology; Academic Press Inc.: Cambridge, MA, USA, 2014; Volume 96, pp. 1–37. [Google Scholar]
- Bajorath, J. Progress in Computational Medicinal Chemistry. J. Med. Chem. 2012, 55, 3593–3594. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report 2022; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- World Health Organization. Tuberculosis. Available online: https://www.who.int/health-topics/tuberculosis#tab=tab_1 (accessed on 14 February 2024).
- Silva Miranda, M.; Breiman, A.; Allain, S.; Deknuydt, F.; Altare, F. The Tuberculous Granuloma: An Unsuccessful Host Defence Mechanism Providing a Safety Shelter for the Bacteria? Clin. Dev. Immunol. 2012, 2012, 139127. [Google Scholar] [CrossRef]
- Kolloli, A.; Subbian, S. Host-Directed Therapeutic Strategies for Tuberculosis. Front. Med. 2017, 4, 171. [Google Scholar] [CrossRef]
- Migliori, G.B.; Ong, C.W.M.; Petrone, L.; D’ambrosio, L.; Centis, R.; Goletti, D. The Definition of Tuberculosis Infection Based on the Spectrum of Tuberculosis Disease. Breathe 2021, 17, 17210079. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2020; World Health Organization: Geneva, Switzerland, 2020; ISBN 9789240013131. [Google Scholar]
- Kumar, V.; Patel, S.; Jain, R. New Structural Classes of Antituberculosis Agents. Med. Res. Rev. 2018, 38, 684–740. [Google Scholar] [CrossRef] [PubMed]
- Trenado-Uribe, M.; Silva-Miranda, M.; Rivero-Cruz, J.F.; Rodríguez-Peña, K.; Espitia-Pinzón, C.I.; Rodríguez-Sanoja, R.; Sánchez, S. Antimycobacterial Activity of an Anthracycline Produced by an Endophyte Isolated from Amphipterygium Adstringens. Mol. Biol. Rep. 2018, 45, 2563–2570. [Google Scholar] [CrossRef]
- Collins, L.A.; Franzblau, S.G. Microplate Alamar Blue Assay versus BACTEC 460 System for High-Throughput Screening of Compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob. Agents Chemother. 1997, 41, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Lmmunological. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Martínez, R.; Espitia-Pinzón, C.I.; Silva Miranda, M.; Chávez-Santos, R.M.; Pretelin-Castillo, G.; Ramos-Orea, A.; Hernández-Báez, Á.M.; Cotlame-Pérez, S.; Pedraza-Rodríguez, R. Synthesis and Antituberculosis Activity of New Acylthiosemicarbazides Designed by Structural Modification. Drug Dev. Res. 2020, 81, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Pretelín-Castillo, G.; Miranda, M.S.; Espitia, C.; Chávez-Santos, R.M.; Suárez-Castro, A.; Chacón-García, L.; Aguayo-Ortiz, R.; Martinez, R. (2z)-3-Hydroxy-3-(4-r-Phenyl)-Prop-2-Enedithioic Acids as New Antituberculosis Compounds. Infect. Drug Resist. 2021, 14, 4323–4332. [Google Scholar] [CrossRef] [PubMed]
- Martínez, R.; Zamudio, G.J.N.; Pretelin-Castillo, G.; Torres-Ochoa, R.O.; Medina-Franco, J.L.; Espitia Pinzón, C.I.; Miranda, M.S.; Hernández, E.; Alanís-Garza, B. Synthesis and Antitubercular Activity of New N-[5-(4-Chlorophenyl)-1,3,4-Oxadiazol-2-Yl]-(Nitroheteroaryl)Carboxamides. Heterocycl. Commun. 2019, 25, 52–59. [Google Scholar] [CrossRef]
- Rassi, A.; Rassi, A.; Marin-Neto, J.A. Chagas Disease. Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention Parasites—American Trypanosomiasis (Also Known as Chagas Disease). Available online: https://www.cdc.gov/chagas/prevention/?CDC_AAref_Val=https://www.cdc.gov/parasites/chagas/prevent.html (accessed on 9 July 2024).
