Design, Development, and Evaluation of Constant Voltage Iontophoresis for the Transungual Delivery of Efinaconazole
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Determination of Efinaconazole by Reverse Phase-High-Performance Liquid Chromatography (RP-HPLC)
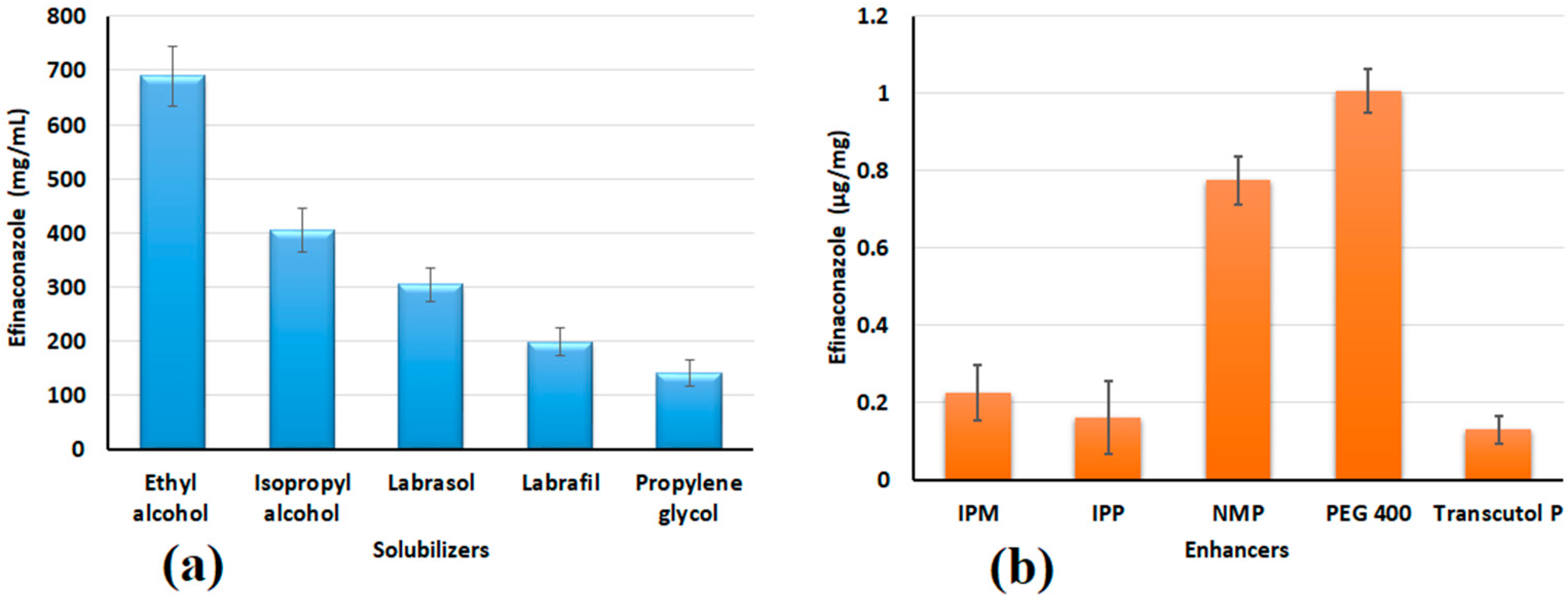
2.3. Drug Solubility in Solubilizers
2.4. Screening of Permeation Enhancers
2.5. Preparation of Primary Formulations
