Abstract
Surfactant-assisted spontaneous imbibition is an important mechanism in enhanced oil recovery by capillary pressure in low permeability and tight oil reservoirs. Though many experiments have been conducted to study the mechanism of enhanced oil recovery by surfactant-assisted spontaneous imbibition, the effects of surfactant type, concentration, and temperature have not been well studied. Using tight sandstone outcrop core samples with similar permeability and porosity, this paper experimentally studies surfactant-assisted spontaneous imbibition using three different surfactant types, i.e., sodium dodecylbenzene sulfonate (SDBS), cocamidopropyl betaine (CAB), and C12–14 fatty alcohol glycoside (APG). In addition to the type of surfactant, the effect of the surfactant concentration and the temperature is also investigated. The study results show that the ultimate oil recovery of spontaneous imbibition with formation water and denoised water is about 10%. Surfactant can significantly improve the oil recovery of spontaneous imbibition by reducing the interfacial tension between oil and water, emulsifying crude oil and improving oil mobility. APG showed better performance compared to SDBS and CAB, with a maximum oil recovery factor of 36.19% achieved with formation water containing 0.05% APG surfactant. Lower concentrations (0.05% APG) in the formation water resulted in a higher oil recovery factor compared to 0.1% APG. Increasing temperature also improves oil recovery by reducing oil viscosity. This empirical study contributes to a better understanding of the mechanism of surfactant-assisted spontaneous imbibition and enhanced oil recovery in tight oil reservoirs.
1. Introduction
The development of unconventional oil reservoirs has attracted significant attention following conventional oil reservoirs. Spontaneous imbibition, as a crucial process for enhancing oil recovery in shale and tight formations, has gradually gained recognition. Imbibition is a prevalent phenomenon across various natural sciences, referring to the process wherein a wetting liquid, driven by capillary forces, displaces a non-wetting phase as it enters a porous media [1,2,3,4]. Imbibition can be classified into co-current imbibition and counter-current imbibition based on the flow direction of the wetting and non-wetting phases [5,6]. Co-current imbibition relies primarily on gravitational effects, resulting in a consistent flow direction of oil and water. Conversely, counter-current imbibition involves opposite flow directions of oil and water [7].
Researchers have been building on this foundation with studies of the mechanisms of imbibition [8,9,10]. Current research indicates that rock permeability, fracture density, interfacial tension (IFT), and crude oil viscosity are the primary factors influencing the imbibition processes [11,12]. Alvarez et al. [13] analyzed the impact of lithology, oil, and surfactant types on wettability and changes in IFT, finding that anionic surfactants were most effective in reducing the IFT between two phases. According to Chen et al. [14], nonionic surfactants generally outperformed ionic surfactants in terms of changing wettability and improving oil recovery. Through simulation methods and theoretical analysis, Sheng [15] showed that surfactants need to change the wettability and maintain a high IFT at the same time to effectively change the wettability in shale and tight rock reservoirs. Additionally, they found that slow diffusion rates negatively impacted the enhancement of oil recovery through spontaneous imbibition. Liu et al. [16], focusing on shale formations in the Sichuan Basin of China, analyzed the mechanisms underlying the changes in wettability induced by non-ionic and anionic surfactants. They also clarified the impact of wettability alteration and reduced IFT on the spontaneous imbibition process in oil-wet shale.
Surfactants are surface active agents that spontaneously aggregate or adsorb at the oil–water interface at low concentrations. The molecules of surfactants are divided into hydrophilic and hydrophobic ends. The hydrophilic end is oriented towards the aqueous phase, while the hydrophobic end is oriented towards the oil phase. These ends are arranged directionally at the interface and are adsorbed to form an orderly monolayer [17]. This reduces the surface tension and IFT by lowering the molecular weight of water at the oil–water interface [18]. Surfactants are utilized to improve oil recovery. Adding an appropriate amount of surfactant can significantly reduce the IFT between oil and water [19]. Surfactants can also change the wettability of the rock surface [3], and emulsified crude oil forms such as O/W or W/O emulsions. To date, the various surfactants studied include anionic surfactants [20,21,22,23,24], cationic surfactants [25], nonionic surfactants [26,27,28,29], and zwitterionic surfactants [30,31,32,33]. Among all surfactants, anionic surfactants have been the most extensively studied due to their low adsorption loss on sandstone surfaces. Nonionic surfactants have a wider pH range of application compared to ionic surfactants. They can be used in combination with other ionic surfactants to improve the surface activity of the system [16,34]. Zwitterionic surfactants have two active groups, which can be anionic, cationic, nonionic, or anionic. They are hydrophilic and have excellent temperature and salt tolerance. Nevertheless, their high prices limit their usage in enhanced oil recovery [35].
Drawing on extensive research in the literature [3,14,36,37], three surfactants, including sodium dodecylbenzene sulfonate (SDBS), cocamidopropyl betaine (CAB), and C12–14 fatty alcohol glycoside (APG), were selected for experimentation. Among them, SDBS is a commonly used anionic surfactant, CAB is a zwitterionic surfactant, and APG is a kind of non-ionic surfactant. APG can be widely used in high temperature and high salinity reservoirs because of its good ecological properties [37]. Moreover, compared with other surfactants, APG is a safer and less polluting surfactant. Tight sand outcrop cores were specifically chosen due to their closely matched porosity and permeability, minimizing the interference of pore-throat structure in imbibition results. This experiment concluded that, among the three different surfactants, the APG solution with a mass fraction of 0.05% had the lowest IFT and the best emulsification during the imbibition process. This was established through analyzing the core imbibition conditions under different surfactant solution types, concentrations, and temperatures. This surfactant solution effectively reduced the flow resistance of crude oil, resulting in enhanced oil recovery. Moreover, the study pioneered the exploration of the promoting effect of temperature on the imbibition process.
2. Materials and Methods
2.1. Experimental Materials
For this investigation, eight tight sandstone cores were collected from the Yanchang Formation, Ordos Basin, NW China, as shown in Figure 1. Prior to the experiments, a thorough cleaning process involving oil washing and drying was conducted on all cores to ensure the absence of oil and gas impurities. Table 1 details the essential physical parameters of the cores, including permeability ranging from 0.317 mD to 0.345 mD, and an average permeability of 0.328 mD. The porosity spanned from 15.67% to 16.03%, with an average porosity of 15.87%.

Figure 1.
Outcrop sandstone cores.

Table 1.
Basic physical parameters of outcrop tight sandstone cores.
As shown in Table 2, the formation water used in the experiments exhibited a pH value of 7.03. Analysis of its mineralization and ion content revealed a total mineralization of 5976.8 mg/L. The formation water contained the cations K+, Na+, Ca2+, and Mg2+, and the anions Cl−, SO42−, and HCO3−. Notably, the combined concentration of K+ and Na+ was as high as 1853.3 mg/L, Ca2+ was present at a concentration of 133.4 mg/L, Mg2+ at 37.4 mg/L, Cl− at 11,936.7 mg/L, SO42− at 560.3 mg/L, and HCO3− at 1455.7 mg/L.

Table 2.
Ion content in formation water.