- Bern, C. Chagas’ Disease. N. Engl. J. Med. 2015, 373, 456–466. [Google Scholar] [CrossRef]
- Murillo-Godínez, G. Enfermedad de Chagas (Tripanosomiasis Americana). Med. Interna Mexico 2018, 34, 959–970. [Google Scholar] [CrossRef]
- Pino-Marín, A.; José Medina-Rincón, G.; Gallo-Bernal, S.; Duran-Crane, A.; Duque, Á.I.A.; Rodríguez, M.J.; Medina-Mur, R.; Manrique, F.T.; Forero, J.F.; Medina, H.M. Chagas Cardiomyopathy: From Romaña Sign to Heart Failure and Sudden Cardiac Death. Pathogens 2021, 10, 505. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.R.; Kelly, J.M. Trypanocidal Drugs: Mechanisms, Resistance and New Targets. Expert Rev. Mol. Med. 2009, 11, e31. [Google Scholar] [CrossRef] [PubMed]
- Docampoz, R.; Stoppan, A.M. Generation of Superoxide Anion and Hydrogen Peroxide Induced by Nifurtimox in Trypanosoma Cruzil. Arch. Biochem. Biophys. 1979, 19, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Martínez, I.; Nogueda, B.; Martínez-Hernández, F.; Espinoza, B. Microsatellite and Mini-Exon Analysis of Mexican Human DTU I Trypanosoma Cruzi Strains and Their Susceptibility to Nifurtimox and Benznidazole. Vector-Borne Zoonotic Dis. 2013, 13, 181–187. [Google Scholar] [CrossRef]
- De Castro, E.; Reus, T.L.; de Aguiar, A.M.; Ávila, A.R.; de Arruda Campos Brasil de Souza, T. Procaspase-Activating Compound-1 Induces Apoptosis in Trypanosoma Cruzi. Apoptosis 2017, 22, 1564–1577. [Google Scholar] [CrossRef] [PubMed]
- Mazzeti, A.L.; Capelari-Oliveira, P.; Bahia, M.T.; Mosqueira, V.C.F. Review on Experimental Treatment Strategies against Trypanosoma Cruzi. J. Exp. Pharmacol. 2021, 13, 409–432. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, K.D.; Martínez, I.; Agredano-Moreno, L.T.; Jiménez-García, L.F.; Reyes-Chilpa, R.; Espinoza, B. Coumarins Isolated from Calophyllum Brasiliense Produce Ultrastructural Alterations and Affect in Vitro Infectivity of Trypanosoma Cruzi. Phytomedicine 2019, 61, 152827. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, K.D.; Martínez, I.; Reyes-Chilpa, R.; Espinoza, B. Mammea Type Coumarins Isolated from Calophyllum Brasiliense Induced Apoptotic Cell Death of Trypanosoma Cruzi through Mitochondrial Dysfunction, ROS Production and Cell Cycle Alterations. Bioorganic Chem. 2020, 100, 103894. [Google Scholar] [CrossRef]
- Cortés-Ruiz, E.M.; Palomino-Hernández, O.; Rodríguez-Hernández, K.D.; Espinoza, B.; Medina-Franco, J.L. Computational Methods to Discover Compounds for the Treatment of Chagas Disease. In Advances in Protein Chemistry and Structural Biology; Academic Press Inc.: Cambridge, MA, USA, 2018; Volume 113, pp. 119–142. ISBN 9780128139165. [Google Scholar]
- López-Huerta, F.A.; Nieto-Camacho, A.; Morales-Flores, F.; Hernández-Ortega, S.; Chávez, M.I.; Méndez Cuesta, C.A.; Martínez, I.; Espinoza, B.; Espinosa-García, F.J.; Delgado, G. Hopane-Type Triterpenes from Cnidoscolus Spinosus and Their Bioactivities. Bioorganic Chem. 2020, 100, 103919. [Google Scholar] [CrossRef]
- Sepúlveda-Robles, O.; Espinoza-Gutiérrez, B.; Gomez-Verjan, J.C.; Guzmán-Gutiérrez, S.L.; De Ita, M.; Silva-Miranda, M.; Espitia-Pinzón, C.I.; Fernández-Ramírez, F.; Herrera-Salazar, A.; Mata-Rocha, M.; et al. Trypanocidal and Toxicological Assessment in Vitro and in Silico of Three Sesquiterpene Lactones from Asteraceae Plant Species. Food Chem. Toxicol. 2019, 125, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Santiago, L.; Martínez, I.; Arroyo-Olarte, R.; Díaz-Garrido, P.; Cuevas-Hernandez, R.I.; Espinoza, B. Structural New Data for Mitochondrial Peroxiredoxin from Trypanosoma Cruzi Show High Similarity with Human Peroxiredoxin 3: Repositioning Thiostrepton as Antichagasic Drug. Front. Cell. Infect. Microbiol. 2022, 12, 907043. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, B.; Martínez-Martínez, I.; de Rodríguez-Fragoso, M.L.; Rodríguez-López, Y.A.; Regla-Contreras, J.I.; López-Ortiz, M.; Fernández-Zertuche, M.; Ortega-Blake, I. Composición Farmacéutica Conteniendo Benznidazol y N-(L)-Histidinamida de Anfotericina B Para El Tratamiento de Tripanosomiasis. Mexican Patent 390,731, 2022. [Google Scholar]
- Murray, C.J.L.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-Adjusted Life Years (DALYs) for 291 Diseases and Injuries in: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef] [PubMed]
- Secretaría de Salud Histórico Boletín Epidemiológico. Available online: https://www.gob.mx/salud/acciones-y-programas/historico-boletin-epidemiologico (accessed on 26 February 2024).
