Tubular C3N4 Nanotubes as Metal-Free Sulfur Hosts toward Stable Lithium–Sulfur Batteries
Abstract
:1. Introduction
2. Materials and Methods
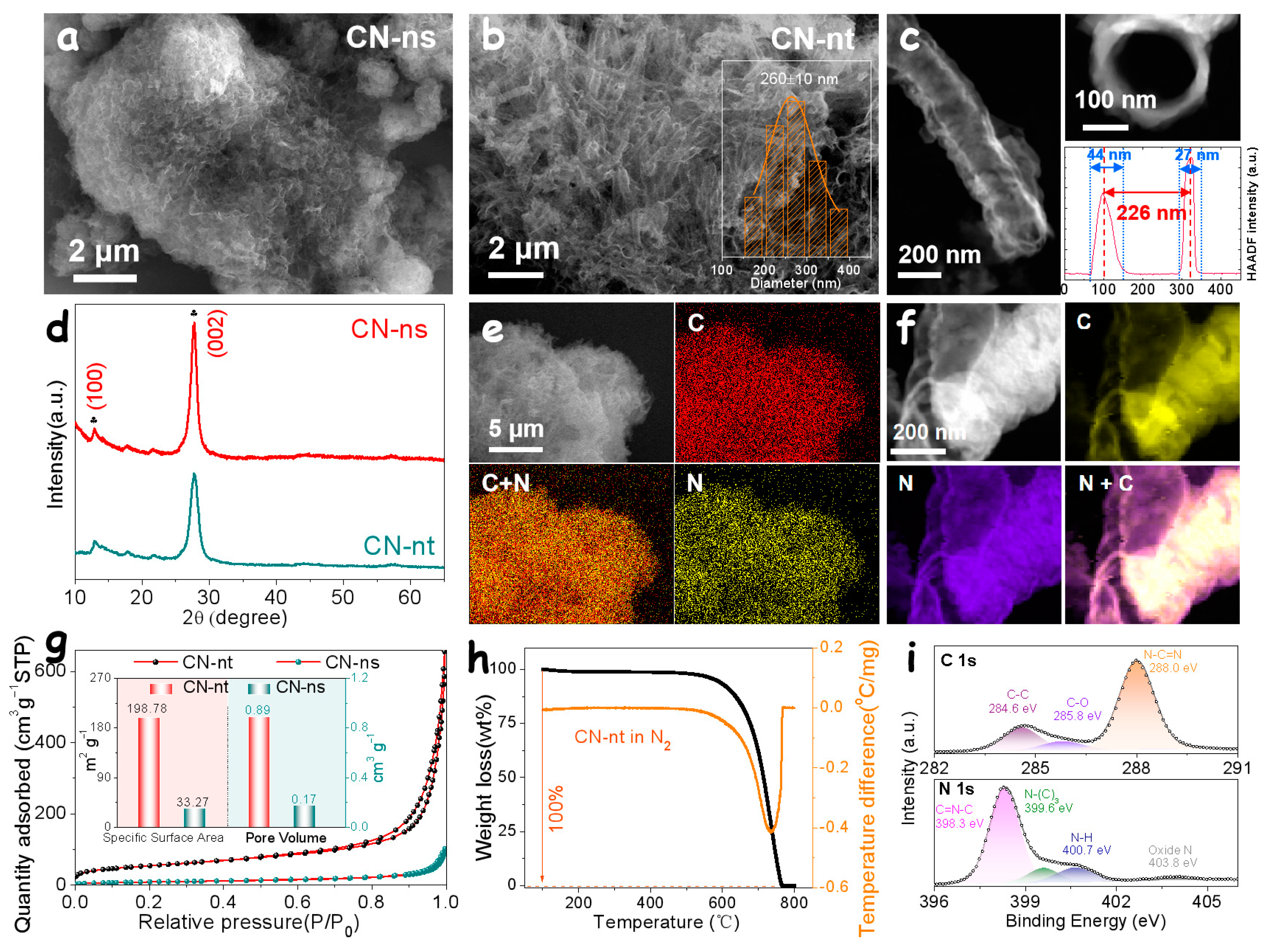
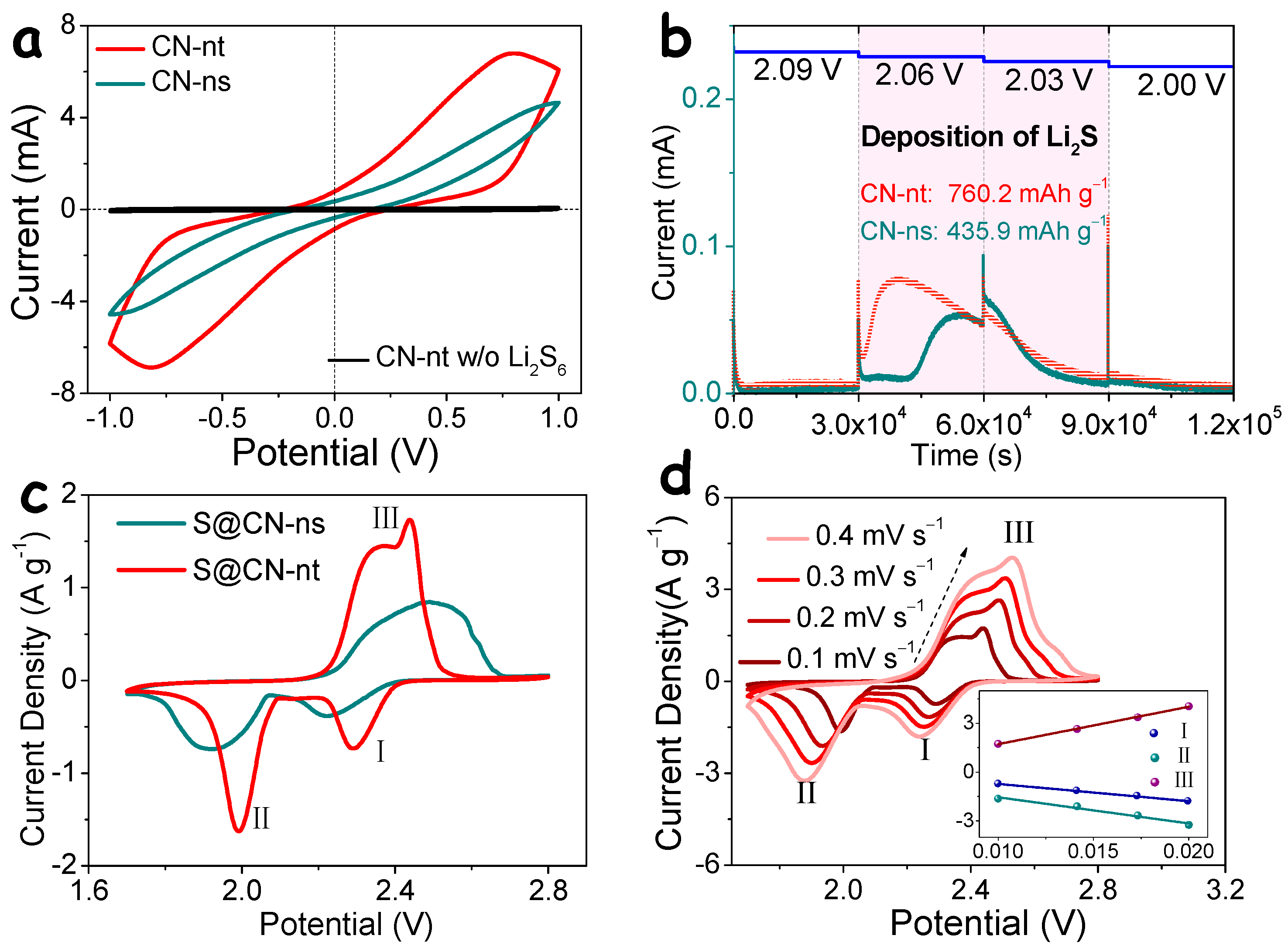
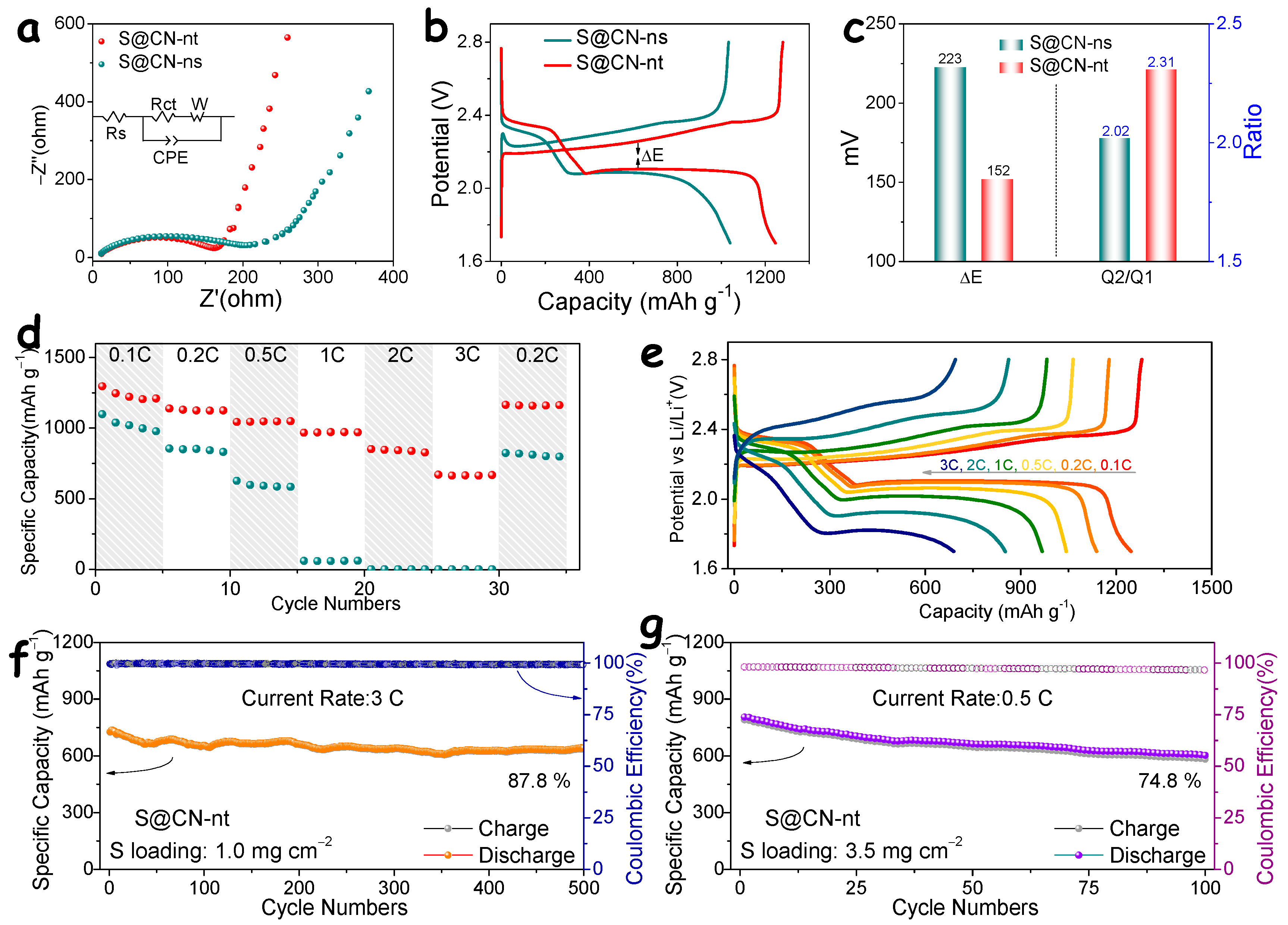
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Blomgren, G.E. The Development and Future of Lithium Ion Batteries. J. Electrochem. Soc. 2016, 164, A5019. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Lu, Y.-C. A Retrospective on Lithium-Ion Batteries. Nat. Commun. 2020, 11, 2499. [Google Scholar] [CrossRef]
- Seh, Z.W.; Sun, Y.; Zhang, Q.; Cui, Y. Designing High-Energy Lithium–Sulfur Batteries. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [Green Version]
- Manthiram, A.; Chung, S.-H.; Zu, C. Lithium–Sulfur Batteries: Progress and Prospects. Adv. Mater. 2015, 27, 1980–2006. [Google Scholar] [CrossRef]
- Eftekhari, A.; Kim, D.-W. Cathode Materials for Lithium–Sulfur Batteries: A Practical Perspective. J. Mater. Chem. A 2017, 5, 17734–17776. [Google Scholar] [CrossRef]
- Yang, D.; Li, M.; Zheng, X.; Han, X.; Zhang, C.; Jacas Biendicho, J.; Llorca, J.; Wang, J.; Hao, H.; Li, J.; et al. Phase Engineering of Defective Copper Selenide toward Robust Lithium–Sulfur Batteries. ACS Nano 2022, 16, 11102–11114. [Google Scholar] [CrossRef]
- Fei, B.; Zhang, C.; Cai, D.; Zheng, J.; Chen, Q.; Xie, Y.; Zhu, L.; Cabot, A.; Zhan, H. Hierarchical Nanoreactor with Multiple Adsorption and Catalytic Sites for Robust Lithium–Sulfur Batteries. ACS Nano 2021, 15, 6849–6860. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, D.; Biendicho, J.J.; Han, X.; Zhang, C.; Liu, K.; Diao, J.; Li, J.; Wang, J.; Heggen, M.; et al. Enhanced Polysulfide Conversion with Highly Conductive and Electrocatalytic Iodine-Doped Bismuth Selenide Nanosheets in Lithium–Sulfur Batteries. Adv. Funct. Mater. 2022, 32, 2200529. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Zhang, C.; Pan, J.L.; Sun, G.W.; Shi, Z.; Li, C.; Chang, X.; Sun, G.Z.; Zhou, J.Y.; Cabot, A. Surface Strain-Enhanced MoS2 as a High-Performance Cathode Catalyst for Lithium–Sulfur Batteries. eScience 2022, 2, 405–415. [Google Scholar] [CrossRef]
