Pre- versus Post-Menopausal Onset of Overactive Bladder and the Response to Vaginal Estrogen Therapy: A Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
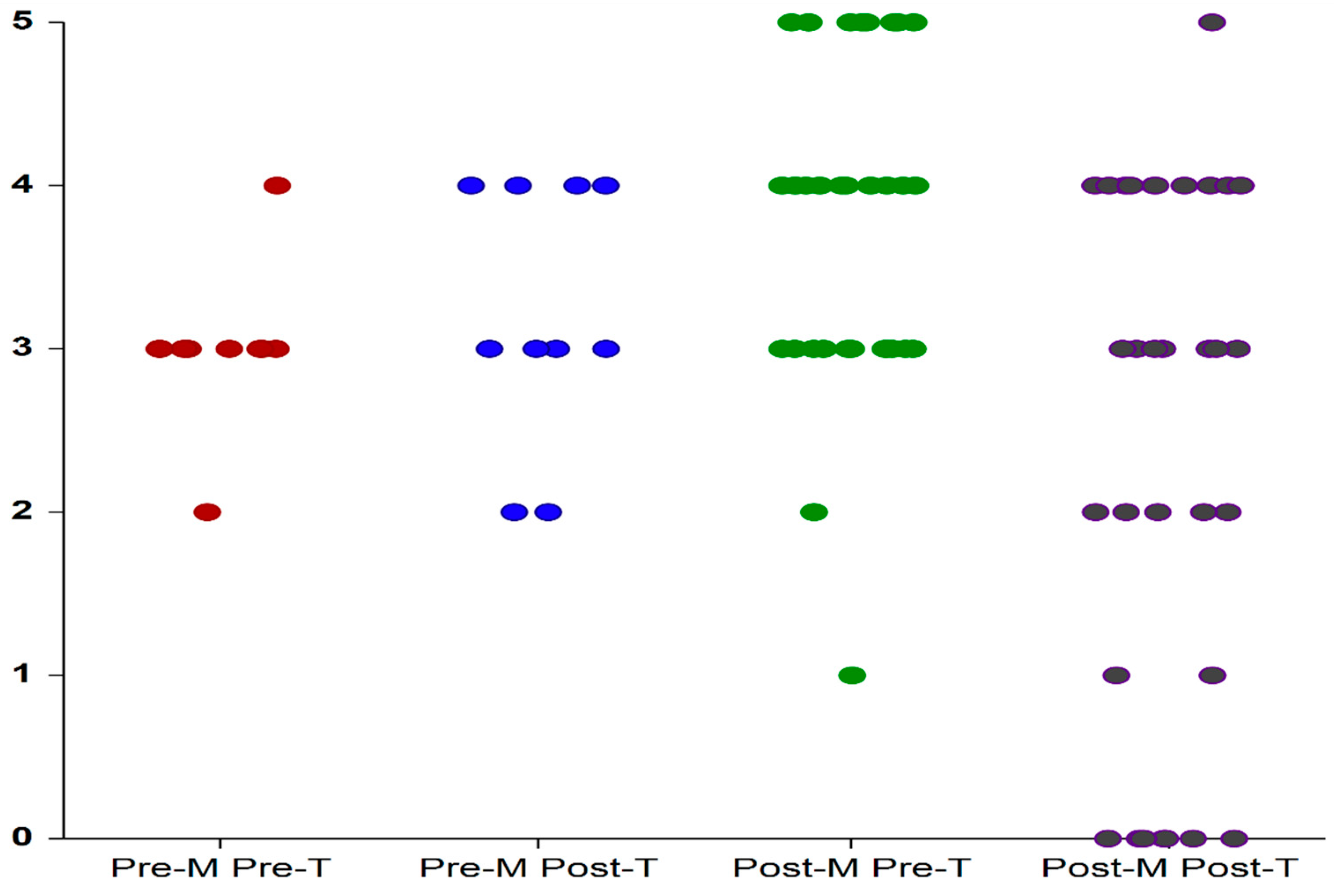
3.1. VHI Scores
3.2. GPFSBQ
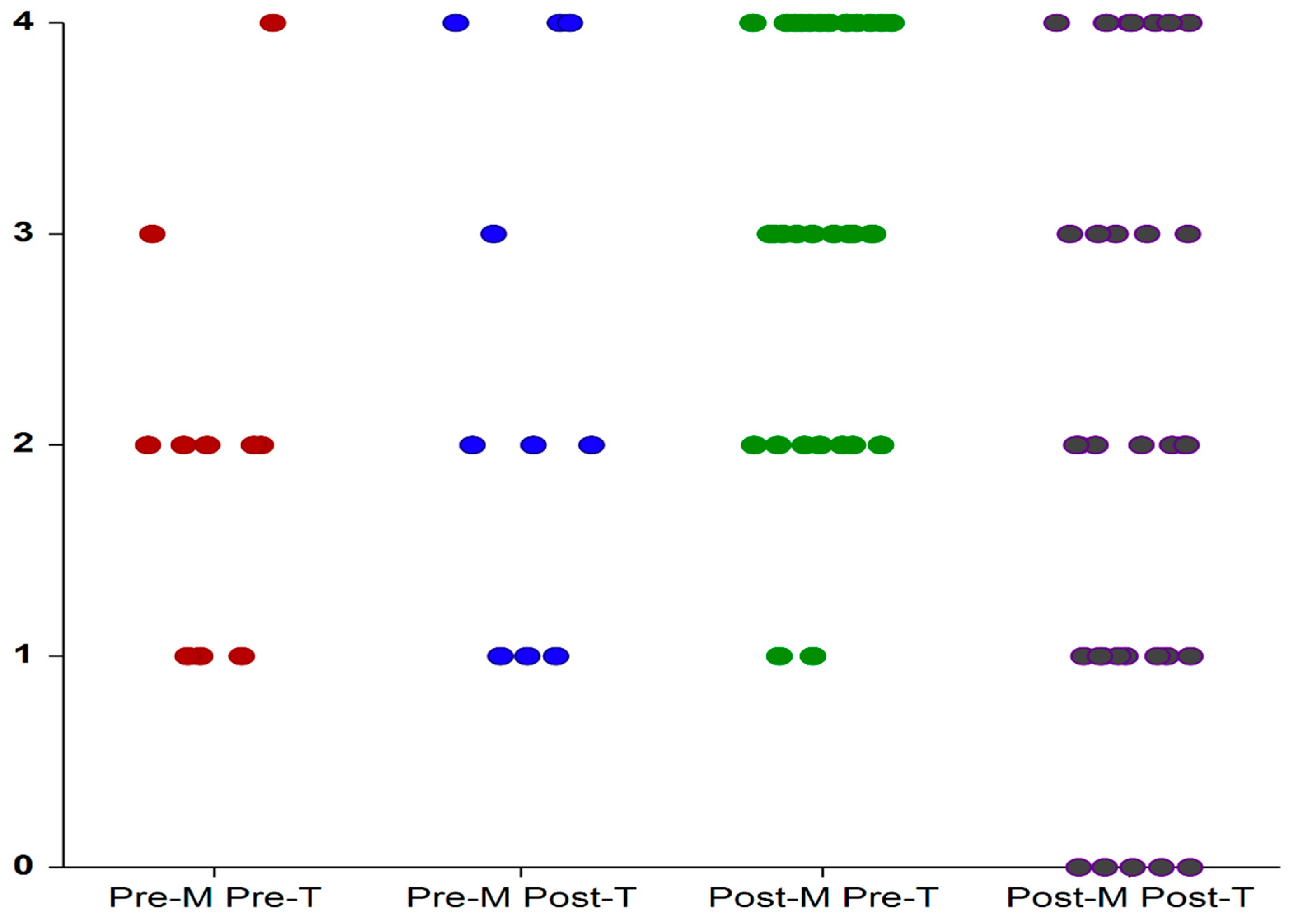
3.3. PPIUS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robinson, D.; Cardozo, L. Estrogens and the lower urinary tract. Neurourol. Urodyn. 2011, 30, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Portman, D.J.; Gass, M.L. Genitourinary syndrome of menopause: New terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause 2014, 21, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Nik-Ahd, F.; Lenore Ackerman, A.; Anger, J. Recurrent Urinary Tract Infections in Females and the Overlap with Overactive Bladder. Curr. Urol. Rep. 2018, 19, 94. [Google Scholar] [CrossRef] [PubMed]
- Banakhar, M.A.; Al-Shaiji, T.F.; Hassouna, M.M. Pathophysiology of overactive bladder. Int. Urogynecol. J. 2012, 23, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; van Kerrebroeck, P.; Victor, A.; Wein, A.J.; Standardisation Sub-Committee of the International Continence Society. The standardisation of terminology of lower urinary tractfunction: Report from the Standardisation Subcommittee of the International Continence Society. Neurourol. Urodyn. 2002, 21, 167–178. [Google Scholar] [CrossRef]
- Raju, R.; Linder, B.J. Evaluation and Treatment of Overactive Bladder in Women. Mayo Clin. Proc. 2020, 95, 370–377. [Google Scholar] [CrossRef]
- Burgio, K.L.; Locher, J.L.; Goode, P.S.; Hardin, J.M.; McDowell, B.J.; Dombrowski, M.; Candib, D. Behavioral vs. drug treatment for urge urinary incontinence in older women: A randomized controlled trial. JAMA 1998, 280, 1995–2000. [Google Scholar] [CrossRef] [Green Version]
- Gormley, E.A.; Lightner, D.J.; Burgio, K.L.; Chai, T.C.; Clemens, J.Q.; Culkin, D.J.; Das, A.K.; Foster, H.E.; Scarpero, H.M.; Tessier, C.D.; et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. J. Urol. 2012, 188, 2455–2463. [Google Scholar] [CrossRef]
- Khizer, Z.; Sadia, A.; Sharma, R.; Farhaj, S.; Nirwan, J.; Kakadia, P.; Hussain, T.; Yousaf, A.; Shahzad, Y.; Conway, B.; et al. Drug delivery approaches for managing overactive bladder (OAB): A Systematic Review. Pharmaceuticals 2021, 14, 409. [Google Scholar] [CrossRef]
