Exploring the Antibacterial Potential of Konjac Glucomannan in Periodontitis: Animal and In Vitro Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Carboxymethylcellulose (CMC) Suspension
2.2. Preparation of KGM
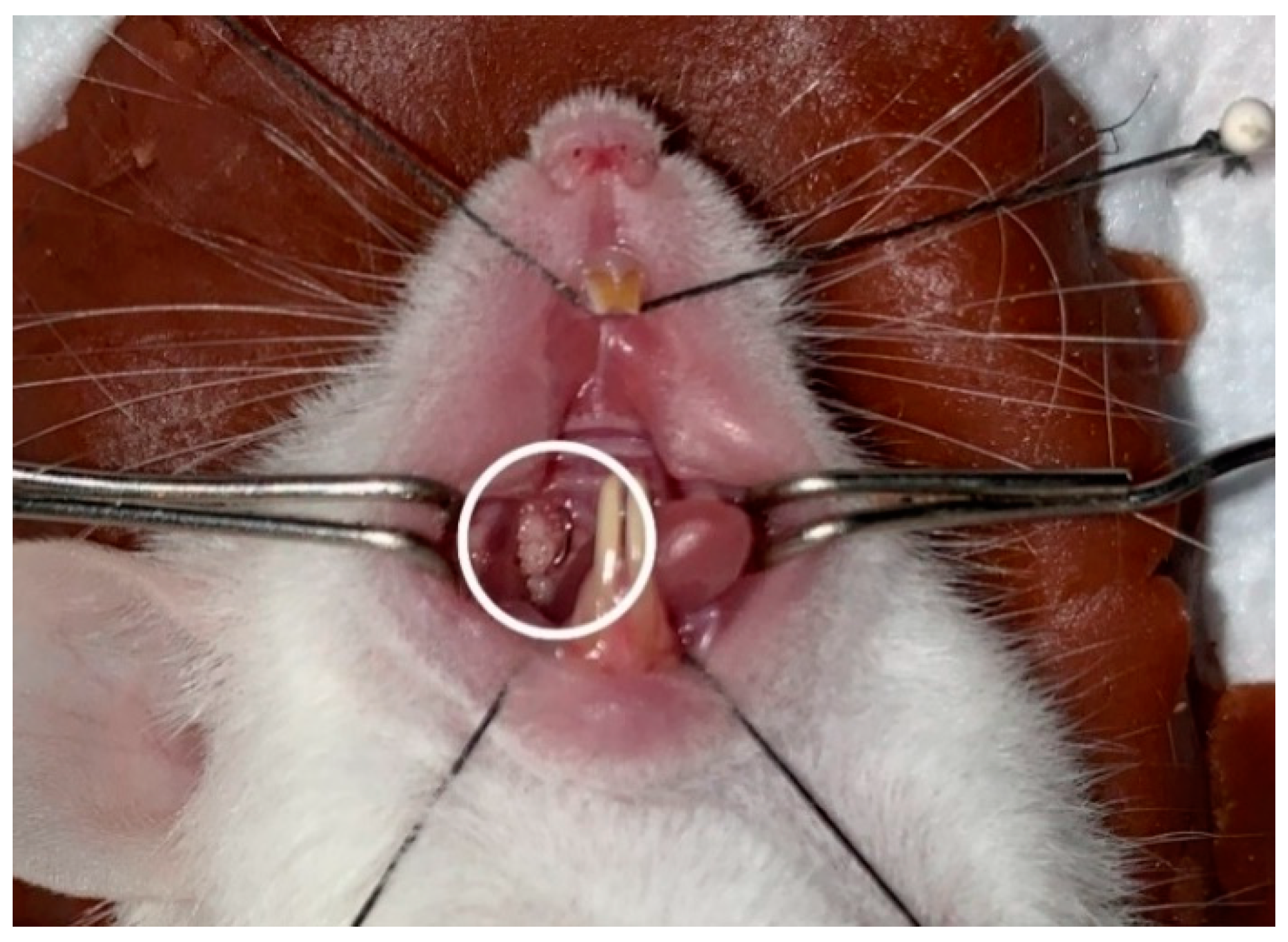
2.3. Preparation of Experimental Animals
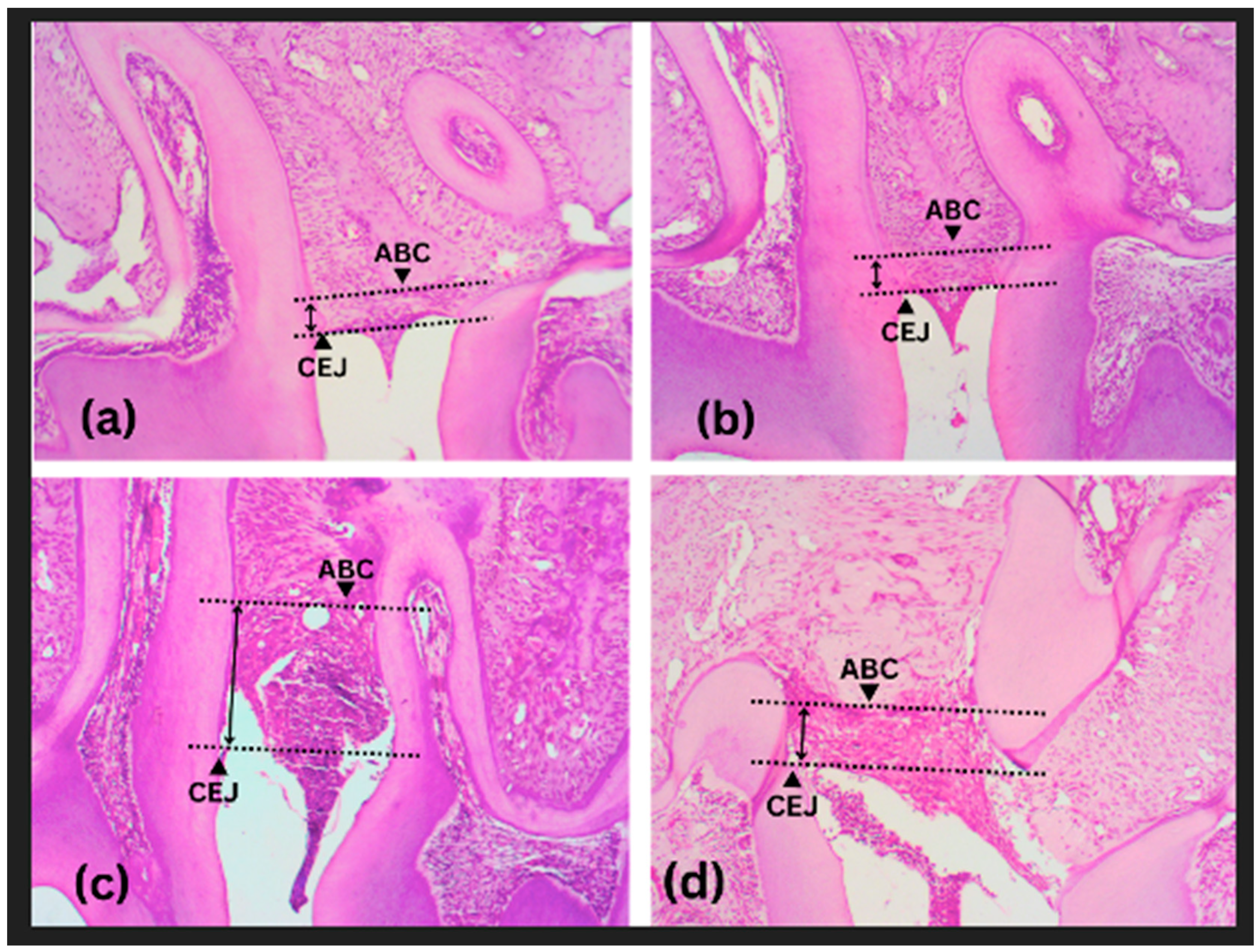
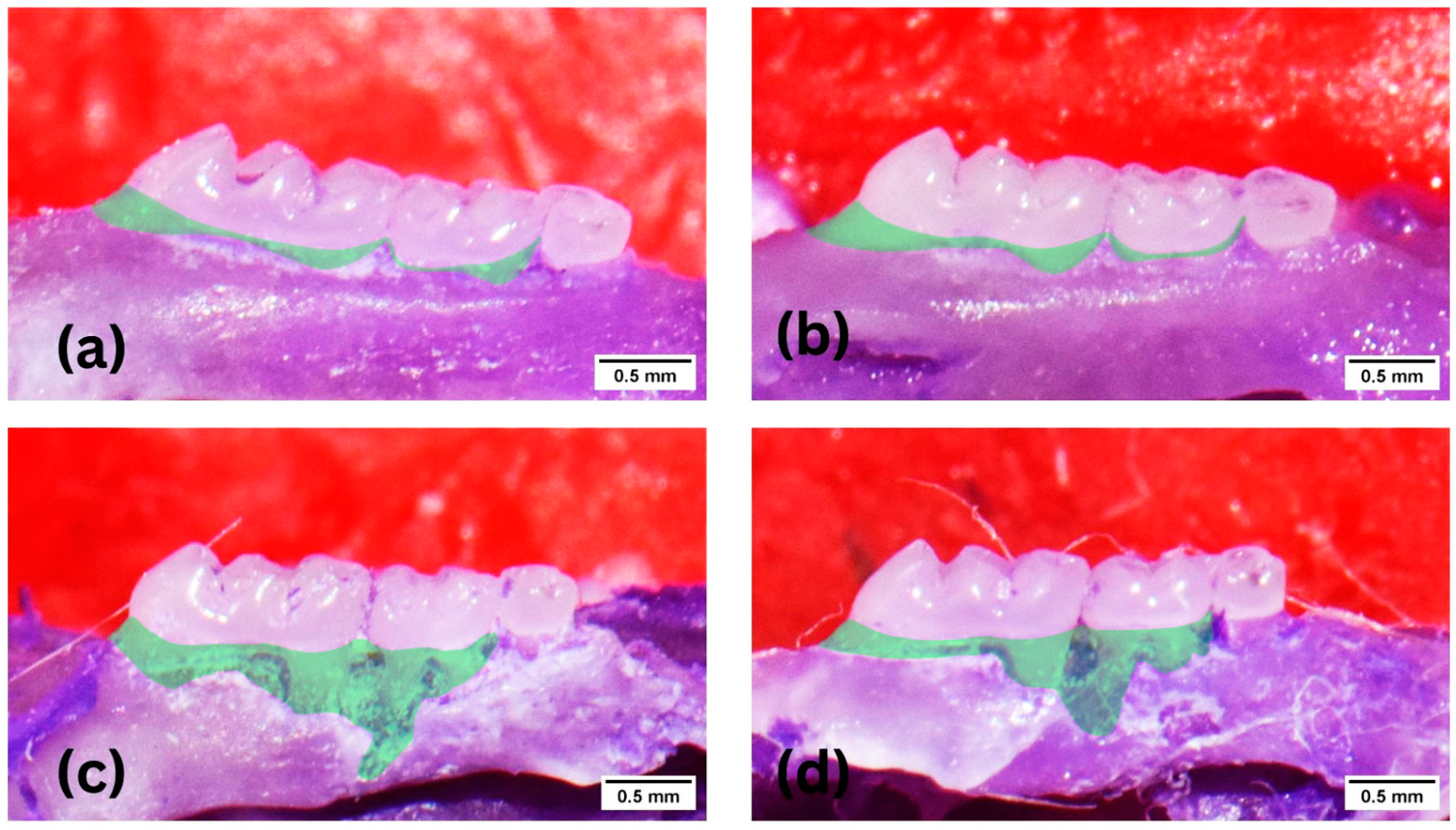
2.4. Histological and Morphometric Preparations
2.5. Antibacterial Activity of P. gingivalis in vitro
2.6. Statistical Analysis
3. Results
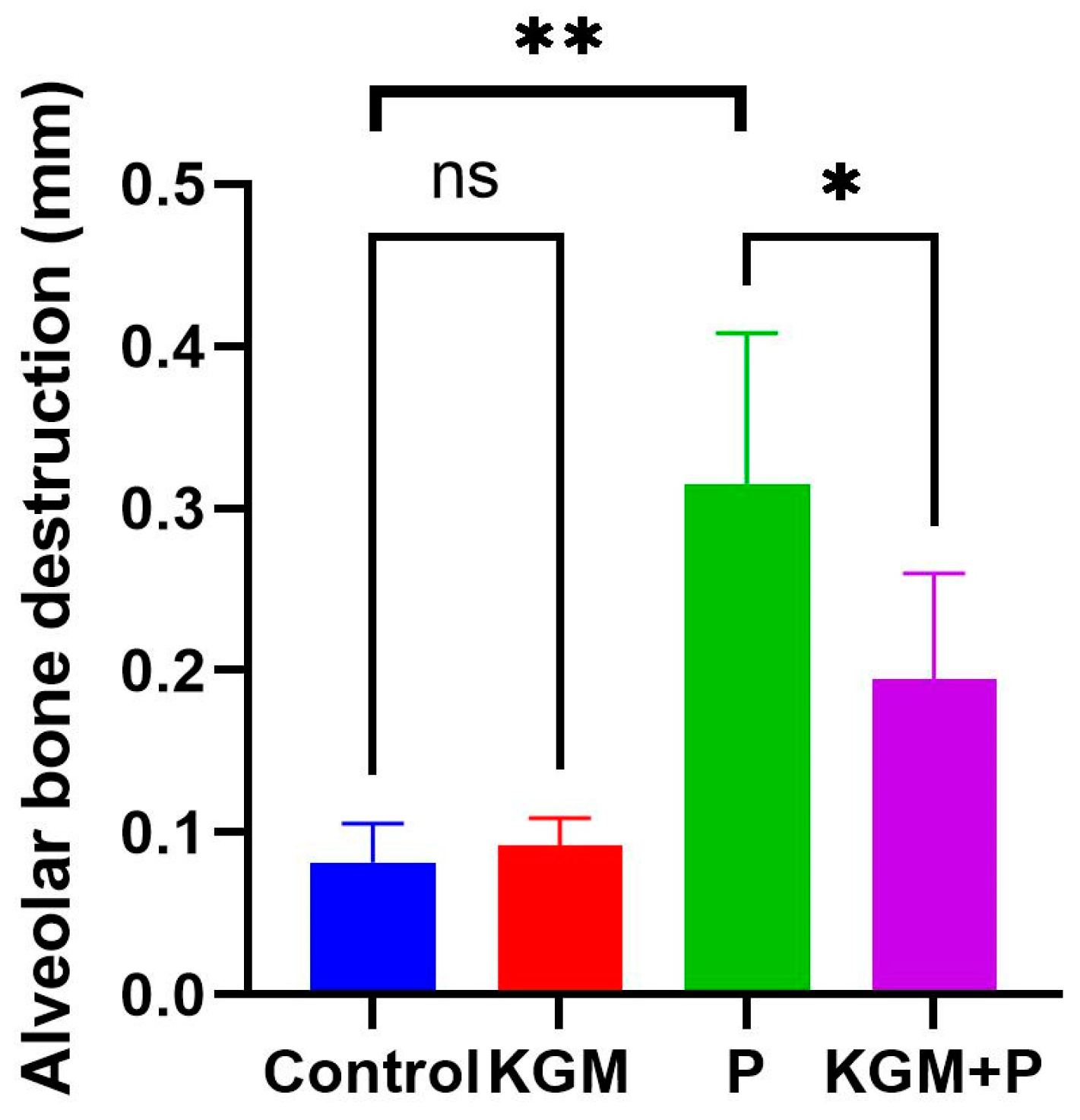
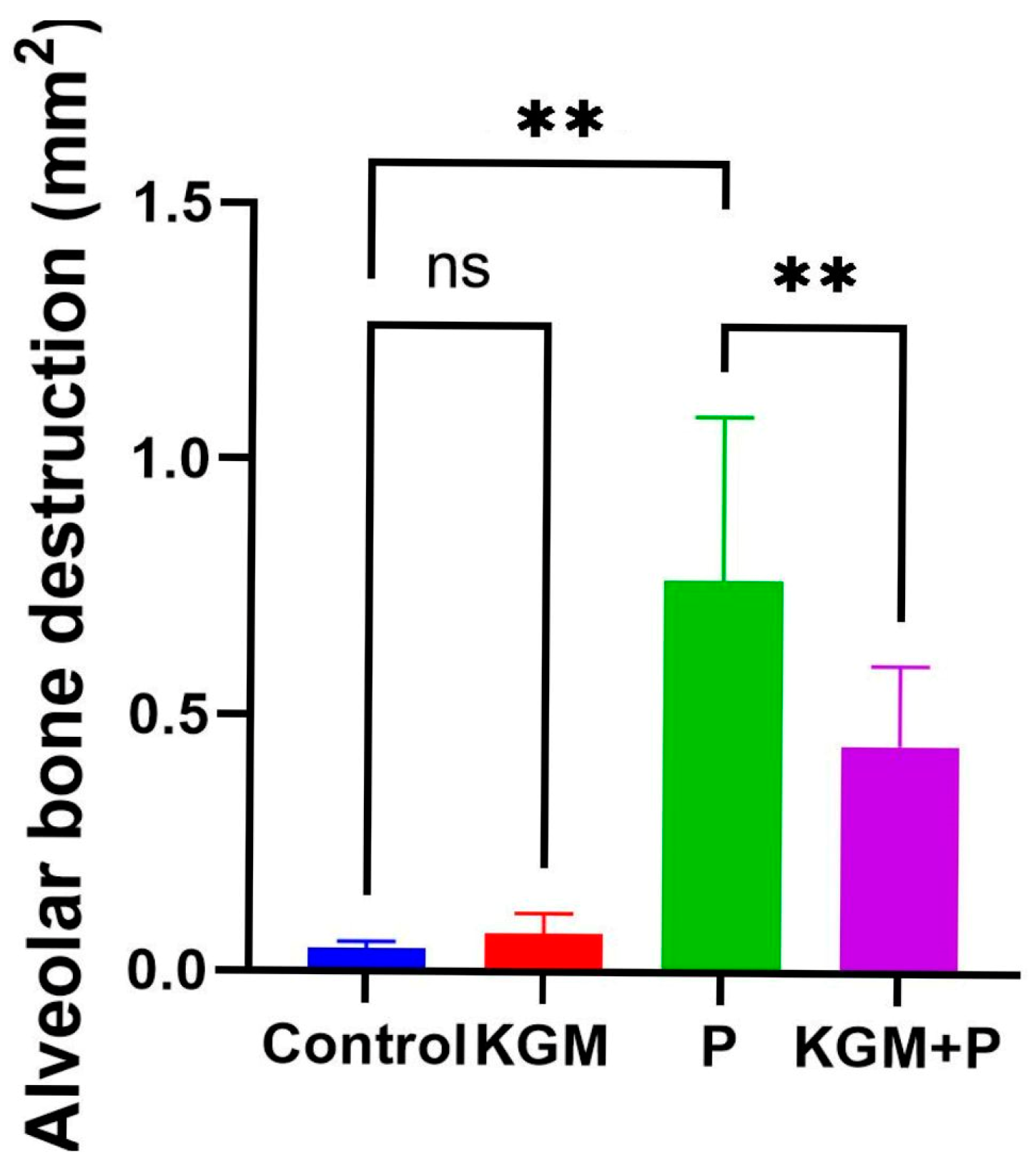
3.1. Effect of KGM on Alveolar Bone Destruction (Animal Study)
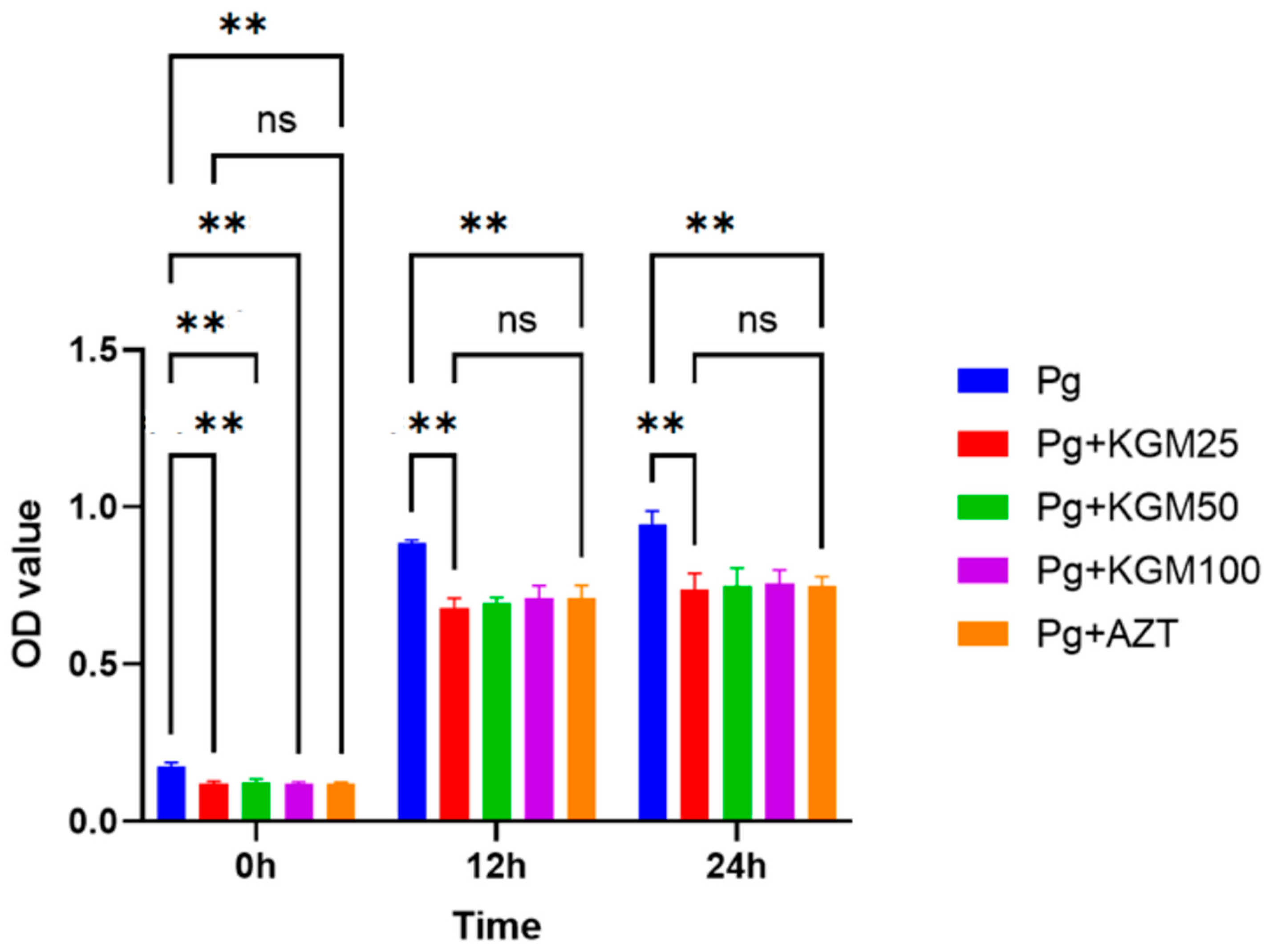
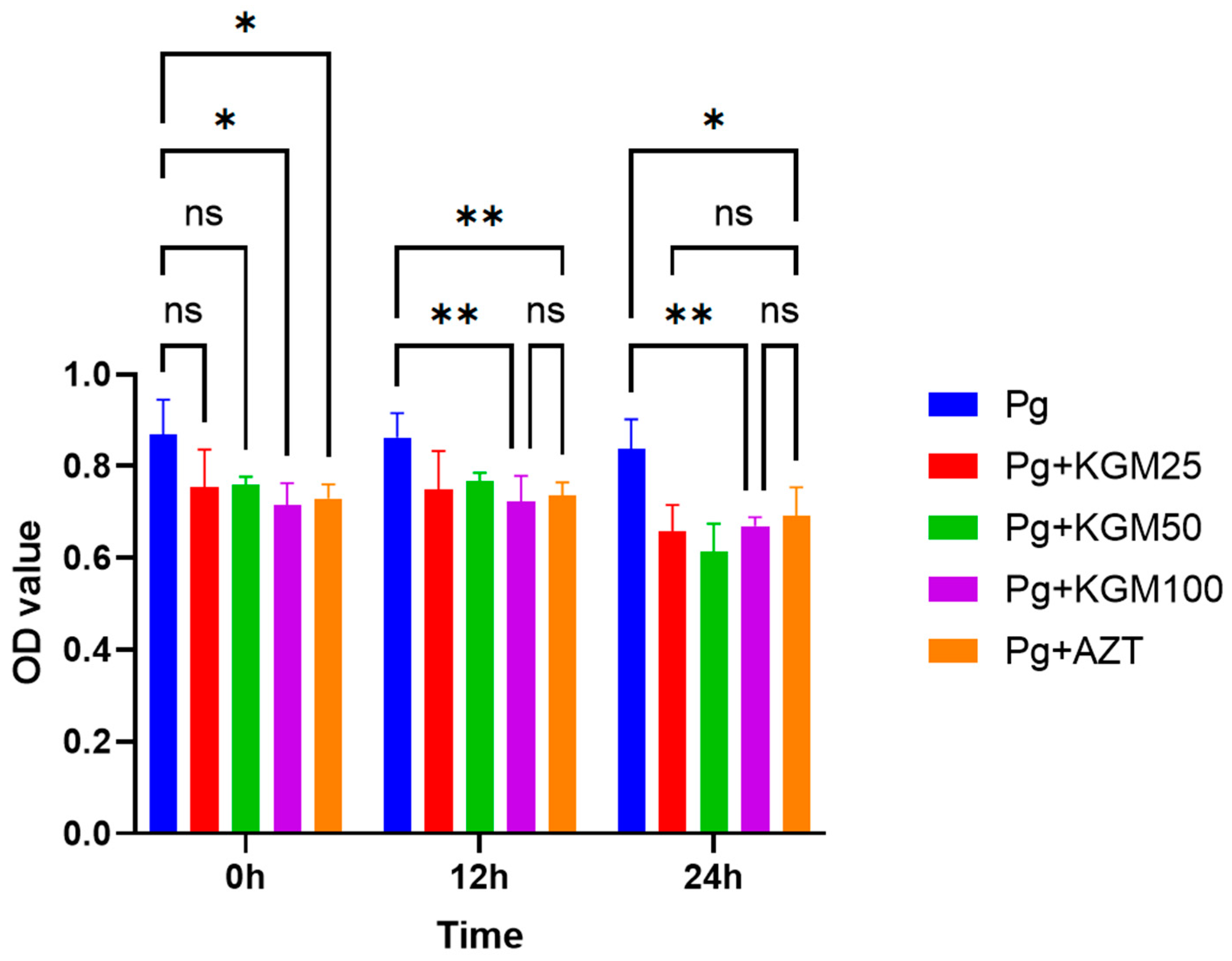
3.2. Antibacterial Activity of KGM (In Vitro)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saini, R.; Marawar, P.; Shete, S.; Saini, S. Periodontitis, a true infection. J. Glob. Infect. Dis. 2009, 1, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, S.; Hijazi, K. Modulatory Mechanisms of Pathogenicity in Porphyromonas gingivalis and Other Periodontal Pathobionts. Microorganisms 2022, 11, 15. [Google Scholar] [CrossRef]
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72–80. [Google Scholar]
- Chapple, I.L.C.; Matthews, J.B. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol. 2000 2007, 43, 160–232. [Google Scholar] [CrossRef] [PubMed]
- Llambés, F. Relationship between diabetes and periodontal infection. World J. Diabetes. 2015, 6, 927. [Google Scholar] [CrossRef]
- Bascones Martínez, A.; Figuero Ruiz, E. Periodontal diseases as bacterial infection. Av. En Periodoncia Implantol. Oral 2005, 17, 111–118. [Google Scholar] [CrossRef]