- Xu, H.; Kong, Z.; Siegenthaler, J.; Zheng, B.; Tong, Y.; Li, J.; Schuelke, T.; Fan, Q.H.; Wang, K.; Xu, H.; et al. Review on Recent Advances in Two-Dimensional Nanomaterials-Based Cathodes for Lithium-Sulfur Batteries. EcoMat 2023, 5, e12286. [Google Scholar] [CrossRef]
- Xu, Z.-L.; Kim, J.-K.; Kang, K. Carbon Nanomaterials for Advanced Lithium Sulfur Batteries. Nano Today 2018, 19, 84–107. [Google Scholar] [CrossRef]
- Wang, J.; Han, W.-Q. A Review of Heteroatom Doped Materials for Advanced Lithium–Sulfur Batteries. Adv. Funct. Mater. 2022, 32, 2107166. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Ma, T.; Zhang, Y.; Huang, H. 2D Graphitic Carbon Nitride for Energy Conversion and Storage. Adv. Funct. Mater. 2021, 31, 2102540. [Google Scholar] [CrossRef]
- Zuo, Y.; Xu, X.; Zhang, C.; Li, J.; Du, R.; Wang, X.; Han, X.; Arbiol, J.; Llorca, J.; Liu, J.; et al. SnS2/g-C3N4/Graphite Nanocomposites as Durable Lithium-Ion Battery Anode with High Pseudocapacitance Contribution. Electrochim. Acta 2020, 349, 136369. [Google Scholar] [CrossRef]
- Sun, W.; Song, Z.; Feng, Z.; Huang, Y.; Xu, Z.J.; Lu, Y.-C.; Zou, Q. Carbon-Nitride-Based Materials for Advanced Lithium–Sulfur Batteries. Nano-Micro Lett. 2022, 14, 222. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Yang, D.; Tang, P.; Zhang, C.; Biendicho, J.J.; Zhang, Y.; Llorca, J.; Wang, X.; Li, J.; Heggen, M.; et al. Atomically Dispersed Fe in a C2N Based Catalyst as a Sulfur Host for Efficient Lithium–Sulfur Batteries. Adv. Energy Mater. 2020, 11, 2003507. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, F.; Qin, Y.; Wang, N. Exfoliated Graphitic Carbon Nitride Nanosheets/Gold Nanoparticles/Spherical Montmorillonite Ternary Porous Heterostructures for the Degradation of Organic Dyes. ACS Appl. Nano Mater. 2020, 3, 7847–7857. [Google Scholar] [CrossRef]
- Du, R.; Xiao, K.; Li, B.; Han, X.; Zhang, C.; Wang, X.; Zuo, Y.; Guardia, P.; Li, J.; Chen, J.; et al. Controlled Oxygen Doping in Highly Dispersed Ni-Loaded g-C3N4 Nanotubes for Efficient Photocatalytic H2O2 Production. Chem. Eng. J. 2022, 441, 135999. [Google Scholar] [CrossRef]