- Aguilar-Díaz, H.; Carrero, J.C.; Argüello-García, R.; Laclette, J.P.; Morales-Montor, J. Cyst and Encystment in Protozoan Parasites: Optimal Targets for New Life-Cycle Interrupting Strategies? Trends Parasitol. 2011, 27, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Al-Areeqi, M.A.; Sady, H.; Al-Mekhlafi, H.M.; Anuar, T.S.; Al-Adhroey, A.H.; Atroosh, W.M.; Dawaki, S.; Elyana, F.N.; Nasr, N.A.; Ithoi, I.; et al. First Molecular Epidemiology of Entamoeba Histolytica, E. Dispar and E. Moshkovskii Infections in Yemen: Different Species-Specific Associated Risk Factors. Trop. Med. Int. Health 2017, 22, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.F.; Siddiqui, S.M.; Agarwal, S.M.; Vikramdeo, K.S.; Mondal, N.; Azam, A. Metronidazole Hydrazone Conjugates: Design, Synthesis, Antiamoebic and Molecular Docking Studies. Bioorganic Med. Chem. Lett. 2015, 25, 3545–3549. [Google Scholar] [CrossRef] [PubMed]
- Marie, C.; Petri, W.A. Amoebic Dysentery. BMJ Clin. Evid. 2013, 2013, 0918. [Google Scholar] [PubMed]
- Calzada, F.; Yépez-Mulia, L.; Aguilar, A. In Vitro Susceptibility of Entamoeba Histolytica and Giardia Lamblia to Plants Used in Mexican Traditional Medicine for the Treatment of Gastrointestinal Disorders. J. Ethnopharmacol. 2006, 108, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Sisson, G.; Goodwin, A.; Raudonikiene, A.; Hughes, N.J.; Mukhopadhyay, A.K.; Berg, D.E.; Hoffman, P.S. Enzymes Associated with Reductive Activation and Action of Nitazoxanide, Nitrofurans, and Metronidazole in Helicobacter Pylori. Antimicrob. Agents Chemother. 2002, 46, 2116–2123. [Google Scholar] [CrossRef]
- Ávila-Blanco, M.E.; Rodríguez, M.G.; Moreno Duque, J.L.; Muñoz-Ortega, M.; Ventura-Juárez, J. Amoebicidal Activity of Essential Oil of Dysphania ambrosioides (L.) Mosyakin & Clemants in an Amoebic Liver Abscess Hamster Model. Evid. Based Complement. Altern. Med. 2014, 2014, 930208. [Google Scholar] [CrossRef]
- Seifert, K.; Duchêne, M.; Wernsdorfer, W.H.; Kollaritsch, H.; Scheiner, O.; Wiedermann, G.; Hottkowitz, T.; Eibl, H. Effects of Miltefosine and Other Alkylphosphocholines on Human Intestinal Parasite Entamoeba histolytica. Antimicrob. Agents Chemother. 2001, 45, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, M.L.M.; Dans, L.F.; Sio-Aguilar, J. Antiamoebic Drugs for Treating Amoebic Colitis. Cochrane Database Syst. Rev. 2019, 2019, CD006085. [Google Scholar] [CrossRef]
- Rossignol, J.-F.; Ayoub, A.; Ayers, M.S. Treatment of Diarrhea Caused by Giardia Intestinalis and Entamoeba Histolytica or E. Dispar: A Randomized, Double-Blind, Placebo-Controlled Study of Nitazoxanide. J. Infect. Dis. 2001, 184, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Speich, B.; Marti, H.; Ame, S.M.; Ali, S.M.; Bogoch, I.I.; Utzinger, J.; Albonico, M.; Keiser, J. Prevalence of Intestinal Protozoa Infection among School-Aged Children on Pemba Island, Tanzania, and Effect of Single-Dose Albendazole, Nitazoxanide and Albendazole-Nitazoxanide. Parasites Vectors 2013, 6, 3. [Google Scholar] [CrossRef]