- Olsen, I.; Lambris, J.D.; Hajishengallis, G. Porphyromonas gingivalis disturbs host–commensal homeostasis by changing complement function. J. Oral Microbiol. 2017, 9, 1340085. [Google Scholar] [CrossRef]
- Mulhall, H.; Huck, O.; Amar, S. Porphyromonas gingivalis, a Long-Range Pathogen: Systemic Impact and Therapeutic Implications. Microorganisms 2020, 8, 869. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef]
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyromonas gingivalis: An overview of periodontopathic pathogen below the gum line. Front. Microbiol. 2016, 7, 53. [Google Scholar] [CrossRef]
- Cekici, A.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol. 2000 2014, 64, 57–80. [Google Scholar] [CrossRef]
- Bezerra, B.; Monajemzadeh, S.; Silva, D.; Pirih, F.Q. Modulating the Immune Response in Periodontitis. Front. Dent. Med. 2022, 3, 879131. [Google Scholar] [CrossRef]
- Jepsen, S.; Caton, J.G.; Albandar, J.M.; Bissada, N.F.; Bouchard, P.; Cortellini, P.; Demirel, K.; de Sanctis, M.; Ercoli, C.; Fan, J.; et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S219–S229. [Google Scholar] [CrossRef]
- Sulijaya, B.; Takahashi, N.; Yamazaki, K. Host modulation therapy using anti-inflammatory and antioxidant agents in periodontitis: A review to a clinical translation. Arch. Oral Biol. 2019, 105, 72–80. [Google Scholar] [CrossRef]
- Mann, J.; Bernstein, Y.; Findler, M. Periodontal disease and its prevention, by traditional and new avenues (Review). Exp. Ther. Med. 2019, 19, 1504–1506. [Google Scholar] [CrossRef] [PubMed]
- Dental Scaling and Root Planing for Periodontal Health: A Review of the Clinical Effectiveness, Cost-Effectiveness, and Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2016. [PubMed]