The simulated oil used in the experiment was concocted by blending crude oil from the Chang 8 layer in Ordos Basin, NW China with kerosene in a 1:1 volumetric ratio. Under room temperature conditions (25 °C), the measured density and viscosity of the simulated oil were 0.8205 g/cm3 and 9.33 mPa·s, respectively. The imbibition fluids comprised solutions prepared with the formation water, incorporating SDBS, CAB, and APG. The specific sources of each reagent are shown in Table 3.

Table 3.
Experimental reagents.
2.2. Experimental Equipment
The experimental setup involved the utilization of the following instruments: the APL-1 vacuum saturation apparatus (Jiangsu Lianyou Scientific Research Instrument Co., Nantong, China), designed for evacuating air from rock cores and saturating them with the prepared simulated oil under the specified pressure; the SVT-20 spinning drop tensiometer (Data Physics Instruments GmbH, Filderstadt, Germany), employed for determining the IFT between crude oil and the imbibition fluid; the KH-55A (250 °C/136 L) constant temperature chamber (Shanghai K&M Measurement Technology Co., Shanghai, China), responsible for controlling the experimentally set temperature; and a high-precision imbibition cell with an accuracy of 0.01 mL. Additionally, other experimental instruments such as 250 mL glass bottles, an electronic balance (Shanghai Zhuojing Electronic Technology Co., Shanghai, China) with a precision of 1 × 10−4 g, and an OS20-S magnetic stirrer (Da Long Xing Chuang Experimental Instrument (Beijing) Co., Ltd., Beijing, China) were also utilized.
2.3. Experimental Design and Procedure
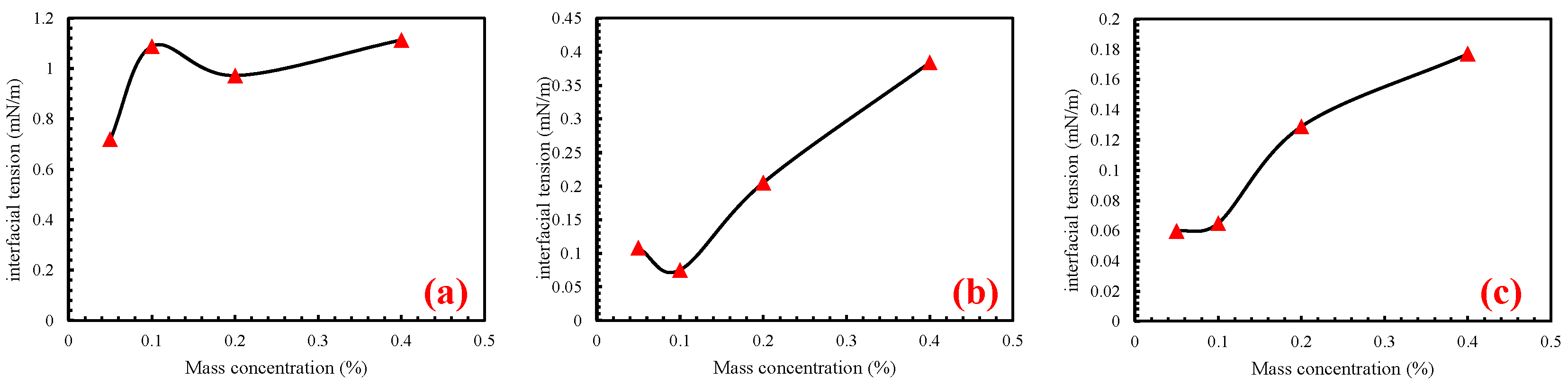
The IFT of the three surfactants at different mass concentrations was measured. It can be seen from Table 4 and Figure 2 that the overall IFT increased with an increase in mass concentration. Overall, the IFT decreased initially, but then increased with an increase in mass concentration. For SDBS, at a mass concentration of 0.05%, the minimum IFT was 0.719 mN/m. However, as the mass concentration increased from 0.1% to 0.2%, the IFT of SDBS solution decreased from 1.088 mN/m to 0.972 mN/m, showing a relatively small decrease.

Table 4.
IFT of three surfactant solutions at different mass concentrations.
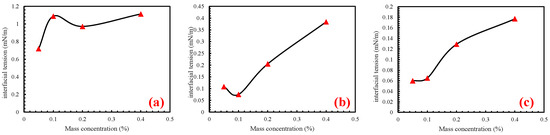
Figure 2.
The IFT of three surfactant solutions at different mass concentrations. (a) is the interfacial pressure of SDBS at different mass concentrations, (b) is the interfacial pressure of CAB at different mass concentrations, (c) is the interfacial pressure of APG at different mass concentrations.
In the case of CAB solution, when the mass concentration ranged from 0.05% to 0.1%, the IFT decreased from 0.108 mN/m to 0.075 mN/m, indicating a slight decrease with limited variation. For APG solutions, IFT increased with increasing mass concentration. Notably, the change in IFT was minimal as the mass concentration increased from 0.05% to 0.1%, increasing only from 0.060 mN/m to 0.065 mN/m. However, beyond a mass concentration of 0.1%, the IFT rapidly increased with increasing concentration.
The surfactant solutions used for imbibition liquid included a 0.05% mass concentration of SDBS, 0.05% and 0.1% mass concentrations of CAB, and 0.05% and 0.1% mass concentrations of APG. These solutions were mixed with formation water to form imbibition fluids. Control experiments were also conducted using only formation water and deionized water for comparison.
Surfactant molecules aggregate with increasing concentration to form micelles. These micelles have a hydrophilic head group outward and a lipophilic head group inward. The concentration at which this occurs is called the critical micelle concentration (CMC) [38,39]. Once the concentration surpasses this threshold, the number of monomer molecules in the solution will no longer increase. Additionally, the adsorption capacity at the interface will reach saturation, and the IFT will cease to decrease. Furthermore, any excess surfactant molecules will exist in the form of micelles.
Table 5 revealed that the IFT between the crude oil and imbibition fluids ranged from 0.060 mN/m to 14.759 mN/m. Notably, the IFT of crude oil in surfactant solutions was significantly lower than that in the formation water solution. Specifically, the 0.1% APG solution prepared with formation water exhibited the lowest IFT at 0.06 mN/m. Therefore, surfactant concentration had a potential impact on the imbibition process in the core by simulating the IFT between the oil and imbibition fluid.

Table 5.
IFT of crude oil in different imbibed fluids.
In order to reveal the oil-displacing effect of different types of surfactants, three surfactants, SDBS, CAB, and APG, were selected. Based on the three surfactants selected above, different concentrations of surfactant solutions were configured for the spontaneous imbibition experiments. The experimental plan is shown in Table 6.

Table 6.
Scheme of imbibition experiments.
Prior to conducting the imbibition experiment, it was essential to perform a saturation experiment on the tight sandstone cores to measure their dry and wet weights. This procedure ensured that the tight sandstone cores were fully saturated with the simulated oil, providing a basis for subsequent imbibition experiments. The steps for vacuuming and pressurizing crude oil were executed as follows:
- The dry and weighed core were placed into the core chamber. The simulated oil was injected into the storage tank, and the storage tank and core chamber were sealed.
- The vacuum pump was turned on in order for 48 h to evacuate the sandstone core and simulated oil, until the pressure within the core stabilized.
- After the core and simulated oil had been evacuated, the vacuum pump was turned off.