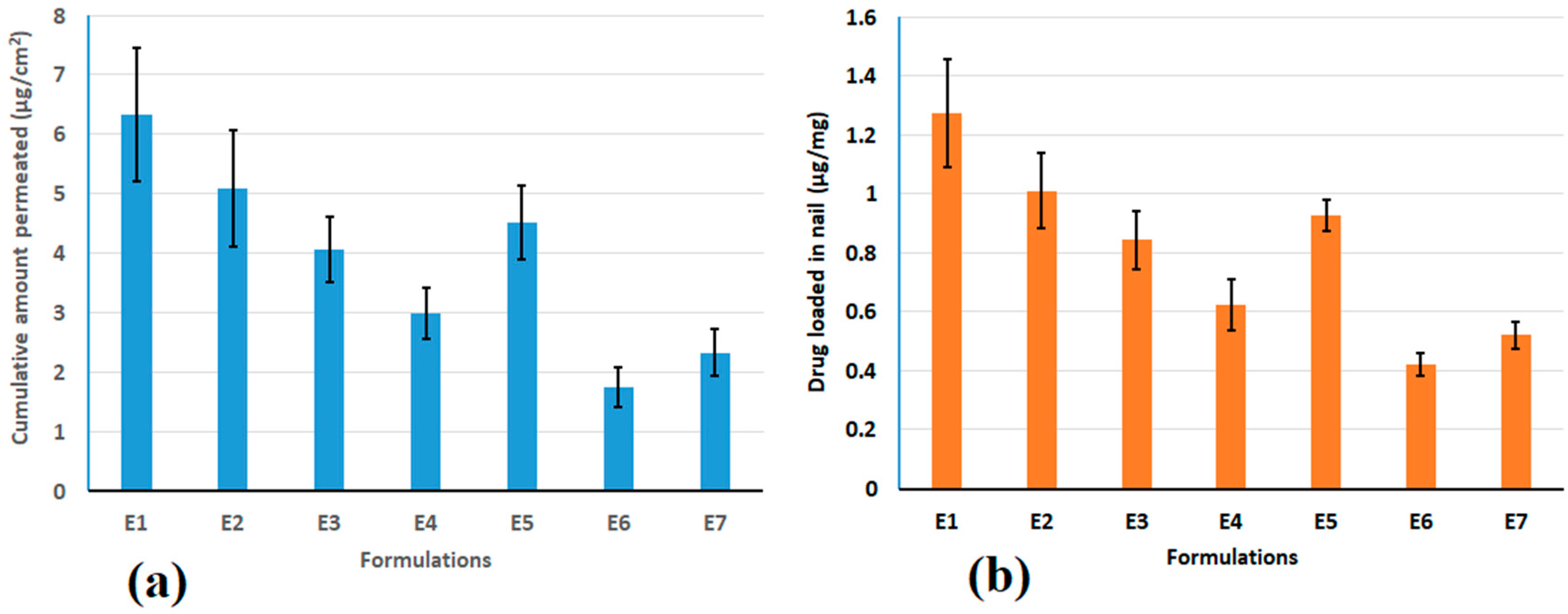
2.6. Permeation and Drug Loading in Nail
2.7. Characterization of Preliminary Formulations
2.8. Custom Design
Data Analysis and Interpretation of Results
