Apremilast as a Potential Targeted Therapy for Metabolic Syndrome in Patients with Psoriasis: An Observational Analysis
Abstract
:1. Introduction
2. Results
2.1. Demographic Features of Enrolled Patients
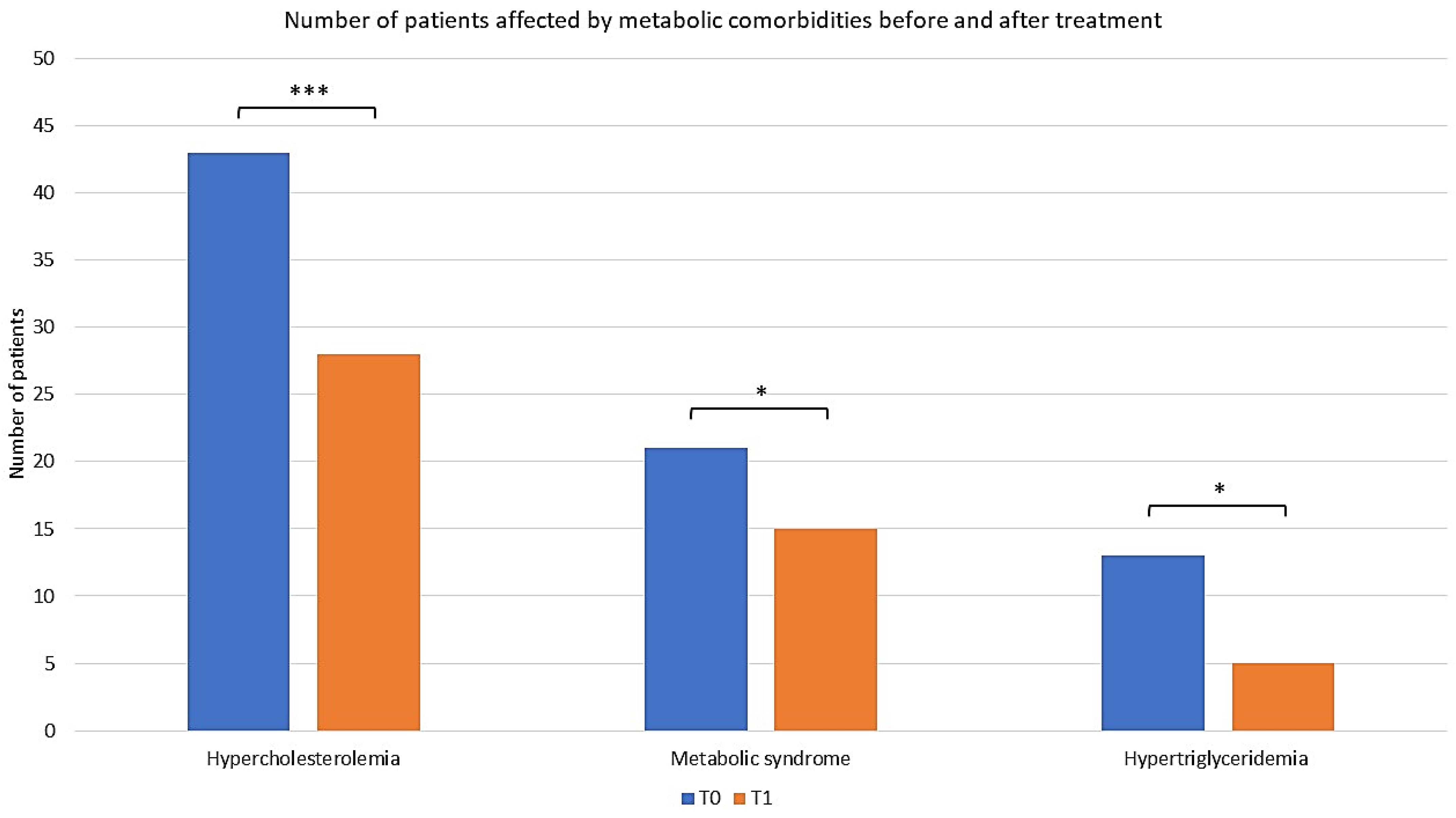
2.2. Comorbidities of Enrolled Patients
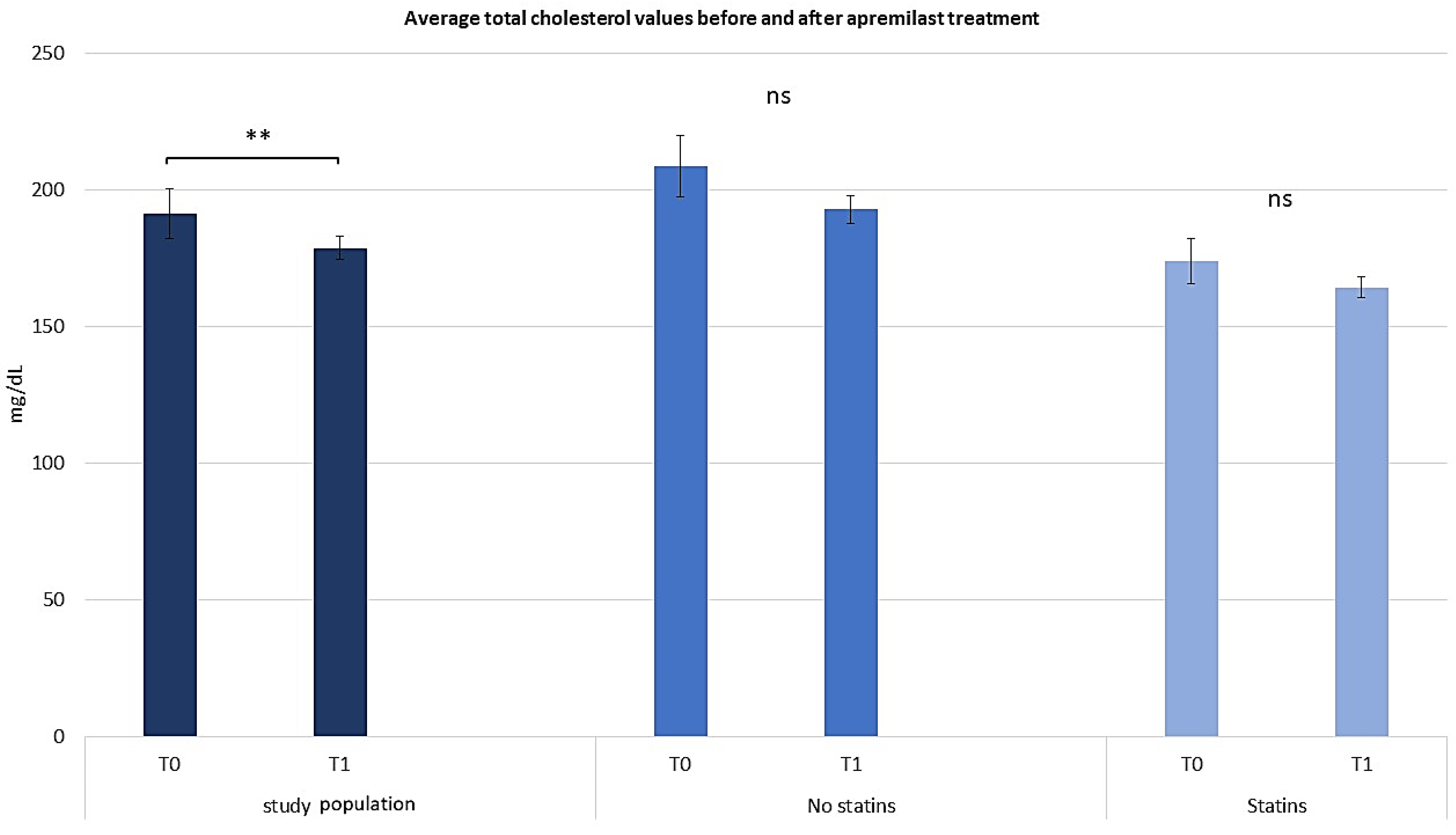
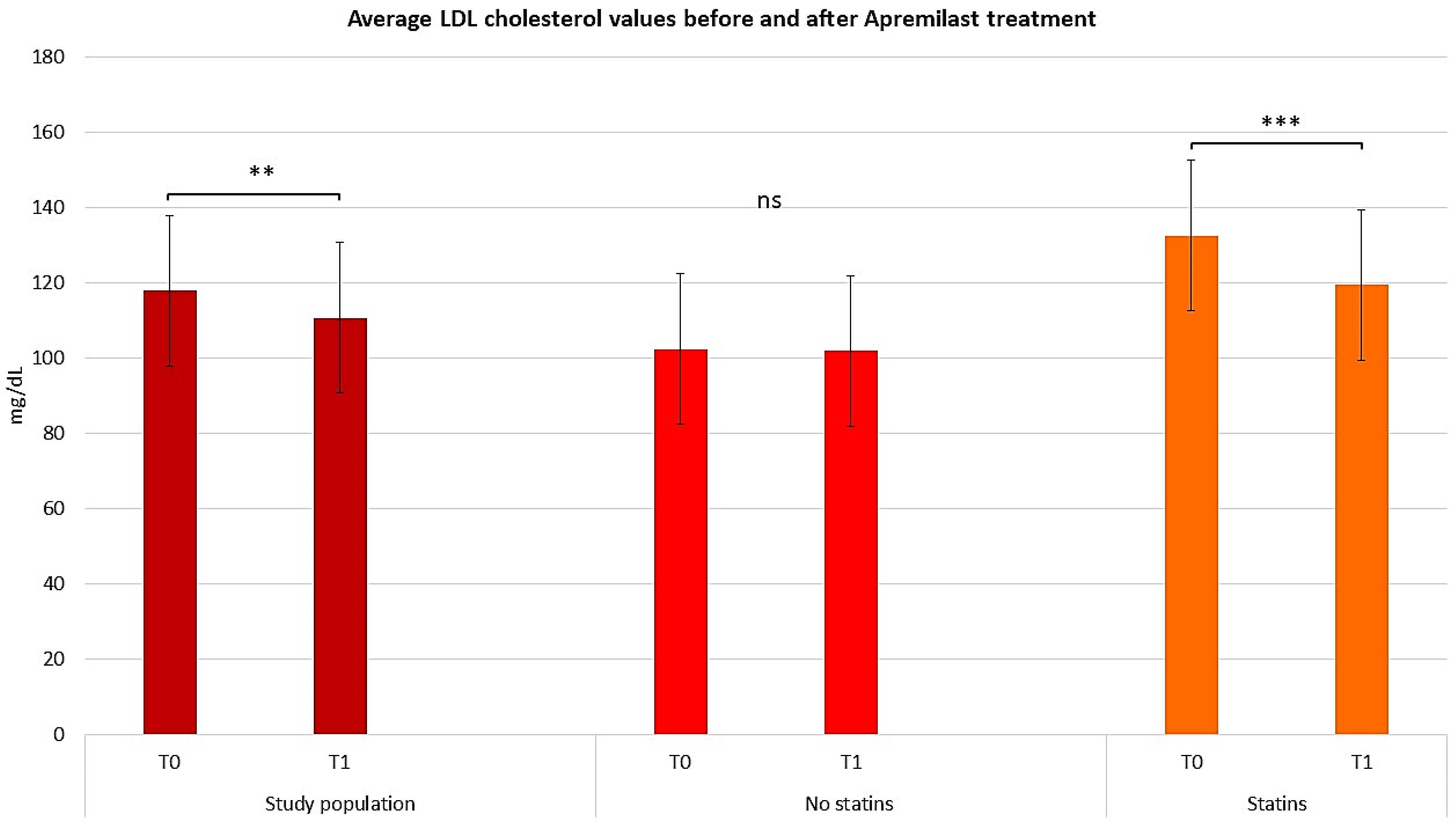
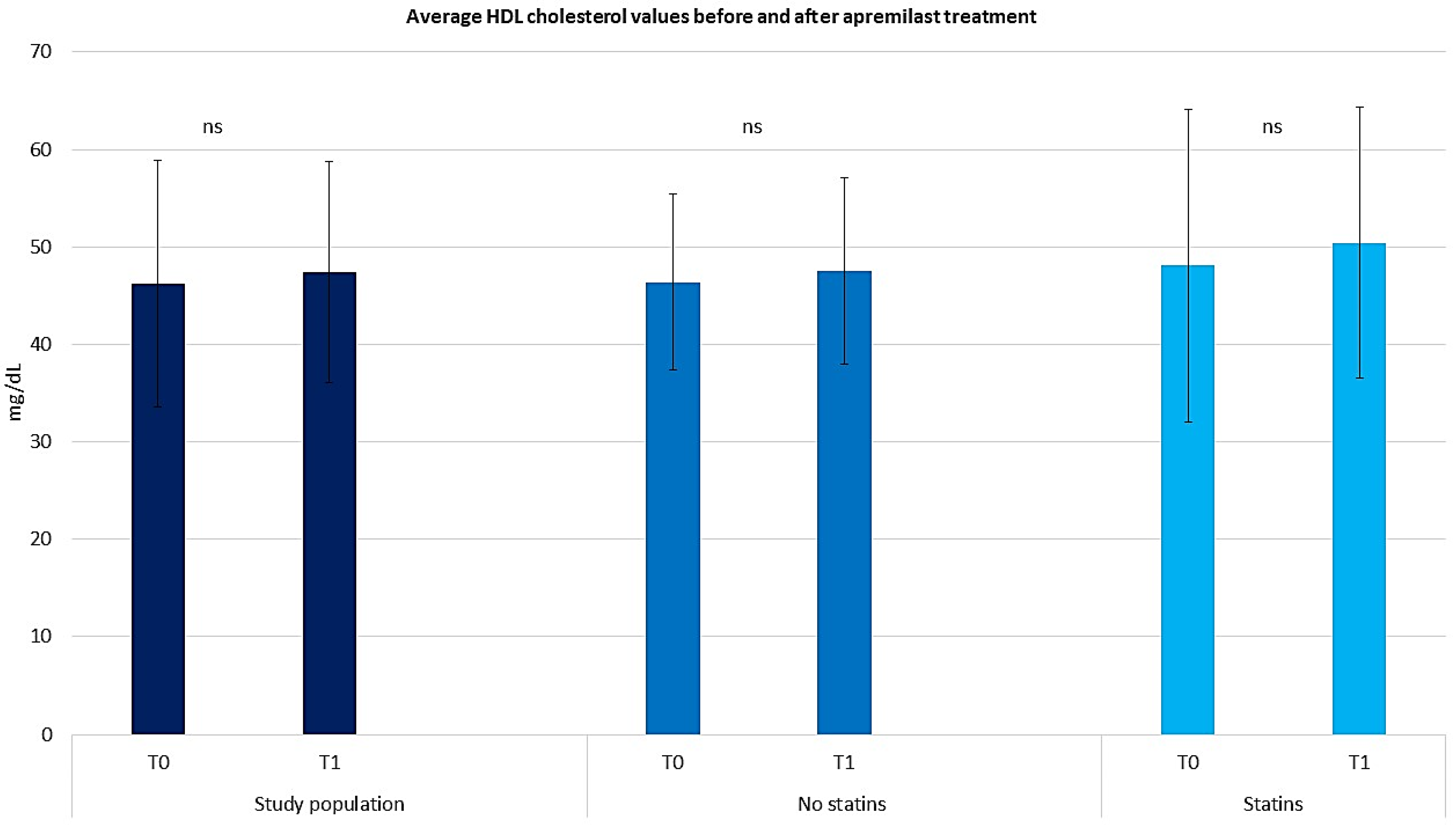
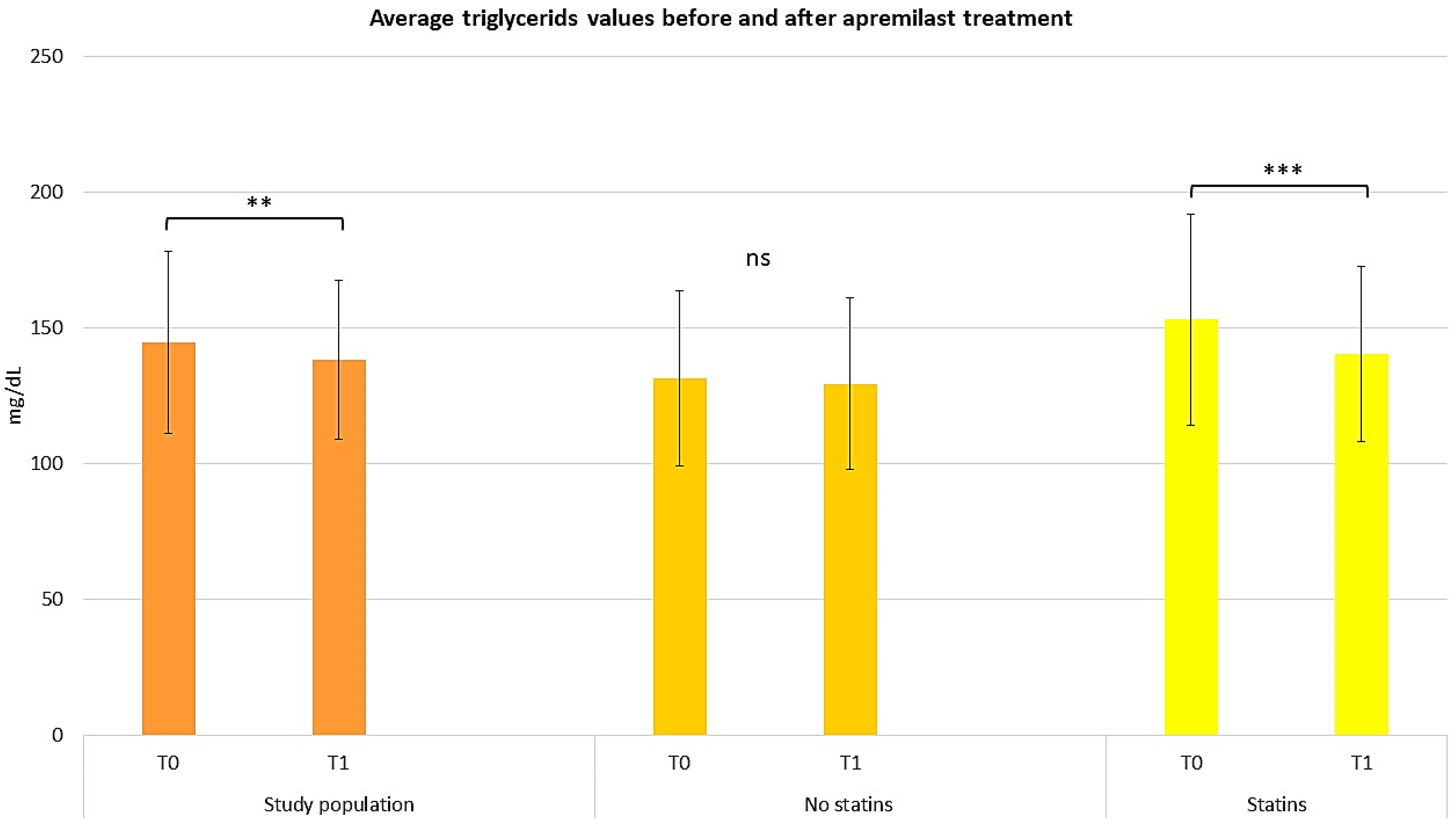
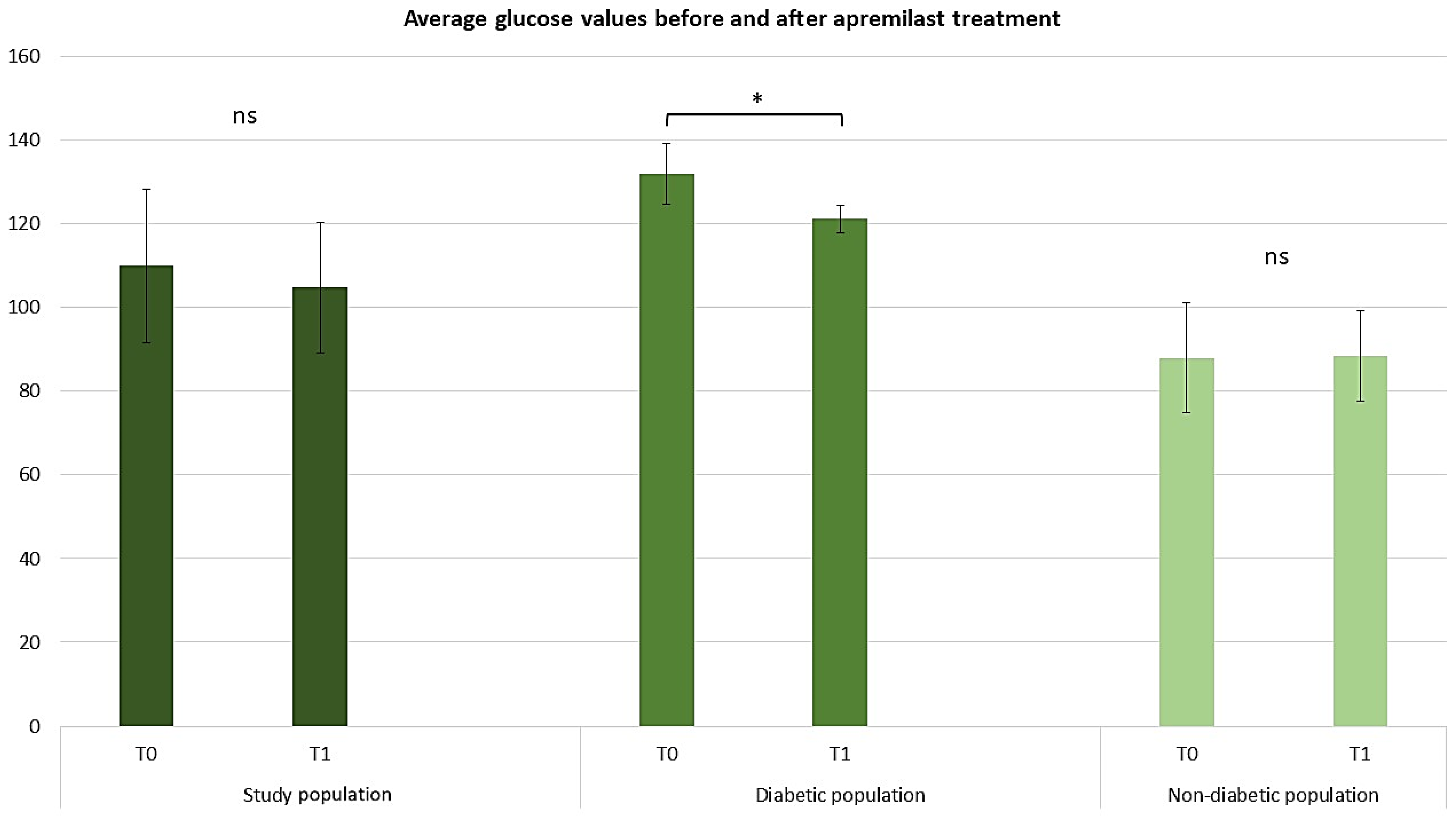
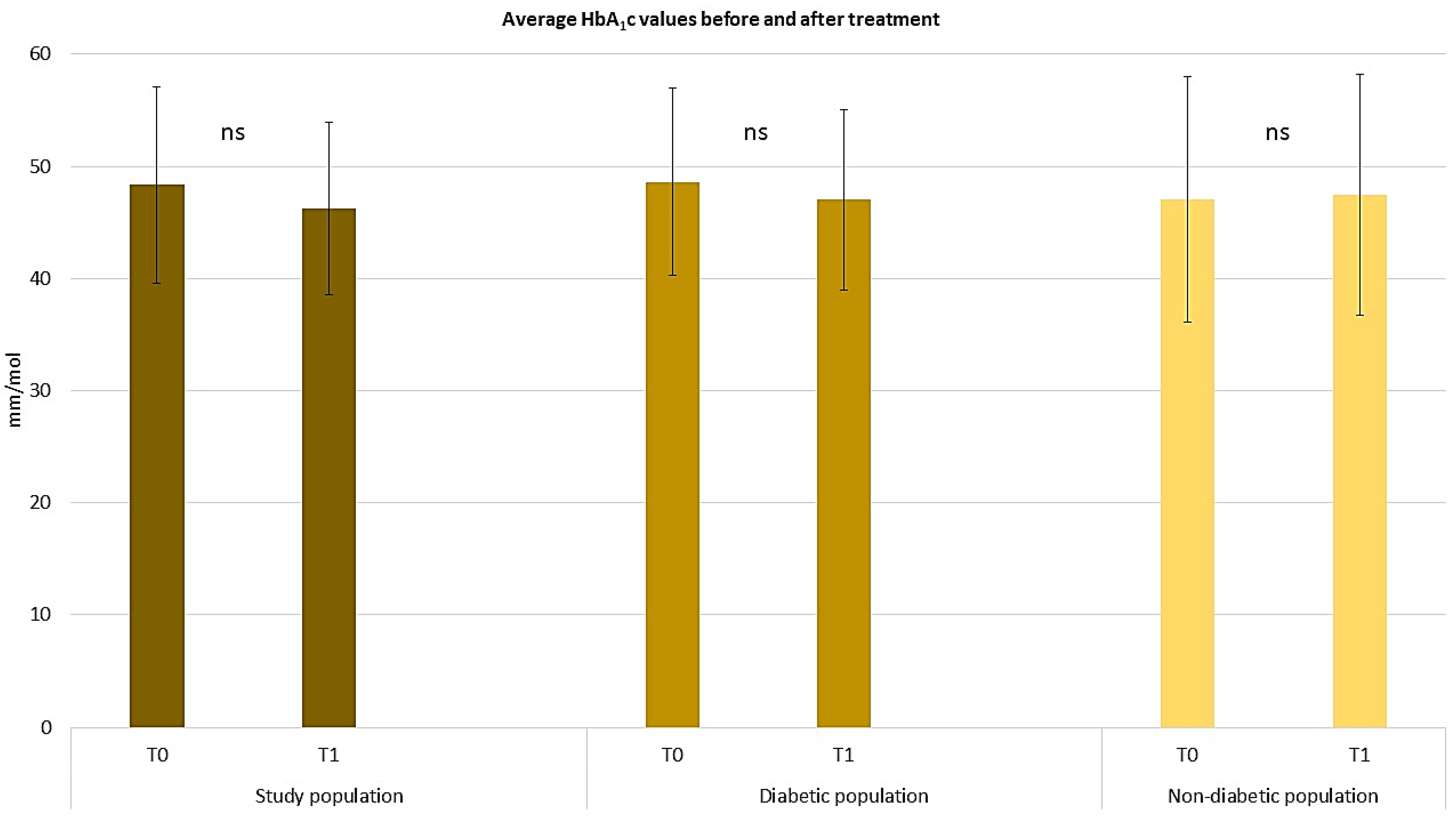
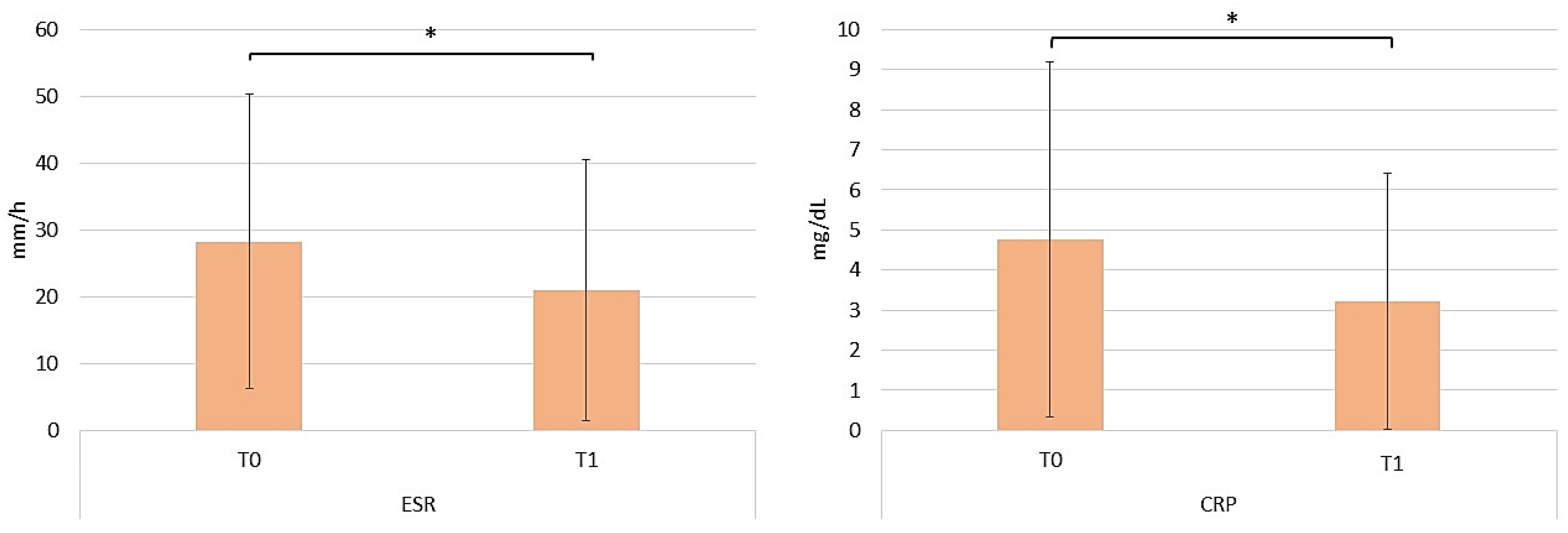
2.3. Assessing the Efficacy of Apremilast in the Context of Comorbid Conditions
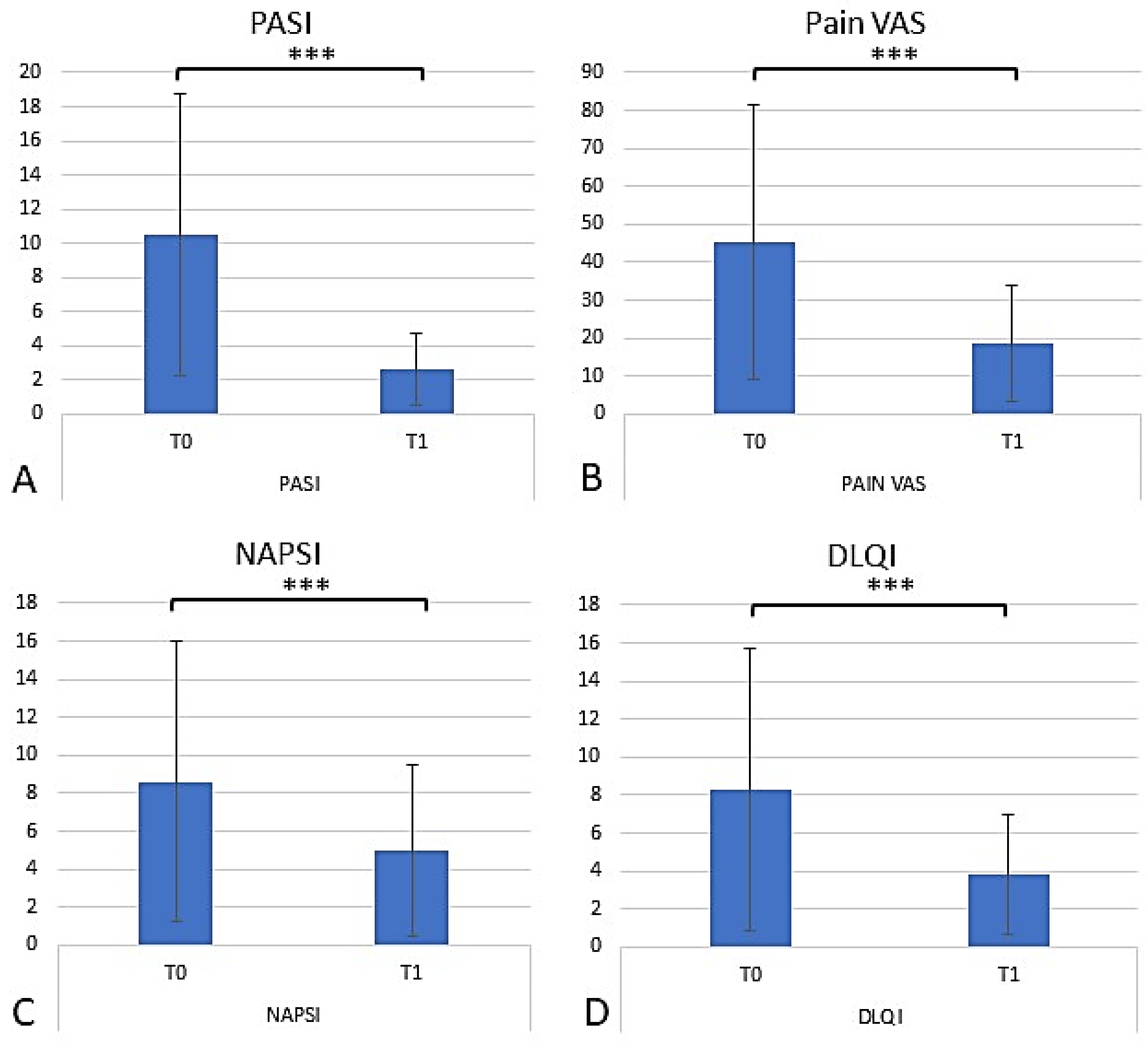
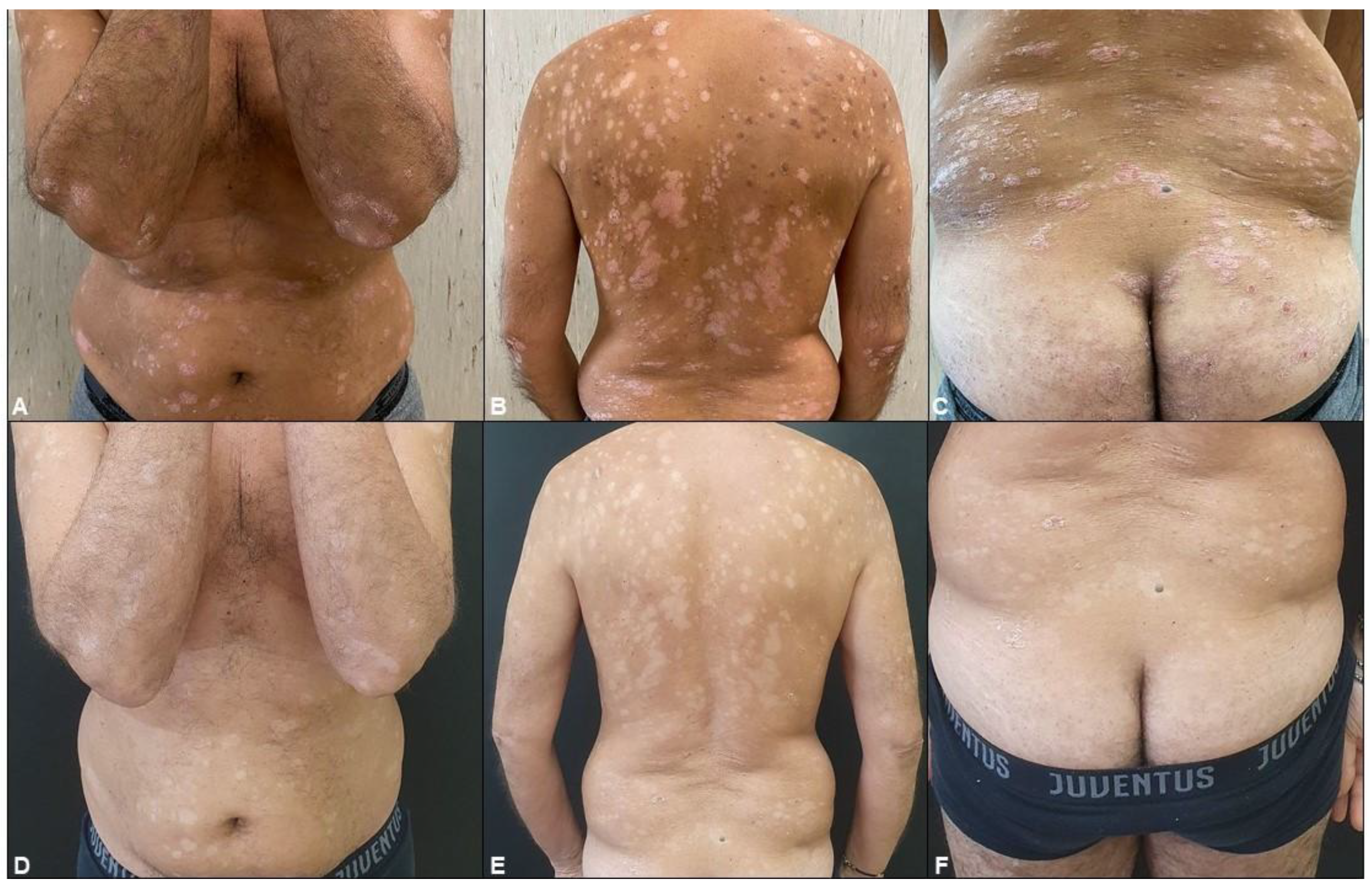
2.4. Assessing the Efficacy of Apremilast in Psoriasis Management
2.5. Safety and Tolerability
3. Discussion
4. Materials and Methods
Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Griffiths, C.E.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Campanati, A.; Marani, A.; Martina, E.; Diotallevi, F.; Radi, G.; Offidani, A. Psoriasis as an Immune-Mediated and Inflammatory Systemic Disease: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2021, 9, 1511. [Google Scholar] [CrossRef]
- Barrea, L.; Nappi, F.; Di Somma, C.; Savanelli, M.C.; Falco, A.; Balato, A.; Balato, N.; Savastano, S. Environmental Risk Factors in Psoriasis: The Point of View of the Nutritionist. Int. J. Environ. Res. Public Health 2016, 13, 743. [Google Scholar] [CrossRef]
- Kimmel, G.W.; Lebwohl, M. Psoriasis: Overview and Diagnosis. In Evidence-Based Psoriasis: Diagnosis and Treatment; Springer: Cham, Switzerland, 2018; pp. 1–16. [Google Scholar] [CrossRef]
- de Oliveira, M.; de Rocha, B.; Duarte, G.V. Psoriasis: Classical and emerging comorbidities. An. Bras. De Dermatol. 2015, 90, 9–20. [Google Scholar] [CrossRef]