- Jun, Y.-S.; Park, J.; Lee, S.U.; Thomas, A.; Hong, W.H.; Stucky, G.D. Three-Dimensional Macroscopic Assemblies of Low-Dimensional Carbon Nitrides for Enhanced Hydrogen Evolution. Angew. Chem. 2013, 125, 11289–11293. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Liu, L.-L.; Wu, J.-H.; Rui, X.-H.; Chen, J.-J.; Yu, Y. Single-Atom Iron Anchored Tubular g-C3N4 Catalysts for Ultrafast Fenton-Like Reaction: Roles of High-Valency Iron-Oxo Species and Organic Radicals. Adv. Mater. 2022, 34, 2202891. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, Y.; Chen, M.; Shi, X.; Zhang, Y.; Cao, J.; Ho, W.; Lee, S.C. Roles of N-Vacancies over Porous g-C3N4 Microtubes during Photocatalytic NOx Removal. ACS Appl. Mater. Interfaces 2019, 11, 10651–10662. [Google Scholar] [CrossRef]
- Iqbal, O.; Ali, H.; Li, N.; Ansari, M.Z.; Al-Sulami, A.I.; Alshammari, K.F.; Abd-Rabboh, H.S.M.; Al-Hadeethi, Y.; Taha, T.A.; Zada, A.; et al. A Review on the Synthesis, Properties, and Characterizations of Graphitic Carbon Nitride (g-C3N4) for Energy Conversion and Storage Applications. Mater. Today Phys. 2023, 34, 101080. [Google Scholar] [CrossRef]
- Han, Z.; Wang, N.; Fan, H.; Ai, S. Ag Nanoparticles Loaded on Porous Graphitic Carbon Nitride with Enhanced Photocatalytic Activity for Degradation of Phenol. Solid State Sci. 2017, 65, 110–115. [Google Scholar] [CrossRef]
- Xue, J.; Ma, S.; Zhou, Y.; Zhang, Z.; He, M. Facile Photochemical Synthesis of Au/Pt/g-C3N4 with Plasmon-Enhanced Photocatalytic Activity for Antibiotic Degradation. ACS Appl. Mater. Interfaces 2015, 7, 9630–9637. [Google Scholar] [CrossRef]
- Liu, Y.; Han, J.; Zeng, X.; Tian, Z.; Yu, F.; Sun, X.; Liu, Q.; Wang, W. G-C3N4 Homophase Junction with High Crystallinity Using MoS2 as Cocatalyst for Robust Visible-Light-Driven Photocatalytic Pollutant Degradation. ChemistrySelect 2022, 7, e202103884. [Google Scholar] [CrossRef]
- Zhang, C.; Du, R.; Biendicho, J.J.; Yi, M.; Xiao, K.; Yang, D.; Zhang, T.; Wang, X.; Arbiol, J.; Llorca, J.; et al. Tubular CoFeP@CN as a Mott–Schottky Catalyst with Multiple Adsorption Sites for Robust Lithium−Sulfur Batteries. Adv. Energy Mater. 2021, 11, 2100432. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, S.; Hu, R.; Gu, J.; Cui, Y.; Li, B.; Chen, W.; Liu, C.; Shang, J.; Yang, S. Catalytic Conversion of Polysulfides on Single Atom Zinc Implanted MXene toward High-Rate Lithium–Sulfur Batteries. Adv. Funct. Mater. 2020, 30, 2002471. [Google Scholar] [CrossRef]