- Robinson, D.; Cardozo, L.; Milsom, I.; Pons, M.E.; Kirby, M.; Koelbl, H.; Vierhout, M. Oestrogens and Overactive Bladder. Neurourol. Urodyn. 2014, 33, 1086–1091. [Google Scholar] [CrossRef]
- Cardozo, L.D.; Wise, B.G.; Benness, C.J. Vaginal oestradiol for the treatment of lower urinary tract symptoms in postmenopausal women -a double-blind placebo-controlled study. J. Obstet. Gynaecol. 2001, 21, 383–385. [Google Scholar]
- Weber, M.A.; Kleijn, M.H.; Langendam, M.; Limpens, J.; Heineman, M.J.; Roovers, J.P. Local Oestrogen for Pelvic Floor Disorders: A Systematic Review. PLoS ONE 2015, 10, e0136265. [Google Scholar] [CrossRef] [Green Version]
- Rahn, D.D.; Carberry, C.; Sanses, T.V.; Mamik, M.M.; Ward, R.M.; Meriwether, K.V.; Olivera, C.K.; Abed, H.; Balk, E.M.; Murphy, M.; et al. Vaginal estrogen for genitourinary syndrome of menopause: A systemic review. Obstet. Gyncol. 2014, 124, 1147–1156. [Google Scholar] [CrossRef] [Green Version]
- Cameron, A.P. Systematic review of lower urinary tract symptoms occurring with pelvic organ prolapse. Arab. J. Urol. 2019, 17, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Bachmann, G.A.; Notelovitz, M.; Kelly, S.J.; Thompson, C.; Owens, A. Long-term non-hormonal treatment of vaginal dryness. Clin. Pract. Sex. 1992, 8, 3–8. [Google Scholar]
- Notte, S.M.; Marshall, T.S.; Lee, M.; Hakimi, Z.; Odeyemi, I.; Chen, W.H.; Revicki, D.A. Content Validity and Test-Retest Reliability of Patient Perception of Intensity of Urgency Scale (PPIUS) for Overactive Bladder. BMC Urol. 2012, 12, 26. [Google Scholar] [CrossRef] [Green Version]
- Milsom, I.; Abrams, P.; Cardozo, L.; Roberts, R.G.; Thuroff, J.; Wein, A.J. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001, 87, 760–766. [Google Scholar] [CrossRef]
- Cartwright, R.; Panayi, D.; Cardozo, L.; Khullar, V. Reliability and normal ranges for the Patient’s Perception of Intensity of Urgency Scale in asymptomatic women. BJU Int. 2010, 105, 832–836. [Google Scholar] [CrossRef]
- Peterson, T.V.; Karp, D.R.; Aguilar, V.C.; Davila, G.W. Validation of a global pelvic floor symptom bother questionnaire. Int. Urogynecol. J. 2010, 21, 1129–1135. [Google Scholar] [CrossRef]