- Ferguson, L.D.; Siebert, S.; McInnes, I.B.; Sattar, N. Cardiometabolic Comorbidities in ra and PSA: Lessons Learned and future directions. Nat. Rev. Rheumatol. 2019, 15, 461–474. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Cetkovska, P.; Dediol, I.; Sola, M.; Kojanova, M.; Trcko, K.; Carija, A.; Ceovic, R.; Ledic-Drvar, D.; Kastelan, M.; Hrabar, A.; et al. Apremilast use in severe psoriasis: Real-world data from Central and Eastern Europe—Advances in therapy. Adv. Ther. 2023, 40, 1787–1802. Available online: https://link.springer.com/article/10.1007/s12325-023-02468-3 (accessed on 22 July 2024). [CrossRef]
- Schafer, P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem. Pharmacol. 2012, 83, 1583–1590. [Google Scholar] [CrossRef]
- Tadros, J.; Fernandez, K. 28463 Aprelimast and psoriasis: Significant hemoglobin A1c reduction and improved insulin resistance independent of changes in body mass index. J. Am. Acad. Dermatol. 2021, 85, AB192. [Google Scholar] [CrossRef]
- De Brandt, E.; Hillary, T. Comorbid Psoriasis and Metabolic Syndrome: Clinical Implications and Optimal Management. Psoriasis 2022, 12, 113–126. [Google Scholar] [CrossRef]
- Ferguson, L.D.; Cathcart, S.; Rimmer, D.; Semple, G.; Brooksbank, K.; Paterson, C.; Brown, R.; Harvie, J.; Gao, X.; Radjenovic, A.; et al. Effect of the phosphodiesterase 4 inhibitor apremilast on cardiometabolic outcomes in psoriatic disease—Results of the immune metabolic associations in psoriatic arthritis study. Rheumatology 2021, 61, 1026–1034. [Google Scholar] [CrossRef]
- Ong, W.; Gribble, F.; Reimann, F.; Lynch, M.; Houslay, M.; Baillie, G.; Furman, B.; Pyne, N. The role of the PDE4D camp phosphodiesterase in the regulation of glucagon-like peptide-1 release. Br. J. Pharmacol. 2009, 157, 633–644. [Google Scholar] [CrossRef]
- Lanna, C.; Cesaroni, G.M.; Mazzilli, S.; Bianchi, L.; Campione, E. Small Molecules, Big Promises: Improvement of Psoriasis Severity and Glucidic Markers with Apremilast: A Case Report. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 2685–2688. [Google Scholar] [CrossRef]