- Zhang, C.; Biendicho, J.J.; Zhang, T.; Du, R.; Li, J.; Yang, X.; Arbiol, J.; Zhou, Y.; Morante, J.R.; Cabot, A. Combined High Catalytic Activity and Efficient Polar Tubular Nanostructure in Urchin-Like Metallic NiCo2Se4 for High-Performance Lithium–Sulfur Batteries. Adv. Funct. Mater. 2019, 29, 1903842. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Zhang, C.; Zhou, G.; Lv, W.; Ling, G.; Zhi, L.; Yang, Q.-H. Catalytic Effects in Lithium–Sulfur Batteries: Promoted Sulfur Transformation and Reduced Shuttle Effect. Adv. Sci. 2018, 5, 1700270. [Google Scholar] [CrossRef]
- Wang, P.; Xi, B.; Huang, M.; Chen, W.; Feng, J.; Xiong, S. Emerging Catalysts to Promote Kinetics of Lithium–Sulfur Batteries. Adv. Energy Mater. 2021, 11, 2002893. [Google Scholar] [CrossRef]
- Yang, D.; Liang, Z.; Zhang, C.; Biendicho, J.J.; Botifoll, M.; Spadaro, M.C.; Chen, Q.; Li, M.; Ramon, A.; Moghaddam, A.O.; et al. NbSe2 Meets C2N: A 2D-2D Heterostructure Catalysts as Multifunctional Polysulfide Mediator in Ultra-Long-Life Lithium–Sulfur Batteries. Adv. Energy Mater. 2021, 11, 2101250. [Google Scholar] [CrossRef]
- Zhang, T.; Shao, W.; Liu, S.; Song, Z.; Mao, R.; Jin, X.; Jian, X.; Hu, F. A Flexible Design Strategy to Modify Ti3C2Tx MXene Surface Terminations via Nucleophilic Substitution for Long-Life Li-S Batteries. J. Energy Chem. 2022, 74, 349–358. [Google Scholar] [CrossRef]
- Chen, Z.-X.; Zhang, Y.-T.; Bi, C.-X.; Zhao, M.; Zhang, R.; Li, B.-Q.; Huang, J.-Q. Premature Deposition of Lithium Polysulfide in Lithium–Sulfur Batteries. J. Energy Chem. 2023, 82, 507–512. [Google Scholar] [CrossRef]
- Fan, F.Y.; Carter, W.C.; Chiang, Y.-M. Mechanism and Kinetics of Li2S Precipitation in Lithium-Sulfur Batteries. Adv. Mater. 2015, 27, 5203–5209. [Google Scholar] [CrossRef]
- Yuan, H.; Peng, H.-J.; Li, B.-Q.; Xie, J.; Kong, L.; Zhao, M.; Chen, X.; Huang, J.-Q.; Zhang, Q. Conductive and Catalytic Triple-Phase Interfaces Enabling Uniform Nucleation in High-Rate Lithium-Sulfur Batteries. Adv. Energy Mater. 2018, 9, 1802768. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Q.; Zhan, H. Supercapacitors Based on Reduced Graphene Oxide Nanofibers Supported Ni(OH)2 Nanoplates with Enhanced Electrochemical Performance. ACS Appl. Mater. Interfaces 2016, 8, 22977–22987. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Ma, X.; Han, X.; Xing, C.; Qi, X.; He, R.; Arbiol, J.; Pan, H.; Zhao, J.; et al. Selective Ethylene Glycol Oxidation to Formate on Nickel Selenide with Simultaneous Evolution of Hydrogen. Adv. Sci. 2023, 10, 2300841. [Google Scholar] [CrossRef]