- Blessmann, J.; Le Van, A.; Tannich, E. Epidemiology and Treatment of Amebiasis in Hué, Vietnam. Arch Med. Res. 2006, 37, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Blessmann, J.; Tannich, E. Treatment of Asymptomatic Intestinal Entamoeba Histolytica Infection. N. Engl. J. Med. 2002, 347, 1861–1874. [Google Scholar] [CrossRef] [PubMed]
- Padilla, N.; Díaz, R.; Muñoz, M. Efficacy and Safety of Quinfamide versus Secnidazole in the Management of Amoebic Non-Dysenteric Colitis in Children. Clin. Drug Investig. 2000, 20, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Padilla, N.; Diaz, R.; Alarcon, A.; Barreda, R. Antiamoebic Chemoprophylaxis Using Quinfamide in Children: A Comparative Study. Sci. World J. 2002, 2, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.K.; Walter, F.G.; Szabo, S. Iodoquinol Associated Seizures and Radiopacity. J. Toxicol. Clin. Toxicol. 1993, 31, 113–120. [Google Scholar] [CrossRef]
- Debnath, A.; Parsonage, D.; Andrade, R.M.; He, C.; Cobo, E.R.; Hirata, K.; Chen, S.; García-Rivera, G.; Orozco, E.; Martínez, M.B.; et al. A High-Throughput Drug Screen for Entamoeba Histolytica Identifies a New Lead and Target. Nat. Med. 2012, 18, 956–960. [Google Scholar] [CrossRef]
- Capparelli, E.V.; Bricker-Ford, R.; Rogers, M.J.; McKerrow, J.H.; Reed, S.L. Phase I Clinical Trial Results of Auranofin, a Novel Antiparasitic Agent. Antimicrob. Agents Chemother. 2017, 61, 10–128. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.C.; Contreras-Rojas, A.; Sánchez-Hernández, B.; Petrosyan, P.; Bobes, R.J.; Ortiz-Ortiz, L.; Laclette, J.P. Protection against Murine Intestinal Amoebiasis Induced by Oral Immunization with the 29kDa Antigen of Entamoeba Histolytica and Cholera Toxin. Exp. Parasitol. 2010, 126, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Meneses-Ruiz, D.M.; Aguilar-Diaz, H.; Bobes, R.J.; Sampieri, A.; Vaca, L.; Laclette, J.P.; Carrero, J.C. Protection against Amoebic Liver Abscess in Hamster by Intramuscular Immunization with an Autographa Californica Baculovirus Driving the Expression of the Gal-Lectin LC3 Fragment. BioMed Res. Int. 2015, 2015, 760598. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.C.; Curay-Herrera, V.; Chacón-Niño, L.; Krengel, F.; Guzmán-Gutiérrez, S.-L.; Silva-Miranda, M.; González-Ramírez, L.-C.; Bobes, R.J.; Espitia, C.; Reyes-Chilpa, R.; et al. Potent Anti-Amoebic Effects of Ibogaine, Voacangine and the Root Bark Alkaloid Fraction of Tabernaemontana arborea. Planta Med. 2023, 89, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Godínez, C.; Ontiveros-Rodríguez, J.C.; Ríos-Valencia, D.G.; Herbert-Pucheta, J.E.; Zepeda-Vallejo, L.G.; Carrero, J.C. Anti-Amoebic Activity of Leaf Extracts and Aporphine Alkaloids Obtained from Annona purpurea. Planta Med. 2020, 86, 425–433. [Google Scholar] [CrossRef] [PubMed]