- De Lorenzo, A.; Romano, L.; Di Renzo, L.; Di Lorenzo, N.; Cenname, G.; Gualtieri, P. Obesity: A preventable, treatable, but relapsing disease. Nutrition 2020, 71, 110615. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Myerson, G.E.; Fleischmann, R.M.; Lioté, F.; Díaz-González, F.; Van den Bosch, F.; Marzo-Ortega, H.; Feist, E.; Shah, K.; Hu, C.; et al. A Phase III, Randomized, Controlled Trial of Apremilast in Patients with Psoriatic Arthritis: Results of the PALACE 2 Trial. J. Rheumatol. 2016, 43, 1724–1734. [Google Scholar] [CrossRef]
- Lemieux, I.; Després, J.P. Metabolic Syndrome: Past, Present and Future. Nutrients 2020, 12, 3501. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Parhofer, K.G. Diabetic dyslipidemia. Metab. Clin. Exp. 2014, 63, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Khan, M.U.; Virani, S.S.; Khan, M.S.; Khan, M.Z.; Rashid, M.; Kalra, A.; Alkhouli, M.; Blaha, M.J.; Blumenthal, R.S.; et al. Efficacy and safety for the achievement of guideline-recommended lower low-density lipoprotein cholesterol levels: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2022, 28, 2001–2009. [Google Scholar] [CrossRef]
- Parhofer, K.G.; Laufs, U. The Diagnosis and Treatment of Hypertriglyceridemia. Dtsch. Ärzteblatt Int. 2019, 116, 825–832. [Google Scholar] [CrossRef]
- Bray, C.; Bell, L.N.; Liang, H.; Haykal, R.; Kaiksow, F.; Mazza, J.J.; Yale, S.H. Erythrocyte Sedimentation Rate and C-reactive Protein Measurements and Their Relevance in Clinical Medicine. WMJ Off. Publ. State Med. Soc. Wis. 2016, 115, 317–321. [Google Scholar]
- Agarwal, K.; Das, S.; Kumar, R.; De, A. Psoriasis and its Association with Metabolic Syndrome. Indian J. Dermatol. 2023, 68, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Malara, G.; Politi, C.; Trifirò, C.; Verduci, C.; D’Arrigo, G.; Testa, A.; Tripepi, G. Effectiveness of apremilast in real life in patients with psoriasis: A longitudinal study. Acta Derm. Venereol. 2021, 101, adv00545. [Google Scholar] [CrossRef] [PubMed]
