Impact of Airborne Exposure to PM10 Increases Susceptibility to P. aeruginosa Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Whole-Body Exposure to PM10
2.3. SKQ1 and Moxifloxacin Treatment
2.4. Bacterial Culture and Corneal Infection
2.5. Ocular Response to Bacterial Infection
2.6. RT-PCR
2.7. Western Blot
2.8. Bacterial Plate Count
2.9. P. aeruginosa and SKQ1 Killing
2.10. Myeloperoxidase (MPO) Assay
2.11. Statistical Analysis
3. Results
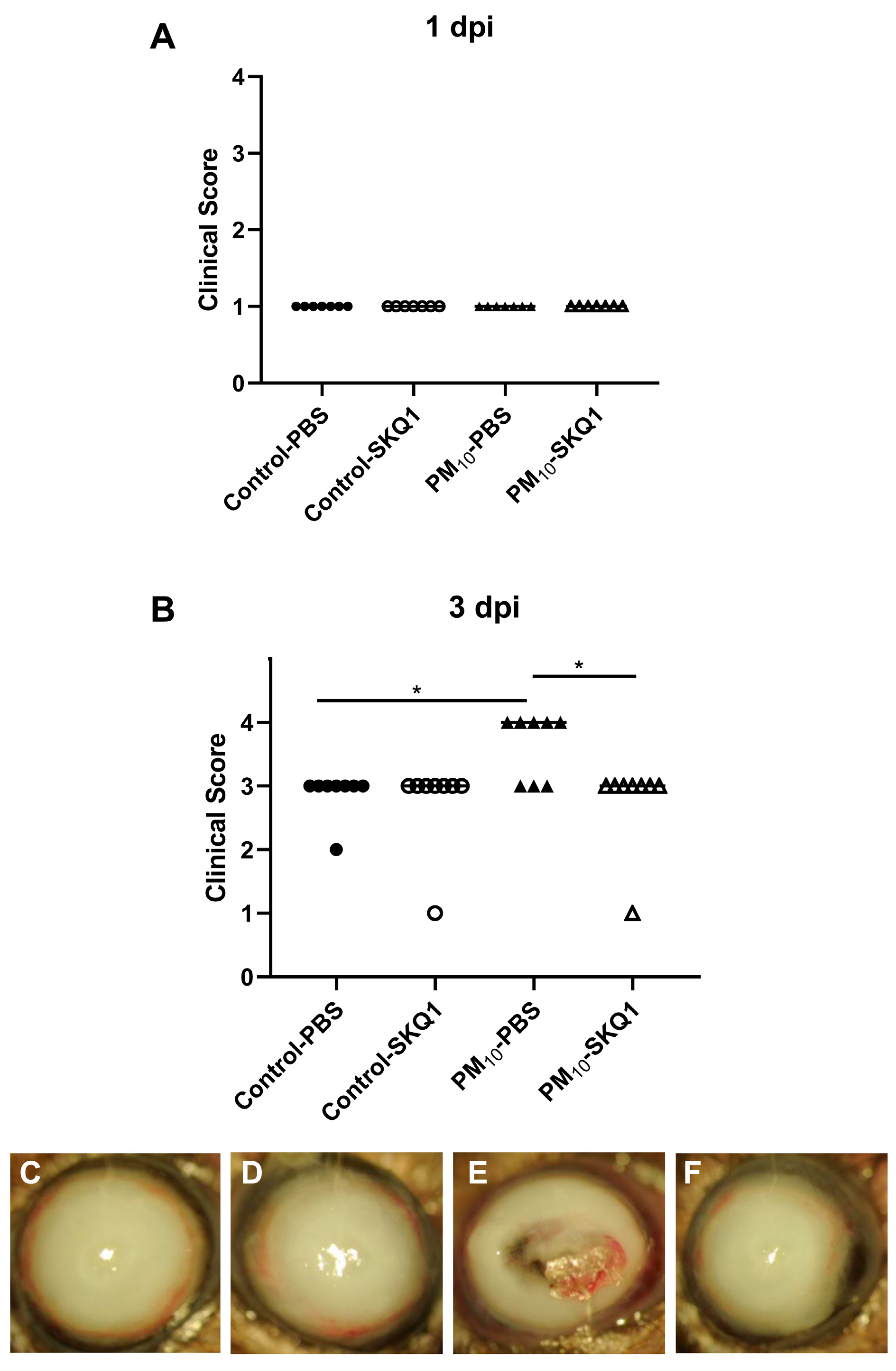
3.1. Clinical Score and Slit lamp
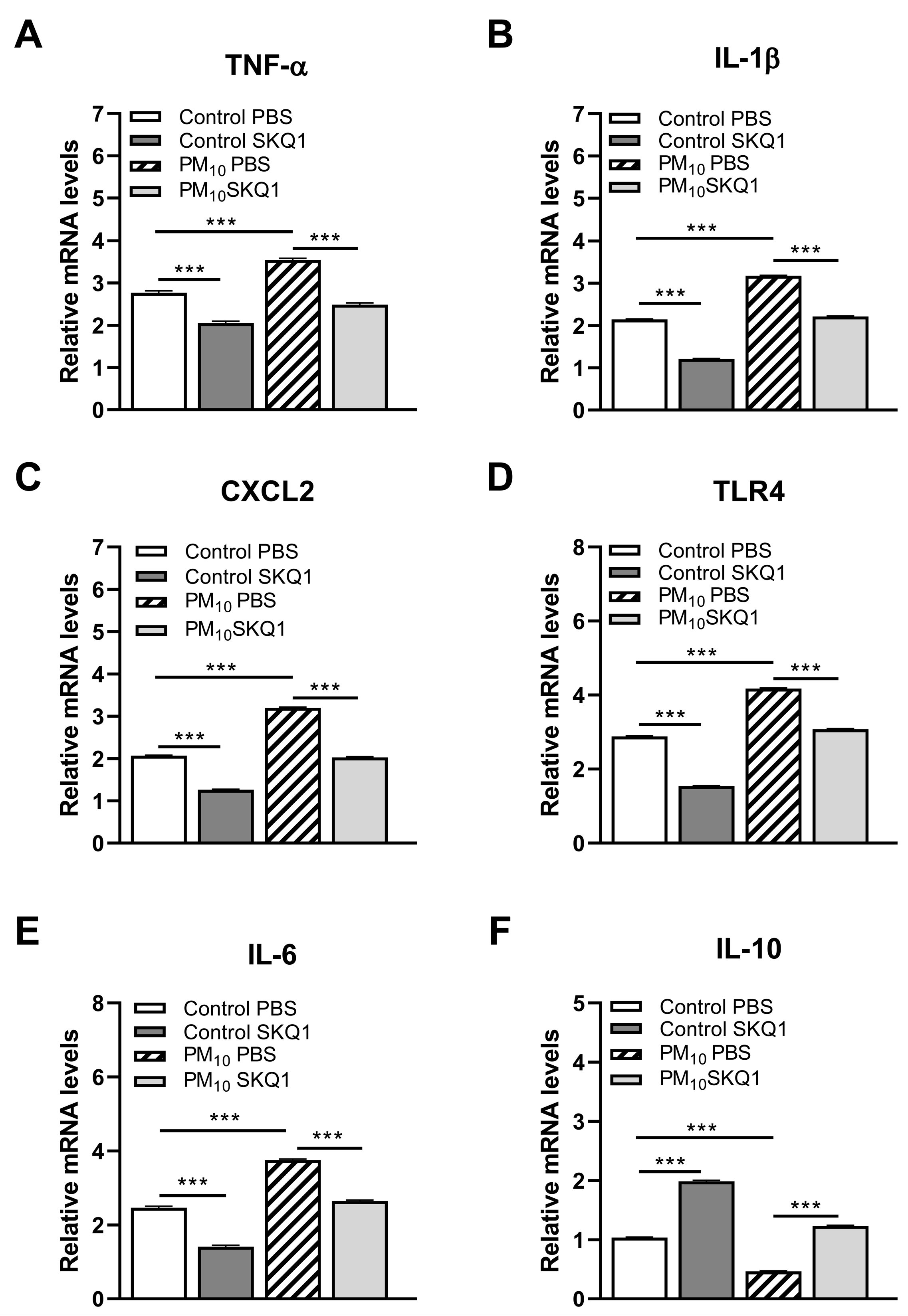
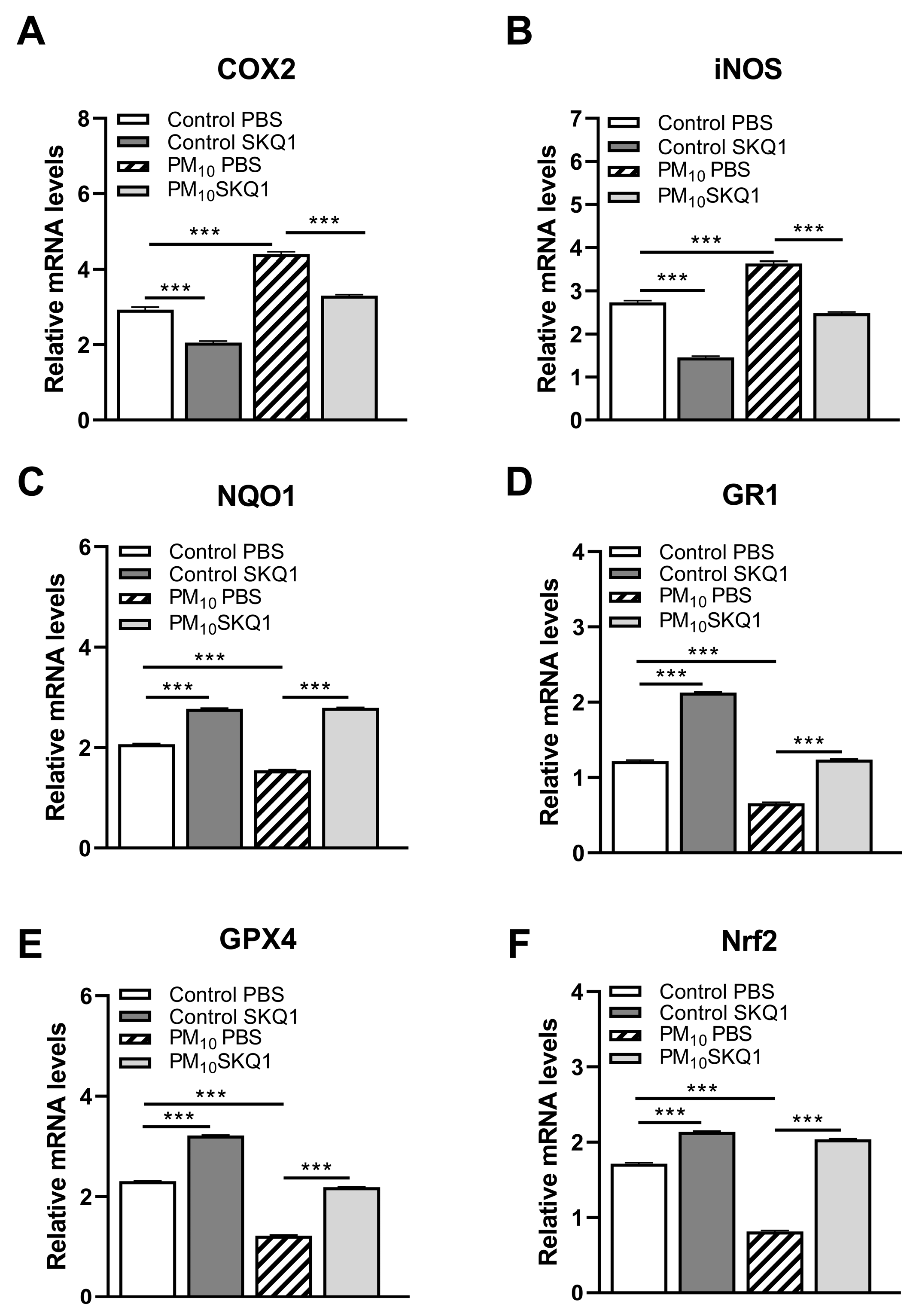
3.2. RT-PCR
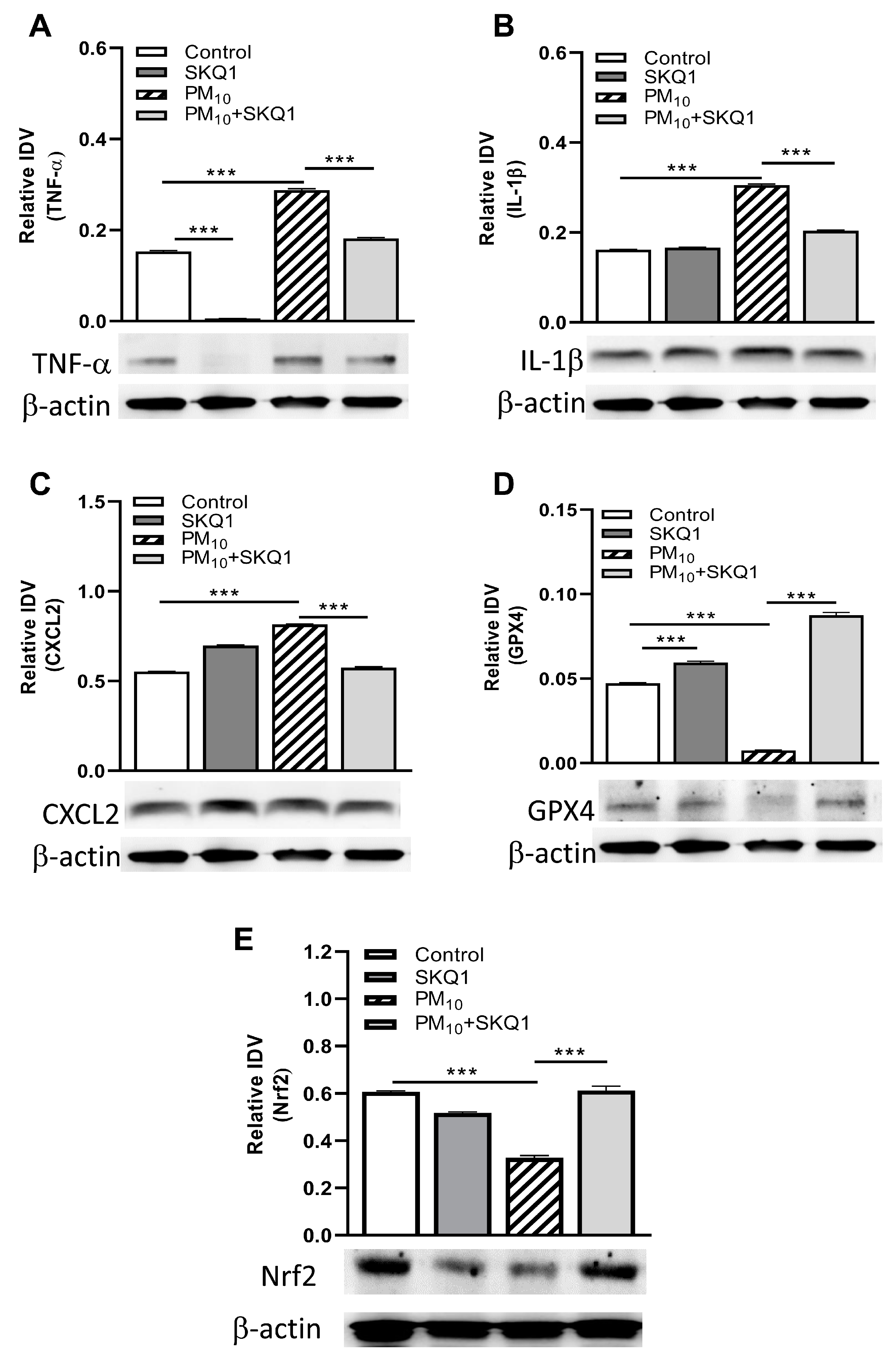