- The liquid storage tank valve and the liquid inlet valve were opened to allow the simulated oil in a vacuum state to enter the core chamber. The hand pump was turned clockwise to increase the pressure in the core chamber to 25 MPa and the core was pressurized and saturated for 72 h, until the pressure within the core stabilized.
- The hand pump was turned counterclockwise to relieve the pressure in the core chamber. The core chamber was opened and the core was removed. An electronic balance was used to measure the wet weight of the core.
- The volume of core saturated with simulated oil was calculated.
The dry weight and wet weight of the core before and after saturation are shown in Table 7. The volume of oil saturation in the tight sandstone cores ranged from 4.804 mL to 4.923 mL. Overall, there was minimal variation in the volume of oil saturation across the tight sandstone cores. This is because these outcrop cores had similar physical properties, and the difference in porosity and permeability variations was not significant. Notably, the CQ2–6 tight sandstone core exhibited the highest oil saturation, reaching up to 4.923 mL. This outcome could be attributed to the favorable physical properties of the CQ2–6 core, boasting the highest permeability among all cores and possessing superior mass characteristics.

Table 7.
Dry and wet weight of cores before and after oil saturation.
To elucidate the mechanism by which cores enhance oil recovery through spontaneous imbibition, imbibition experiments were conducted using the imbibition cell (as shown in Figure 3). The experimental steps were as follows:

Figure 3.
Imbibition bottle used in the experiments.
- Before the experiment, the imbibition cell was rinsed with the prepared imbibition solution, and then the weighed core was put into the imbibition cell.
- The imbibition liquid from the rubber tube was injected into the imbibition cell until the imbibition liquid rose to 1/3~2/3 of the scale tube of the imbibition cell. The openings of the scale tube and rubber tube were sealed with plastic wrap.
- After ensuring that there was no leakage at the interface of the imbibition cell, the imbibition experiment was initiated.
- The initial time of core imbibition was recorded. Then, the amount of oil imbibed at intervals was recorded according to experimental conditions, and photos were taken to observe the adsorption state of crude oil.
3. Results and Discussion
To investigate the influence of different surfactant types, concentrations, and temperatures on the imbibition efficiency of tight sandstone cores, static spontaneous imbibition experiments were conducted on eight outcrop sandstone cores. The experimental results are summarized in Table 8.

Table 8.
Parameters of core samples and imbibition liquid, and ultimate oil recovery factors.
Table 8 shows that the produced oil volumes for these eight cores ranged from 0.47 mL to 1.755 mL, resulting in final recovery rates between 9.63% and 36.19%. Notably, cores CQ2–4 and CQ2–6 exhibited the lowest recovery rates. This can be attributed to the fact that the imbibition fluids for these cores were formation water and deionized water, respectively, leading to higher IFT and increased flow resistance. Consequently, crude oil struggled to detach from the core surfaces. On the other hand, the ultimate oil recovery of the cores percolated in the surfactant solution ranged from 18.94% to 36.19%, which illustrates that adding surfactant into the imbibition liquid significantly improved the ultimate oil recovery.
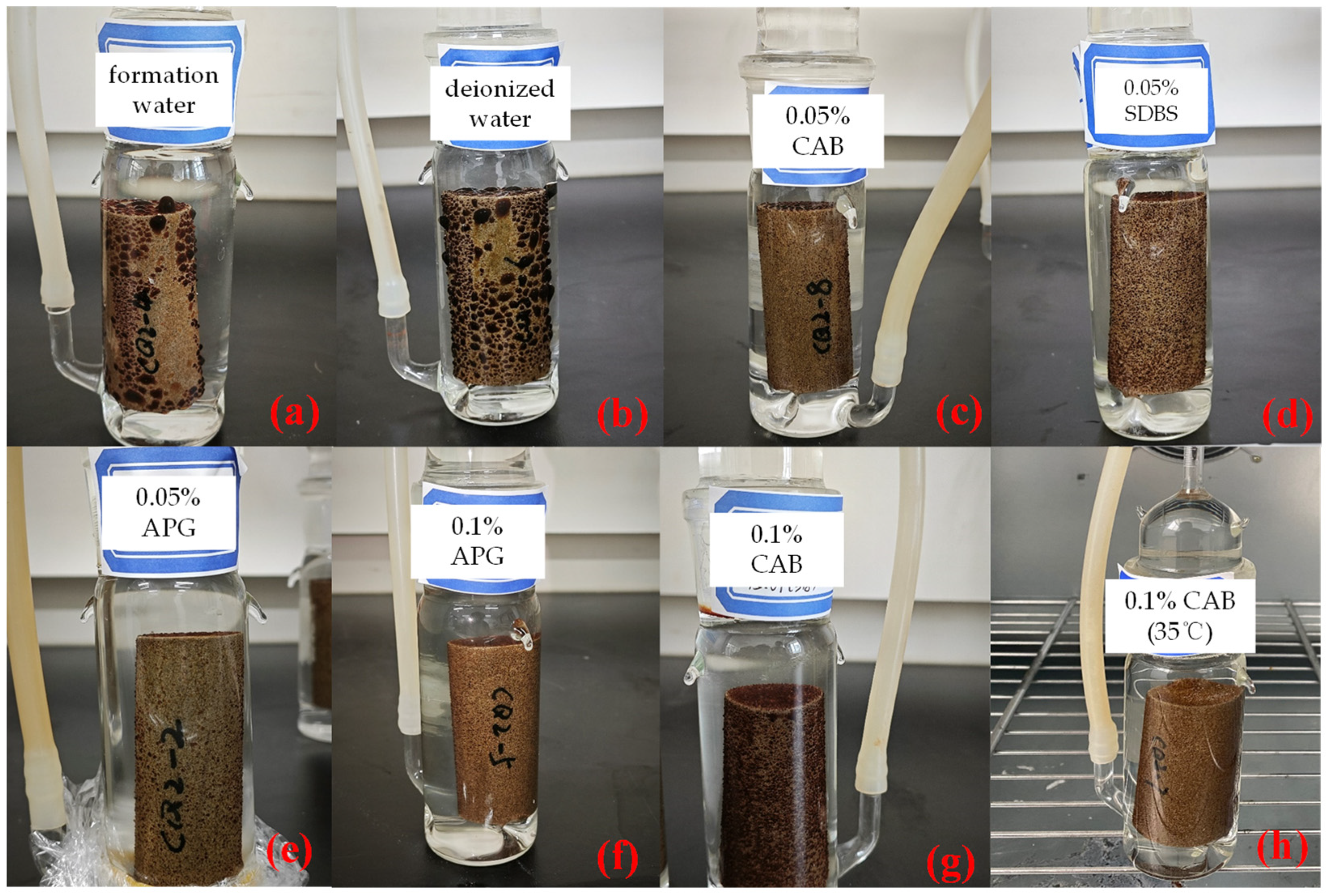
As illustrated in Figure 4, it is evident that in imbibition liquids with higher IFT, such as formation water and deionized water, crude oil adsorbed onto the core surfaces in larger oil droplets. In contrast, when there is surfactant in the imbibition fluid, numerous small oil droplets dispersed across the sidewalls of the core, accompanied by a few larger oil droplets on the core surface. This observation suggests that surfactant can reduce IFT and emulsify crude oil, making large oil droplets more prone to dispersion into numerous smaller droplets that detach from the core surface. Consequently, this enhances the oil mobility. Furthermore, for imbibition solutions with the same surfactant type but different concentrations, at lower concentrations, i.e., 0.05% mass concentration of surfactant, the adsorbed oil on the core surfaces tended to be finer.