- Meehan, E.V.; Wang, K. Interleukin-17 Family Cytokines in Metabolic Disorders and Cancer. Genes 2022, 13, 1643. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhu, Y.J.; Zou, S.; Zhou, P.; Hu, Y.W.; Zhao, Q.X.; Gu, L.N.; Zhang, H.Z.; Wang, Z.; Li, J. Metabolic Syndrome and Psoriasis: Mechanisms and Future Directions. Front. Immunol. 2021, 12, 711060. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga, L.A.; Shen, W.J.; Joyce-Shaikh, B.; Pyatnova, E.A.; Richards, A.G.; Thom, C.; Andrade, S.M.; Cua, D.J.; Kraemer, F.B.; Butcher, E.C. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J. Immunol. 2010, 185, 6947–6959. [Google Scholar] [CrossRef]
- Kanemaru, K.; Matsuyuki, A.; Nakamura, Y.; Fukami, K. Obesity exacerbates imiquimod-induced psoriasis-like epidermal hyperplasia and interleukin-17 and interleukin-22 production in mice. Exp. Dermatol. 2015, 24, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Gozashti, M.H.; Aghadavood, M.; Mehdizadeh, M.R.; Hayatbakhsh, M.M. Clinical Significance of Serum IL-6 and TNF-α Levels in Patients with Metabolic Syndrome. Rep. Biochem. Mol. Biol. 2017, 6, 74–79. [Google Scholar] [PubMed]
- Ullah, M.I.; Alzahrani, B.; Alsrhani, A.; Atif, M.; Alameen, A.A.M.; Ejaz, H. Determination of serum tumor necrosis factor-alpha (TNF-α) levels in metabolic syndrome patients from Saudi population. Pak. J. Med. Sci. 2021, 37, 700–705. [Google Scholar] [CrossRef]
- Feingold, K.R.; Soued, M.; Staprans, I.; Gavin, L.A.; Donahue, M.E.; Huang, B.J.; Moser, A.H.; Gulli, R.; Grunfeld, C. Effect of tumor necrosis factor (TNF) on lipid metabolism in the diabetic rat. Evidence that inhibition of adipose tissue lipoprotein lipase activity is not required for TNF-induced hyperlipidemia. J. Clin. Investig. 1989, 83, 1116–1121. [Google Scholar] [CrossRef]
- Sethi, J.K.; Hotamisligil, G.S. Metabolic Messengers: Tumour necrosis factor. Nat. Metab. 2021, 3, 1302–1312. [Google Scholar] [CrossRef]
- Perry, R.J.; Camporez, J.G.; Kursawe, R.; Titchenell, P.M.; Zhang, D.; Perry, C.J.; Jurczak, M.J.; Abudukadier, A.; Han, M.S.; Zhang, X.M.; et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 2015, 160, 745–758. [Google Scholar] [CrossRef]
- Han, M.S.; White, A.; Perry, R.J.; Camporez, J.P.; Hidalgo, J.; Shulman, G.I.; Davis, R.J. Regulation of adipose tissue inflammation by interleukin 6. Proc. Natl. Acad. Sci. USA 2020, 117, 2751–2760. [Google Scholar] [CrossRef]
