Positive Effects of Physical Activity on Insulin Signaling
Abstract
1. Introduction
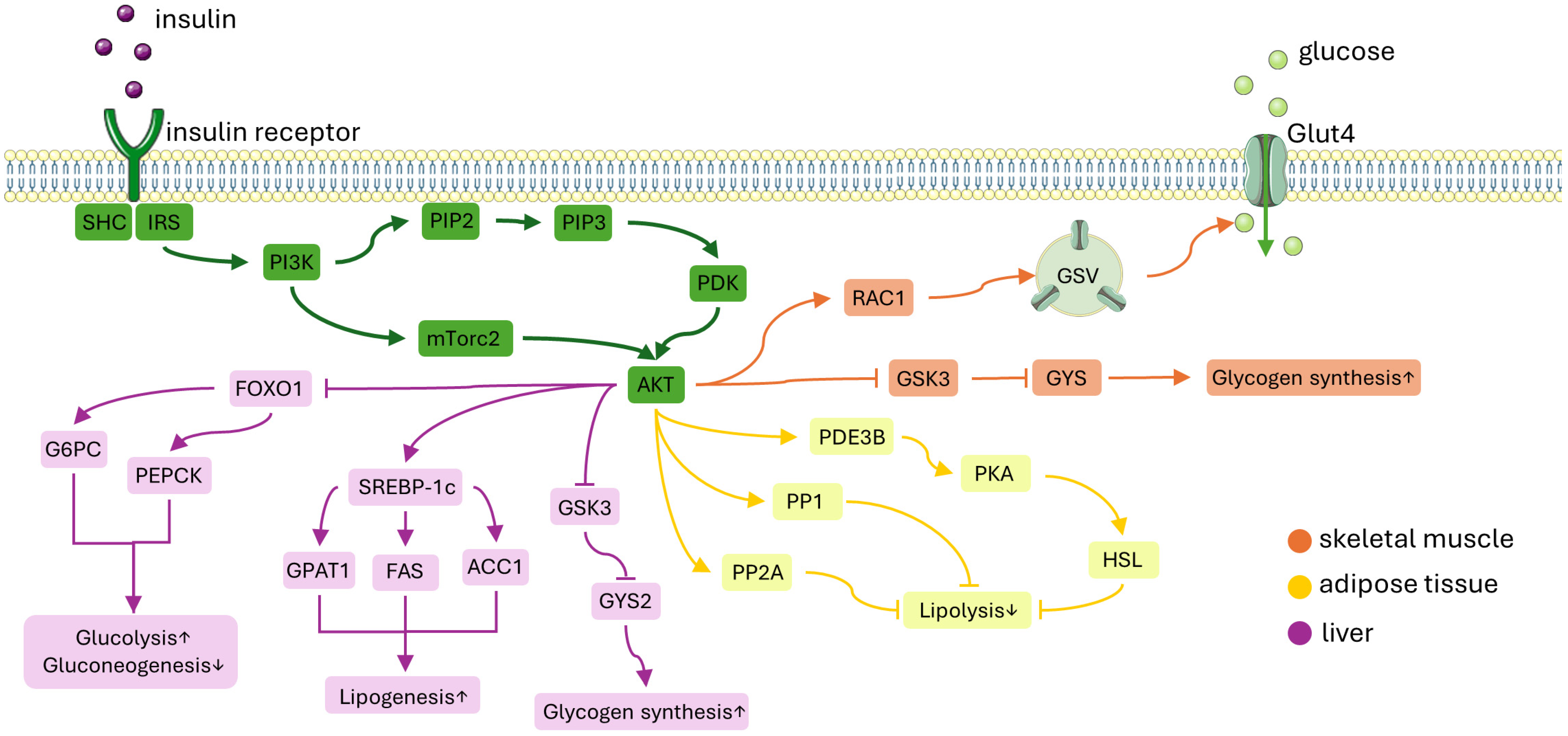
2. Insulin Signaling Pathway
2.1. Insulin Signaling Pathway in Skeletal Muscle
2.2. Insulin Signaling Pathway in WAT
2.3. Insulin Signaling Pathway in Liver
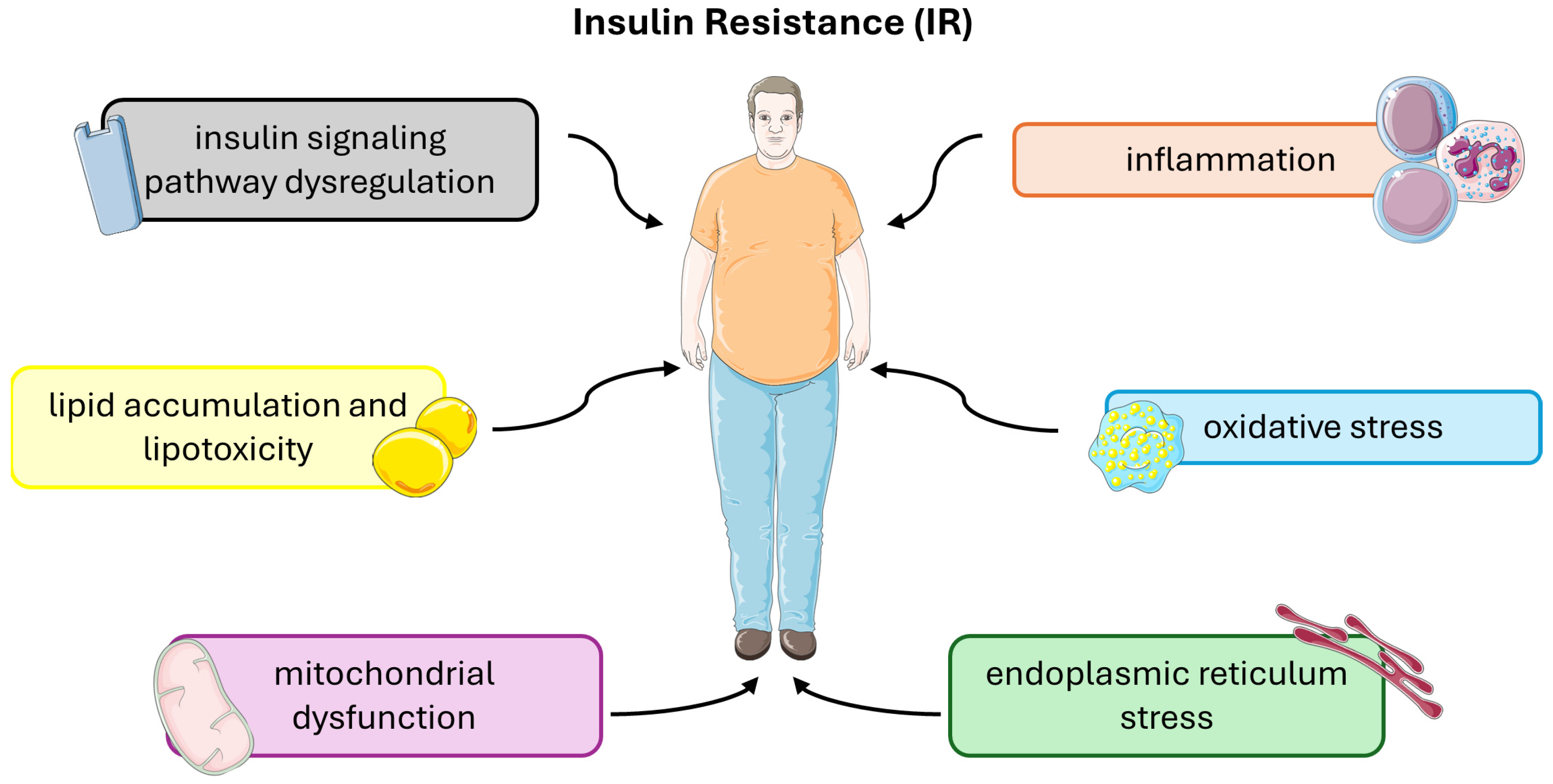
3. Mechanisms of Insulin Resistance
3.1. Insulin Signaling Pathway Dysregulation
3.2. Inflammation and Oxidative Stress
3.3. Lipid Accumulation and Lipotoxicity
3.4. Mitochondrial Dysfunction
4. Physical Activity and Insulin Resistance
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Park, S.-Y.; Choi, C.S. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; McCullough, A.J.; Marchesini, G. Insulin Resistance: A Metabolic Pathway to Chronic Liver Disease. Hepatology 2005, 42, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; An, X.; Yang, C.; Sun, W.; Ji, H.; Lian, F. The Crucial Role and Mechanism of Insulin Resistance in Metabolic Disease. Front. Endocrinol. 2023, 14, 1149239. [Google Scholar] [CrossRef]
- Kasuga, M. Insulin Resistance and Pancreatic Beta Cell Failure. J. Clin. Investig. 2006, 116, 1756–1760. [Google Scholar] [CrossRef]
- Kahn, S.E. The Relative Contributions of Insulin Resistance and Beta-Cell Dysfunction to the Pathophysiology of Type 2 Diabetes. Diabetologia 2003, 46, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Morigny, P.; Houssier, M.; Mouisel, E.; Langin, D. Adipocyte Lipolysis and Insulin Resistance. Biochimie 2016, 125, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Kosmas, C.E.; Bousvarou, M.D.; Kostara, C.E.; Papakonstantinou, E.J.; Salamou, E.; Guzman, E. Insulin Resistance and Cardiovascular Disease. J. Int. Med. Res. 2023, 51, 03000605231164548. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.-C.; Lee, M.Y.; Kim, Y.-H.; Huh, J.-H.; Kim, J.-Y.; Wild, S.H.; Byrne, C.D. Obesity and Incidence of Diabetes: Effect of Absence of Metabolic Syndrome, Insulin Resistance, Inflammation and Fatty Liver. Atherosclerosis 2018, 275, 50–57. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Atkin, S.L.; Simental-Mendía, L.E.; Sahebkar, A. Molecular Mechanisms by Which Aerobic Exercise Induces Insulin Sensitivity. J. Cell Physiol. 2019, 234, 12385–12392. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Maleki, M.; Sathyapalan, T.; Jamialahmadi, T.; Sahebkar, A. Pathophysiology of Physical Inactivity-Dependent Insulin Resistance: A Theoretical Mechanistic Review Emphasizing Clinical Evidence. J. Diabetes Res. 2021, 2021, 7796727. [Google Scholar] [CrossRef] [PubMed]
- Lee-Ødegård, S.; Olsen, T.; Norheim, F.; Drevon, C.A.; Birkeland, K.I. Potential Mechanisms for How Long-Term Physical Activity May Reduce Insulin Resistance. Metabolites 2022, 12, 208. [Google Scholar] [CrossRef]
- Hubbard, S.R. The Insulin Receptor: Both a Prototypical and Atypical Receptor Tyrosine Kinase. Cold Spring Harb. Perspect. Biol. 2013, 5, a008946. [Google Scholar] [CrossRef]
- Belfiore, A.; Malaguarnera, R.; Vella, V.; Lawrence, M.C.; Sciacca, L.; Frasca, F.; Morrione, A.; Vigneri, R. Insulin Receptor Isoforms in Physiology and Disease: An Updated View. Endocr. Rev. 2017, 38, 379–431. [Google Scholar] [CrossRef] [PubMed]
- De Meyts, P. The Insulin Receptor: A Prototype for Dimeric, Allosteric Membrane Receptors? Trends Biochem. Sci. 2008, 33, 376–384. [Google Scholar] [CrossRef]
- Youngren, J.F. Regulation of Insulin Receptor Function. Cell. Mol. Life Sci. 2007, 64, 873–891. [Google Scholar] [CrossRef]
- Khalid, M.; Alkaabi, J.; Khan, M.A.B.; Adem, A. Insulin Signal Transduction Perturbations in Insulin Resistance. Int. J. Mol. Sci. 2021, 22, 8590. [Google Scholar] [CrossRef] [PubMed]
- Leto, D.; Saltiel, A.R. Regulation of Glucose Transport by Insulin: Traffic Control of GLUT4. Nat. Rev. Mol. Cell Biol. 2012, 13, 383–396. [Google Scholar] [CrossRef]
- Chiu, T.T.; Jensen, T.E.; Sylow, L.; Richter, E.A.; Klip, A. Rac1 Signalling towards GLUT4/Glucose Uptake in Skeletal Muscle. Cell. Signal. 2011, 23, 1546–1554. [Google Scholar] [CrossRef]
- Sylow, L.; Kleinert, M.; Pehmøller, C.; Prats, C.; Chiu, T.T.; Klip, A.; Richter, E.A.; Jensen, T.E. Akt and Rac1 Signaling Are Jointly Required for Insulin-Stimulated Glucose Uptake in Skeletal Muscle and Downregulated in Insulin Resistance. Cell. Signal. 2014, 26, 323–331. [Google Scholar] [CrossRef]
- Belman, J.P.; Habtemichael, E.N.; Bogan, J.S. A Proteolytic Pathway That Controls Glucose Uptake in Fat and Muscle. Rev. Endocr. Metab. Disord. 2014, 15, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Sylow, L.; Jensen, T.E.; Kleinert, M.; Højlund, K.; Kiens, B.; Wojtaszewski, J.; Prats, C.; Schjerling, P.; Richter, E.A. Rac1 Signaling Is Required for Insulin-Stimulated Glucose Uptake and Is Dysregulated in Insulin-Resistant Murine and Human Skeletal Muscle. Diabetes 2013, 62, 1865–1875. [Google Scholar] [CrossRef]