2.9. Evaluation of Hydrogels
2.9.1. Conductivity
2.9.2. FTIR Spectroscopy
2.9.3. DSC Analysis
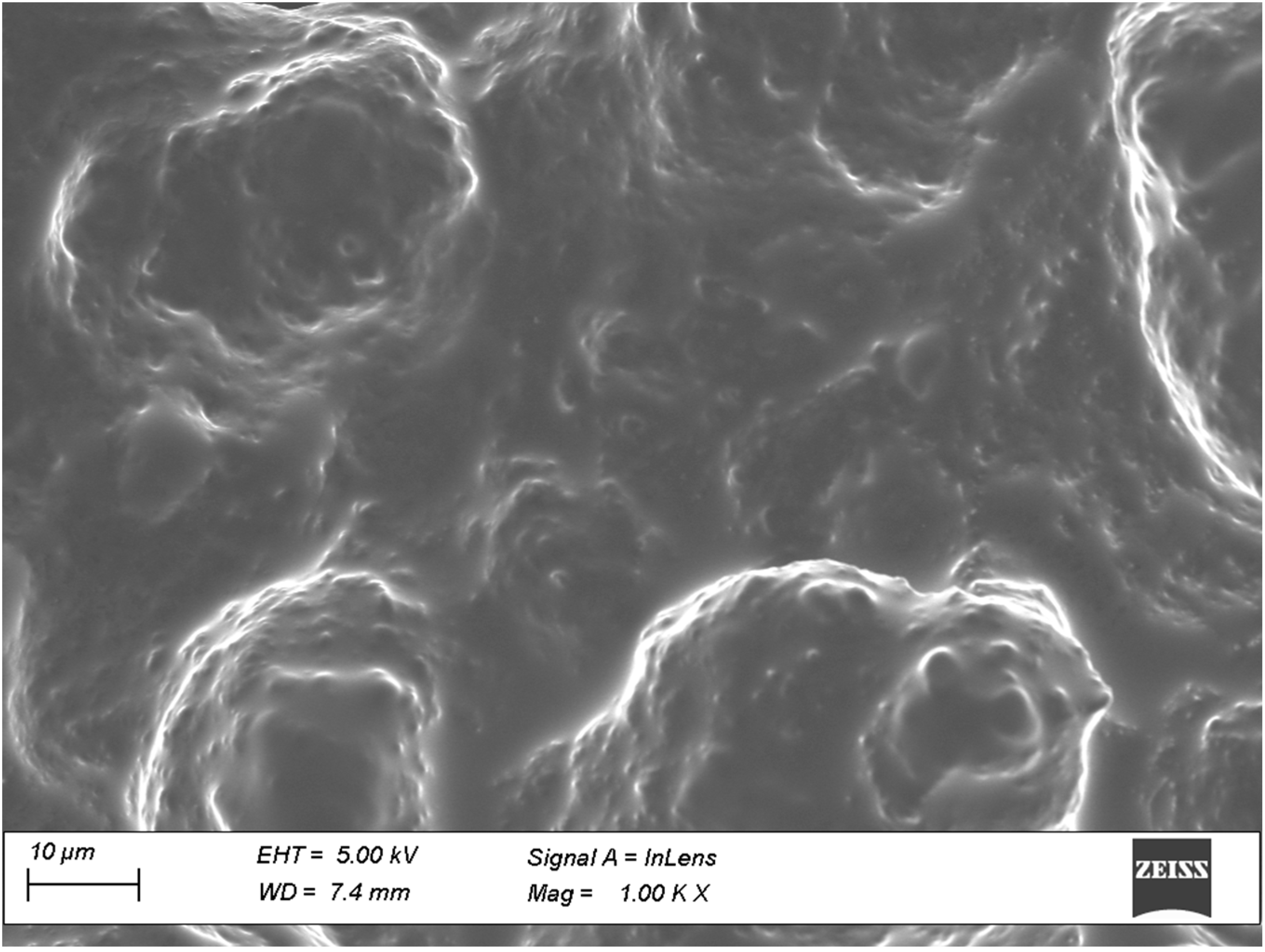
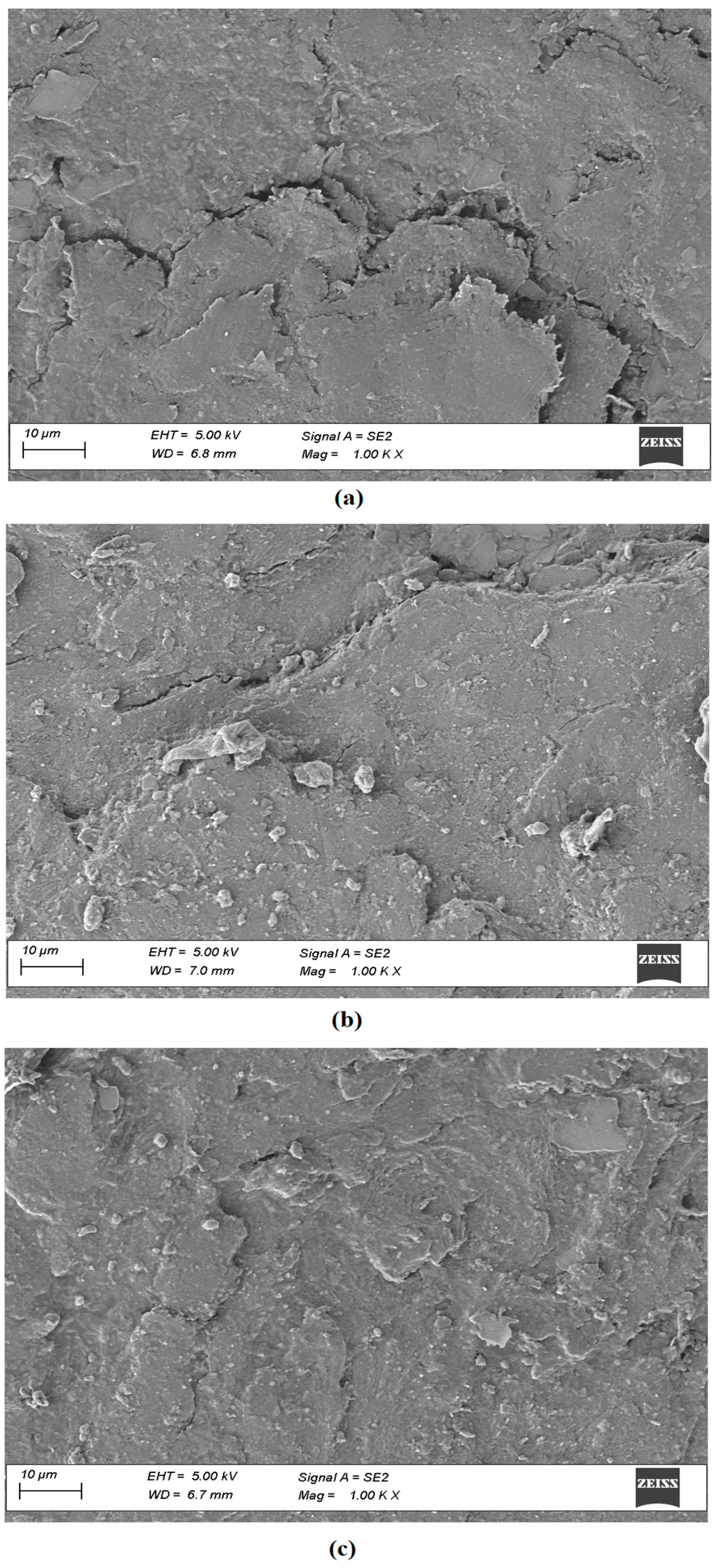
2.9.4. Field Emission Scanning Electron Microscopy (SEM)