- Esposito, K.; Pontillo, A.; Giugliano, F.; Giugliano, G.; Marfella, R.; Nicoletti, G.; Giugliano, D. Association of low interleukin-10 levels with the metabolic syndrome in obese women. J. Clin. Endocrinol. Metab. 2003, 88, 1055–1058. [Google Scholar] [CrossRef]
- Kelly, B.; O’Neill, L.A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015, 25, 771–784. [Google Scholar] [CrossRef]
- Filippi, F.; Patrizi, A.; Iezzi, L.; Carpanese, M.A.; Conti, A.; Lasagni, C.; Tabanelli, M.; D’Adamio, S.; DI Nuzzo, S.; Cortelazzi, C.; et al. Use of apremilast® in the psoriasis treatment: A real-life multicenter Italian experience. Ital. J. Dermatol. Venereol. 2022, 157, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Guerra, P.; Di Cesare, A.; Rosi, E.; Scandagli, I.; Silvi, G.; Nunziati, G.; Prignano, F. Effects on Lipid Profile after One Year of Apremilast Therapy in Patients with Psoriasis: A Monocentric Experience. Life 2024, 14, 395. [Google Scholar] [CrossRef] [PubMed]
- Gelfand, J.M.; Shin, D.B.; Armstrong, A.W.; Tyring, S.K.; Blauvelt, A.; Gottlieb, S.; Lockshin, B.N.; Kalb, R.E.; Fitzsimmons, R.; Rodante, J.; et al. Association of Apremilast with Vascular Inflammation and Cardiometabolic Function in Patients with Psoriasis: The VIP-A Phase 4, Open-label, Nonrandomized Clinical Trial. JAMA Dermatol. 2022, 158, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Arias de la Rosa, I.; López-Montilla, M.D.; Román-Rodríguez, C.; Pérez-Sánchez, C.; Gómez-García, I.; López-Medina, C.; Ladehesa-Pineda, M.L.; Ábalos-Aguilera, M.D.C.; Ruiz, D.; Patiño-Trives, A.M.; et al. The clinical and molecular cardiometabolic fingerprint of an exploratory psoriatic arthritis cohort is associated with the disease activity and differentially modulated by methotrexate and apremilast. J. Intern. Med. 2022, 291, 676–693. [Google Scholar] [CrossRef] [PubMed]
- Sui, D.; Yu, H. Protective roles of apremilast via Sirtuin 1 in atherosclerosis. Bioengineered 2022, 13, 13872–13881. [Google Scholar] [CrossRef]