- Ueda, S.; Kitazawa, S.; Ishida, K.; Nishikawa, Y.; Matsui, M.; Matsumoto, H.; Aoki, T.; Nozaki, S.; Takeda, T.; Tamori, Y.; et al. Crucial Role of the Small GTPase Rac1 in Insulin-Stimulated Translocation of Glucose Transporter 4 to the Mouse Skeletal Muscle Sarcolemma. FASEB J. 2010, 24, 2254–2261. [Google Scholar] [CrossRef] [PubMed]
- Cross, D.A.; Alessi, D.R.; Cohen, P.; Andjelkovich, M.; Hemmings, B.A. Inhibition of Glycogen Synthase Kinase-3 by Insulin Mediated by Protein Kinase B. Nature 1995, 378, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; Brady, M.J.; O’Doherty, R.M.; Saltiel, A.R. Organizing Glucose Disposal: Emerging Roles of the Glycogen Targeting Subunits of Protein Phosphatase-1. Diabetes 2000, 49, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Jurczak, M.J.; Danos, A.M.; Rehrmann, V.R.; Brady, M.J. The Role of Protein Translocation in the Regulation of Glycogen Metabolism. J. Cell Biochem. 2008, 104, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Brady, M.J.; Saltiel, A.R. The Role of Protein Phosphatase-1 in Insulin Action. Recent Prog. Horm. Res. 2001, 56, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Delibegovic, M.; Armstrong, C.G.; Dobbie, L.; Watt, P.W.; Smith, A.J.H.; Cohen, P.T.W. Disruption of the Striated Muscle Glycogen Targeting Subunit PPP1R3A of Protein Phosphatase 1 Leads to Increased Weight Gain, Fat Deposition, and Development of Insulin Resistance. Diabetes 2003, 52, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Lanner, C.; Kim, J.H.; Vilardo, P.G.; Zhang, H.; Yang, J.; Cooper, L.D.; Steele, M.; Kennedy, A.; Bock, C.B.; et al. Insulin Control of Glycogen Metabolism in Knockout Mice Lacking the Muscle-Specific Protein Phosphatase PP1G/RGL. Mol. Cell. Biol. 2001, 21, 2683–2694. [Google Scholar] [CrossRef]
- Cohen, P.; Frame, S. The Renaissance of GSK3. Nat. Rev. Mol. Cell Biol. 2001, 2, 769–776. [Google Scholar] [CrossRef]
- Agius, L. Role of Glycogen Phosphorylase in Liver Glycogen Metabolism. Mol. Asp. Med. 2015, 46, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Rebrin, K.; Steil, G.M.; Mittelman, S.D.; Bergman, R.N. Causal Linkage between Insulin Suppression of Lipolysis and Suppression of Liver Glucose Output in Dogs. J. Clin. Investig. 1996, 98, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Camporez, J.-P.G.; Kursawe, R.; Titchenell, P.M.; Zhang, D.; Perry, C.J.; Jurczak, M.J.; Abudukadier, A.; Han, M.S.; Zhang, X.-M.; et al. Hepatic Acetyl CoA Links Adipose Tissue Inflammation to Hepatic Insulin Resistance and Type 2 Diabetes. Cell 2015, 160, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. Banting Lecture 1988. Role of Insulin Resistance in Human Disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef]
- Choi, Y.H.; Park, S.; Hockman, S.; Zmuda-Trzebiatowska, E.; Svennelid, F.; Haluzik, M.; Gavrilova, O.; Ahmad, F.; Pepin, L.; Napolitano, M.; et al. Alterations in Regulation of Energy Homeostasis in Cyclic Nucleotide Phosphodiesterase 3B–Null Mice. J. Clin. Investig. 2006, 116, 3240–3251. [Google Scholar] [CrossRef]
- Jaworski, K.; Sarkadi-Nagy, E.; Duncan, R.E.; Ahmadian, M.; Sul, H.S. Regulation of Triglyceride Metabolism. IV. Hormonal Regulation of Lipolysis in Adipose Tissue. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G1–G4. [Google Scholar] [CrossRef] [PubMed]
- Holm, C. Molecular Mechanisms Regulating Hormone-Sensitive Lipase and Lipolysis. Biochem. Soc. Trans. 2003, 31, 1120–1124. [Google Scholar] [CrossRef]
- Sztalryd, C.; Xu, G.; Dorward, H.; Tansey, J.T.; Contreras, J.A.; Kimmel, A.R.; Londos, C. Perilipin A Is Essential for the Translocation of Hormone-Sensitive Lipase during Lipolytic Activation. J. Cell Biol. 2003, 161, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Albert, J.S.; Yerges-Armstrong, L.M.; Horenstein, R.B.; Pollin, T.I.; Sreenivasan, U.T.; Chai, S.; Blaner, W.S.; Snitker, S.; O’Connell, J.R.; Gong, D.-W.; et al. Null Mutation in Hormone-Sensitive Lipase Gene and Risk of Type 2 Diabetes. N. Engl. J. Med. 2014, 370, 2307. [Google Scholar] [CrossRef]
- Brasaemle, D.L. Thematic Review Series: Adipocyte Biology. The Perilipin Family of Structural Lipid Droplet Proteins: Stabilization of Lipid Droplets and Control of Lipolysis. J. Lipid Res. 2007, 48, 2547–2559. [Google Scholar] [CrossRef]
- Zechner, R. FAT FLUX: Enzymes, Regulators, and Pathophysiology of Intracellular Lipolysis. EMBO Mol. Med. 2015, 7, 359–362. [Google Scholar] [CrossRef]
- Marcinkiewicz, A.; Gauthier, D.; Garcia, A.; Brasaemle, D.L. The Phosphorylation of Serine 492 of Perilipin a Directs Lipid Droplet Fragmentation and Dispersion. J. Biol. Chem. 2006, 281, 11901–11909. [Google Scholar] [CrossRef] [PubMed]
- Begum, N. Stimulation of Protein Phosphatase-1 Activity by Insulin in Rat Adipocytes. Evaluation of the Role of Mitogen-Activated Protein Kinase Pathway. J. Biol. Chem. 1995, 270, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Resjö, S.; Göransson, O.; Härndahl, L.; Zolnierowicz, S.; Manganiello, V.; Degerman, E. Protein Phosphatase 2A Is the Main Phosphatase Involved in the Regulation of Protein Kinase B in Rat Adipocytes. Cell. Signal. 2002, 14, 231–238. [Google Scholar] [CrossRef]