- Zhao, M.; Tan, P.; Cai, D.; Liu, Y.; Zhang, C.; Fei, B.; Sa, B.; Chen, Q.; Zhan, H. Customizing Component Regulated Dense Heterointerfaces for Crafting Robust Lithium-Sulfur Batteries. Adv. Funct. Mater. 2023, 33, 2211505. [Google Scholar] [CrossRef]
- Fu, J.; Shen, Z.; Cai, D.; Fei, B.; Zhang, C.; Wang, Y.; Chen, Q.; Zhan, H. A Hierarchical VN/Co3ZnC@NCNT Composite as a Multifunctional Integrated Host for Lithium–Sulfur Batteries with Enriched Adsorption Sites and Accelerated Conversion Kinetics. J. Mater. Chem. A 2022, 10, 20525–20534. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Zhang, C.; Sun, G.W.; Pan, J.L.; Gong, L.; Sun, G.Z.; Biendicho, J.J.; Balcells, L.; Fan, X.L.; Morante, J.R.; et al. Spin Effect to Promote Reaction Kinetics and Overall Performance of Lithium-Sulfur Batteries under External Magnetic Field. Angew. Chem. Int. Ed. 2022, 61, e202211570. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, C.; Luo, Z.; Zhang, T.; Liu, J.; Li, J.; Zuo, Y.; Biendicho, J.J.; Llorca, J.; Arbiol, J.; et al. A Low Temperature Solid State Reaction to Produce Hollow MnxFe3-XO4 Nanoparticles as Anode for Lithium-Ion Batteries. Nano Energy 2019, 66, 104199. [Google Scholar] [CrossRef]
- Zhang, C.; Fei, B.; Yang, D.; Zhan, H.; Wang, J.; Diao, J.; Li, J.; Henkelman, G.; Cai, D.; Biendicho, J.J.; et al. Robust Lithium–Sulfur Batteries Enabled by Highly Conductive WSe2-Based Superlattices with Tunable Interlayer Space. Adv. Funct. Mater. 2022, 32, 2201322. [Google Scholar] [CrossRef]
- Qie, L.; Zu, C.; Manthiram, A. A High Energy Lithium-Sulfur Battery with Ultrahigh-Loading Lithium Polysulfide Cathode and Its Failure Mechanism. Adv. Energy Mater. 2016, 6, 1502459. [Google Scholar] [CrossRef]
- Yang, J.; Xu, L.; Li, S.; Peng, C. The Role of Titanium-Deficient Anatase TiO2 Interlayers in Boosting Lithium–Sulfur Battery Performance: Polysulfide Trapping, Catalysis and Enhanced Lithium Ion Transport. Nanoscale 2020, 12, 4645–4654. [Google Scholar] [CrossRef]