- Kitada, M.; Ogura, Y.; Koya, D. The protective role of Sirt1 in vascular tissue: Its relationship to vascular aging and atherosclerosis. Aging 2016, 8, 2290–2307. [Google Scholar] [CrossRef]
- Schick, M.A.; Schlegel, N. Clinical Implication of Phosphodiesterase-4-Inhibition. Int. J. Mol. Sci. 2022, 23, 1209. [Google Scholar] [CrossRef] [PubMed]
- Egan, J.J.; Greenberg, A.S.; Chang, M.K.; Wek, S.A.; Moos, M.C., Jr.; Londos, C. Mechanism of hormone-stimulated lipolysis in adipocytes: Translocation of hormone-sensitive lipase to the lipid storage droplet. Proc. Natl. Acad. Sci. USA 1992, 89, 8537–8541. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.J.; Blanco, F.J.; Crowley, J.; Birbara, C.A.; Jaworski, J.; Aelion, J.; Stevens, R.M.; Vessey, A.; Zhan, X.; Bird, P. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: A phase III, randomised, controlled trial (PALACE 3). Ann. Rheum. Dis. 2016, 75, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Puig, L.; Korman, N.; Greggio, C.; Cirulli, J.; Teng, L.; Chandran, V.; Khraishi, M.; Paris, M.; Mehta, N.N. Long-term hemoglobin A1C changes with apremilast in patients with psoriasis and psoriatic arthritis: Pooled analysis of phase 3 esteem and palace trials and phase 3b liberate trial. J. Am. Acad. Dermatol. 2019, 81, AB89. [Google Scholar] [CrossRef]
- Mazzilli, S.; Lanna, C.; Chiaramonte, C.; Cesaroni, G.M.; Zangrilli, A.; Palumbo, V.; Cosio, T.; Dattola, A.; Gaziano, R.; Galluzzo, M.; et al. Real life experience of apremilast in psoriasis and arthritis psoriatic patients: Preliminary results on metabolic biomarkers. J. Dermatol. 2020, 47, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Pavlidis, G.; Kadoglou, N.; Makavos, G.; Katogiannis, K.; Kountouri, A.; Thymis, J.; Kostelli, G.; Kapniari, I.; Theodoropoulos, K.; et al. Apremilast Improves Endothelial Glycocalyx Integrity, Vascular and Left Ventricular Myocardial Function in Psoriasis. Pharmaceuticals 2022, 15, 172. [Google Scholar] [CrossRef]
