A Novel Approach to Cardiac Magnetic Resonance Scar Characterization in Patients Affected by Cardiac Amyloidosis: A Pilot Study
Abstract
:1. Introduction
1.1. Amyloid Cardiomyopathy
1.2. Cardiac Magnetic Resonance Imaging
1.3. A Novel CMR Tool
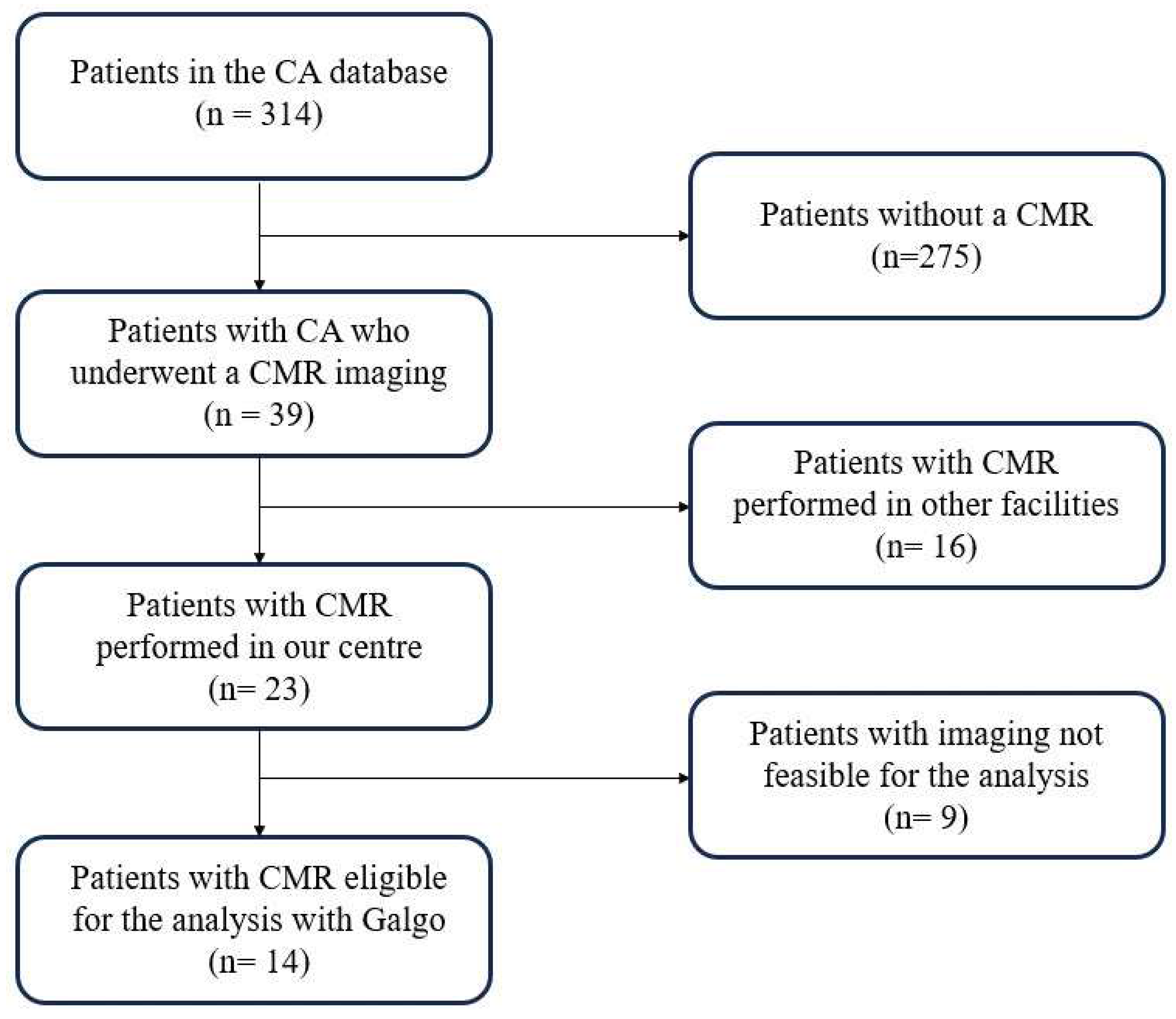
2. Materials and Methods
2.1. Cardiological Assessment
2.2. Endpoints
2.3. Statistical Analysis
2.4. CMR Protocol
2.5. Software Application
3. Results
3.1. Population
3.2. 3D Software Analysis
3.3. Outcomes
4. Discussion
4.1. CMR Overview
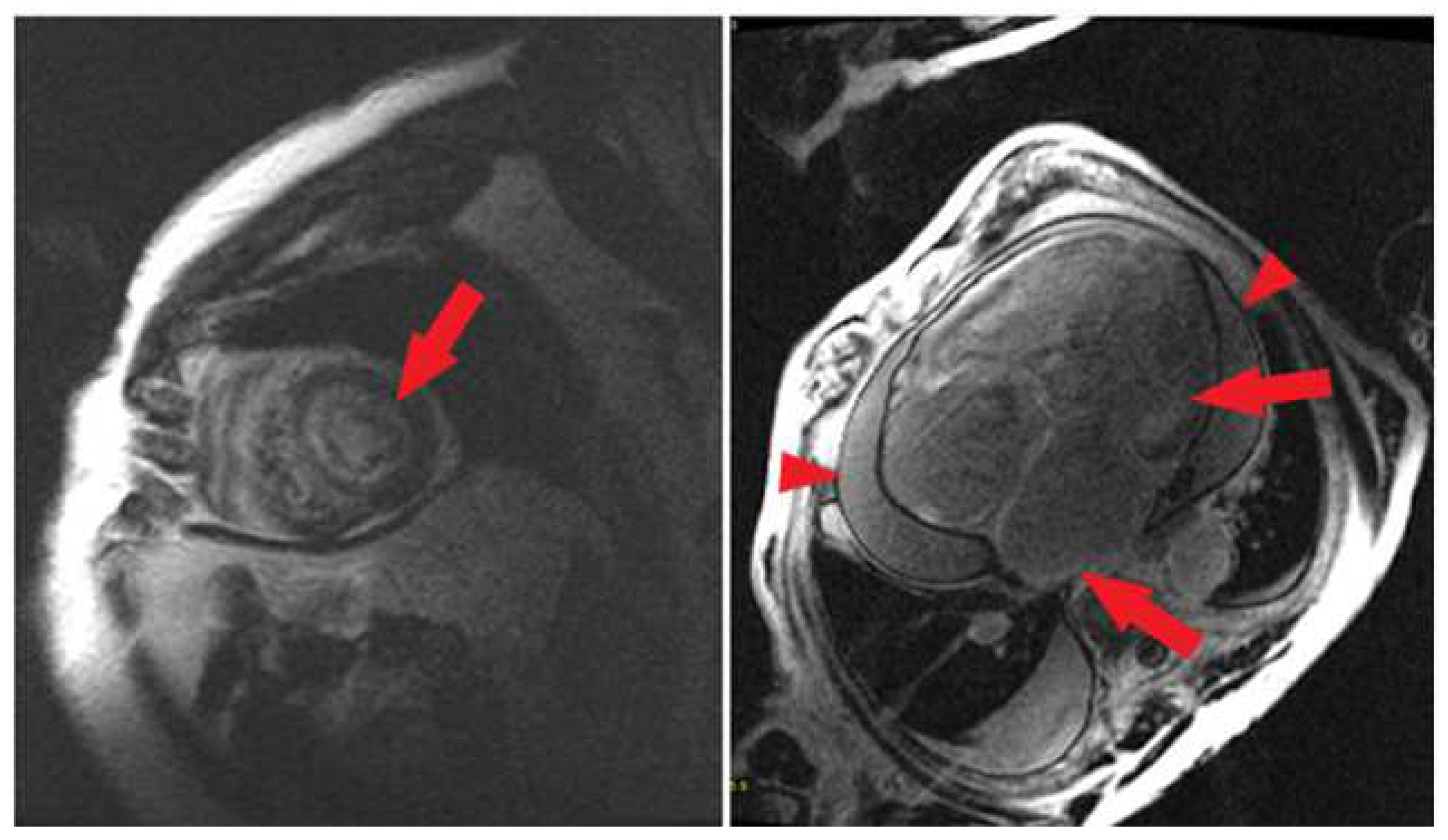
4.2. Ventricular LGE
4.3. Atrial Amyloidosis
4.4. Arrhythmia Prevention
4.5. ADAS 3D
4.6. Ventricular Corridors and Scar Analysis
4.7. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies: Developed by the task force on the management of cardiomyopathies of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef]
- Vogelsberg, H.; Mahrholdt, H.; Deluigi, C.C.; Yilmaz, A.; Kispert, E.M.; Greulich, S.; Klingel, K.; Kandolf, R.; Sechtem, U. Cardiovascular magnetic resonance in clinically suspected cardiac amyloidosis: Noninvasive imaging compared to endomyocardial biopsy. J. Am. Coll. Cardiol. 2008, 51, 1022–1030. [Google Scholar] [CrossRef]
- Syed, I.S.; Glockner, J.F.; Feng, D.; Araoz, P.A.; Martinez, M.W.; Edwards, W.D.; Gertz, M.A.; Dispenzieri, A.; Oh, J.K.; Bellavia, D.; et al. Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. JACC Cardiovasc. Imaging 2010, 3, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Tang, C.; Ren, G.; Guo, J.; Zhao, L.; Xu, W.; Zhou, X.; Zhang, L.; Huang, X. The prognostic value of multiparametric cardiac magnetic resonance in patients with systemic light chain amyloidosis. Front. Oncol. 2023, 13, 1069788. [Google Scholar] [CrossRef]
- Saad, J.M.; Ahmed, A.I.; Han, Y.; Malahfji, M.; Aljizeeri, A.; Al-Mallah, M.H. Cardiovascular magnetic resonance for suspected cardiac amyloidosis: Where are we now? Heart Fail. Rev. 2022, 27, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Naharro, A.; Treibel, T.A.; Abdel-Gadir, A.; Bulluck, H.; Zumbo, G.; Knight, D.S.; Kotecha, T.; Francis, R.; Hutt, D.F.; Rezk, T.; et al. Magnetic Resonance in Transthyretin Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2017, 70, 466–477. [Google Scholar] [CrossRef]