3.3. Western Blot
3.4. Viable Plate Count and MPO Assay
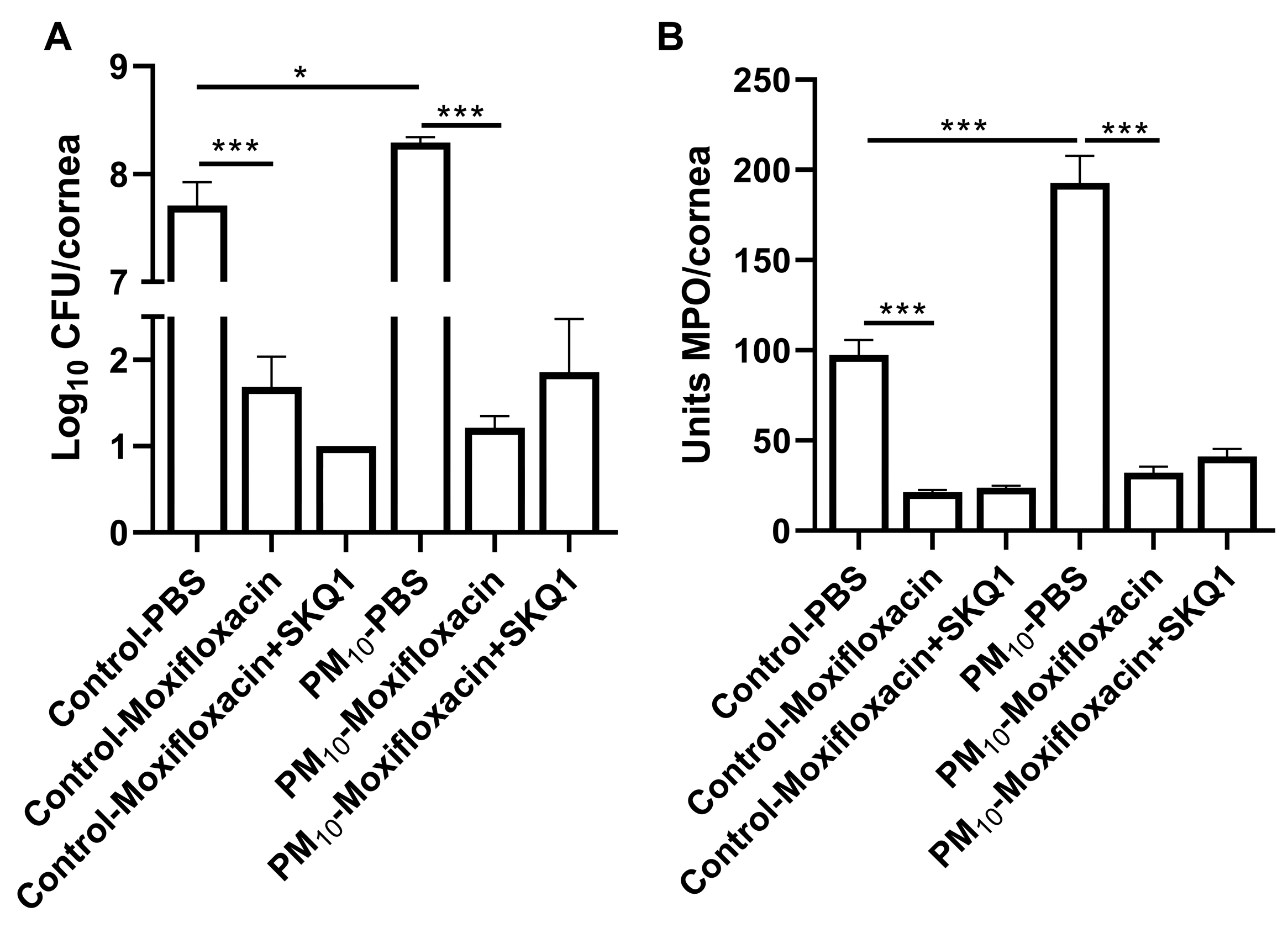
3.5. SKQ1 Antibiotic Effect
3.6. Clinical Score and Slit Lamp after Moxifloxacin/SKQ1 Treatment
3.7. Viable Plate Count and MPO Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torricelli, A.A.; Novaes, P.; Matsuda, M.; Alves, M.R.; Monteiro, M.L. Ocular surface adverse effects of ambient levels of air pollution. Arq. Bras. Oftalmol. 2011, 74, 377–381. [Google Scholar] [CrossRef]
- World Health Organization. Air Pollution. Available online: https://www.who.int/news-room/factsheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 30 June 2021).