- Strålfors, P.; Honnor, R.C. Insulin-Induced Dephosphorylation of Hormone-Sensitive Lipase. Correlation with Lipolysis and cAMP-Dependent Protein Kinase Activity. Eur. J. Biochem. 1989, 182, 379–385. [Google Scholar] [CrossRef]
- Virtanen, K.A.; Lönnroth, P.; Parkkola, R.; Peltoniemi, P.; Asola, M.; Viljanen, T.; Tolvanen, T.; Knuuti, J.; Rönnemaa, T.; Huupponen, R.; et al. Glucose Uptake and Perfusion in Subcutaneous and Visceral Adipose Tissue during Insulin Stimulation in Nonobese and Obese Humans. J. Clin. Endocrinol. Metab. 2002, 87, 3902–3910. [Google Scholar] [CrossRef]
- Kersten, S. Mechanisms of Nutritional and Hormonal Regulation of Lipogenesis. EMBO Rep. 2001, 2, 282–286. [Google Scholar] [CrossRef]
- Rieusset, J.; Andreelli, F.; Auboeuf, D.; Roques, M.; Vallier, P.; Riou, J.P.; Auwerx, J.; Laville, M.; Vidal, H. Insulin Acutely Regulates the Expression of the Peroxisome Proliferator-Activated Receptor-Gamma in Human Adipocytes. Diabetes 1999, 48, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Cherrington, A.D.; Edgerton, D.; Sindelar, D.K. The Direct and Indirect Effects of Insulin on Hepatic Glucose Production In Vivo. Diabetologia 1998, 41, 987–996. [Google Scholar] [CrossRef]
- Dong, X.C.; Copps, K.D.; Guo, S.; Li, Y.; Kollipara, R.; DePinho, R.A.; White, M.F. Inactivation of Hepatic Foxo1 by Insulin Signaling Is Required for Adaptive Nutrient Homeostasis and Endocrine Growth Regulation. Cell Metab. 2008, 8, 65–76. [Google Scholar] [CrossRef]
- Tzivion, G.; Dobson, M.; Ramakrishnan, G. FoxO Transcription Factors; Regulation by AKT and 14-3-3 Proteins. Biochim. Biophys. Acta 2011, 1813, 1938–1945. [Google Scholar] [CrossRef] [PubMed]
- Langlet, F.; Haeusler, R.A.; Lindén, D.; Ericson, E.; Norris, T.; Johansson, A.; Cook, J.R.; Aizawa, K.; Wang, L.; Buettner, C.; et al. Selective Inhibition of FOXO1 Activator/Repressor Balance Modulates Hepatic Glucose Handling. Cell 2017, 171, 824–835.e18. [Google Scholar] [CrossRef] [PubMed]
- Ros, S.; García-Rocha, M.; Domínguez, J.; Ferrer, J.C.; Guinovart, J.J. Control of Liver Glycogen Synthase Activity and Intracellular Distribution by Phosphorylation. J. Biol. Chem. 2009, 284, 6370–6378. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the Complete Program of Cholesterol and Fatty Acid Synthesis in the Liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Krycer, J.R.; Sharpe, L.J.; Luu, W.; Brown, A.J. The Akt-SREBP Nexus: Cell Signaling Meets Lipid Metabolism. Trends Endocrinol. Metab. 2010, 21, 268–276. [Google Scholar] [CrossRef]
- Jelenik, T.; Kaul, K.; Séquaris, G.; Flögel, U.; Phielix, E.; Kotzka, J.; Knebel, B.; Fahlbusch, P.; Hörbelt, T.; Lehr, S.; et al. Mechanisms of Insulin Resistance in Primary and Secondary Nonalcoholic Fatty Liver. Diabetes 2017, 66, 2241–2253. [Google Scholar] [CrossRef] [PubMed]
- Eberlé, D.; Hegarty, B.; Bossard, P.; Ferré, P.; Foufelle, F. SREBP Transcription Factors: Master Regulators of Lipid Homeostasis. Biochimie 2004, 86, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Brown, M.S.; Goldstein, J.L. Bifurcation of Insulin Signaling Pathway in Rat Liver: mTORC1 Required for Stimulation of Lipogenesis, but Not Inhibition of Gluconeogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 3441–3446. [Google Scholar] [CrossRef] [PubMed]
- Horita, S.; Nakamura, M.; Suzuki, M.; Satoh, N.; Suzuki, A.; Seki, G. Selective Insulin Resistance in the Kidney. Biomed Res. Int. 2016, 2016, 5825170. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Palakkott, A.; Ayoub, M.A. Anti-Insulin Receptor Antibodies in the Pathology and Therapy of Diabetes Mellitus. Curr. Diabetes Rev. 2021, 17, 198–206. [Google Scholar] [CrossRef]
- Hall, C.; Yu, H.; Choi, E. Insulin Receptor Endocytosis in the Pathophysiology of Insulin Resistance. Exp. Mol. Med. 2020, 52, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Rivers, S.L.; Klip, A.; Giacca, A. NOD1: An Interface Between Innate Immunity and Insulin Resistance. Endocrinology 2019, 160, 1021–1030. [Google Scholar] [CrossRef]
- Copps, K.D.; White, M.F. Regulation of Insulin Sensitivity by Serine/Threonine Phosphorylation of Insulin Receptor Substrate Proteins IRS1 and IRS2. Diabetologia 2012, 55, 2565–2582. [Google Scholar] [CrossRef]
- Carvalho-Filho, M.A.; Carvalho, B.M.; Oliveira, A.G.; Guadagnini, D.; Ueno, M.; Dias, M.M.; Tsukumo, D.M.; Hirabara, S.M.; Reis, L.F.; Curi, R.; et al. Double-Stranded RNA-Activated Protein Kinase Is a Key Modulator of Insulin Sensitivity in Physiological Conditions and in Obesity in Mice. Endocrinology 2012, 153, 5261–5274. [Google Scholar] [CrossRef] [PubMed]
- Hage Hassan, R.; Pacheco de Sousa, A.C.; Mahfouz, R.; Hainault, I.; Blachnio-Zabielska, A.; Bourron, O.; Koskas, F.; Górski, J.; Ferré, P.; Foufelle, F.; et al. Sustained Action of Ceramide on the Insulin Signaling Pathway in Muscle Cells: IMPLICATION OF THE DOUBLE-STRANDED RNA-ACTIVATED PROTEIN KINASE. J. Biol. Chem. 2016, 291, 3019–3029. [Google Scholar] [CrossRef] [PubMed]