- Yang, D.; Liang, Z.; Tang, P.; Zhang, C.; Tang, M.; Li, Q.; Biendicho, J.J.; Li, J.; Heggen, M.; Dunin-Borkowski, R.E.; et al. A High Conductivity 1D π–d Conjugated Metal–Organic Framework with Efficient Polysulfide Trapping-Diffusion-Catalysis in Lithium–Sulfur Batteries. Adv. Mater. 2022, 34, 2108835. [Google Scholar] [CrossRef]
- Chu, R.; Nguyen, T.T.; Bai, Y.; Kim, N.H.; Lee, J.H. Uniformly Controlled Treble Boundary Using Enriched Adsorption Sites and Accelerated Catalyst Cathode for Robust Lithium–Sulfur Batteries. Adv. Energy Mater. 2022, 12, 2102805. [Google Scholar] [CrossRef]
- Lv, D.; Zheng, J.; Li, Q.; Xie, X.; Ferrara, S.; Nie, Z.; Mehdi, L.B.; Browning, N.D.; Zhang, J.-G.; Graff, G.L.; et al. High Energy Density Lithium–Sulfur Batteries: Challenges of Thick Sulfur Cathodes. Adv. Energy Mater. 2015, 5, 1402290. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, X.; Ju, Z.; Wang, L.; Hui, Z.; Mayilvahanan, K.; Takeuchi, K.J.; Marschilok, A.C.; West, A.C.; Takeuchi, E.S.; et al. From Fundamental Understanding to Engineering Design of High-Performance Thick Electrodes for Scalable Energy-Storage Systems. Adv. Mater. 2021, 33, 2101275. [Google Scholar] [CrossRef]
- Zheng, H.; Li, J.; Song, X.; Liu, G.; Battaglia, V.S. A Comprehensive Understanding of Electrode Thickness Effects on the Electrochemical Performances of Li-Ion Battery Cathodes. Electrochim. Acta 2012, 71, 258–265. [Google Scholar] [CrossRef]
- Dong, F.; Sun, Y.; Wu, L.; Fu, M.; Wu, Z. Facile Transformation of Low Cost Thiourea into Nitrogen-Rich Graphitic Carbon Nitride Nanocatalyst with High Visible Light Photocatalytic Performance. Catal. Sci. Technol. 2012, 2, 1332–1335. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Ho, W.; Zhang, Y.; Huang, H.; Cai, Q.; Dong, F. Highly Enhanced Visible-Light Photocatalytic NOx Purification and Conversion Pathway on Self-Structurally Modified g-C3N4 Nanosheets. Sci. Bull. 2018, 63, 609–620. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Zhang, G.; Lin, Z.; Wang, X. Condensed and Low-Defected Graphitic Carbon Nitride with Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation. Appl. Catal. B Environ. 2016, 181, 413–419. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Wu, G.; Chen, W. Porous Graphitic Carbon Nitride Synthesized via Direct Polymerization of Urea for Efficient Sunlight-Driven Photocatalytic Hydrogen Production. Nanoscale 2012, 4, 5300–5303. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Zhang, H.; Yu, Y.; Chen, Y.; Yan, J.; Li, X.; Zhang, H. Trithiocyanuric Acid Derived g–C3N4 for Anchoring the Polysulfide in Li–S Batteries Application. J. Energy Chem. 2020, 43, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Hartley, G.; Ward, A.J.; Young, P.A.; Masters, A.F.; Maschmeyer, T. Hydrogenated Defects in Graphitic Carbon Nitride Nanosheets for Improved Photocatalytic Hydrogen Evolution. J. Phys. Chem. C 2015, 119, 14938–14946. [Google Scholar] [CrossRef]
- Xu, H.; Yan, J.; She, X.; Xu, L.; Xia, J.; Xu, Y.; Song, Y.; Huang, L.; Li, H. Graphene-Analogue Carbon Nitride: Novel Exfoliation Synthesis and Its Application in Photocatalysis and Photoelectrochemical Selective Detection of Trace Amount of Cu2+. Nanoscale 2014, 6, 1406–1415. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Li, Y.; Li, S.; Liu, N. Novel PO Codoped G-C3N4 with Large Specific Surface Area: Hydrothermal Synthesis Assisted by Dissolution–Precipitation Process and Their Visible Light Activity under Anoxic Conditions. Appl. Surf. Sci. 2015, 357, 131–138. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Antonietti, M.; Zhang, Y. Facile One-Pot Synthesis of Nanoporous Carbon Nitride Solids by Using Soft Templates. ChemSusChem 2010, 3, 435–439. [Google Scholar] [CrossRef]
- Li, X.-H.; Wang, X.; Antonietti, M. Mesoporous G-C3N4 Nanorods as Multifunctional Supports of Ultrafine Metal Nanoparticles: Hydrogen Generation from Water and Reduction of Nitrophenol with Tandem Catalysis in One Step. Chem. Sci. 2012, 3, 2170–2174. [Google Scholar] [CrossRef]
- Lee, E.Z.; Jun, Y.-S.; Hong, W.H.; Thomas, A.; Jin, M.M. Cubic Mesoporous Graphitic Carbon(IV) Nitride: An All-in-One Chemosensor for Selective Optical Sensing of Metal Ions. Angew. Chem. 2010, 122, 9900–9904. [Google Scholar] [CrossRef]