- Pope, C.A., 3rd; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002, 287, 1132–1141. [Google Scholar] [CrossRef]
- Nurkiewicz, T.R.; Porter, D.W.; Barger, M.; Millecchia, L.; Rao, K.M.; Marvar, P.J.; Hubbs, A.F.; Castranova, V.; Boegehold, M.A. Systemic microvascular dysfunction and inflammation after pulmonary particulate matter exposure. Environ. Health Perspect. 2006, 114, 412–419. [Google Scholar] [CrossRef]
- Miller, M.R.; Borthwick, S.J.; Shaw, C.A.; McLean, S.G.; McClure, D.; Mills, N.L.; Duffin, R.; Donaldson, K.; Megson, I.L.; Hadoke, P.W.; et al. Direct impairment of vascular function by diesel exhaust particulate through reduced bioavailability of endothelium-derived nitric oxide induced by superoxide free radicals. Environ. Health Perspect. 2009, 117, 611–616. [Google Scholar] [CrossRef]
- Liu, L.; Poon, R.; Chen, L.; Frescura, A.M.; Montuschi, P.; Ciabattoni, G.; Wheeler, A.; Dales, R. Acute effects of air pollution on pulmonary function, air-way inflammation, and oxidative stress in asthmatic children. Environ. Health Perspect. 2009, 117, 668–674. [Google Scholar] [CrossRef]
- Churg, A.; Brauer, M.; del Carmen Avial-Casado, M.; Fortoul, T.I.; Wright, J.L. Chronic exposure to high levels of particulate air pollution and small airway remodeling. Environ. Health Perspect. 2003, 111, 714–718. [Google Scholar] [CrossRef]
- Brunekreef, B.; Holgate, S.T. Air pollution and health. Lancet 2002, 360, 1233–1242. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, J.W.; Kim, E.J.; Lee, M.Y.; Nam, C.W.; Chung, I.S. Spatial analysis between particulate matter and emergency room visits for conjunctivitis and keratitis. Ann. Occup. Environ. Med. 2018, 30, 41. [Google Scholar] [CrossRef]
- Sendra, V.G.; Tau, J.; Zapata, G.; Lasagni Vitar, R.M.; Illian, E.; Chiaradía, P.; Berra, A. Polluted air exposure compromises corneal immunity and exacerbates inflammation in acute herpes simplex keratitis. Front. Immunol. 2021, 12, 618597. [Google Scholar] [CrossRef]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and health impacts of air pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef]
- Mo, Z.; Fu, Q.; Lyu, D.; Zhang, L.; Qin, Z.; Tang, Q.; Yin, H.; Xu, P.; Wu, L.; Wang, X.; et al. Impacts of air pollution on dry eye disease among residents in Hangzhou, China: A case-crossover study. Environ. Pollut. 2019, 246, 183–189. [Google Scholar] [CrossRef]
- Klopfer, J. Effects of environmental air pollution on the eye. J. Am. Optom. Assoc. 1989, 60, 773–778. [Google Scholar]
- Torricelli, A.A.; Matsuda, M.; Novaes, P.; Braga, A.L.; Saldiva, P.H.; Alves, M.R.; Monteiro, M.L. Effects of ambient levels of traffic-derived air pollution on the ocular surface: Analysis of symptoms, conjunctival goblet cell count and mucin 5AC gene expression. Environ. Res. 2014, 131, 59–63. [Google Scholar] [CrossRef]
- Chang, C.J.; Yang, H.H.; Chang, C.A.; Tsai, H.Y. Relationship between air pollution and outpatient visits for nonspecific conjunctivitis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 429–433. [Google Scholar] [CrossRef]
- Chang, C.J.; Yang, H.H. Impact on eye health regarding gaseous and particulate pollutants. Aerosol Air Qual. Res. 2020, 20, 1695–1699. [Google Scholar] [CrossRef]
- Li, X.Y.; Gilmour, P.S.; Donaldson, K.; MacNee, W. Free radical activity and pro-inflammatory effects of particulate air pollution (PM10) in vivo and in vitro. Thorax 1996, 51, 1216–1222. [Google Scholar] [CrossRef]
- Sánchez-Pérez, Y.; Chirino, Y.I.; Osornio-Vargas, Á.R.; Morales-Bárcenas, R.; Gutiérrez-Ruíz, C.; Vázquez-López, I.; García-Cuellar, C.M. DNA damage response of A549 cells treated with particulate matter (PM10) of urban air pollutants. Cancer Lett. 2009, 278, 192–200. [Google Scholar] [CrossRef]
- Brown, D.M.; Donaldson, K.; Borm, P.J.; Schins, R.P.; Dehnhardt, M.; Gilmour, P.; Jimenez, L.A.; Stone, V. Calcium and ROS-mediated activation of transcription factors and TNF-alpha cytokine gene expression in macrophages exposed to ultrafine particles. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L344–L353. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlahoyianni, T.; Fiotakis, K. Comparative study of the formation of oxidative damage marker 8-hydroxy-2’deoxyguanosine (8-OHdG) adduct from the nucleoside 2’-deoxyguanosine by transition metals and suspensions of particulate matter in relation to metal content and redox reactivity. Free Radic. Res. 2005, 39, 1071–1081. [Google Scholar] [CrossRef]
- Li, Y.J.; Takizawa, H.; Azuma, A.; Kohyama, T.; Yamauchi, Y.; Takahashi, S.; Yamamoto, M.; Kawada, T.; Kudoh, S.; Sugawara, I. Disruption of Nrf2 enhances susceptibility to airway inflammatory responses induced by low-dose diesel exhaust particles in mice. Clin. Immunol. 2008, 128, 366–373. [Google Scholar] [CrossRef]
- Somayajulu, M.; Ekanayaka, S.; McClellan, S.; Bessert, D.; Pitchaikannu, A.; Zhang, K.; Hazlett, L.D. Airborne particulates affect corneal homeostasis and immunity. Investig. Ophthalmol. Vis. Sci. 2020, 61, 23. [Google Scholar] [CrossRef]
- Ko, R.; Hayashi, M.; Tanaka, M.; Okuda, T.; Nishita-Hara, C.; Ozaki, H.; Uchio, E. Effects of ambient particulate matter on a reconstructed human corneal epithelium model. Sci. Rep. 2021, 11, 3417. [Google Scholar] [CrossRef]
- Song, S.J.; Hyun, S.W.; Lee, T.G.; Park, B.; Jo, K.; Kim, C.S. New application for assessment of dry eye syndrome induced by particulate matter exposure. Ecotoxicol. Environ. Saf. 2020, 205, 111125. [Google Scholar] [CrossRef]
- Li, J.; Tan, G.; Ding, X.; Wang, Y.; Wu, A.; Yang, Q.; Ye, L.; Shao, Y. A mouse dry eye model induced by topical administration of the air pollutant particulate matter 10. Biomed. Pharmacother. 2017, 96, 524–534. [Google Scholar] [CrossRef]
- Baird, L.; Dinkova-Kostova, A.T. The cytoprotective role of the Keap1-Nrf2 pathway. Arch. Toxicol. 2011, 85, 241–272. [Google Scholar] [CrossRef]
- The Economist. Available online: https://www.economist.com/the-economist-explains/2021/03/15/why-is-beijings-air-quality-so-bad-again (accessed on 24 January 2024).