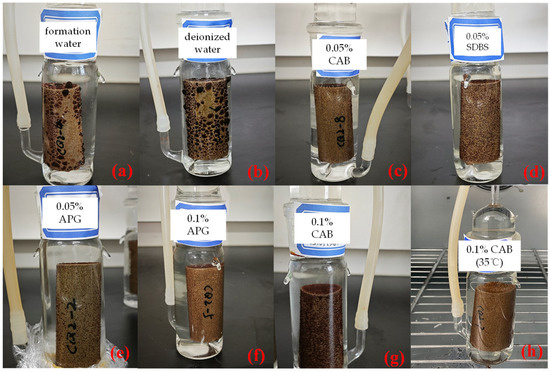
Figure 4.
Adsorption state of crude oil in different types of imbibition liquids. (a) CQ2–4: formation water, (b) CQ2–6: deionized water, (c) CQ2–8: formation water with 0.05% CAB, (d) CQ2–9: formation water with 0.05% SDBS, (e) CQ2–2: formation water with 0.05% APG, (f) CQ2–5: formation water with 0.1% APG, (g) CQ2–3: formation water with 0.1% CAB, and (h) CQ2–7: formation water with 0.1% CAB (30 °C).
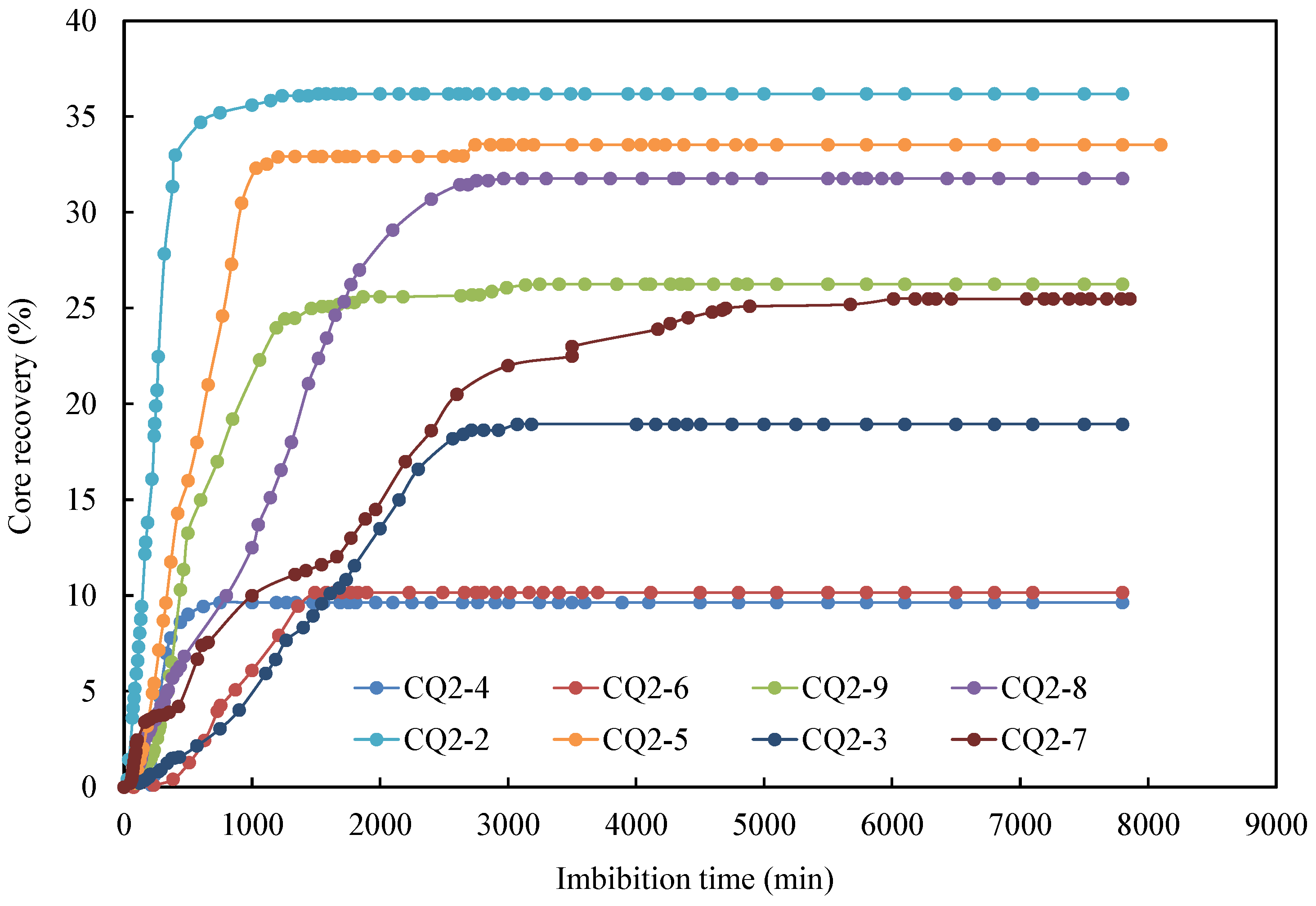
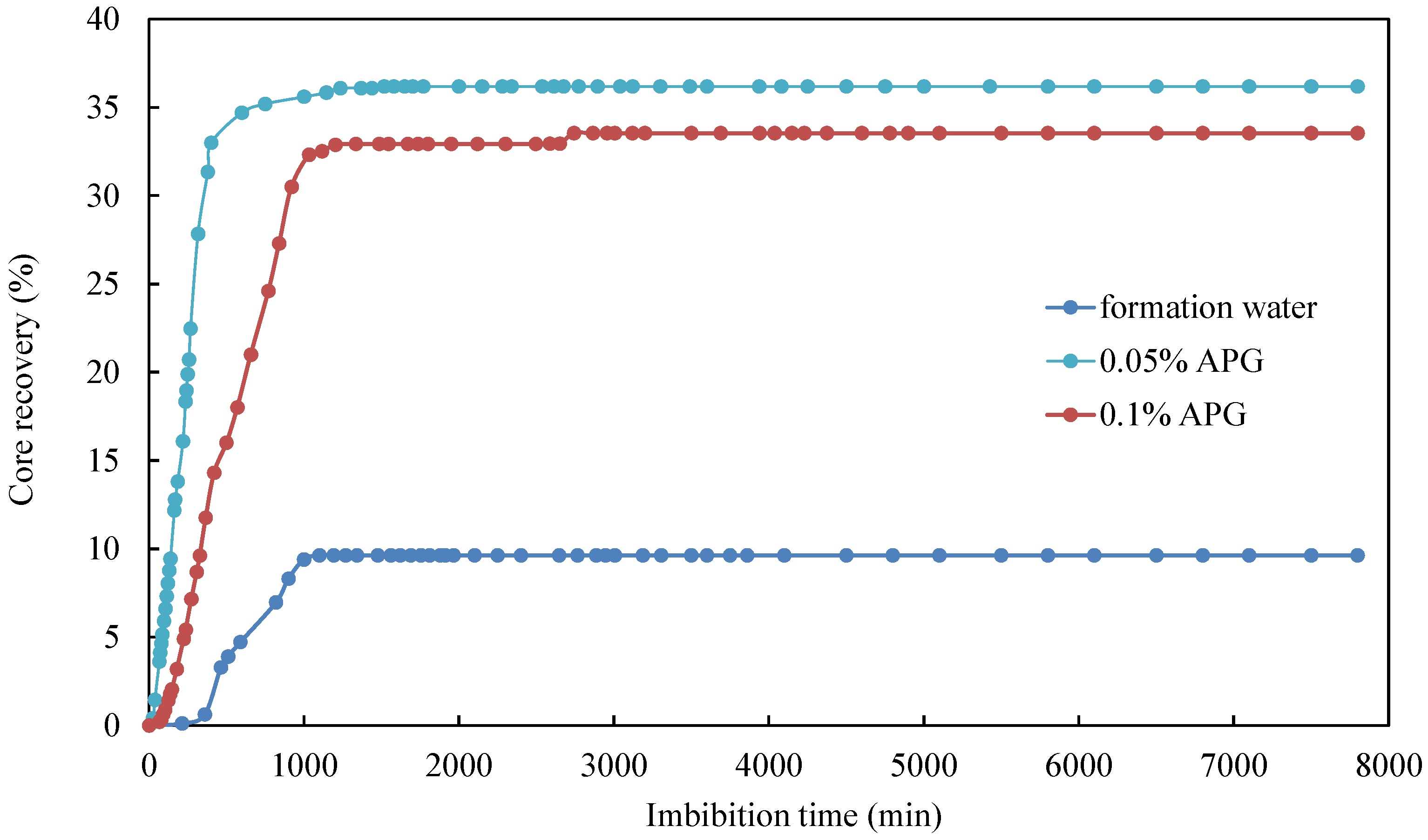
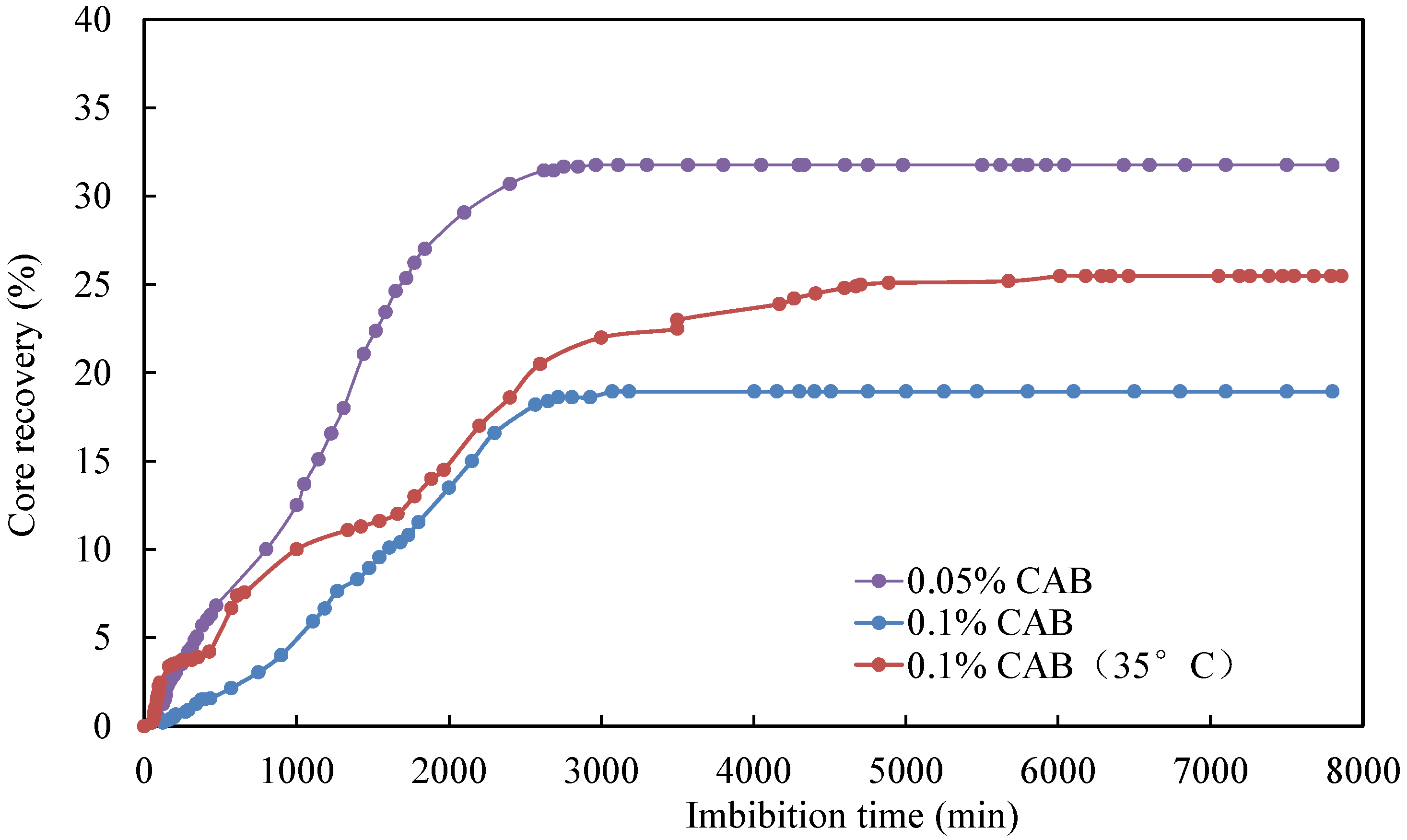
The oil recovery factor of different cores over time is illustrated in Figure 5. Overall, the core imbibition process can be divided into two stages. The first stage was characterized by a rapid increase in the imbibition