- Wang, H.; Yang, G.; Zhang, Q.; Liang, X.; Liu, Y.; Gao, M.; Guo, Y.; Chen, L. Apremilast ameliorates ox-LDL-induced endothelial dysfunction mediated by KLF6. Aging 2020, 12, 19012–19021. [Google Scholar] [CrossRef] [PubMed]
- Gualtierotti, R.; De Lucia, O. Efficacy and Metabolic Effect on Serum Lipids of Apremilast in Psoriatic Arthritis: A Case Report. J. Clin. Med. 2019, 8, 398. [Google Scholar] [CrossRef]
- Rich, P.; Gooderham, M.; Bachelez, H.; Goncalves, J.; Day, R.M.; Chen, R.; Crowley, J. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with difficult-to-treat nail and scalp psoriasis: Results of 2 phase III randomized, controlled trials (ESTEEM 1 and ESTEEM 2). J. Am. Acad. Dermatol. 2016, 74, 134–142. [Google Scholar] [CrossRef]
- Lanna, C.; Cesaroni, G.M.; Mazzilli, S.; Vollono, L.; Gaziano, R.; Marino, D.; Bianchi, L.; Campione, E. Apremilast as a target therapy for nail psoriasis: A real-life observational study proving its efficacy in restoring the nail unit. J. Dermatol. Treat. 2022, 33, 1097–1101. [Google Scholar] [CrossRef]
- Muñoz-Santos, C.; Sola-Ortigosa, J.; Vidal, D.; Guilabert, A. Apremilast improves quality of life and ultrasonography parameters in patients with nail psoriasis: A prospective cohort study. J. Dermatol. 2021, 48, 1593–1596. [Google Scholar] [CrossRef]
- Galluzzo, M.; Talamonti, M.; Cioni, A.; Maffei, V.; Shumak, R.G.; Tofani, L.; Bianchi, L.; Campione, E. Efficacy of Tildrakizumab for the Treatment of Difficult-to-Treat Areas: Scalp, Nail, Palmoplantar and Genital Psoriasis. J. Clin. Med. 2022, 11, 2631. [Google Scholar] [CrossRef]
| Parameter | Number | Percentage of Patients |
|---|---|---|
| Demographics | ||
| Male | 80 | 58.4% |
| Female | 57 | 41.6% |
| Average Age (range), years | 63.7 (22–87) | |
| Average Height (range), cm | 169 (150–206) | |
| Average Weight (range), kg | 78.4 (48–155) | |
| 27.16 (16.7–50.6) | ||
| Number of Patients at T0 | 137 | 100% |
| Number of Patients at T1 | 120 | 87.59% |
| Patient Dropout | 17 | 12.40% |
| Previous Dermatological Treatments | 122 | 89.05% |
| Topical treatments | 40 | 32.79% |
| Cyclosporine | 36 | 29.51% |