- Ucci, A.; Aimo, A.; Musetti, V.; Barison, A.; Vergaro, G.; Genovesi, D.; Giorgetti, A.; Masotti, S.; Arzilli, C.; Prontera, C.; et al. Amyloid Deposits and Fibrosis on Left Ventricular Endomyocardial Biopsy Correlate With Extracellular Volume in Cardiac Amyloidosis. J. Am. Heart Assoc. 2021, 10, e020358. [Google Scholar] [CrossRef]
- Boretto, P.; Patel, N.H.; Patel, K.; Rana, M.; Saglietto, A.; Soni, M.; Ahmad, M.; Sin Ying Ho, J.; de Filippo, O.; Providencia, R.A.; et al. Prognosis prediction in cardiac amyloidosis by cardiac magnetic resonance imaging: A systematic review with meta-analysis. Eur. Heart J. Open 2023, 3, oead092. [Google Scholar] [CrossRef] [PubMed]
- Dungu, J.N.; Valencia, O.; Pinney, J.H.; Gibbs, S.D.; Rowczenio, D.; Gilbertson, J.A.; Lachmann, H.J.; Wechalekar, A.; Gillmore, J.D.; Whelan, C.J.; et al. CMR-based differentiation of AL and ATTR cardiac amyloidosis. JACC Cardiovasc. Imaging 2014, 7, 133–142. [Google Scholar] [CrossRef]
- Fontana, M.; Pica, S.; Reant, P.; Abdel-Gadir, A.; Treibel, T.A.; Banypersad, S.M.; Maestrini, V.; Barcella, W.; Rosmini, S.; Bulluck, H.; et al. Prognostic Value of Late Gadolinium Enhancement Cardiovascular Magnetic Resonance in Cardiac Amyloidosis. Circulation 2015, 132, 1570–1579. [Google Scholar] [CrossRef]
- Aquaro, G.D.; de Gori, C.; Faggioni, L.; Parisella, M.L.; Cioni, D.; Lencioni, R.; Neri, E. Diagnostic and prognostic role of late gadolinium enhancement in cardiomyopathies. Eur. Heart J. Suppl. 2023, 25 (Suppl. C), C130–C136. [Google Scholar] [CrossRef] [PubMed]
- Kwong, R.Y.; Heydari, B.; Abbasi, S.; Steel, K.; Al-Mallah, M.; Wu, H.; Falk, R.H. Characterization of Cardiac Amyloidosis by Atrial Late Gadolinium Enhancement Using Contrast-Enhanced Cardiac Magnetic Resonance Imaging and Correlation With Left Atrial Conduit and Contractile Function. Am. J. Cardiol. 