- Wang, J.; Meng, Z.; Yang, W.; Yan, X.; Guo, R.; Han, W.-Q. Facile Synthesis of RGO/g-C3N4/CNT Microspheres via an Ethanol-Assisted Spray-Drying Method for High-Performance Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2019, 11, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Nazar, L.F. Long-Life and High-Areal-Capacity Li–S Batteries Enabled by a Light-Weight Polar Host with Intrinsic Polysulfide Adsorption. ACS Nano 2016, 10, 4111–4118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Z.; Hou, Y.-N.; Tang, Y.; Dong, Y.; Wang, S.; Hu, X.; Zhang, Z.; Wang, X.; Qiu, J. Nanopore-Confined g-C3N4 Nanodots in N, S Co-Doped Hollow Porous Carbon with Boosted Capacity for Lithium–Sulfur Batteries. J. Mater. Chem. A 2018, 6, 7133–7141. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, X.; Li, J.; Han, T.; Zhu, M.; Xu, X.; Hu, C.; Liu, J. A Binder-Free Lithium-Sulfur Battery Cathode Using Three-Dimensional Porous g-C3N4 Nanoflakes as Sulfur Host Displaying High Binding Energies with Lithium Polysulfides. J. Alloys Compd. 2021, 881, 160629. [Google Scholar] [CrossRef]
- Wang, W.; Dong, W.; Hong, X.; Liu, Y.; Yang, S. Preparation of G-C3N4/CNTs Composite by Dissolution-Precipitation Method as Sulfur Host for High-Performance Lithium-Sulfur Batteries. Mater. Chem. Phys. 2022, 283, 126014. [Google Scholar] [CrossRef]
- Moon, S.-H.; Shin, J.-H.; Kim, J.-H.; Jang, J.-S.; Kim, S.-B.; Park, Y.-Y.; Lee, S.-N.; Park, K.-W. Polypyrrole Coated G-C3N4/RGO/S Composite as Sulfur Host for High Stability Lithium-Sulfur Batteries. Mater. Chem. Phys. 2022, 287, 126267. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Du, R.; Martí-Sánchez, S.; Xiao, K.; Yang, D.; Zhang, C.; Li, C.; Zeng, G.; Chang, X.; He, R.; et al. Tubular C3N4 Nanotubes as Metal-Free Sulfur Hosts toward Stable Lithium–Sulfur Batteries. Energies 2023, 16, 4545. https://doi.org/10.3390/en16124545
Zhang C, Du R, Martí-Sánchez S, Xiao K, Yang D, Zhang C, Li C, Zeng G, Chang X, He R, et al. Tubular C3N4 Nanotubes as Metal-Free Sulfur Hosts toward Stable Lithium–Sulfur Batteries. Energies. 2023; 16(12):4545. https://doi.org/10.3390/en16124545
Chicago/Turabian StyleZhang, Chaoqi, Ruifeng Du, Sara Martí-Sánchez, Ke Xiao, Dawei Yang, Chaoyue Zhang, Canhuang Li, Guifang Zeng, Xingqi Chang, Ren He, and et al. 2023. "Tubular C3N4 Nanotubes as Metal-Free Sulfur Hosts toward Stable Lithium–Sulfur Batteries" Energies 16, no. 12: 4545. https://doi.org/10.3390/en16124545