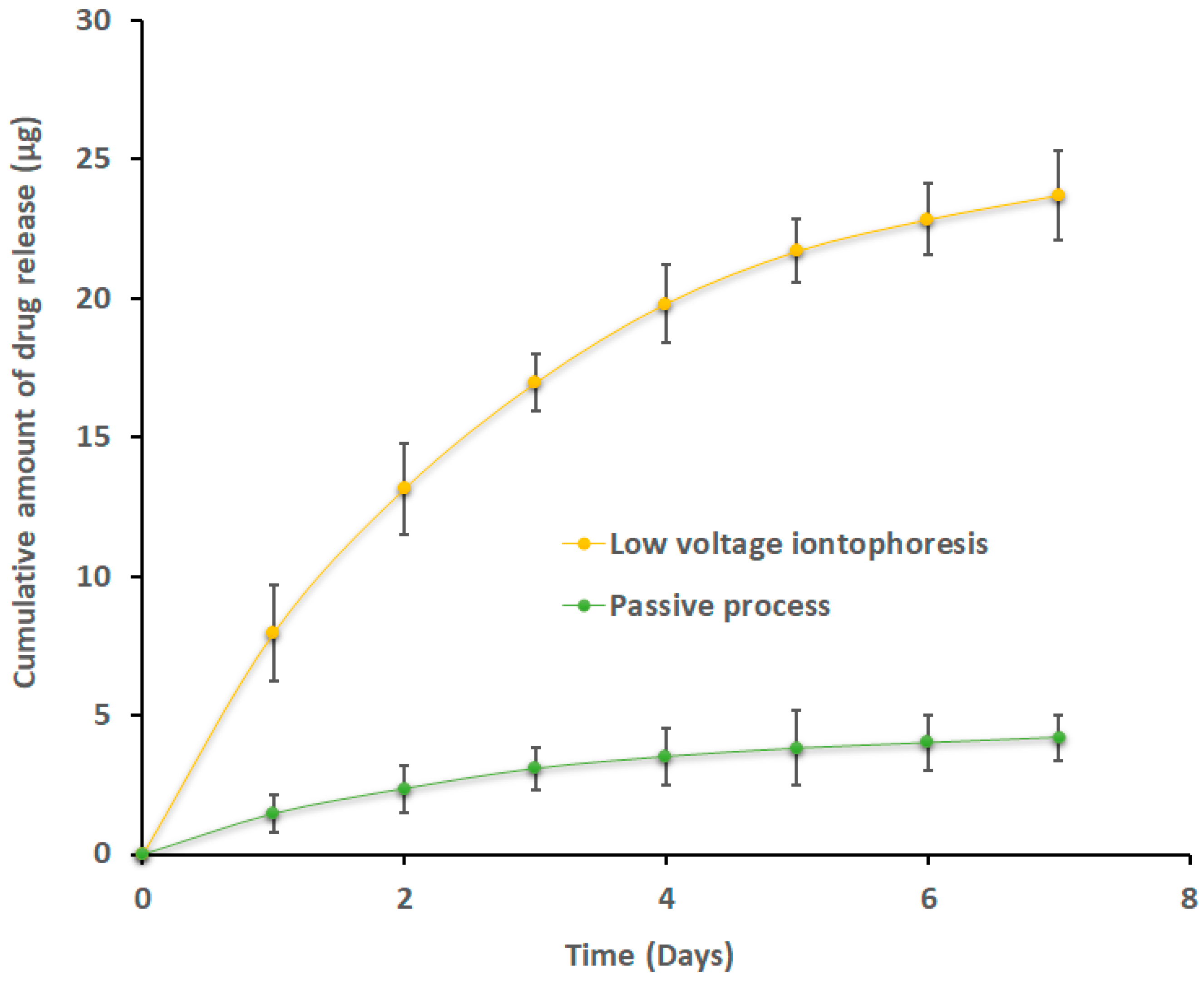
2.10. Release of Efinaconazole from Loaded Nails
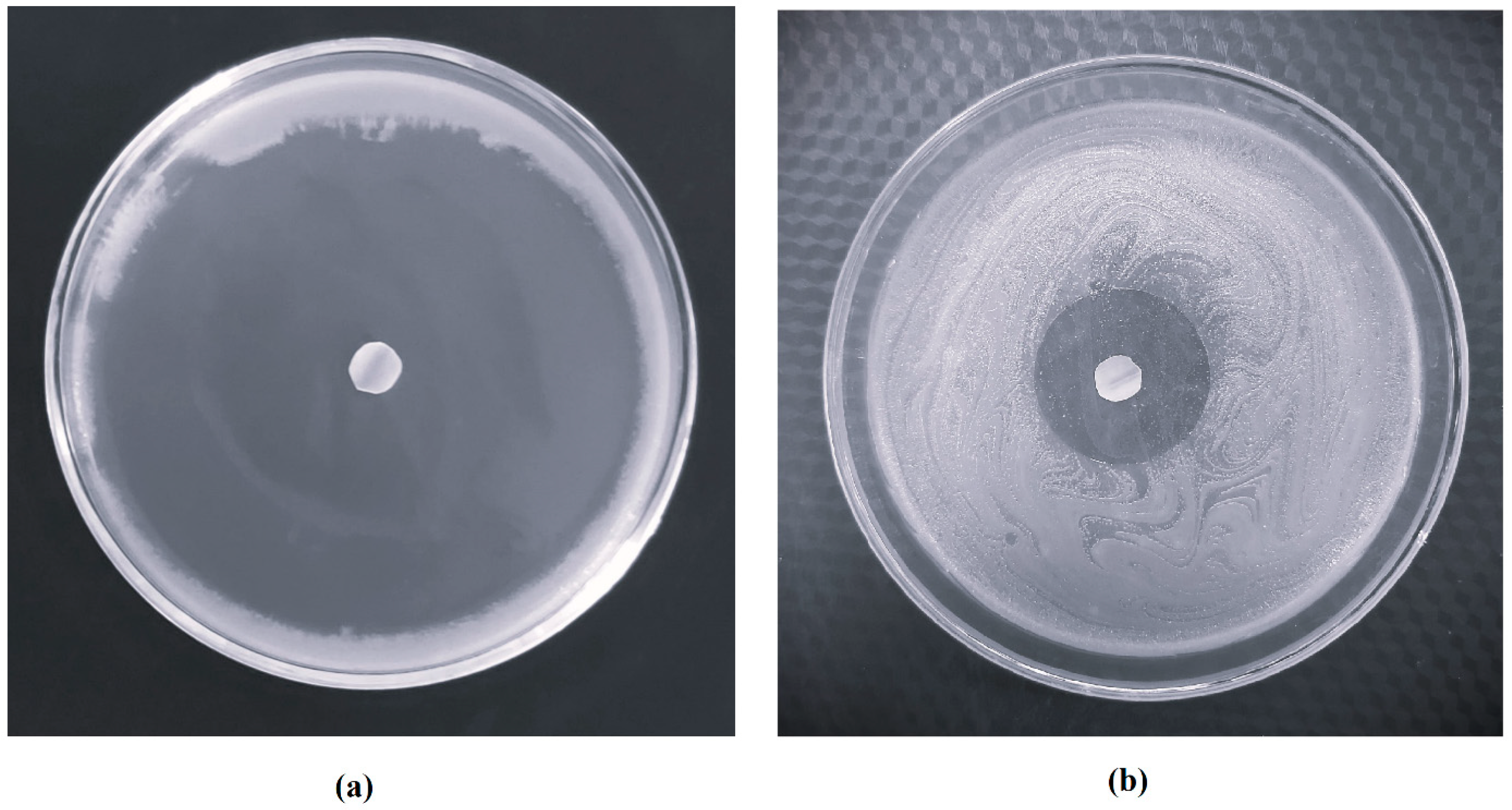
2.11. Antifungal Study
2.12. Formulation Stability