- Allaker, R.P.; Stephen, A.S. Use of Probiotics and Oral Health. Curr. Oral Health Rep. 2017, 4, 309–318. [Google Scholar] [CrossRef]
- Brookes, Z.L.S.; Bescos, R.; Belfield, L.A.; Ali, K.; Roberts, A. Current uses of chlorhexidine for management of oral disease: A narrative review. J. Dent. 2020, 103, 103497. [Google Scholar] [CrossRef] [PubMed]
- Slots, J.; Rams, T.E. Antibiotics in periodontal therapy: Advantages and disadvantages. J. Clin. Periodontol. 1990, 17, 479–493. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C. Konjac glucomannan, a promising polysaccharide of Amorphophallus konjac K. Koch in health care. Int. J. Biol. Macromol. 2016, 92, 942–956. [Google Scholar] [CrossRef]
- Gamboa-Gómez, C.I.; Guerrero-Romero, F.; Sánchez-Meraz, M.A.; Simental-Mendía, L.E. Hypoglycemic and antioxidant properties of konjac (Amorphophallus konjac) in vitro and in vivo. J. Food Biochem. 2020, 44, e13503. [Google Scholar] [CrossRef]
- Devaraj, R.D.; Reddy, C.K.; Xu, B. Health-promoting effects of konjac glucomannan and its practical applications: A critical review. Int. J. Biol. Macromol. 2019, 126, 273–281. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Q.; Fang, F.; Liu, J.; Wang, Z.; Chen, H.; Zhang, F. The influence of konjac glucomannan on the physicochemical and rheological properties and microstructure of canna starch. Foods 2021, 10, 422. [Google Scholar] [CrossRef]
- Zhao, Y.; Jayachandran, M.; Xu, B. In vivo antioxidant and anti-inflammatory effects of soluble dietary fiber Konjac glucomannan in type-2 diabetic rats. Int. J. Biol. Macromol. 2020, 159, 1186–1196. [Google Scholar] [CrossRef]