2015, 116, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Alcaine, A.; Jáuregui, B.; Soto-Iglesias, D.; Acosta, J.; Penela, D.; Fernández-Armenta, J.; Linhart, M.; Andreu, D.; Mont, L.; Laguna, P.; et al. Automatic Detection of Slow Conducting Channels during Substrate Ablation of Scar-Related Ventricular Arrhythmias. J. Interv. Cardiol. 2020, 2020, 4386841. [Google Scholar] [CrossRef] [PubMed]
- Vergaro, G.; Aimo, A.; Rapezzi, C.; Castiglione, V.; Fabiani, I.; Pucci, A.; Buda, G.; Passino, C.; Lupón, J.; Bayes-Genis, A.; et al. Atrial amyloidosis: Mechanisms and clinical manifestations. Eur. J. Heart Fail. 2022, 24, 2019–2028. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Holmes, B.B.; Huang, S.; Lugo, R.; Al Aboud, A.; Goodman, S.; Hung, R.R.; Slosky, D.; Stevenson, W.G.; Michaud, G.F.; et al. Outcomes in patients with cardiac amyloidosis and implantable cardioverter-defibrillator. Europace 2020, 22, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Higgins, A.Y.; Annapureddy, A.R.; Wang, Y.; Minges, K.E.; Lampert, R.; Rosenfeld, L.E.; Jacoby, D.L.; Curtis, J.P.; Miller, E.J.; Freeman, J.V. Survival Following Implantable Cardioverter-Defibrillator Implantation in Patients With Amyloid Cardiomyopathy. J. Am. Heart Assoc. 2020, 9, e016038. [Google Scholar] [CrossRef] [PubMed]
- Chopra, N.; Tokuda, M.; Ng, J.; Reichlin, T.; Nof, E.; John, R.M.; Tedrow, U.B.; Stevenson, W.G. Relation of the unipolar low-voltage penumbra surrounding the endocardial low-voltage scar to ventricular tachycardia circuit sites and ablation outcomes in ischemic cardiomyopathy. J. Cardiovasc. Electrophysiol. 2014, 25, 602–608. [Google Scholar] [CrossRef]
- Robbers, L.F.; Delewi, R.; Nijveldt, R.; Hirsch, A.; Beek, A.M.; Kemme, M.J.; van Beurden, Y.; van der Laan, A.M.; van der Vleuten, P.A.; Tio, R.A.; et al. Myocardial infarct heterogeneity assessment by late gadolinium enhancement cardiovascular magnetic resonance imaging shows predictive value for ventricular arrhythmia development after acute myocardial infarction. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Perez-David, E.; Arenal, A.; Rubio-Guivernau, J.L.; del Castillo, R.; Atea, L.; Arbelo, E.; Caballero, E.; Celorrio, V.; Datino, T.; Gonzalez-Torrecilla, E.; et al. Noninvasive identification of ventricular tachycardia-related conducting channels using contrast-enhanced magnetic resonance imaging in patients with chronic myocardial infarction: Comparison of signal intensity scar mapping and endocardial voltage mapping. J. Am. Coll. Cardiol. 2011, 57, 184–194. [Google Scholar] [CrossRef]
- Khanna, S.; Lo, P.; Cho, K.; Subbiah, R. Ventricular Arrhythmias in Cardiac Amyloidosis: A Review of Current Literature. Clin. Med. Insights Cardiol. 2020, 14, 1179546820963055. [Google Scholar] [CrossRef]
- Cappelli, F.; Vignini, E.; Martone, R.; Perlini, S.; Mussinelli, R.; Sabena, A.; Morini, S.; Gabriele, M.; Taborchi, G.; Bartolini, S.; et al. Baseline ECG Features and Arrhythmic Profile in Transthyretin Versus Light Chain Cardiac Amyloidosis. Circ. Heart Fail. 2020, 13, e006619. [Google Scholar] [CrossRef] [PubMed]
- Briasoulis, A.; Kourek, C.; Papamichail, A.; Loritis, K.; Bampatsias, D.; Repasos, E.; Xanthopoulos, A.; Tsougos, E.; Paraskevaidis, I. Arrhythmias in Patients with Cardiac Amyloidosis: A Comprehensive Review on Clinical Management and Devices. J. Cardiovasc. Dev. Dis. 2023, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Martini, N.; Sinigiani, G.; de Michieli, L.; Mussinelli, R.; Perazzolo Marra, M.; Iliceto, S.; Zorzi, A.; Perlini, S.; Corrado, D.; Cipriani, A. Electrocardiographic features and rhythm disorders in cardiac amyloidosis. Trends Cardiovasc. Med. 2023; in press. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Somonte, P.; Garre, P.; Vázquez-Calvo, S.; Quinto, L.; Borràs, R.; Prat, S.; Ortiz-Perez, J.T.; Steghöfer, M.; Figueras i Ventura, R.M.; Guasch, E.; et al. Scar conducting channel characterization to predict arrhythmogenicity during ventricular tachycardia ablation. Europace 2023, 25, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Bhagirath, P. Post-ablation cardiac magnetic resonance in ventricular tachycardia ablation: Shining light on dark cores and corridors. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 199–200. [Google Scholar] [CrossRef] [PubMed]