- León-Sicairos, N.; Martínez-Pardo, L.; Sánchez-Hernández, B.; De La Garza, M.; Carrero, J.Ć. Oral Lactoferrin Treatment Resolves Amoebic Intracecal Infection in C3H/HeJ Mice. Biochem. Cell Biol. 2012, 90, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Godínez, C.; González-Galindo, X.; Meza-Menchaca, T.; Bobes, R.J.; de la Garza, M.; León-Sicairos, N.; Laclette, J.P.; Carrero, J.C. Synthetic Bovine Lactoferrin Peptide Lfampin Kills Entamoeba Histolytica Trophozoites by Necrosis and Resolves Amoebic Intracecal Infection in Mice. Biosci. Rep. 2019, 39, BSR20180850. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS Global HIV & AIDS Statistics—Fact Sheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 11 January 2024).
- Connell, B.J.; Hermans, L.E.; Wensing, A.M.J.; Schellens, I.; Schipper, P.J.; van Ham, P.M.; de Jong, D.T.C.M.; Otto, S.; Mathe, T.; Moraba, R.; et al. Immune Activation Correlates with and Predicts CXCR4 Co-Receptor Tropism Switch in HIV-1 Infection. Sci. Rep. 2020, 10, 15866. [Google Scholar] [CrossRef] [PubMed]
- Huerta, L. Editorial: Anti-Infective 2020: HIV—From Pathogenesis to Treatment. Curr. Opin. Pharmacol. 2020, 54, x–xii. [Google Scholar] [CrossRef]
- Kutsch, O.; Levy, D.N.; Bates, P.J.; Decker, J.; Kosloff, B.R.; Shaw, G.M.; Priebe, W.; Benveniste, E.N. Bis-Anthracycline Antibiotics Inhibit Human Immunodeficiency Virus Type 1 Transcription. Antimicrob. Agents Chemother. 2004, 48, 1652–1663. [Google Scholar] [CrossRef]
- Himmel, D.M.; Das, K.; Clark, A.D.; Hughes, S.H.; Benjahad, A.; Oumouch, S.; Guillemont, J.; Coupa, S.; Poncelet, A.; Csoka, I.; et al. Crystal Structures for HIV-1 Reverse Transcriptase in Complexes with Three Pyridinone Derivatives: A New Class of Non-Nucleoside Inhibitors Effective against a Broad Range of Drug-Resistant Strains. J. Med. Chem. 2005, 48, 7582–7591. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Tian, Y.; Song, Y.; Zhan, P.; Liu, X. Novel HIV-1 Non-Nucleoside Reverse Transcriptase Inhibitors: A Patent Review (2011–2014). Expert Opin. Ther. Pat. 2014, 24, 1199–1227. [Google Scholar] [CrossRef] [PubMed]
- Medina-Franco, J.L.; Rodríguez-Morales, S.; Juárez-Gordiano, C.; Hernández-Campos, A.; Castillo, R. Docking-Based CoMFA and CoMSIA Studies of Non-Nucleoside Reverse Transcriptase Inhibitors of the Pyridinone Derivative Type. J. Comput. Aided Mol. Des. 2004, 18, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Medina-Franco, J.L.; Golbraikh, A.; Oloff, S.; Castillo, R.; Tropsha, A. Quantitative Structure-Activity Relationship Analysis of Pyridinone HIV-1 Reverse Transcriptase