- Molina-Torres, G.; Amiano-López, L.; Córdoba-Peláez, M.M.; Ibáñez-Vera, A.J.; Diaz-Mohedo, E. Analysis of the Structural Characteristics and Psychometric Properties of the Pelvic Floor Bother Questionnaire (PFBQ): A Systematic Review. J. Clin. Med. 2022, 11, 7075. [Google Scholar] [CrossRef]
- Milsom, I.; Stewart, W.; Thuroff, J. The prevalence of overactive bladder. Am. J. Manag. Care. 2000, 6, S565–S573. [Google Scholar] [PubMed]
- Temml, C.; Heidler, S.; Ponholzer, A.; Madersbacher, S. Prevalence of the overactive bladder syndrome by applying the International Continence Society definition. Eur. Urol. 2005, 48, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Pratt, T.S.; Suskind, A.M. Management of Overactive Bladder in Older Women. Curr. Urol. Rep. 2018, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Hazra, A. Using the confidence interval confidently. J. Thorac. Dis. 2017, 9, 4125–4130. [Google Scholar] [CrossRef] [Green Version]
- Sexton, C.C.; Coyne, K.S.; Thompson, C.; Bavendam, T.; Chen, C.I.; Markland, A. Prevalence and effect on health-related quality of life of overactive bladder in older americans: Results from the epidemiology of lower urinary tract symptoms study. J. Am. Geriatr. Soc. 2011, 59, 1465–1470. [Google Scholar] [CrossRef]
- Stewart, W.F.; Van Rooyen, J.B.; Cundiff, G.W.; Abrams, P.; Herzog, A.R.; Corey, R.; Hunt, T.; Wein, A. Prevalence and burden of overactive bladder in the United States. World J. Urol. 2003, 20, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Dmochowski, R.R.; Newman, D.K. Impact of overactive bladder on women in the United States: Results of a national survey. Curr. Med. Res. Opin. 2007, 23, 65–76. [Google Scholar] [CrossRef]
- Iosif, C.S.; Batra, S.; Ek, A.; Åstedt, B. Estrogen receptors in the human female lower urinary tract. Am. J. Obstet. Gynecol. 1981, 141, 817–820. [Google Scholar] [CrossRef]
- Griebling, T.L.; Liao, Z.; Smith, P.G. Systemic and topical hormone therapies reduce vaginal innervations density in postmenopausal women. Menopause 2010, 19, 630–635. [Google Scholar] [CrossRef] [Green Version]
- Tincello, D.; Taylor, A.; Spurling, S.; Bell, S.C. Receptor isoforms that mediate estrogen and progesterone action in the female lower urinary tract. J. Urol. 2009, 181, 1474–1482. [Google Scholar] [CrossRef] [Green Version]