rate, where the initial oil saturation is high, leading to a high oil production rate. In the second phase, the rise in imbibition rate tapered off and eventually leveled off. During this stage, the produced oil mainly originated from the small pores, where the flow resistance was high, resulting in a deceleration of the oil production rate. Among these, core CQ2–2 demonstrated the fastest imbibition rate and the highest oil recovery. After 600 min, the oil recovery factor reached an impressive 34.7%. A comparison between core CQ2–3 (20 °C) and CQ2–7 (35 °C) revealed a significantly lower imbibition rate and oil recovery factor for the core at 20 °C compared to the one at 35 °C.
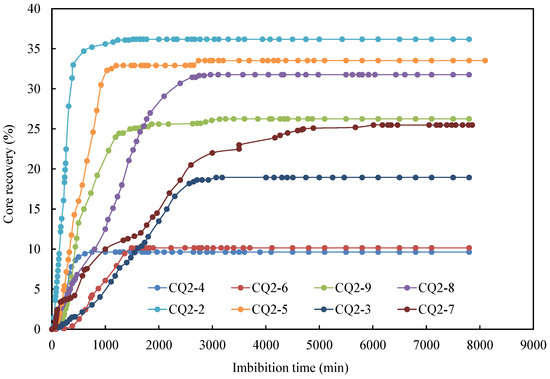
Figure 5.
Oil recovery factor of different cores versus time.