- Zhai, X.; Lin, D.; Zhao, Y.; Li, W.; Yang, X. Enhanced anti-obesity effects of bacterial cellulose combined with konjac glucomannan in high-fat diet-fed C57BL/6J mice. Food Funct. 2018, 9, 5260–5272. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Fan, Y.H.; Chen, M.E.; Chan, Y. Unhydrolyzed and hydrolyzed konjac glucomannans modulated cecal and fecal microflora in Balb/c mice. Nutrition 2005, 21, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Sun, F.; Zhou, H.F.; Yang, J.; Huang, C.; Fan, H. A systematic review exploring the anticancer activity and mechanisms of glucomannan. Front. Pharmacol. 2019, 10, 930. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Yang, L.; Ye, X.; Li, B. Antibacterial activity of konjac glucomannan/chitosan blend films and their irradiation-modified counterparts. Carbohydr. Polym. 2013, 92, 1302–1307. [Google Scholar] [CrossRef]
- Avrill, A.; Lennart, B. Effect of short-term ingestion of konjac glucomannan on serum cholesterol in healthy men. Am. J. Clin. Nutr. 1995, 61, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Felix da Silva, D.; Barbosa de Souza Ferreira, S.; Bruschi, M.L.; Britten, M.; Matumoto-Pintro, P.T. Effect of commercial konjac glucomannan and konjac flours on textural, rheological and microstructural properties of low fat processed cheese. Food Hydrocoll. 2016, 60, 308–316. [Google Scholar] [CrossRef]
- Kaats, G.R.; Bagchi, D.; Preuss, H.G. Konjac Glucomannan Dietary Supplementation Causes Significant Fat Loss in Compliant Overweight Adults. J. Am. Coll. Nutr. 2015, 22, 1–7. [Google Scholar] [CrossRef]