Inhibitors Using the k Nearest Neighbor Method and QSAR-Based Database Mining. J. Comput. Aided Mol. Des. 2005, 19, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Medina-Franco, J.L.; Martínez-Mayorga, K.; Juárez-Gordiano, C.; Castillo, R. Pyridin-2(1H)-Ones: A Promising Class of HIV-1 Non-Nucleoside Reverse Transcriptase Inhibitors. ChemMedChem 2007, 2, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Reyes, H. Síntesis de Híbridos de Piridinona-UC781 y Análogos Para Estudios de Acoplamiento Molecular y Evaluación de La Replicación de VIH-1. Ph.D. Thesis, Instituto Tecnológico de Tijuana, Tijuana, Mexico, 2014. [Google Scholar]
- Cabrera, A.; Miranda, L.D.; Reyes, H.; Aguirre, G.; Chávez, D. Crystal Structure of Ethyl 2,4-Dichloroquinoline-3-Carboxylate. Acta Crystallogr. Sect. Crystallogr. Commun. 2015, 71, o939. [Google Scholar] [CrossRef] [PubMed]
- Vite-Caritino, H.; Méndez-Lucio, O.; Reyes, H.; Cabrera, A.; Chávez, D.; Medina-Franco, J.L. Advances in the Development of Pyridinone Derivatives as Non-Nucleoside Reverse Transcriptase Inhibitors. RSC Adv. 2016, 6, 2119–2130. [Google Scholar] [CrossRef]
- Rodríguez-Vega, G.; Abarca-Magaña, J.C.; Castro-Perea, N.V.; Ruiz-Rivera, M.B.; Medina-Franco, J.L.; Huerta-Hernández, L.; Chávez, D. Synthesis and biological evaluation of cyclohexanpyridin-2 (1H)-one analogues as novel HIV-1 NNRTIs. World J. Adv. Res. Rev. 2024, 22, 369–385. [Google Scholar] [CrossRef]
- Cáceres, G.; Calderon, R.; Ugarte-Gil, C. Tuberculosis and Comorbidities: Treatment Challenges in Patients with Comorbid Diabetes Mellitus and Depression. Ther. Adv. Infect. Dis. 2022, 9, 204993612210958. [Google Scholar] [CrossRef]
- Haque, R.; Petri, W.A. Diagnosis of Amebiasis in Bangladesh. Arch Med. Res. 2006, 37, 272–275. [Google Scholar] [CrossRef]
- Ribeiro, A.L.; Nunes, M.P.; Teixeira, M.M.; Rocha, M.O.C. Diagnosis and Management of Chagas Disease and Cardiomyopathy. Nat. Rev. Cardiol. 2012, 9, 576–589. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Klimova, E.; Centeno-Leija, S.; Sánchez, S. The Impact of Genome-Mining in the Development of New Anti-Infectives. In Frontiers in Clinical Drug Research—Anti Infectives; Rahman, A., Ed.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2014; pp. 3–54. ISBN 9781608058549. [Google Scholar]
- Gubbens, J.; Zhu, H.; Girard, G.; Song, L.; Florea, B.I.; Aston, P.; Ichinose, K.; Filippov, D.V.; Choi, Y.H.; Overkleeft, H.S.; et al. Natural Product Proteomining, a Quantitative Proteomics Platform, Allows Rapid Discovery of Biosynthetic Gene Clusters for Different Classes of Natural Products. Chem. Biol. 2014, 21, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Guerra, R.E.; Moreno-Gutierrez, D.S.; de Vargas-Dorantes, O.J.; Espinoza, B.; Hernandez-Garcia, A. Delivery of Antisense DNA into Pathogenic Parasite Trypanosoma Cruzi Using Virus-Like Protein-Based Nanoparticles. Nucleic Acid Ther. 2020, 30, 392–401. [Google Scholar] [CrossRef] [PubMed]