3.1. Effect of Surfactant Type
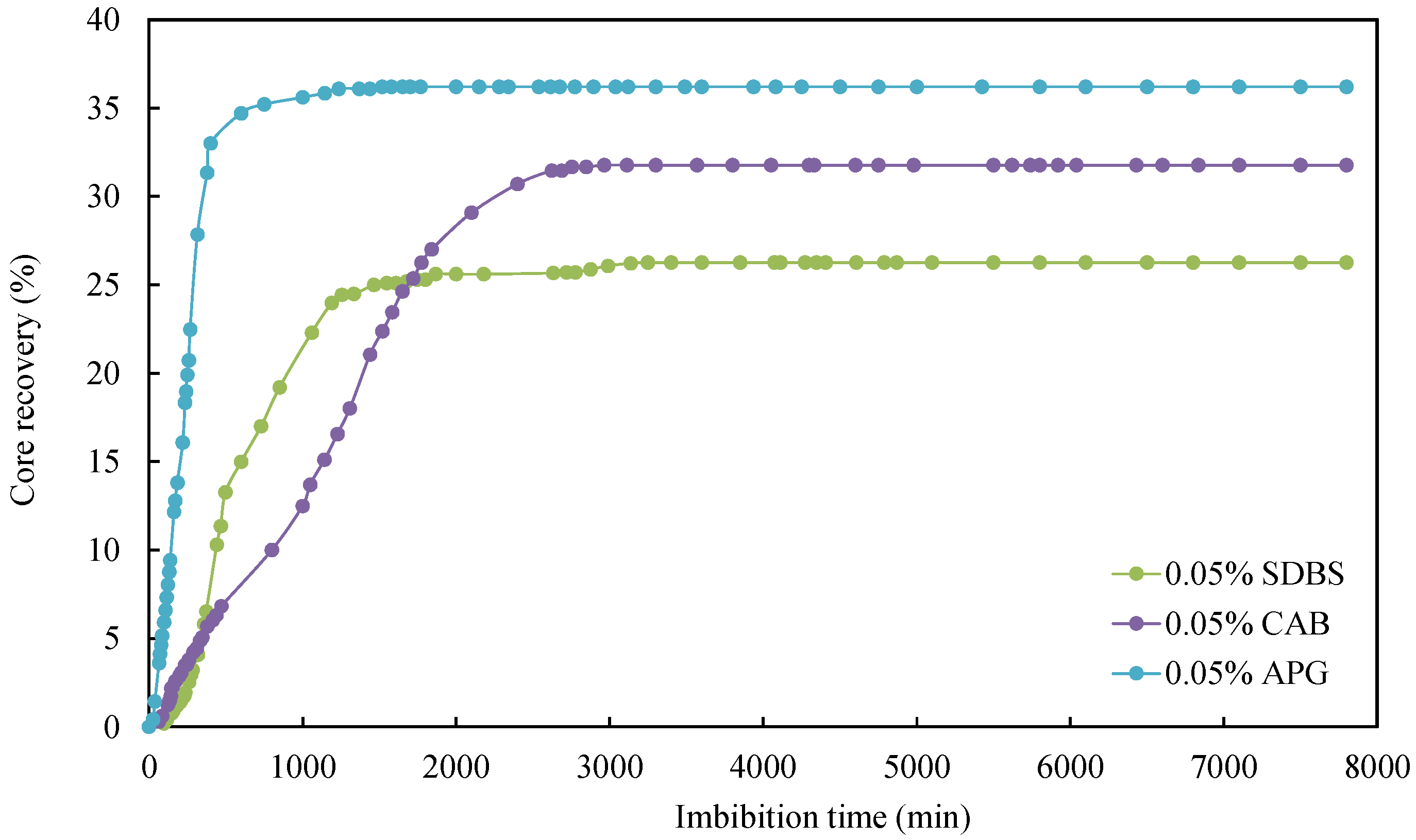
As can be seen from Table 4, the IFT between simulated oil and surfactant solution ranged from 0.0598 mN/m to 0.6821 mN/m, which was much smaller than the IFT between simulated oil and formation water. Consequently, introducing surfactants was observed to significantly reduce IFT. Secondly, the degree of improvement in imbibition recovery varied depending on the type of surfactant. Comparing the relationship curves between different types of surfactants shows variations in core imbibition rates and imbibition oil recovery factors, as shown in Figure 6 and Figure 7.
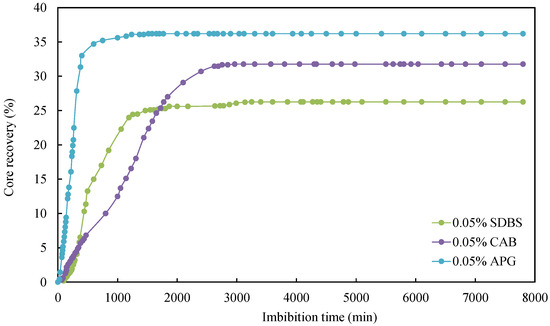
Figure 6.
Relationship between core imbibition recovery factor and surfactant.

Figure 7.
The state of imbibition of crude oil in different surfactant solutions. From left to right are the APG solution with a mass fraction of 0.05%, CAB solution with a mass fraction of 0.05%, and SDBS solution with a mass fraction of 0.05%.
The surfactant concentration of the imbibition liquid for the cores CQ2–2, CQ2–8, and CQ2–9 were the same, i.e., 0.05%, but the surfactant type was different. They were APG, CAB, and SDBS respectively. As shown in Figure 6, it was evident that the formation water with 0.05% APG achieved the highest ultimate oil recovery factor, reaching 36.19%, followed by the formation water with 0.05% CAB, with an ultimate oil recovery factor of 31.76%. Although the formation water with 0.5% SDBS exhibited a higher initial imbibition rate than formation water with 0.05% APG, its ultimate oil recovery factor was the lowest compared with the other two surfactants. Secondly, formation water with 0.05% APG had strong oil emulsification ability, and the emulsification effect lead to turbidity. At an imbibition time of 600 min, 34.7% of the oil was recovered. As the imbibition time increased, the imbibition rate of the core slowed down. This is because the APG solution with a concentration of 0.05% had the lowest IFT, and large oil droplets were easily dispersed into fine oil droplets and desorbed from the core wall. During the imbibition process, crude oil and surfactant emulsified, making the 0.05% APG solution turbid (as shown in Figure 7). Thus, emulsification can improve oil mobility and enhance oil recovery.
3.2. Effect of Surfactant Concentration
For the same surfactant, taking APG as an example, the imbibition rate and ultimate oil recovery factor of the formation water with 0.05% APG were the highest in the early stages of imbibition, followed by the formation water with 0.1% APG, and the formation water without surfactant had the lowest oil recovery factor (Figure 8). This indicates that adding surfactants to imbibition liquid promoted the imbibition oil recovery rate. Although the 0.1% APG solution exhibited an ultra-low IFT, the ultimate oil recovery factor was lower than that of the 0.05% APG solution. The IFT of the 0.1% APG solution was the lowest. However, lower IFT does not always yield better results, as an ultra-low IFT significantly reduces capillary force, which is the driving factor behind imbibition flow. An excessive concentration of surfactant can lead to dense micellar structure, and this micellar structure encapsulates oil and water, causing oil to become trapped and unable to flow. Overall, the difference in the ultimate oil recovery factor was minimal between the 0.05% and 0.1% APG solutions, amounting to 2.65% of oil recovery.