- Wei, Y.; Guo, Y.; Li, R.; Ma, A.; Zhang, H. Rheological characterization of polysaccharide thickeners oriented for dysphagia management: Carboxymethylated curdlan, konjac glucomannan and their mixtures compared to xanthan gum. Food Hydrocoll. 2021, 110, 106198. [Google Scholar] [CrossRef]
- Yang, D.; Yuan, Y.; Wang, L.; Wang, X.; Mu, R.; Pang, J.; Xiao, J.; Zheng, Y. A Review on Konjac Glucomannan Gels: Microstructure and Application. Int. J. Mol. Sci. 2017, 18, 2250. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zheng, S.; Sun, H.; Xi, R.; Sun, Y.; Luo, D.; Xu, W.; Jin, W.; Shah, B.R. Structural characterization and antibacterial properties of konjac glucomannan/soluble green tea powder blend films for food packaging. J. Food Sci. Technol. 2021, 59, 562–571. [Google Scholar] [CrossRef]
- Al-Ghazzewi, F.H.; Tester, R.F.; Alvani, K. The synbiotic effects of konjac glucomannan hydrolysates (GMH) and lactobacilli on the growth of Staphylococcus aureus and Salmonella typhimurium. Nutr. Food Sci. 2012, 42, 97–101. [Google Scholar] [CrossRef]
- Tester, R.F.; Al-Ghazzewi, F.H. A preliminary study of the synbiotic effects of konjac glucomannan hydrolysates (GMH) and lactobacilli on the growth of the oral bacterium Streptococcus mutans. Nutr. Food Sci. 2011, 41, 234–237. [Google Scholar] [CrossRef]
- Santonocito, S.; Giudice, A.; Polizzi, A.; Troiano, G.; Merlo, E.M.; Sclafani, R.; Grosso, G.; Isola, G. A Cross-Talk between Diet and the Oral Microbiome: Balance of Nutrition on Inflammation and Immune System’s Response during Periodontitis. Nutrients 2022, 14, 2426. [Google Scholar] [CrossRef] [PubMed]
- Hohlbaum, K.; Bert, B.; Dietze, S.; Palme, R.; Fink, H.; Thöne-Reineke, C. Impact of repeated anesthesia with ketamine and xylazine on the well-being of C57BL/6JRj mice. PLoS ONE 2018, 13, e0203559. [Google Scholar] [CrossRef] [PubMed]