- Roca-Luque, I.; Vázquez-Calvo, S.; Garre, P.; Ortiz-Perez, J.T.; Prat-Gonzalez, S.; Sanchez-Somonte, P.; Ferro, E.; Quinto, L.; Alarcón, F.; Althoff, T.; et al. Post-Ablation cardiac Magnetic resonance to assess Ventricular Tachycardia recurrence (PAM-VT study). Eur. Heart J. Cardiovasc. Imaging 2024, 25, 188–198, Erratum in Eur. Heart J. Cardiovasc. Imaging 2024, 25, e100. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Somonte, P.; Quinto, L.; Garre, P.; Zaraket, F.; Alarcón, F.; Borràs, R.; Caixal, G.; Vázquez, S.; Prat, S.; Ortiz-Perez, J.T.; et al. Scar channels in cardiac magnetic resonance to predict appropriate therapies in primary prevention. Heart Rhythm 2021, 18, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Casella, M.; Compagnucci, P.; Ciliberti, G.; Falanga, U.; Barbarossa, A.; Valeri, Y.; Cipolletta, L.; Volpato, G.; Stronati, G.; Rizzo, S.; et al. Characteristics and Clinical Value of Electroanatomical Voltage Mapping in Cardiac Amyloidosis. Can. J. Cardiol. 2024, 40, 372–384. [Google Scholar] [CrossRef]
- Betensky, B.P.; Dong, W.; D’Souza, B.A.; Zado, E.S.; Han, Y.; Marchlinski, F.E. Cardiac magnetic resonance imaging and electroanatomic voltage discordance in non-ischemic left ventricle ventricular tachycardia and premature ventricular depolarizations. J. Interv. Card. Electrophysiol. 2017, 49, 11–19. [Google Scholar] [CrossRef]



| Characteristic | Total (n = 14) |
|---|---|
| Age, years, mean ± SD | 66.9 ± 14.8 |
| Male gender, n (%) | 11 (78.6) |
| CA variant, n (%) | |
| - ATTR-wt | 8 (57.1) |
| - ATTR-m | 1 (7.1) |
| - AL | 5 (35.7) |
| Baseline EF, %, mean ± SD | 54.7 ± 11.4 |
| Anamnesis | |
| - Previous MI, n (%) | - |
| - Hypertension, n (%) | 8 (57.1) |
| - Dyslipidemia, n (%) | 7 (50.0) |
| - Diabetes, n (%) | 3 (21.4) |
| - Smoking history | 4 (28.6) |
| - Previous stroke, n (%) | - |
| - Previous VTs, n (%) | 4 (28.6) |
| - Previous SVAs, n (%) | 6 (42.9) |
| ○ AF, n (%) | 4 (28.6) |