2.13. Statistical Evaluation
3. Results and Discussion
3.1. Selection of Solubilizer and Permeation Enhancer
3.2. Preparation of Primary Formulations
3.3. Permeation and Drug Loading in Nail
3.4. Custom Design
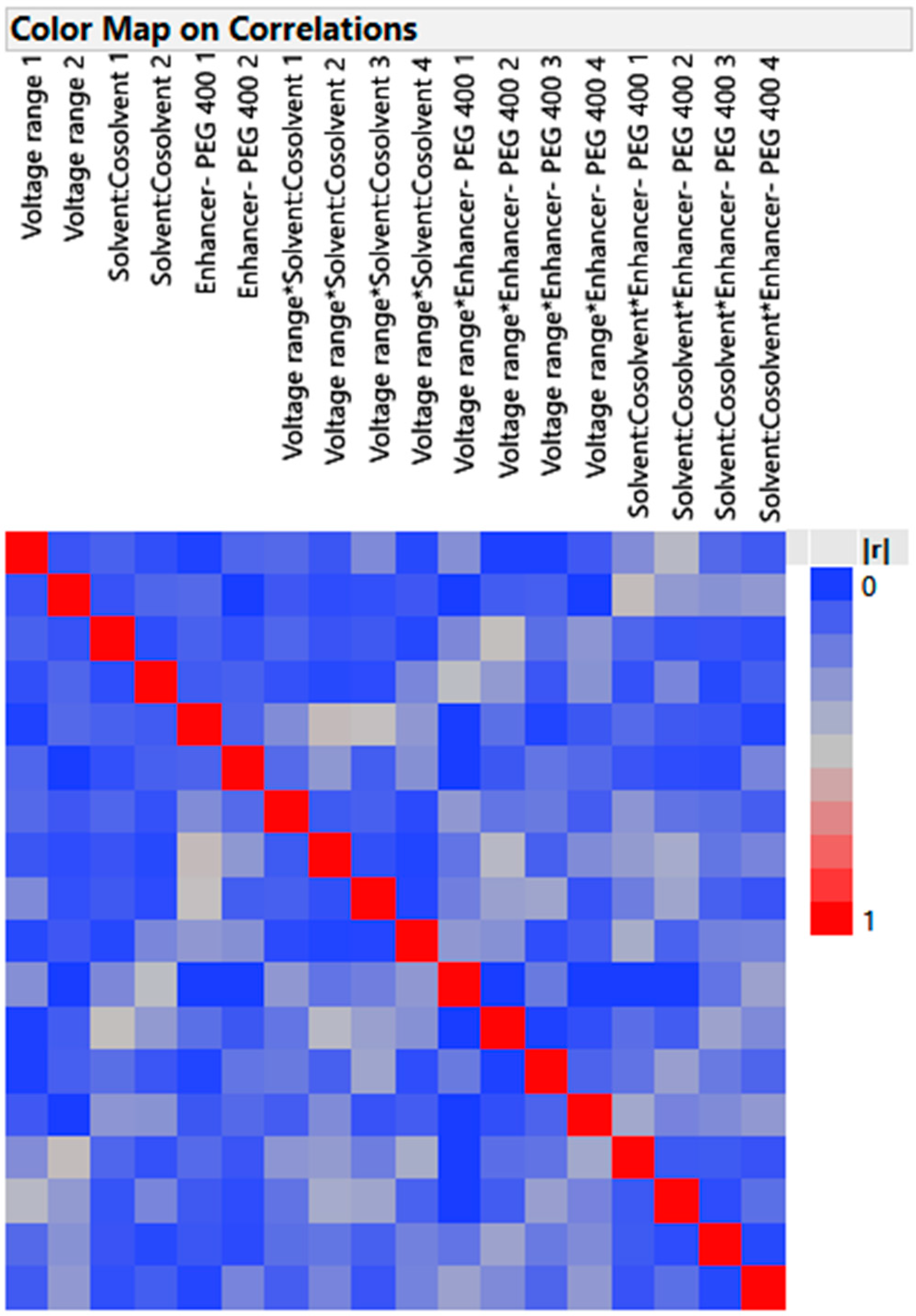
3.4.1. Design Verification by Color Map and Design Diagnostic
- Color Map
- 2.
- Design Diagnostics
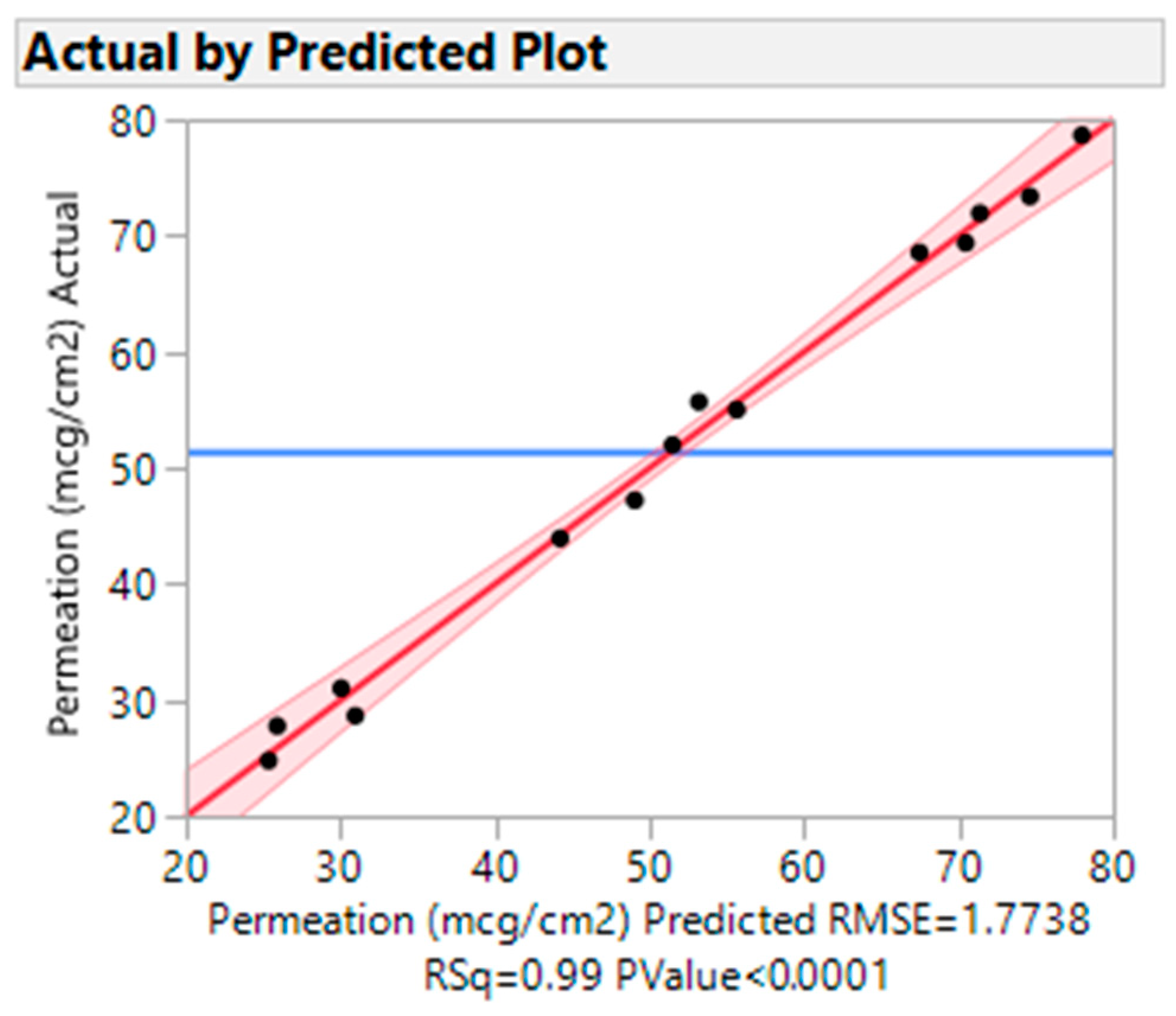
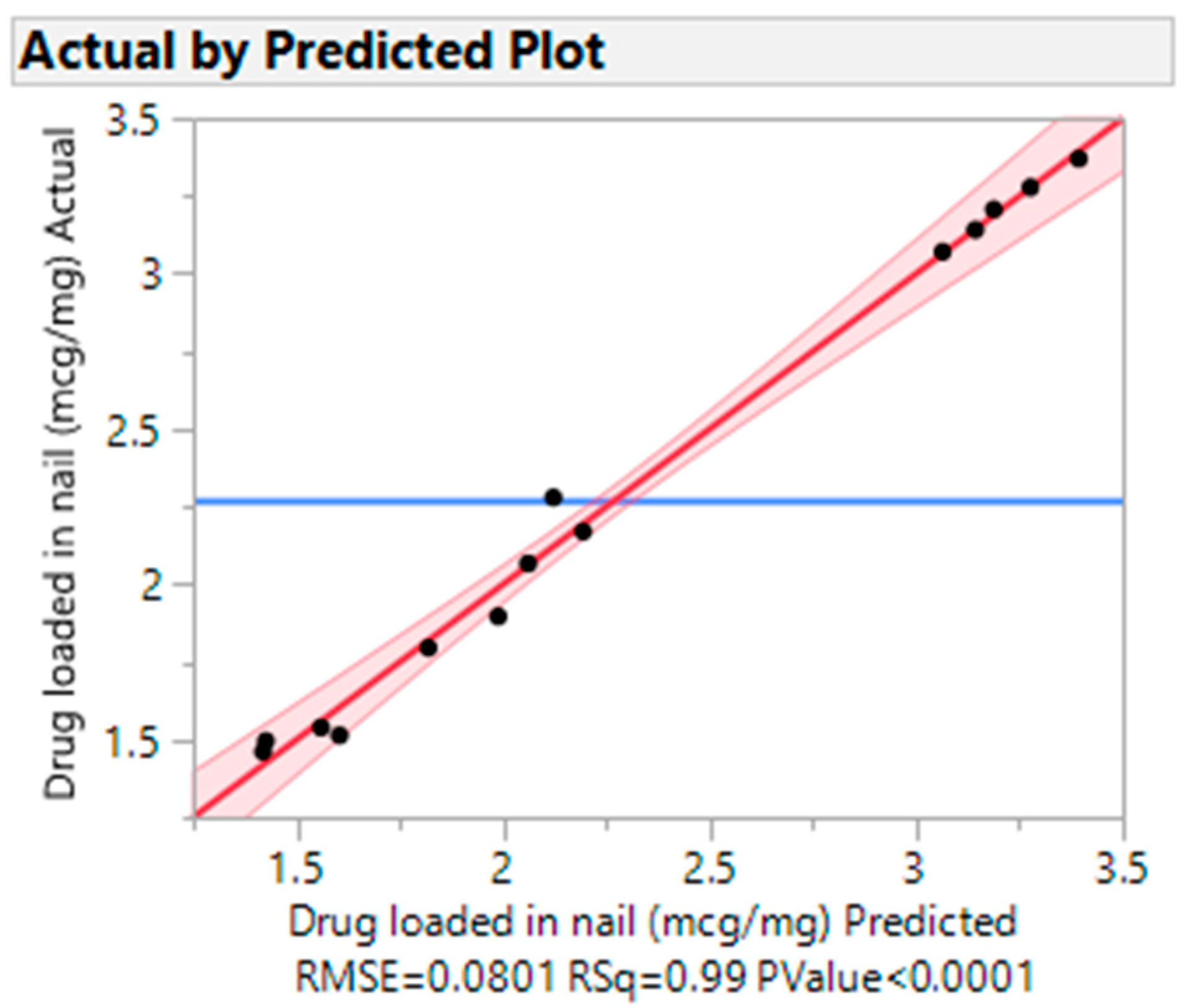