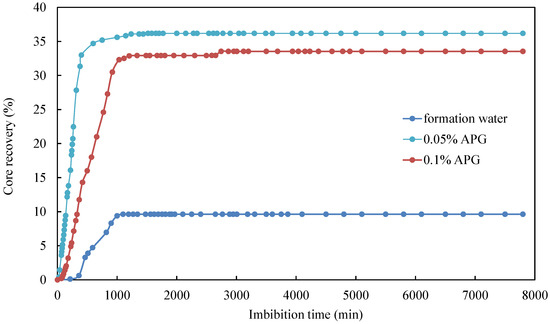
Figure 8.
Effect of concentration of surfactant solutions on core imbibition recovery rate.
3.3. Effect of Temperature
Comparing the impact of different temperatures on the oil recovery factor (Figure 9), it is evident that the oil recovery factor for the CQ2–7 core at 35 °C was significantly higher than that of the CQ2–3 core at 20 °C, even with the same surfactant concentration in the imbibition fluid. This can be attributed to the fact that an increase in temperature resulted in a reduction in the oil viscosity, subsequently lowering the resistance of fluid flow and increasing oil mobility. For other surfactants, an increase in temperature may result in enhanced oil recovery for similar reasons. However, excessively high temperatures can also cause surfactants to lose their effectiveness.
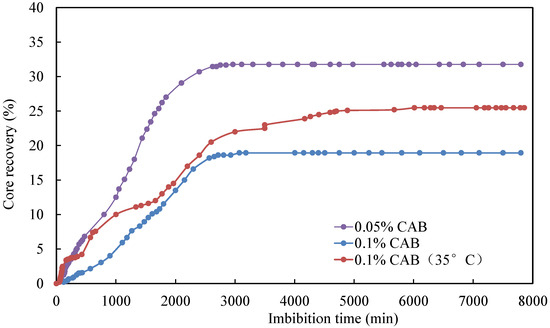
Figure 9.
Effect of temperature on core imbibition recovery rate.
4. Conclusions
This paper examines the mechanism of enhanced oil recovery through surfactant-assisted spontaneous imbibition using three different types of surfactants: SDBS, CAB, and APG. The study analyzes the effects of surfactant type, concentration, and temperature on oil recovery.
The results indicate that surfactant solutions significantly reduce the IFT between oil and water, emulsify oil, and improve oil mobility. Surfactant can significantly enhance oil recovery by spontaneous imbibition, compared to formation water and deionized water without surfactant. Cores with formation water and deionized water have an oil recovery factor of approximately 10%. However, when surfactants are present in the imbibition liquid, the ultimate oil recovery factor ranges from 18.94% to 36.19%. For the same surfactant concentration, APG exhibits better performance compared to SDBS and CAB.
The spontaneous imbibition experiments indicate that formation water with a 0.05% concentration of APG yields the highest oil recovery factor. It is observed that elevating the temperature can decrease oil viscosity, thereby improving oil recovery. Surfactant solutions containing 0.05% APG or 0.05% CAB have demonstrated superior efficacy in enhancing oil recovery when compared to their 0.1% counterparts. Consequently, these findings suggest that tight oil reservoirs could be effectively developed for enhanced recovery by employing the optimal surfactant concentration identified.
Author Contributions
Conceptualization, F.W.; methodology, F.W., L.W. and H.H.; validation, F.W., L.W. and H.H.; formal analysis, F.W., L.W. and H.H.; investigation, F.W., L.W. and H.H.; resources, F.W.; data curation, F.W.; writing—original draft preparation, L.W. and H.H.; writing—review and editing, F.W., L.W. and H.H.; project administration, F.W.; funding acquisition, F.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Sinopec Key Laboratory of Shale Oil/Gas Exploration and Production Technology. Grant Number: 33550000-22-ZC0613-0212.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
Author Lu Wang was employed by the company No. 7 Oil Production Plant, PetroChina Changqing Oilfield Company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Li, Z.; Yao, Y.; Zhang, C.; Liu, Y. A novel method for evaluation of the spontaneous imbibition process in tight reservoir rocks: Mathematical model and experimental verification. Geoenergy Sci. Eng. 2023, 223, 211554. [Google Scholar] [CrossRef]
- Wang, F.; Fu, J. A capillary bundle model for the forced imbibition in the shale matrix with dual-wettability. Can. J. Chem. Eng. 2023, 101, 2330–2340. [Google Scholar] [CrossRef]
- Wang, F.; Zeng, F.; Wang, L.; Yue, H.; Chang, S. Experimental Study of Surfactant-Aided Dynamic Spontaneous Imbibition in Tight Oil Reservoirs: The Effect of Fluid Flow, Displacement Pressure, Temperature, and Fracture. Energy Fuels 2023, 37, 18632–18641. [Google Scholar] [CrossRef]
- Morrow, N.R.; Mason, G. Recovery of oil by spontaneous imbibition. Curr. Opin. Colloid. 2001, 6, 321–337. [Google Scholar] [CrossRef]
- Chen, Y.; Zhi, D.; Qin, J.; Song, P.; Zhao, H.; Wang, F. Experimental study of spontaneous imbibition and CO2 huff and puff in shale oil reservoirs with NMR. J. Pet. Sci. Eng. 2022, 209, 109883. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, J. A mathematical model for co-current spontaneous water imbibition into oil-saturated tight sandstone: Upscaling from pore-scale to core-scale with fractal approach. J. Pet. Sci. Eng. 2019, 178, 376–388. [Google Scholar] [CrossRef]
- Wu, R.; Yang, S.; Xie, J.; Wang, M.; Yan, J. Experiment and mechanism of spontaneous imbibition of matrix core in tight oil-gas reservoirs. Pet. Geol. Recovery Effic. 2017, 24, 98–104. [Google Scholar]
- Cheng, H.; Wang, F. Mathematical model of the spontaneous imbibition of water into oil-saturated fractured porous media with gravity. Chem. Eng. Sci. 2021, 231, 116317. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, J. Mathematical model of liquid spontaneous imbibition into gas-saturated porous media with dynamic contact angle and gravity. Chem. Eng. Sci. 2021, 229, 116139. [Google Scholar] [CrossRef]
- Wang, F.; Yue, H. A comprehensive mathematical model for spontaneous imbibition in oil-saturated fractured tight sandstones: Incorporating fracture distribution, displacement pressure, gravity, and buoyancy effects. Phys. Fluids 2023, 35, 062122. [Google Scholar]
- Dou, L.; Xiao, Y.; Gao, H.; Wang, R.; Liu, C.; Sun, H. The study of enhanced displacement efficiency in tight sandstone from the combination of spontaneous and dynamic imbibition. J. Pet. Sci. Eng. 2021, 199, 108327. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, C.; Zou, C.; You, Q.; Zhao, M.; Zhao, G.; Sun, Y. Impact of flow rate on dynamic imbibition in fractured tight sandstone cores. Pet. Sci. 2022, 19, 2895–2904. [Google Scholar] [CrossRef]
- Alvarez, J.O.; Schechter, D.S. Improving oil recovery in the Wolfcamp unconventional liquid reservoir using surfactants in completion fluids. J. Pet. Sci. Eng. 2017, 157, 806–815. [Google Scholar] [CrossRef]