- Sulijaya, B.; Yamada-Hara, M.; Yokoji-Takeuchi, M.; Matsuda-Matsukawa, Y.; Yamazaki, K.; Matsugishi, A.; Tsuzuno, T.; Sato, K.; Aoki-Nonaka, Y.; Takahashi, N.; et al. Antimicrobial function of the polyunsaturated fatty acid KetoC in an experimental model of periodontitis. J. Periodontol. 2019, 90, 1470–1480. [Google Scholar] [CrossRef]
- Li, D.; Feng, Y.; Tang, H.; Huang, L.; Tong, Z.; Hu, C.; Chen, X.; Tan, J. A Simplified and Effective Method for Generation of Experimental Murine Periodontitis Model. Front. Bioeng. Biotechnol. 2020, 8, 444. [Google Scholar] [CrossRef]
- Wiedmeyer, C.E.; Ruben, D.; Franklin, C. Complete blood count, clinical chemistry, and serology profile by using a single tube of whole blood from mice. J. Am. Assoc. Lab. Anim. Sci. 2007, 46, 59–64. [Google Scholar]
- Abe, T.; Hajishengallis, G. Optimization of the ligature-induced periodontitis model in mice. J. Immunol. Methods 2013, 394, 49–54. [Google Scholar] [CrossRef]
- Chun, M.J.; Shim, E.; Kho, E.H.; Park, K.J.; Jung, J.; Kim, J.M.; Kim, B.; Lee, K.-H.; Cho, D.-L.; Bai, D.-H.; et al. Surface modification of orthodontic wires with photocatalytic titanium oxide for its antiadherent and antibacterial properties. Angle Orthod. 2007, 77, 483–488. [Google Scholar] [CrossRef]
- Nie, H.; Shen, X.; Zhou, Z.; Jiang, Q.; Chen, Y.; Xie, A.; Wang, Y.; Han, C.C. Electrospinning and characterization of konjac glucomannan/chitosan nanofibrous scaffolds favoring the growth of bone mesenchymal stem cells. Carbohydr. Polym. 2011, 85, 681–686. [Google Scholar] [CrossRef]