- Arkan, M.C.; Hevener, A.L.; Greten, F.R.; Maeda, S.; Li, Z.-W.; Long, J.M.; Wynshaw-Boris, A.; Poli, G.; Olefsky, J.; Karin, M. IKK-β Links Inflammation to Obesity-Induced Insulin Resistance. Nat. Med. 2005, 11, 191–198. [Google Scholar] [CrossRef]
- Yung, J.H.M.; Giacca, A. Role of C-Jun N-Terminal Kinase (JNK) in Obesity and Type 2 Diabetes. Cells 2020, 9, 706. [Google Scholar] [CrossRef]
- Hilder, T.L.; Tou, J.C.L.; Grindeland, R.E.; Wade, C.E.; Graves, L.M. Phosphorylation of Insulin Receptor Substrate-1 Serine 307 Correlates with JNK Activity in Atrophic Skeletal Muscle. FEBS Lett. 2003, 553, 63–67. [Google Scholar] [CrossRef]
- Yan, H.; He, L.; Lv, D.; Yang, J.; Yuan, Z. The Role of the Dysregulated JNK Signaling Pathway in the Pathogenesis of Human Diseases and Its Potential Therapeutic Strategies: A Comprehensive Review. Biomolecules 2024, 14, 243. [Google Scholar] [CrossRef]
- James, D.E.; Stöckli, J.; Birnbaum, M.J. The Aetiology and Molecular Landscape of Insulin Resistance. Nat. Rev. Mol. Cell Biol. 2021, 22, 751–771. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin Signalling and the Regulation of Glucose and Lipid Metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Schenk, S.; Saberi, M.; Olefsky, J.M. Insulin Sensitivity: Modulation by Nutrients and Inflammation. J. Clin. Investig. 2008, 118, 2992–3002. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H. Obese Visceral Fat Tissue Inflammation: From Protective to Detrimental? BMC Med. 2022, 20, 494. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose Tissue Inflammation and Metabolic Dysfunction in Obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, A.J.P.O.; de Oliveira, J.C.P.L.; da Silva Pontes, L.V.; de Souza Júnior, J.F.; Gonçalves, T.A.F.; Dantas, S.H.; de Almeida Feitosa, M.S.; Silva, A.O.; de Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and Its Implications in Aging Pathways. Oxidative Med. Cell. Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Besse-Patin, A.; Estall, J.L. An Intimate Relationship between ROS and Insulin Signalling: Implications for Antioxidant Treatment of Fatty Liver Disease. Int. J. Cell Biol. 2014, 2014, 519153. [Google Scholar] [CrossRef] [PubMed]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Yoon, H.; Shaw, J.L.; Haigis, M.C.; Greka, A. Lipid Metabolism in Sickness and in Health: Emerging Regulators of Lipotoxicity. Mol. Cell 2021, 81, 3708–3730. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, C.; Kong, X.; Xia, Z.; Kong, W.; Si, K.; Han, P.; Vivian Liu, W.; Li, X. Pancreatic Fat Infiltration, β-Cell Function and Insulin Resistance: A Study of the Young Patients with Obesity. Diabetes Res. Clin. Pract. 2022, 187, 109860. [Google Scholar] [CrossRef] [PubMed]
- Snel, M.; Jonker, J.T.; Schoones, J.; Lamb, H.; de Roos, A.; Pijl, H.; Smit, J.W.A.; Meinders, A.E.; Jazet, I.M. Ectopic Fat and Insulin Resistance: Pathophysiology and Effect of Diet and Lifestyle Interventions. Int. J. Endocrinol. 2012, 2012, 983814. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Mottillo, E.P. Adipocyte Lipolysis: From Molecular Mechanisms of Regulation to Disease and Therapeutics. Biochem. J. 2020, 477, 985–1008. [Google Scholar] [CrossRef] [PubMed]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr. Physiol. 2020, 10, 785–809. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Seo, Y.-K. Excess Accumulation of Lipid Impairs Insulin Sensitivity in Skeletal Muscle. Int. J. Mol. Sci. 2020, 21, 1949. [Google Scholar] [CrossRef] [PubMed]
- Perreault, L.; Newsom, S.A.; Strauss, A.; Kerege, A.; Kahn, D.E.; Harrison, K.A.; Snell-Bergeon, J.K.; Nemkov, T.; D’Alessandro, A.; Jackman, M.R.; et al. Intracellular Localization of Diacylglycerols and Sphingolipids Influences Insulin Sensitivity and Mitochondrial Function in Human Skeletal Muscle. JCI Insight 2018, 3, e96805. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Peiffer, C.; Biden, T.J. Protein Kinase C Function in Muscle, Liver, and Beta-Cells and Its Therapeutic Implications for Type 2 Diabetes. Diabetes 2008, 57, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Faber, K.N.; de Meijer, V.E.; Blokzijl, H.; Moshage, H. How Does Hepatic Lipid Accumulation Lead to Lipotoxicity in Non-Alcoholic Fatty Liver Disease? Hepatol. Int. 2021, 15, 21–35. [Google Scholar] [CrossRef] [PubMed]
- London, A.; Lundsgaard, A.-M.; Kiens, B.; Bojsen-Møller, K.N. The Role of Hepatic Fat Accumulation in Glucose and Insulin Homeostasis—Dysregulation by the Liver. J. Clin. Med. 2021, 10, 390. [Google Scholar] [CrossRef]
- Lipke, K.; Kubis-Kubiak, A.; Piwowar, A. Molecular Mechanism of Lipotoxicity as an Interesting Aspect in the Development of Pathological States—Current View of Knowledge. Cells 2022, 11, 844. [Google Scholar] [CrossRef]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose Tissue and Insulin Resistance in Obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial Dysfunction and Oxidative Stress in Metabolic Disorders—A Step towards Mitochondria Based Therapeutic Strategies. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Sobenin, I.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. Mitochondrial Aging and Age-Related Dysfunction of Mitochondria. Biomed Res. Int. 2014, 2014, 238463. [Google Scholar] [CrossRef]