- Isali, I.; McClellan, P.; Wong, T.R.; Sun, C.; Stout, A.C.; Schumacher, F.R.; Markt, S.; Wu, C.-H.W.; Penney, K.L.; El-Nashar, S.; et al. A systematic review and in silico study of potential genetic markers implicated in cases of overactive bladder. Am. J. Obstet Gynecol. 2022, 4, S0002-9378(22)00616-0, Online ahead of print. [Google Scholar] [CrossRef]
- Lightner, D.J.; Gomelsky, A.; Souter, L.; Vasavada, S.P. Diagnosis and Treatment of Overactive Bladder (Non-Neurogenic) in Adults: AUA/SUFU Guideline Amendment 2019. J. Urol. 2019, 202, 558–563. [Google Scholar] [CrossRef] [Green Version]
| Pre-Menopausal | Post-Menopausal | p | |
|---|---|---|---|
| Age | 65.4 ± 8 | 62.45 ± 7.1 | 0.356 |
| VHI | |||
| Pre-treatment | 11.6 ± 2 | 11.6 ± 3.1 | 0.989 |
| Post-treatment | 17.8 ± 1.2 | 14.12 ± 3.5 | <0.001 |
| delta VHI | 6.2 ± 2.6 | 2.48 ± 3.5 | 0.009 |
| pwithin groups | 0.005 | 0.004 | |
| GPFSB-3 | |||
| Pre-treatment | 3.0 ± 0.47 | 3.79 ± 1 | 0.013 |
| Post-treatment | 3.2 ± 0.79 | 2.48 ± 1.6 | 0.341 |
| delta GPFSB-3 | 0.2 ± 0.79 | −1.3 ± 2 | 0.026 |
| pwithin groups | 0.414 | 0.002 | |
| PPIUS | |||
| Pre-treatment | 2.1 ± 0.87 | 3.06 ± 1.03 | 0.015 |
| Post-treatment | 2.3 ± 1.33 | 2.33 ± 1.47 | 0.944 |
| delta PPIUS | 0.2 ± 1.31 | −0.7 ± 1.77 | 0.184 |
| pwithin groups | 0.680 | 0.036 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baruch, Y.; Torella, M.; De Bastiani, S.; Meschia, M.; Candiani, M.; Colacurci, N.; Salvatore, S. Pre- versus Post-Menopausal Onset of Overactive Bladder and the Response to Vaginal Estrogen Therapy: A Prospective Study. Medicina 2023, 59, 245. https://doi.org/10.3390/medicina59020245
Baruch Y, Torella M, De Bastiani S, Meschia M, Candiani M, Colacurci N, Salvatore S. Pre- versus Post-Menopausal Onset of Overactive Bladder and the Response to Vaginal Estrogen Therapy: A Prospective Study. Medicina. 2023; 59(2):245. https://doi.org/10.3390/medicina59020245
Chicago/Turabian StyleBaruch, Yoav, Marco Torella, Sarah De Bastiani, Michele Meschia, Massimo Candiani, Nicola Colacurci, and Stefano Salvatore. 2023. "Pre- versus Post-Menopausal Onset of Overactive Bladder and the Response to Vaginal Estrogen Therapy: A Prospective Study" Medicina 59, no. 2: 245. https://doi.org/10.3390/medicina59020245