- Express News Service. Available online: https://indianexpress.com/article/cities/delhi/delhi-weather-dust-pm10-levels-low-visibility-8611601/ (accessed on 24 January 2024).
- Krasnov, H.; Katra, I.; Koutrakis, P.; Friger, M.D. Contribution of dust storms to PM10 levels in an urban arid environment. J. Air Waste Manag. Assoc. 2014, 64, 89–94. [Google Scholar] [CrossRef]
- Zernii, E.Y.; Gancharova, O.S.; Baksheeva, V.E.; Golovastova, M.O.; Kabanova, E.I.; Savchenko, M.S.; Tiulina, V.V.; Sotnikova, L.F.; Zamyatnin, A.A., Jr.; Philippov, P.P.; et al. Mitochondria-Targeted antioxidant SkQ1 prevents anesthesia-induced dry eye syndrome. Oxid. Med. Cell. Longev. 2017, 2017, 9281519. [Google Scholar] [CrossRef]
- Kwon, B.; Hazlett, L.D. Association of CD4+ T cell-dependent keratitis with genetic susceptibility to Pseudomonas aeruginosa ocular infection. J. Immunol. 1997, 159, 6283–6290. [Google Scholar] [CrossRef]
- Hazlett, L.D.; Moon, M.M.; Strejc, M.; Berk, R.S. Evidence for N-acetylmannosamine as an ocular receptor for P. aeruginosa adherence to scarified cornea. Investig. Ophthalmol. Vis. Sci. 1987, 28, 1978–1985. [Google Scholar]
- Huang, X.; Barrett, R.P.; McClellan, S.A.; Hazlett, L.D. Silencing Toll-like receptor-9 in Pseudomonas aeruginosa keratitis. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4209–4216. [Google Scholar] [CrossRef]
- Somayajulu, M.; McClellan, S.A.; Wright, R.; Pitchaikannu, A.; Croniger, B.; Zhang, K.; Hazlett, L.D. Airborne exposure of the cornea to PM10 induces oxidative stress and disrupts Nrf2 mediated anti-oxidant defenses. Int. J. Mol. Sci. 2023, 24, 3911. [Google Scholar] [CrossRef]
- Nazarov, P.A.; Kotova, E.A.; Skulachev, V.P.; Antonenko, Y.N. Genetic variability of the AcrAB-TolC multidrug efflux pump underlies SkQ1 resistance in gram-negative bacteria. Acta Nat. 2019, 11, 93–98. [Google Scholar] [CrossRef]
- Williams, R.N.; Paterson, C.A.; Eakins, K.E.; Bhattacherjee, P. Quantification of ocular inflammation: Evaluation of polymorphonuclear leucocyte infiltration by measuring myeloperoxidase activity. Curr. Eye Res. 1982, 2, 465–470. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Al-Kindi, S.G.; Brook, R.D. Air pollution and cardiovascular disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2054–2070. [Google Scholar] [CrossRef]
- de Bont, J.; Jaganathan, S.; Dahlquist, M.; Persson, Å.; Stafoggia, M.; Ljungman, P. Ambient air pollution and cardiovascular diseases: An umbrella review of systematic reviews and meta-analyses. J. Intern. Med. 2022, 291, 779–800. [Google Scholar] [CrossRef]
- Krittanawong, C.; Qadeer, Y.K.; Hayes, R.B.; Wang, Z.; Virani, S.; Thurston, G.D.; Lavie, C.J. PM2.5 and cardiovascular health risks. Curr. Probl. Cardiol. 2023, 48, 101670. [Google Scholar] [CrossRef]
- Zhang, S.; Qian, Z.M.; Chen, L.; Zhao, X.; Cai, M.; Wang, C.; Zou, H.; Wu, Y.; Zhang, Z.; Li, H.; et al. Exposure to air pollution during pre-hypertension and subsequent hypertension, cardiovascular disease, and death: A trajectory analysis of the UK Biobank Cohort. Environ. Health Perspect. 2023, 131, 17008, Erratum in Environ. Health Perspect. 2023, 131, 29001. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, M.; Ji, M.; Fan, J.; Xie, J.; Wei, X.; Jiang, X.; Xu, J.; Chen, L.; Yin, R.; et al. Air pollution, genetic factors, and the risk of lung cancer: A prospective study in the UK Biobank. Am. J. Respir. Crit. Care Med. 2021, 204, 817–825, Erratum in Am. J. Respir. Crit. Care Med. 2022, 205, 1254. [Google Scholar] [CrossRef]
- Zare Sakhvidi, M.J.; Lequy, E.; Goldberg, M.; Jacquemin, B. Air pollution exposure and bladder, kidney and urinary tract cancer risk: A systematic review. Environ. Pollut. 2020, 267, 115328. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K.; Loridas, S. Pulmonary oxidative stress, inflammation and cancer: Respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int. J. Environ. Res. Public Health 2013, 10, 3886–3907. [Google Scholar] [CrossRef]