- Putri, D.G.A.D. Pengaruh Ekstrak Lidah Buaya (Aloe chinensis Baker) Terhadap Ketebalan Tulang Alveolar pada Rattus norvegicus Strain Wistar Jantan Yang Diinduksi Lipopolisakarida; Universitas Brawijaya: Malang, Indonesia, 2018. [Google Scholar]
- Susanto, C.; Lokanata, S.; Ningrum, J.W. The Effect of Hydrogel Aloe vera (Aloe vera (L.) Burm) on the Number of Neutrophil Cells in Aggressive Periodontitis Induced by Aggregatibacter actinomycetemcomitans (In Vivo Study on Wistar Rats). Biosci. Med. J. Biomed. Transl. Res. 2021, 5, 657–663. [Google Scholar] [CrossRef]
- Ipshita, S.; Kurian, I.G.; Dileep, P.; Kumar, S.; Singh, P.; Pradeep, A.R. One percent alendronate and aloe vera gel local host modulating agents in chronic periodontitis patients with class II furcation defects: A randomized, controlled clinical trial. J. Investig. Clin. Dent. 2018, 9, e12334. [Google Scholar] [CrossRef]
- Song, L.; Xie, W.; Zhao, Y.; Lv, X.; Yang, H.; Zeng, Q.; Zheng, Z.; Yang, X. Synthesis, Antimicrobial, Moisture Absorption and Retention Activities of Kojic Acid-Grafted Konjac Glucomannan Oligosaccharides. Polymers 2019, 11, 1979. [Google Scholar] [CrossRef]
- Xu, W.; Jia, Y.; Wei, J.; Ning, Y.; Sun, H.; Jiang, L.; Chai, L.; Luo, D.; Cao, S.; Shah, B.R. Characterization and antibacterial behavior of an edible konjac glucomannan/soluble black tea powder hybrid film with ultraviolet absorption. RSC Adv. 2022, 12, 32061–32069. [Google Scholar] [CrossRef]
- Gerits, E.; Verstraeten, N.; Michiels, J. New approaches to combat Porphyromonas gingivalis biofilms. J. Oral Microbiol. 2017, 9, 1300366. [Google Scholar] [CrossRef]