- Sergi, D.; Naumovski, N.; Heilbronn, L.K.; Abeywardena, M.; O’Callaghan, N.; Lionetti, L.; Luscombe-Marsh, N. Mitochondrial (Dys)Function and Insulin Resistance: From Pathophysiological Molecular Mechanisms to the Impact of Diet. Front. Physiol. 2019, 10, 532. [Google Scholar] [CrossRef]
- Hales, K.G. The Machinery of Mitochondrial Fusion, Division, and Distribution, and Emerging Connections to Apoptosis. Mitochondrion 2004, 4, 285–308. [Google Scholar] [CrossRef]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial Fission, Fusion, and Stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical Activity, Exercise, and Physical Fitness: Definitions and Distinctions for Health-Related Research. Public Health Rep. 1985, 100, 126–131. [Google Scholar]
- Schuch, F.B.; Vancampfort, D. Physical Activity, Exercise, and Mental Disorders: It Is Time to Move On. Trends Psychiatry Psychother. 2021, 43, 177–184. [Google Scholar] [CrossRef]
- Nystoriak, M.A.; Bhatnagar, A. Cardiovascular Effects and Benefits of Exercise. Front. Cardiovasc. Med. 2018, 5, 135. [Google Scholar] [CrossRef]
- Benedetti, M.G.; Furlini, G.; Zati, A.; Letizia Mauro, G. The Effectiveness of Physical Exercise on Bone Density in Osteoporotic Patients. Biomed Res. Int. 2018, 2018, 4840531. [Google Scholar] [CrossRef]
- Pinckard, K.; Baskin, K.K.; Stanford, K.I. Effects of Exercise to Improve Cardiovascular Health. Front. Cardiovasc. Med. 2019, 6, 69. [Google Scholar] [CrossRef]
- Conn, V.S.; Koopman, R.J.; Ruppar, T.M.; Phillips, L.J.; Mehr, D.R.; Hafdahl, A.R. Insulin Sensitivity Following Exercise Interventions: Systematic Review and Meta-Analysis of Outcomes Among Healthy Adults. J. Prim. Care Community Health 2014, 5, 211–222. [Google Scholar] [CrossRef]
- Eaton, S.B.; Eaton, S.B. Physical Inactivity, Obesity, and Type 2 Diabetes: An Evolutionary Perspective. Res. Q. Exerc. Sport 2017, 88, 1–8. [Google Scholar] [CrossRef]
- Sampath Kumar, A.; Maiya, A.G.; Shastry, B.A.; Vaishali, K.; Ravishankar, N.; Hazari, A.; Gundmi, S.; Jadhav, R. Exercise and Insulin Resistance in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Ann. Phys. Rehabil. Med. 2019, 62, 98–103. [Google Scholar] [CrossRef]
- Bird, S.R.; Hawley, J.A. Update on the Effects of Physical Activity on Insulin Sensitivity in Humans. BMJ Open Sport Exerc. Med. 2017, 2, e000143. [Google Scholar] [CrossRef]
- Barseem, N.F.; Helwa, M.A. Homeostatic Model Assessment of Insulin Resistance as a Predictor of Metabolic Syndrome: Consequences of Obesity in Children and Adolescents. Egypt. Pediatr. Assoc. Gaz. 2015, 63, 19–24. [Google Scholar] [CrossRef][Green Version]
- Jenkins, N.T.; Hagberg, J.M. Aerobic Training Effects on Glucose Tolerance in Prediabetic and Normoglycemic Humans. Med. Sci. Sports Exerc. 2011, 43, 2231–2240. [Google Scholar] [CrossRef]
- Hays, N.P.; Starling, R.D.; Sullivan, D.H.; Fluckey, J.D.; Coker, R.H.; Evans, W.J. Comparison of Insulin Sensitivity Assessment Indices with Euglycemic-Hyperinsulinemic Clamp Data after a Dietary and Exercise Intervention in Older Adults. Metabolism 2006, 55, 525–532. [Google Scholar] [CrossRef]
- Keshel, T.E.; Coker, R.H. Exercise Training and Insulin Resistance: A Current Review. J. Obes. Weight Loss Ther. 2015, 5, S5-003. [Google Scholar] [CrossRef]
- Hudish, L.I.; Reusch, J.E.B.; Sussel, L. β Cell Dysfunction during Progression of Metabolic Syndrome to Type 2 Diabetes. J. Clin. Investig. 2019, 129, 4001–4008. [Google Scholar] [CrossRef]
- Dela, F.; von Linstow, M.E.; Mikines, K.J.; Galbo, H. Physical Training May Enhance Beta-Cell Function in Type 2 Diabetes. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E1024–E1031. [Google Scholar] [CrossRef]
- Narendran, P.; Solomon, T.P.; Kennedy, A.; Chimen, M.; Andrews, R.C. The Time Has Come to Test the Beta Cell Preserving Effects of Exercise in Patients with New Onset Type 1 Diabetes. Diabetologia 2015, 58, 10–18. [Google Scholar] [CrossRef]
- Slentz, C.A.; Tanner, C.J.; Bateman, L.A.; Durheim, M.T.; Huffman, K.M.; Houmard, J.A.; Kraus, W.E. Effects of Exercise Training Intensity on Pancreatic β-Cell Function. Diabetes Care 2009, 32, 1807–1811. [Google Scholar] [CrossRef]
- Lee, S.F.; Pei, D.; Chi, M.J.; Jeng, C. An Investigation and Comparison of the Effectiveness of Different Exercise Programmes in Improving Glucose Metabolism and Pancreatic β Cell Function of Type 2 Diabetes Patients. Int. J. Clin. Pract. 2015, 69, 1159–1170. [Google Scholar] [CrossRef]
- Bloem, C.J.; Chang, A.M. Short-Term Exercise Improves β-Cell Function and Insulin Resistance in Older People with Impaired Glucose Tolerance. J. Clin. Endocrinol. Metab. 2008, 93, 387–392. [Google Scholar] [CrossRef]