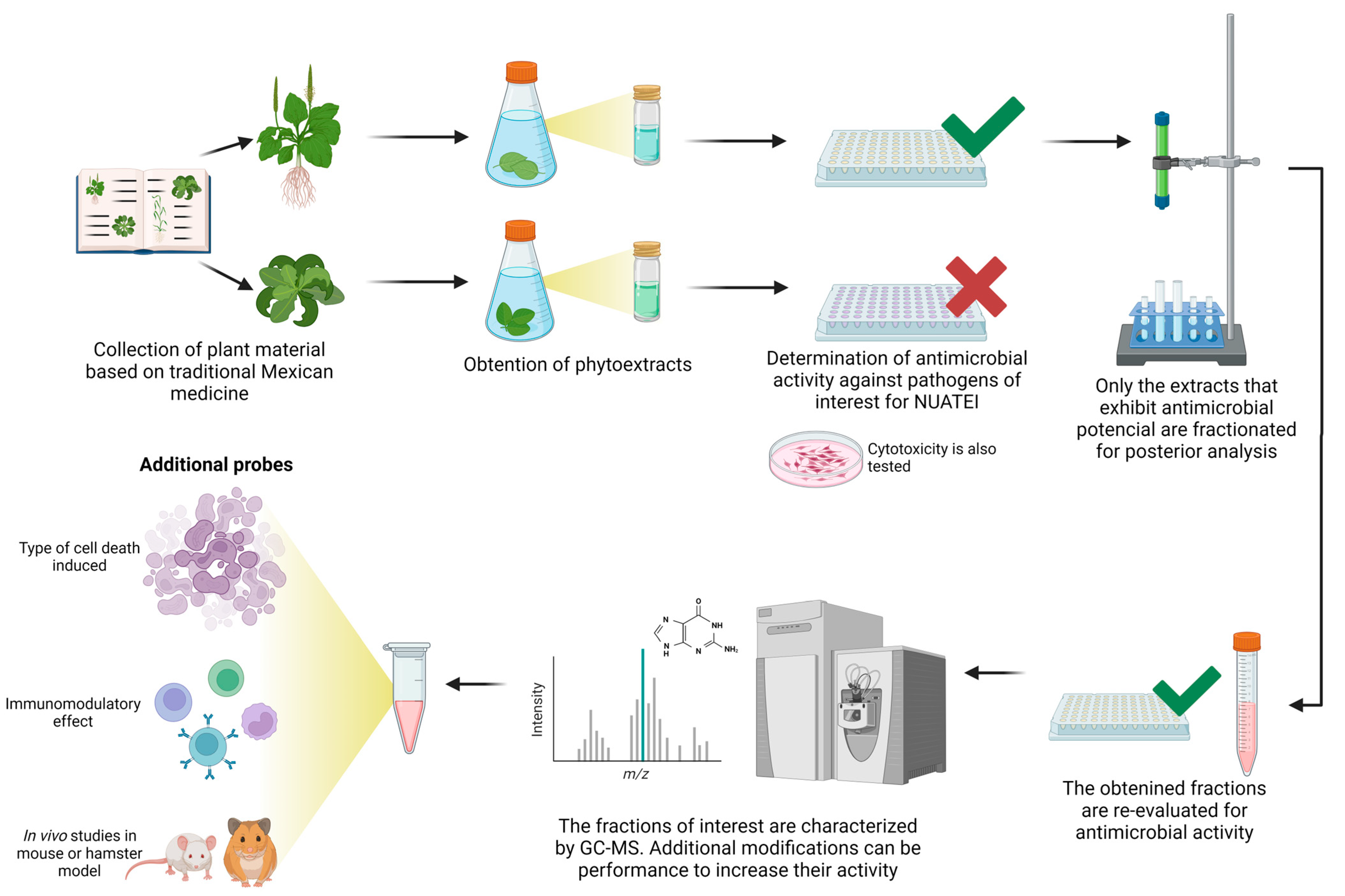
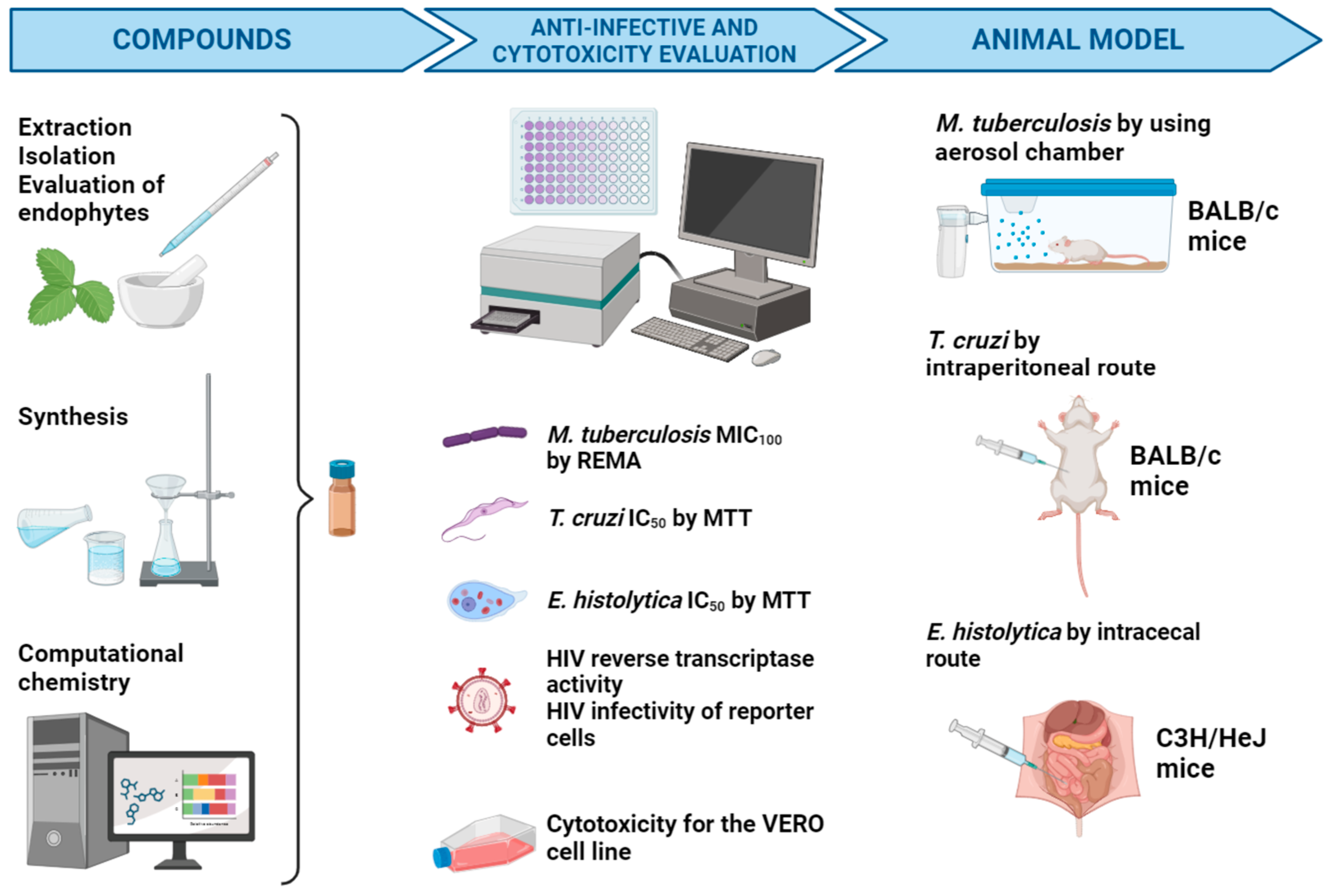
| Infectious Agent | Type | Disease | No. Cases (Mex./Worldwide) | Annual Deaths | Mortality Ranking | Refs. |
|---|---|---|---|---|---|---|
| Mycobacterium tuberculosis | Mainly pulmonary bacteria | Tuberculosis | 25,449 in 2016/ 10.4 million | 1.6 million | 9th worldwide | [3] |
| Trypanosma cruzi | Blood and visceral protozoan | Chagas disease | 1.1 million in 2016/ 6–7 million | 10,000 to 12,000 | 4th among protozoan parasites | [4,5] |
| Entamoeba histolytica | Enteric and hepatic protozoan | Amoebiasis | 200,000 in 2019/ 50 million | 40,000 to 100,000 | 3rd among protozoan parasites | [6] |
| Human immunodeficiency virus (HIV) | Systemic virus | AIDS | 13,489 in 2023/ 39 million | 480,000 to 880,000 | 19th worldwide | [7,8] |
| Compound | Source | M. tuberculosis Strain | MIC100 (mg/mL) | CC50 (mg/mL) | SI | Reference |
|---|---|---|---|---|---|---|
| GPC-1 | Synthetic | H37Rv | 7.8 | 106 | 13.6 | [63] |
| ARO-1 | Synthetic | H37Rv | 7.8 | 129 | 16.5 | [64] |
| SB-38 | Synthetic | H37Rv | 3.9 | 298 | 76.4 | This paper |
| AMBH-4 | Synthetic | H37Rv | 31.25 | 387 | 12.3 | This paper |
| R1-4F-2 | Synthetic | H37Rv | 15.6 | 219 | 14.0 | This paper |
| SB-18 | Synthetic | H37Rv | 7.8 | 138 | 17.7 | This paper |
| Benzo-NO2 | Synthetic | H37Rv | 31.25 | 555 | 17.76 | This paper |
| R1-4Br-2 | Synthetic | H37Rv | 15.6 | 288 | 18.5 | This paper |
| F-NO2 | Synthetic | H37Rv | 31.25 | 696 | 22.2 | This paper |
| R1-4I-2 | Synthetic | H37Rv | 15.6 | 451 | 28.9 | This paper |
| RCS-NO2 | Synthetic | H37Rv | 7.8 | 255 | 32.7 | This paper |
| Anthracycline StefB | Endophyte from Amphipterygium adstringens. | H37Rv | 7.8 | 99.3 | 12.7 | [59] |
| Anthracycline StefB | Endophyte from Amphipterygium adstringens. | Strain 209 * (clinic isolated) | 3.9 | 99.3 | 25.46 | [59] |
| Natural Origin | Compound Name | IC50 (μM) | SI | Reference |
|---|---|---|---|---|
| Cnidoscolus spinosus | GDL-1 | >200 | ND | [78] |
| Mikania sp. | GDL-10 | <50 | ND | [78] |
| GDL-15 | <50 | |||
| Ambrosia sp. | GDL-21 | <50 | ND | [78] |
| Calophyllum brasiliense | Mix of coumarins | 22.5 | ND | [76] |
| Calophyllum brasiliense | Mammea A/BA | 17.6 | 7.7 | [76] |
| Parthenium hysterophorus | Ambrosin | 68.4 | 11.46 | [79] |
| Decachaeta incompta | Incomptine B | 132.3 | 8.42 | [79] |
| Vernonia liatroides | Glaucolide E | 199.7 | 2.37 | [79] |
| Streptomyces spp. | Thiostrepton | 4.5 | 8.8 | [80] |
| Synthetic Compounds | ||||
| Amphotericin B | A21 | <2 | >200 | [81] |
| 5-nitroimidazoles | Secnidazol | >300 | ND | This paper |
| Co-secnidazol | >300 | |||