- Junaid, M.; Syed, J.H.; Abbasi, N.A.; Hashmi, M.Z.; Malik, R.N.; Pei, D.S. Status of indoor air pollution (IAP) through particulate matter (PM) emissions and associated health concerns in South Asia. Chemosphere 2018, 191, 651–663. [Google Scholar] [CrossRef]
- Dutta, A.; Jinsart, W. Air pollution in Delhi, India: It’s status and association with respiratory diseases. PLoS ONE 2022, 17, e0274444. [Google Scholar] [CrossRef]
- Lu, C.W.; Fu, J.; Liu, X.F.; Cui, Z.H.; Chen, W.W.; Guo, L.; Li, X.L.; Ren, Y.; Shao, F.; Chen, L.N.; et al. Impacts of air pollution and meteorological conditions on dry eye disease among residents in a northeastern Chinese metropolis: A six-year crossover study in a cold region. Light. Sci. Appl. 2023, 12, 186. [Google Scholar] [CrossRef]
- Alves, M.; Asbell, P.; Dogru, M.; Giannaccare, G.; Grau, A.; Gregory, D.; Kim, D.H.; Marini, M.C.; Ngo, W.; Nowinska, A.; et al. TFOS Lifestyle Report: Impact of environmental conditions on the ocular surface. Ocul. Surf. 2023, 29, 1–52. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, Y.H.; Kim, M.K.; Paik, H.J.; Kim, D.H. Different adverse effects of air pollutants on dry eye disease: Ozone, PM2.5, and PM10. Environ. Pollut. 2020, 265 Pt B, 115039. [Google Scholar] [CrossRef]
- Brito-Zerón, P.; Retamozo, S.; Ramos-Casals, M. Sjögren syndrome. Med. Clin. 2023, 160, 163–171. [Google Scholar] [CrossRef]
- Tsai, T.Y.; Adiyabazar, D.; Hsiao, C.H.; Pan, L.Y.; Chen, S.Y.; Tsai, Y.J.; Chen, C.B.; Chung, W.H.; Ma, D.H. Microbial keratitis in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis: Experience from a tertiary centre in Taiwan. Cornea 2023, 42, 66–73. [Google Scholar] [CrossRef]
- Somani, S.N.; Ronquillo, Y.; Moshirfar, M. Acanthamoeba Keratitis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Somayajulu, M.; McClellan, S.A.; Muhammed, F.; Wright, R.; Hazlett, L.D. PM10 and Pseudomonas aeruginosa: Effects on corneal epithelium. Front. Cell Infect. Microbiol. 2023, 13, 1240903. [Google Scholar] [CrossRef]
- Skulachev, V.P. Cationic antioxidants as a powerful tool against mitochondrial oxidative stress. Biochem. Biophys. Res. Commun. 2013, 441, 275–279. [Google Scholar] [CrossRef]
- Demianenko, I.A.; Vasilieva, T.V.; Domnina, L.V.; Dugina, V.B.; Egorov, M.V.; Ivanova, O.Y.; Ilinskaya, O.P.; Pletjushkina, O.Y.; Popova, E.N.; Sakharov, I.Y.; et al. Novel mitochondria-targeted antioxidants, “Skulachev-ion” derivatives, accelerate dermal wound healing in animals. Biochemistry 2010, 75, 274–280. [Google Scholar] [CrossRef]
- Skulachev, V.P. SkQ1 treatment and food restriction—Two ways to retard an aging program of organisms. Aging 2011, 3, 1045–1050. [Google Scholar] [CrossRef]
- Genrikhs, E.E.; Stelmashook, E.V.; Popova, O.V.; Kapay, N.A.; Korshunova, G.A.; Sumbatyan, N.V.; Skrebitsky, V.G.; Skulachev, V.P.; Isaev, N.K. Mitochondria-targeted antioxidant SkQT1 decreases trauma-induced neurological deficit in rat and prevents amyloid-β-induced impairment of long-term potentiation in rat hippocampal slices. J. Drug Target. 2015, 23, 347–352. [Google Scholar] [CrossRef]
- Kezic, A.; Spasojevic, I.; Lezaic, V.; Bajcetic, M. Mitochondria-targeted antioxidants: Future perspectives in kidney ischemia reperfusion injury. Oxid. Med. Cell. Longev. 2016, 2016, 2950503. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, N.; Qiu, X.; Zeng, R.; Wang, S.; Hua, M.; Li, Q.; Nan, K.; Lin, S. Mitochondria-targeted SkQ1 nanoparticles for dry eye disease: Inhibiting NLRP3 inflammasome activation by preventing mitochondrial DNA oxidation. J. Control. Release 2023, 365, 1–15. [Google Scholar] [CrossRef]
- Nazarov, P.A.; Osterman, I.A.; Tokarchuk, A.V.; Karakozova, M.V.; Korshunova, G.A.; Lyamzaev, K.G.; Skulachev, M.V.; Kotova, E.A.; Skulachev, V.P.; Antonenko, Y.N. Mitochondria-targeted antioxidants as highly effective antibiotics. Sci. Rep. 2017, 7, 1394. [Google Scholar] [CrossRef]