- Chen, W.; Schechter, D.S. Surfactant selection for enhanced oil recovery based on surfactant molecular structure in unconventional liquid reservoirs. J. Pet. Sci. Eng. 2021, 196, 107702. [Google Scholar] [CrossRef]
- Sheng, J.J. What type of surfactants should be used to enhance spontaneous imbibition in shale and tight reservoirs? J. Pet. Sci. Eng. 2017, 159, 635–643. [Google Scholar] [CrossRef]
- Liu, J.; Sheng, J.J.; Wang, X.; Ge, H.; Yao, E. Experimental study of wettability alteration and spontaneous imbibition in Chinese shale oil reservoirs using anionic and nonionic surfactants. J. Pet. Sci. Eng. 2019, 175, 624–633. [Google Scholar] [CrossRef]
- Sheng, J.J. Modern Chemical Enhanced Oil Recovery: Theory and Practice; Gulf Professional Publishing: Houston, TX, USA, 2010. [Google Scholar]
- Rudin, J.; Bernard, C.; Wasan, D.T. Effect of added surfactant on interfacial tension and spontaneous emulsification in alkali/acidic oil systems. Ind. Eng. Chem. Res. 1994, 33, 1150–1158. [Google Scholar] [CrossRef]
- Rosen, M.J.; Wang, H.; Shen, P.; Zhu, Y. Ultralow interfacial tension for enhanced oil recovery at very low surfactant concentrations. Langmuir 2005, 21, 3749–3756. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, G.; Ge, J.; Jiang, P.; Tang, J.; Liu, Y. Research of the heavy oil displacement mechanism by using alkaline/surfactant flooding system. Colloids Surf. A Physicochem. Eng. Asp. 2013, 434, 63–71. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, G.; Ge, J.; Liao, K.; Pei, H.; Jiang, P.; Li, X. Study on organic alkali-surfactant-polymer flooding for enhanced ordinary heavy oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2016, 508, 230–239. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z.; Liu, X.; Wang, L.; Cui, Z. 1, 3-Dialkyl glyceryl ethers derivatives as surfactants for enhanced oil recovery in high salinity and high temperature reservoir conditions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124425. [Google Scholar] [CrossRef]
- Chen, J.; Hu, X.; Fang, Y.; Liu, H.; Xia, Y. Comparative study of conventional/ethoxylated/extended n-alkylsulfate surfactants. Langmuir 2019, 35, 3116–3125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xu, Y.; Qiao, W.; Li, Z. Interfacial tensions upon the addition of alcohols to phenylalkane sulfonate monoisomer systems. Fuel 2004, 83, 2059–2063. [Google Scholar] [CrossRef]
- Jarrahian, K.; Seiedi, O.; Sheykhan, M.; Sefti, M.V.; Ayatollahi, S. Wettability alteration of carbonate rocks by surfactants: A mechanistic study. Colloids Surf. A Physicochem. Eng. Asp. 2012, 410, 1–10. [Google Scholar] [CrossRef]
- Li, S.; Yang, K.; Li, Z.; Zhang, K.; Jia, N. Properties of CO2 foam stabilized by hydrophilic nanoparticles and nonionic surfactants. Energy Fuels 2019, 33, 5043–5054. [Google Scholar] [CrossRef]
- Yang, K.; Li, S.; Zhang, K.; Wang, Y. Synergy of hydrophilic nanoparticle and nonionic surfactant on stabilization of carbon dioxide-in-brine foams at elevated temperatures and extreme salinities. Fuel 2021, 288, 119624. [Google Scholar] [CrossRef]
- Iglauer, S.; Wu, Y.; Shuler, P.; Tang, Y.; Goddard III, W.A. Alkyl polyglycoside surfactant–alcohol cosolvent formulations for improved oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2009, 339, 48–59. [Google Scholar] [CrossRef]
- Yan, L.; Ma, J.; Cui, Z.; Jiang, J.; Song, B.; Pei, X. A new series of double-chain single-head sulfobetaine surfactants derived from 1, 3-dialkyl glyceryl ether for reducing crude oil/water interfacial tension. J. Surfactants Deterg. 2019, 22, 47–60. [Google Scholar] [CrossRef]
- Ma, J.; Yan, L.; Cui, Z.; Jiang, J.; Pei, X.; Song, B. Synthesis of a new sulfobetaine surfactant with double long alkyl chains and its performances in surfactant-polymer flooding. J. Dispers. Sci. Technol. 2018, 39, 1185–1191. [Google Scholar] [CrossRef]
- Cui, Z.; Du, X.; Pei, X.; Jiang, J.; Wang, F. Synthesis of didodecylmethylcarboxyl betaine and its application in surfactant–polymer flooding. J. Surfactants Deterg. 2012, 15, 685–694. [Google Scholar] [CrossRef]
- Hu, X.; Qi, D.; Yan, L.; Cui, Z.; Song, B.; Pei, X.; Jiang, J. Inhibiting hydrophobization of sandstones via adsorption of alkyl carboxyl betaines in SP flooding by using gentle alkali. Colloids Surf. A Physicochem. Eng. Asp. 2017, 535, 75–82. [Google Scholar] [CrossRef]
- Li, P.; Yang, C.; Cui, Z.; Song, B.; Jiang, J.; Wang, Z. A new type of sulfobetaine surfactant with double alkyl polyoxyethylene ether chains for enhanced oil recovery. J. Surfactants Deterg. 2016, 19, 967–977. [Google Scholar] [CrossRef]
- Yahya, Z.N.M.; Puspaseruni, N.P.; Kurnia, R.; Wahyuningrum, D.; Mulyani, I.; Wijayanto, T.; Kurihara, M.; Waskito, S.S.; Aslam, B.M.; Marhaendrajana, T. The effect of aluminosilicate in anionic–nonionic surfactant mixture on wetness and interfacial tension in its application for enhanced oil recovery. Energy Rep. 2022, 8, 1013–1025. [Google Scholar] [CrossRef]
- Kesarwani, H.; Saxena, A.; Mandal, A.; Sharma, S. Anionic/nonionic surfactant mixture for enhanced oil recovery through the investigation of adsorption, interfacial, rheological, and rock wetting characteristics. Energy Fuels 2021, 35, 3065–3078. [Google Scholar] [CrossRef]
- Zhu, W.; Zhao, C.; Ma, Q.; Li, H.; Song, Z. Effect of Hydrophobic Carbon Chain Length of Alkyl Betaine Surfactant on Spontaneous Imbibition of Low-permeability Core. Spec. Oil Gas. Reserv. 2021, 28, 102–107. [Google Scholar]
- Chen, S.; Han, M.; AlSofi, A.; Fuseni, A. Non-Ionic Surfactant Formulation with Ultra-Low Interfacial Tension at High-Temperature and High-Salinity Conditions; SPE Conference at Oman Petroleum & Energy Show: Muscat, Oman, 2022; p. D022S057R001. [Google Scholar]
- Chan, K.S.; Shah, D.O. The molecular mechanism for achieving ultra low interfacial tension minimum in a petroleum sulfonate/oil/brine system. J. Dispers. Sci. Technol. 1980, 1, 55–95. [Google Scholar] [CrossRef]
- Schramm, L.L.; Stasiuk, E.N.; Marangoni, D.G. 2 Surfactants and their applications. Annu. Rep. Sect. “C” 2003, 99, 3–48. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).