| Methotrexate | 24 | 19.67% |
| Acitretin | 6 | 4.92% |
| Narrowband-UVB | 6 | 4.92% |
| Dimethyl fumarate | 3 | 2.46% |
| Adalimumab | 4 | 3.28% |
| Etanercept | 3 | 2.46% |
| Comorbidities | ||
| Metabolic Syndrome | 21 | 22.1% |
| Hypertension | 52 | 54.7% |
| Diabetes Mellitus | 18 | 18.9% |
| Hypercholesterolemia | 43 | 45.3% |
| Hypertriglyceridemia | 13 | 13.7% |
| Cardiovascular Comorbidities | 28 | 29.5% |
| Psoriatic Arthritis | 80 | 58% |
| Pharmacological Treatments | ||
| Insulin and/or Hypoglycemics | 18 | 18.9% |
| Statins | 40 | 42.1% |
| Anticoagulants | 29 | 30.5% |
| Antihypertensives | 47 | 49.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campione, E.; Zarabian, N.; Cosio, T.; Borselli, C.; Artosi, F.; Cont, R.; Sorge, R.; Shumak, R.G.; Costanza, G.; Rivieccio, A.; et al. Apremilast as a Potential Targeted Therapy for Metabolic Syndrome in Patients with Psoriasis: An Observational Analysis. Pharmaceuticals 2024, 17, 989. https://doi.org/10.3390/ph17080989
Campione E, Zarabian N, Cosio T, Borselli C, Artosi F, Cont R, Sorge R, Shumak RG, Costanza G, Rivieccio A, et al. Apremilast as a Potential Targeted Therapy for Metabolic Syndrome in Patients with Psoriasis: An Observational Analysis. Pharmaceuticals. 2024; 17(8):989. https://doi.org/10.3390/ph17080989
Chicago/Turabian StyleCampione, Elena, Nikkia Zarabian, Terenzio Cosio, Cristiana Borselli, Fabio Artosi, Riccardo Cont, Roberto Sorge, Ruslana Gaeta Shumak, Gaetana Costanza, Antonia Rivieccio, and et al. 2024. "Apremilast as a Potential Targeted Therapy for Metabolic Syndrome in Patients with Psoriasis: An Observational Analysis" Pharmaceuticals 17, no. 8: 989. https://doi.org/10.3390/ph17080989
APA StyleCampione, E., Zarabian, N., Cosio, T., Borselli, C., Artosi, F., Cont, R., Sorge, R., Shumak, R. G., Costanza, G., Rivieccio, A., Gaziano, R., & Bianchi, L. (2024). Apremilast as a Potential Targeted Therapy for Metabolic Syndrome in Patients with Psoriasis: An Observational Analysis. Pharmaceuticals, 17(8), 989. https://doi.org/10.3390/ph17080989