| Variable chemical nature | 1, 2, 3, 5, 6, 7, 12, V-5, M1A, L1A, J1B, 93, F42, A4, lan, Ffan, Fan | All >50 | ND | This paper |
| Biometal compound | PtSO3 | >100 | ND | This paper |
| Biometal compound | Risedronato-Zn | <25 | ND | This paper |
| Biometal compound | Risedronato-CuIA | <2 | 1.09 | This paper |
| Biometal compound | Risedronato-CuIB | <2 | 1.23 | This paper |
| Source | Compound or Fraction | IC50 at 24 h (μg/mL) | SI | References |
|---|---|---|---|---|
| Tabernaemontana arborea (plant) | Alkaloid fraction | 0.2 | 10.2 | [104] |
| Ibogaine | 0.8 | 252.8 | ||
| Voacangine | 0.8 | 13.6 | ||
| Voacamine | 10 | ND | ||
| Annona purpurea (plant) | Alkaloid fraction | 20.8 | ND | [105] |
| Glaziovine | 9.9 | ND | ||
| 3-Hydroxiglaucine | 66.6 | ND | ||
| Norpurpureine | 68.5 | ND | ||
| Cow milk | Lactoferrin (Lf) | 2 | >1000 | [106] |
| Synthetic peptides | Lactoferrampin | 0.5 | ND | [107] |
| Lactoferricin 17–30 | 1 | >1000 | ||
| Lactoferricin B | 1 | >1000 | ||
| Synthetic compound | A21 (amphotericin B derivative) | <1 | >200 | This paper |
| Synthetic compound | PRO54 | 0.8 | 109.25 | This paper |
| Synthetic nitroimidazole | Metronidazol | 1.2 | >854.7 | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrero, J.C.; Espinoza, B.; Huerta, L.; Silva-Miranda, M.; Guzmán-Gutierrez, S.-L.; Dorazco-González, A.; Reyes-Chilpa, R.; Espitia, C.; Sánchez, S. Introducing the NUATEI Consortium: A Mexican Research Program for the Identification of Natural and Synthetic Antimicrobial Compounds for Prevalent Infectious Diseases. Pharmaceuticals 2024, 17, 957. https://doi.org/10.3390/ph17070957
Carrero JC, Espinoza B, Huerta L, Silva-Miranda M, Guzmán-Gutierrez S-L, Dorazco-González A, Reyes-Chilpa R, Espitia C, Sánchez S. Introducing the NUATEI Consortium: A Mexican Research Program for the Identification of Natural and Synthetic Antimicrobial Compounds for Prevalent Infectious Diseases. Pharmaceuticals. 2024; 17(7):957. https://doi.org/10.3390/ph17070957
Chicago/Turabian StyleCarrero, Julio César, Bertha Espinoza, Leonor Huerta, Mayra Silva-Miranda, Silvia-Laura Guzmán-Gutierrez, Alejandro Dorazco-González, Ricardo Reyes-Chilpa, Clara Espitia, and Sergio Sánchez. 2024. "Introducing the NUATEI Consortium: A Mexican Research Program for the Identification of Natural and Synthetic Antimicrobial Compounds for Prevalent Infectious Diseases" Pharmaceuticals 17, no. 7: 957. https://doi.org/10.3390/ph17070957
APA StyleCarrero, J. C., Espinoza, B., Huerta, L., Silva-Miranda, M., Guzmán-Gutierrez, S.-L., Dorazco-González, A., Reyes-Chilpa, R., Espitia, C., & Sánchez, S. (2024). Introducing the NUATEI Consortium: A Mexican Research Program for the Identification of Natural and Synthetic Antimicrobial Compounds for Prevalent Infectious Diseases. Pharmaceuticals, 17(7), 957. https://doi.org/10.3390/ph17070957