- Hazlett, L.D.; Ekanayaka, S.A.; McClellan, S.A.; Francis, R. Glycyrrhizin use for multi-drug resistant Pseudomonas aeruginosa: In vitro and in vivo studies. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2978–2989. [Google Scholar] [CrossRef]
- van Eeden, S.F.; Tan, W.C.; Suwa, T.; Mukae, H.; Terashima, T.; Fujii, T.; Qui, D.; Vincent, R.; Hogg, J.C. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)). Am. J. Respir. Crit. Care Med. 2001, 164, 826–830. [Google Scholar] [CrossRef]
- Foldenauer, M.E.; McClellan, S.A.; Berger, E.A.; Hazlett, L.D. Mammalian target of rapamycin regulates IL-10 and resistance to Pseudomonas aeruginosa corneal infection. J. Immunol. 2013, 190, 5649–5658. [Google Scholar] [CrossRef]
- Hazlett, L.D.; Jiang, X.; McClellan, S.A. IL-10 function, regulation, and in bacterial keratitis. J. Ocul. Pharmacol. Ther. 2014, 30, 373–380. [Google Scholar] [CrossRef]
- Somayajulu, M.; McClellan, S.A.; Bessert, D.A.; Pitchaikannu, A.; Hazlett, L.D. Ocular effects of glycyrrhizin at acidic and neutral pH. Front. Cell Infect. Microbiol. 2022, 11, 782063. [Google Scholar] [CrossRef]



| Gene | Nucleotide Sequence | Primer | GenBank |
|---|---|---|---|
| 18s | 5’-GTA ACC CGT TGA ACC CCA TT-3’ | F | NM_003278.3 |
| 5’-CCA TCC AAT CGG TAG TAG CG-3′ | R | ||
| Inf-a | 5’-ACC CTC ACA CTC AGA TCA TCT T -3′ | F | NM_013693.2 |
| 5’-GGT TGT CTT TGA GAT CCA TGC -3′ | R | ||
| I1-1β | 5’-CGC AGC AGC ACA TCA ACA AGA GC -3′ | F | NM_008361.3 |
| 5′-TGT CCT CAT CCT GGA AGG TCC ACG -3’ | R | ||
| Cxc12 | 5′-TGT CAA TGC CTG AAG ACC CTG CC -3′ | F | NM_009140.2 |
| 5′-AAC TTT TTG ACC GCC CTT GAG AGT GG -3’ | R | ||
| Tlr4 | 5′-CCT GAC ACC AGG AAG CTT G AA -3′ | F | NM_021297.2 |
| 5′-TCT GAT CCA TGC ATT GGT AGG T -3′ | |||
| I1-6 | 5′-CAC AAG TCC GGA GAG GAG AC-3′ | F | NM_031168.1 |
| 5′-CAG AAT TGC CAT TGC ACA AC-3′ | R | ||
| II-10 | 5′-TGC TAA CCG ACT CCT TAA TGC AGG AC-3′ | F | NM_010548.2 |
| 5′-CCT TGA TTT CTG GGC CAT GCT TCT C-3′ | R | ||
| Cox2 | 5′-GCA GTT CCA GTA TCA GAA CCG CAT TG-3′ | F | NM_011198.2 |
| 5′-GAG TGA GTC CAT GTT CCA GGA GGA TG-3’ | R | ||
| inos | 5′-TCC TCA CTG GGA CAG CAC AGA ATG-3′ | F | NM_010927.3 |
| 5′-GTG TCA TGC AAA ATC TCT CCA CTG CC-3′ | R | ||
| Nqo1 | 5′-TTC TGT GGC TTC CAG GTC TT-3′ | F | NM_008706.5 |
| 5′-TCC AGA CGT TTC TTC CAT CC-3′ | R | ||
| Gr1 | 5′-CCA CGG CTA TGC AAC ATT CG-3′ | F | NM_010344.4 |
| 5′-GAT CTG GCT CTC GTG AGG AA-3′ | R | ||
| Gpx4 | 5′-GCA ACC AGT TTG GGA GGC AGG AG-3′ | F | NM_008162.4 |
| 5′-CCT CCA TGG GAC CAT AGC GCT TC-3′ | R | ||
| Nrf2 | 5′-TGC CCC TCA TCA GGC CCA GT-3′ | F | NM_010902.5 |
| 5′-GCT CGG CTG GGA CTC GTG TT-3′ | R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McClellan, S.A.; Wright, R.; Muhammed, F.; Hazlett, L.D. Impact of Airborne Exposure to PM10 Increases Susceptibility to P. aeruginosa Infection. Int. J. Environ. Res. Public Health 2024, 21, 722. https://doi.org/10.3390/ijerph21060722
McClellan SA, Wright R, Muhammed F, Hazlett LD. Impact of Airborne Exposure to PM10 Increases Susceptibility to P. aeruginosa Infection. International Journal of Environmental Research and Public Health. 2024; 21(6):722. https://doi.org/10.3390/ijerph21060722
Chicago/Turabian StyleMcClellan, Sharon A., Robert Wright, Farooq Muhammed, and Linda D. Hazlett. 2024. "Impact of Airborne Exposure to PM10 Increases Susceptibility to P. aeruginosa Infection" International Journal of Environmental Research and Public Health 21, no. 6: 722. https://doi.org/10.3390/ijerph21060722
APA StyleMcClellan, S. A., Wright, R., Muhammed, F., & Hazlett, L. D. (2024). Impact of Airborne Exposure to PM10 Increases Susceptibility to P. aeruginosa Infection. International Journal of Environmental Research and Public Health, 21(6), 722. https://doi.org/10.3390/ijerph21060722