- Gomes, R.M.; Tófolo, L.P.; Rinaldi, W.; Scomparin, D.X.; Grassiolli, S.; Barella, L.F.; de Oliveira, J.C.; Branco, R.C.S.; Agostinho, A.R.; da Silva Ribeiro, T.A.; et al. Moderate Exercise Restores Pancreatic Beta-Cell Function and Autonomic Nervous System Activity in Obese Rats Induced by High-Fat Diet. Cell Physiol. Biochem. 2013, 32, 310–321. [Google Scholar] [CrossRef]
- Delghingaro-Augusto, V.; Décary, S.; Peyot, M.-L.; Latour, M.G.; Lamontagne, J.; Paradis-Isler, N.; Lacharité-Lemieux, M.; Akakpo, H.; Birot, O.; Nolan, C.J.; et al. Voluntary Running Exercise Prevents β-Cell Failure in Susceptible Islets of the Zucker Diabetic Fatty Rat. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E254–E264. [Google Scholar] [CrossRef]
- Biensø, R.S.; Ringholm, S.; Kiilerich, K.; Aachmann-Andersen, N.-J.; Krogh-Madsen, R.; Guerra, B.; Plomgaard, P.; van Hall, G.; Treebak, J.T.; Saltin, B.; et al. GLUT4 and Glycogen Synthase Are Key Players in Bed Rest–Induced Insulin Resistance. Diabetes 2012, 61, 1090–1099. [Google Scholar] [CrossRef]
- Alibegovic, A.C.; Sonne, M.P.; Højbjerre, L.; Bork-Jensen, J.; Jacobsen, S.; Nilsson, E.; Faerch, K.; Hiscock, N.; Mortensen, B.; Friedrichsen, M.; et al. Insulin Resistance Induced by Physical Inactivity Is Associated with Multiple Transcriptional Changes in Skeletal Muscle in Young Men. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E752–E763. [Google Scholar] [CrossRef]
- Hawley, J.A. Exercise as a Therapeutic Intervention for the Prevention and Treatment of Insulin Resistance. Diabetes Metab. Res. Rev. 2004, 20, 383–393. [Google Scholar] [CrossRef]
- Chibalin, A.V.; Yu, M.; Ryder, J.W.; Song, X.M.; Galuska, D.; Krook, A.; Wallberg-Henriksson, H.; Zierath, J.R. Exercise-Induced Changes in Expression and Activity of Proteins Involved in Insulin Signal Transduction in Skeletal Muscle: Differential Effects on Insulin-Receptor Substrates 1 and 2. Proc. Natl. Acad. Sci. USA 2000, 97, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lee, S.; Langleite, T.; Norheim, F.; Pourteymour, S.; Jensen, J.; Stadheim, H.K.; Storås, T.H.; Davanger, S.; Gulseth, H.L.; et al. Subsarcolemmal Lipid Droplet Responses to a Combined Endurance and Strength Exercise Intervention. Physiol. Rep. 2014, 2, e12187. [Google Scholar] [CrossRef] [PubMed]
- Roden, M.; Shulman, G.I. The Integrative Biology of Type 2 Diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef]
- Małkowska, P.; Sawczuk, M. Cytokines as Biomarkers for Evaluating Physical Exercise in Trained and Non-Trained Individuals: A Narrative Review. Int. J. Mol. Sci. 2023, 24, 11156. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.M.; Dillon, C.B.; Perry, I.J. Does Replacing Sedentary Behaviour with Light or Moderate to Vigorous Physical Activity Modulate Inflammatory Status in Adults? Int. J. Behav. Nutr. Phys. Act. 2017, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Højbjerre, L.; Sonne, M.P.; Alibegovic, A.C.; Nielsen, N.B.; Dela, F.; Vaag, A.; Bruun, J.M.; Stallknecht, B. Impact of Physical Inactivity on Adipose Tissue Low-Grade Inflammation in First-Degree Relatives of Type 2 Diabetic Patients. Diabetes Care 2011, 34, 2265–2272. [Google Scholar] [CrossRef] [PubMed]
- Febbraio, M.A.; Pedersen, B.K. Muscle-Derived Interleukin-6: Mechanisms for Activation and Possible Biological Roles. FASEB J. 2002, 16, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Nakaji, S.; Yamada, M.; Totsuka, M.; Sato, K.; Sugawara, K. Systemic Inflammatory Response to Exhaustive Exercise. Cytokine Kinetics. Exerc. Immunol. Rev. 2002, 8, 6–48. [Google Scholar] [PubMed]
- Esposito, K.; Pontillo, A.; Di Palo, C.; Giugliano, G.; Masella, M.; Marfella, R.; Giugliano, D. Effect of Weight Loss and Lifestyle Changes on Vascular Inflammatory Markers in Obese Women: A Randomized Trial. JAMA 2003, 289, 1799–1804. [Google Scholar] [CrossRef]
- Stefan, N.; Stumvoll, M. Adiponectin—Its Role in Metabolism and Beyond. Horm. Metab. Res. 2002, 34, 469–474. [Google Scholar] [CrossRef]
- Shamsuzzaman, A.S.M.; Winnicki, M.; Wolk, R.; Svatikova, A.; Phillips, B.G.; Davison, D.E.; Berger, P.B.; Somers, V.K. Independent Association between Plasma Leptin and C-Reactive Protein in Healthy Humans. Circulation 2004, 109, 2181–2185. [Google Scholar] [CrossRef]
- Adamopoulos, S.; Parissis, J.; Kroupis, C.; Georgiadis, M.; Karatzas, D.; Karavolias, G.; Koniavitou, K.; Coats, A.J.; Kremastinos, D.T. Physical Training Reduces Peripheral Markers of Inflammation in Patients with Chronic Heart Failure. Eur. Heart J. 2001, 22, 791–797. [Google Scholar] [CrossRef]
- Lee, S.; Norheim, F.; Langleite, T.M.; Gulseth, H.L.; Birkeland, K.I.; Drevon, C.A. Effects of Long-Term Exercise on Plasma Adipokine Levels and Inflammation-Related Gene Expression in Subcutaneous Adipose Tissue in Sedentary Dysglycaemic, Overweight Men and Sedentary Normoglycaemic Men of Healthy Weight. Diabetologia 2019, 62, 1048–1064. [Google Scholar] [CrossRef]