- Tang, J.; Liu, J.; Yan, Q.; Gu, Z.; August, A.; Huang, W.; Jiang, Z. Konjac Glucomannan Oligosaccharides Prevent Intestinal Inflammation Through SIGNR1-Mediated Regulation of Alternatively Activated Macrophages. Mol. Nutr. Food Res. 2021, 65, 2001010. [Google Scholar] [CrossRef]
- Deandra, F.A.; Ketherin Rachmasari, R.; Sulijaya, B.; Takahashi, N. Probiotics and metabolites regulate the oral and gut microbiome composition as host modulation agents in periodontitis: A narrative review. Heliyon 2023, 9, e13475. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lestari, K.D.; Dwiputri, E.; Kurniawan Tan, G.H.; Sulijaya, B.; Soeroso, Y.; Natalina, N.; Harsas, N.A.; Takahashi, N. Exploring the Antibacterial Potential of Konjac Glucomannan in Periodontitis: Animal and In Vitro Studies. Medicina 2023, 59, 1778. https://doi.org/10.3390/medicina59101778
Lestari KD, Dwiputri E, Kurniawan Tan GH, Sulijaya B, Soeroso Y, Natalina N, Harsas NA, Takahashi N. Exploring the Antibacterial Potential of Konjac Glucomannan in Periodontitis: Animal and In Vitro Studies. Medicina. 2023; 59(10):1778. https://doi.org/10.3390/medicina59101778
Chicago/Turabian StyleLestari, Kartika Dhipta, Edlyn Dwiputri, Geraldi Hartono Kurniawan Tan, Benso Sulijaya, Yuniarti Soeroso, Natalina Natalina, Nadhia Anindhita Harsas, and Naoki Takahashi. 2023. "Exploring the Antibacterial Potential of Konjac Glucomannan in Periodontitis: Animal and In Vitro Studies" Medicina 59, no. 10: 1778. https://doi.org/10.3390/medicina59101778
APA StyleLestari, K. D., Dwiputri, E., Kurniawan Tan, G. H., Sulijaya, B., Soeroso, Y., Natalina, N., Harsas, N. A., & Takahashi, N. (2023). Exploring the Antibacterial Potential of Konjac Glucomannan in Periodontitis: Animal and In Vitro Studies. Medicina, 59(10), 1778. https://doi.org/10.3390/medicina59101778