| ○ Typical AFL, n (%) | - |
| ○ Atypical AFL, n (%) | 2 (14.3) |
| - Previous PMK implantation | 1 (7.1) |
| - Previous ICD implantation | 1 (7.1) |
| Therapy at baseline assessment | |
| - Beta-blockers, n (%) | 3 (21.4) |
| - ACE inhibitors, n (%) | 2 (14.3) |
| - ARBs, n (%) | 6 (42.9) |
| - MRAs, n (%) | 2 (14.3) |
| - SGLT-2 inhibitors, n (%) | 1 (7.1) |
| - Loop diuretics, n (%) | 10 (71.4) |
| - ARNI, n (%) | - |
| - Tafamidis, n (%) | 1 (7.1) |
| - Amiodarone, n (%) | 4 (28.6) |
| Imaging Feature (n, %) | Correlation (p) |
|---|---|
| - Presence of atrial LGE (n = 13, 92.9%) | p = 0.61 |
| - Mid-ventricular LGE (n = 1, 7.1%) | p = 0.59 |
| - Mid-ventricular + subepicardial LGEs (n = 2, 14.3%) | p = 0.18 |
| - Transmural ventricular LGE (n = 1; 7.1%) | p = 0.72 |
| - Isolated subendocardial ventricular LGE | p = 0.076 |
| - Atrial wall thickening (n = 3; 21.4%) | p = 0.003 |
| - Presence of corridors (n = 5; 35.7%) | p = 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfieri, M.; Guerra, F.; Lofiego, C.; Fogante, M.; Ciliberti, G.; Vagnarelli, F.; Barbarossa, A.; Principi, S.; Stronati, G.; Volpato, G.; et al. A Novel Approach to Cardiac Magnetic Resonance Scar Characterization in Patients Affected by Cardiac Amyloidosis: A Pilot Study. Medicina 2024, 60, 613. https://doi.org/10.3390/medicina60040613
Alfieri M, Guerra F, Lofiego C, Fogante M, Ciliberti G, Vagnarelli F, Barbarossa A, Principi S, Stronati G, Volpato G, et al. A Novel Approach to Cardiac Magnetic Resonance Scar Characterization in Patients Affected by Cardiac Amyloidosis: A Pilot Study. Medicina. 2024; 60(4):613. https://doi.org/10.3390/medicina60040613
Chicago/Turabian StyleAlfieri, Michele, Federico Guerra, Carla Lofiego, Marco Fogante, Giuseppe Ciliberti, Fabio Vagnarelli, Alessandro Barbarossa, Samuele Principi, Giulia Stronati, Giovanni Volpato, and et al. 2024. "A Novel Approach to Cardiac Magnetic Resonance Scar Characterization in Patients Affected by Cardiac Amyloidosis: A Pilot Study" Medicina 60, no. 4: 613. https://doi.org/10.3390/medicina60040613