- Bilet, L.; Phielix, E.; van de Weijer, T.; Gemmink, A.; Bosma, M.; Moonen-Kornips, E.; Jorgensen, J.A.; Schaart, G.; Zhang, D.; Meijer, K.; et al. One-Leg Inactivity Induces a Reduction in Mitochondrial Oxidative Capacity, Intramyocellular Lipid Accumulation and Reduced Insulin Signalling upon Lipid Infusion: A Human Study with Unilateral Limb Suspension. Diabetologia 2020, 63, 1211–1222. [Google Scholar] [CrossRef]
- Alghadir, A.H.; Gabr, S.A.; Anwer, S.; Al-Eisa, E. Fatigue and Oxidative Stress Response to Physical Activity in Type 2 Diabetic Patients. Int. J. Diabetes Dev. Ctries. 2016, 36, 59–64. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Rowiński, R.; Kornatowski, M.; Dąbrowski, A.; Kędziora-Kornatowska, K.; Strachecka, A. Relation of Moderate Physical Activity to Blood Markers of Oxidative Stress and Antioxidant Defense in the Elderly. Oxid. Med. Cell Longev. 2019, 2019, 5123628. [Google Scholar] [CrossRef]
- Powers, S.K.; Ji, L.L.; Leeuwenburgh, C. Exercise Training-Induced Alterations in Skeletal Muscle Antioxidant Capacity: A Brief Review. Med. Sci. Sports Exerc. 1999, 31, 987–997. [Google Scholar] [CrossRef]
- Leeuwenburgh, C.; Hansen, P.A.; Holloszy, J.O.; Heinecke, J.W. Hydroxyl Radical Generation during Exercise Increases Mitochondrial Protein Oxidation and Levels of Urinary Dityrosine. Free Radic. Biol. Med. 1999, 27, 186–192. [Google Scholar] [CrossRef]
- Bejma, J.; Ji, L.L. Aging and Acute Exercise Enhance Free Radical Generation in Rat Skeletal Muscle. J. Appl. Physiol. 1999, 87, 465–470. [Google Scholar] [CrossRef]
- Leeuwenburgh, C.; Fiebig, R.; Chandwaney, R.; Ji, L.L. Aging and Exercise Training in Skeletal Muscle: Responses of Glutathione and Antioxidant Enzyme Systems. Am. J. Physiol. 1994, 267, R439–R445. [Google Scholar] [CrossRef]
- Leeuwenburgh, C.; Hollander, J.; Leichtweis, S.; Griffiths, M.; Gore, M.; Ji, L.L. Adaptations of Glutathione Antioxidant System to Endurance Training Are Tissue and Muscle Fiber Specific. Am. J. Physiol. 1997, 272, R363–R369. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Criswell, D.; Lawler, J.; Martin, D.; Lieu, F.K.; Ji, L.L.; Herb, R.A. Rigorous Exercise Training Increases Superoxide Dismutase Activity in Ventricular Myocardium. Am. J. Physiol. 1993, 265, H2094–H2098. [Google Scholar] [CrossRef]
- Reidy, P.T.; Mahmassani, Z.S.; McKenzie, A.I.; Petrocelli, J.J.; Summers, S.A.; Drummond, M.J. Influence of Exercise Training on Skeletal Muscle Insulin Resistance in Aging: Spotlight on Muscle Ceramides. Int. J. Mol. Sci. 2020, 21, 1514. [Google Scholar] [CrossRef]
- Bergouignan, A.; Trudel, G.; Simon, C.; Chopard, A.; Schoeller, D.A.; Momken, I.; Votruba, S.B.; Desage, M.; Burdge, G.C.; Gauquelin-Koch, G.; et al. Physical Inactivity Differentially Alters Dietary Oleate and Palmitate Trafficking. Diabetes 2009, 58, 367–376. [Google Scholar] [CrossRef]
- Kwon, O.S.; Tanner, R.E.; Barrows, K.M.; Runtsch, M.; Symons, J.D.; Jalili, T.; Bikman, B.T.; McClain, D.A.; O’Connell, R.M.; Drummond, M.J. MyD88 Regulates Physical Inactivity-Induced Skeletal Muscle Inflammation, Ceramide Biosynthesis Signaling, and Glucose Intolerance. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E11–E21. [Google Scholar] [CrossRef]
- Bergman, B.C.; Brozinick, J.T.; Strauss, A.; Bacon, S.; Kerege, A.; Bui, H.H.; Sanders, P.; Siddall, P.; Wei, T.; Thomas, M.K.; et al. Muscle Sphingolipids during Rest and Exercise: A C18:0 Signature for Insulin Resistance in Humans. Diabetologia 2016, 59, 785–798. [Google Scholar] [CrossRef]
- Reidy, P.T.; McKenzie, A.I.; Mahmassani, Z.; Morrow, V.R.; Yonemura, N.M.; Hopkins, P.N.; Marcus, R.L.; Rondina, M.T.; Lin, Y.K.; Drummond, M.J. Skeletal Muscle Ceramides and Relationship with Insulin Sensitivity after 2 Weeks of Simulated Sedentary Behaviour and Recovery in Healthy Older Adults. J. Physiol. 2018, 596, 5217–5236. [Google Scholar] [CrossRef]
- Distefano, G.; Standley, R.A.; Zhang, X.; Carnero, E.A.; Yi, F.; Cornnell, H.H.; Coen, P.M. Physical Activity Unveils the Relationship between Mitochondrial Energetics, Muscle Quality, and Physical Function in Older Adults. J. Cachexia Sarcopenia Muscle 2018, 9, 279–294. [Google Scholar] [CrossRef]
- Figueiredo, P.A.; Powers, S.K.; Ferreira, R.M.; Amado, F.; Appell, H.J.; Duarte, J.A. Impact of Lifelong Sedentary Behavior on Mitochondrial Function of Mice Skeletal Muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 927–939. [Google Scholar] [CrossRef]
- Heinonen, S.; Muniandy, M.; Buzkova, J.; Mardinoglu, A.; Rodríguez, A.; Frühbeck, G.; Hakkarainen, A.; Lundbom, J.; Lundbom, N.; Kaprio, J.; et al. Mitochondria-Related Transcriptional Signature Is Downregulated in Adipocytes in Obesity: A Study of Young Healthy MZ Twins. Diabetologia 2017, 60, 169–181. [Google Scholar] [CrossRef]
- Drew, B.G.; Hamidi, H.; Zhou, Z.; Villanueva, C.J.; Krum, S.A.; Calkin, A.C.; Parks, B.W.; Ribas, V.; Kalajian, N.Y.; Phun, J.; et al. Estrogen Receptor (ER)α-Regulated Lipocalin 2 Expression in Adipose Tissue Links Obesity with Breast Cancer Progression*. J. Biol. Chem. 2015, 290, 5566–5581. [Google Scholar] [CrossRef]
- Zhou, Z.; Moore, T.M.; Drew, B.G.; Ribas, V.; Wanagat, J.; Civelek, M.; Segawa, M.; Wolf, D.M.; Norheim, F.; Seldin, M.M.; et al. Estrogen Receptor α Controls Metabolism in White and Brown Adipocytes by Regulating Polg1 and Mitochondrial Remodeling. Sci. Transl. Med. 2020, 12, eaax8096. [Google Scholar] [CrossRef]
- De Paoli, M.; Zakharia, A.; Werstuck, G.H. The Role of Estrogen in Insulin Resistance: A Review of Clinical and Preclinical Data. Am. J. Pathol. 2021, 191, 1490–1498. [Google Scholar] [CrossRef]
- Pu, D.; Tan, R.; Yu, Q.; Wu, J. Metabolic Syndrome in Menopause and Associated Factors: A Meta-Analysis. Climacteric 2017, 20, 583–591. [Google Scholar] [CrossRef]
- Christakis, M.K.; Hasan, H.; De Souza, L.R.; Shirreff, L. The Effect of Menopause on Metabolic Syndrome: Cross-Sectional Results from the Canadian Longitudinal Study on Aging. Menopause 2020, 27, 999. [Google Scholar] [CrossRef]
- Zarotsky, V.; Huang, M.-Y.; Carman, W.; Morgentaler, A.; Singhal, P.K.; Coffin, D.; Jones, T.H. Systematic Literature Review of the Risk Factors, Comorbidities, and Consequences of Hypogonadism in Men. Andrology 2014, 2, 819–834. [Google Scholar] [CrossRef]
- Caliber, M.; Saad, F. Testosterone Therapy for Prevention and Reversal of Type 2 Diabetes in Men with Low Testosterone. Curr. Opin. Pharmacol. 2021, 58, 83–89. [Google Scholar] [CrossRef]
- Han, S.; Jeon, Y.-J.; Lee, T.Y.; Park, G.-M.; Park, S.; Kim, S.C. Testosterone Is Associated with Abdominal Body Composition Derived from Computed Tomography: A Large Cross Sectional Study. Sci. Rep. 2022, 12, 22528. [Google Scholar] [CrossRef]
- Regensteiner, J.G.; Bauer, T.A.; Huebschmann, A.G.; Herlache, L.; Weinberger, H.D.; Wolfel, E.E.; Reusch, J.E.B. Sex Differences in the Effects of Type 2 Diabetes on Exercise Performance. Med. Sci. Sports Exerc. 2015, 47, 58–65. [Google Scholar] [CrossRef]
- Rehman, D. Sex Differences in Exercise Response in Type 2 Diabetes; Norwegian University of Science and Technology: Trondheim, Norway, 2019. [Google Scholar]
- Niemann, M.J.; Tucker, L.A.; Bailey, B.W.; Davidson, L.E. Strength Training and Insulin Resistance: The Mediating Role of Body Composition. J. Diabetes Res. 2020, 2020, 7694825. [Google Scholar] [CrossRef]
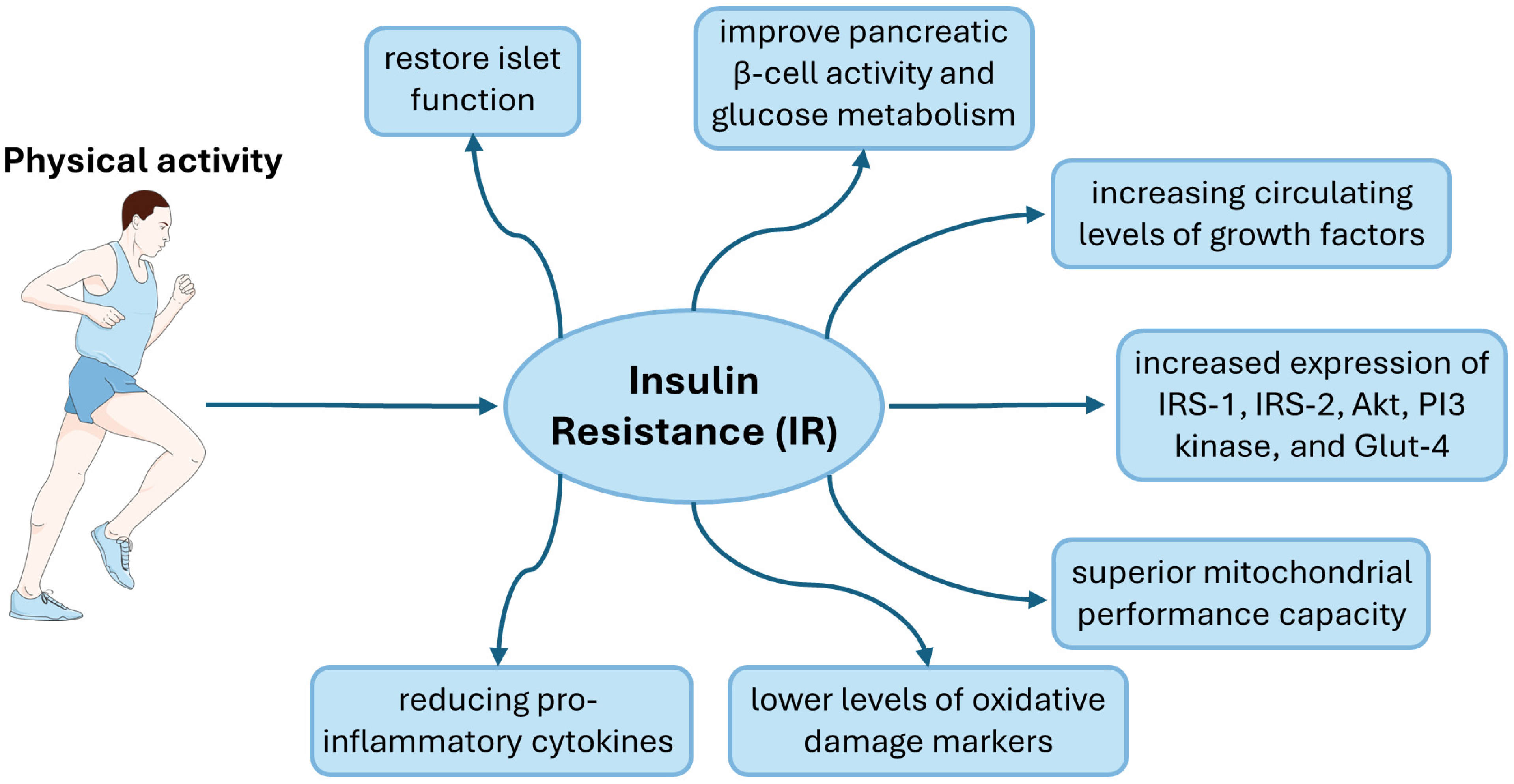
| Mechanism | Research Group | Physical Activity | Impact of Physical Activity | References |
|---|---|---|---|---|
| Islet cells | 12 type 2 diabetic male patients | 12-week programme on ergometer cycle (5 sessions/week) | preserve and even restore islet function, thereby enhancing peripheral insulin sensitivity | [111] |
| 40 type 2 diabetic patients | accumulated million steps for 1 year | even short-term exercise interventions improve pancreatic β-cell activity and glucose metabolism | [113] | |
| Genes and proteins | female Wistar rats | two 3 h exercise bouts, separated by one 45 min rest period | increased expression of IRS-1, IRS-2, Akt, PI3 kinase, and Glut-4 | [121] |
| Inflammation | General data from review articles. A detailed summary was presented in a previous paper [124] | increased levels of anti-inflammatory cytokines and reduced pro-inflammatory ones | [124,127,128] | |
| 100 healthy volunteers (48 men, and 52 women) | lifestyle evaluation | decreased level of leptin | [131] | |
| Oxidative Stress | 75 type 2 diabetic patients | based on Global Physical Activity Questionnaire | lower levels of oxidative damage markers, such as MDA and higher TAC in plasma | [135] |
| 87 older males | engage in any physical activity every day for 12 months | higher activity of key antioxidant enzymes like SOD, CAT and GPx | [136] | |
| 344 male rats | 10 weeks of exercise training | endurance training leads to elevated levels of antioxidants and antioxidant enzymes in skeletal and cardiac muscles | [140,141,142] | |
| Lipid Accumulation and Lipotoxicity | 14 obese sedentary individuals, 15 type 2 diabetic patients and 15 endurance-trained athletes | ergometer cycle for 1.5 h | acute exercise reduces sphingolipid synthesis during recovery periods, leading to improved insulin sensitivity in trained individuals | [146] |
| Mitochondrial Dysfunction | 26 sedentary (<1 exercise session/week) men | 12-week intensive exercise intervention | increased number of mitochondria in adipose tissue | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Małkowska, P. Positive Effects of Physical Activity on Insulin Signaling. Curr. Issues Mol. Biol. 2024, 46, 5467-5487. https://doi.org/10.3390/cimb46060327
Małkowska P. Positive Effects of Physical Activity on Insulin Signaling. Current Issues in Molecular Biology. 2024; 46(6):5467-5487. https://doi.org/10.3390/cimb46060327
Chicago/Turabian StyleMałkowska, Paulina. 2024. "Positive Effects of Physical Activity on Insulin Signaling" Current Issues in Molecular Biology 46, no. 6: 5467-5487. https://doi.org/10.3390/cimb46060327
APA StyleMałkowska, P. (2024). Positive Effects of Physical Activity on Insulin Signaling. Current Issues in Molecular Biology, 46(6), 5467-5487. https://doi.org/10.3390/cimb46060327