3.4.2. Actual vs. Predicted Plots
3.4.3. Effects Summary Report
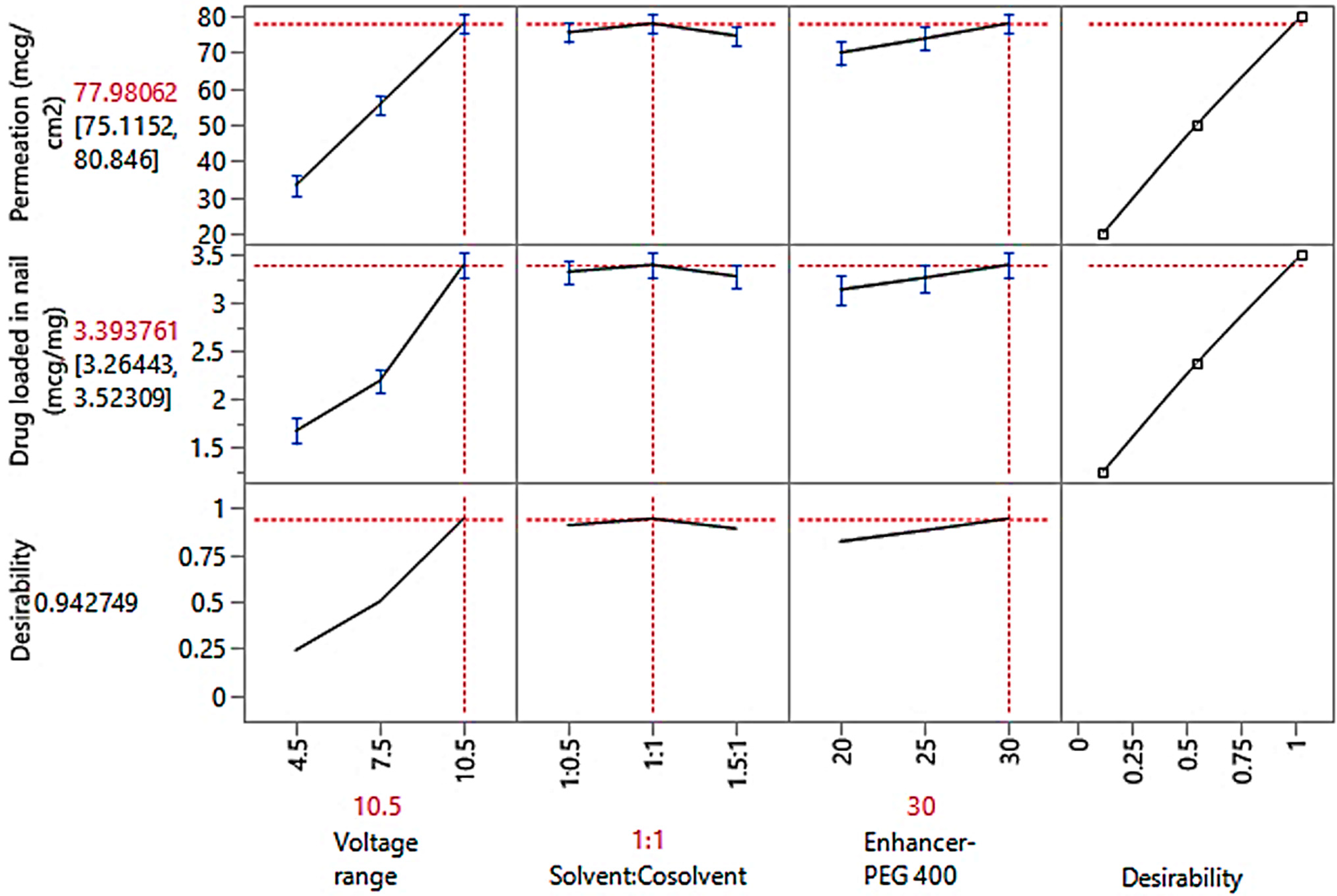
3.4.4. Optimization Study Observations
3.5. Evaluation of Hydrogels
3.5.1. Conductivity and Viscosity
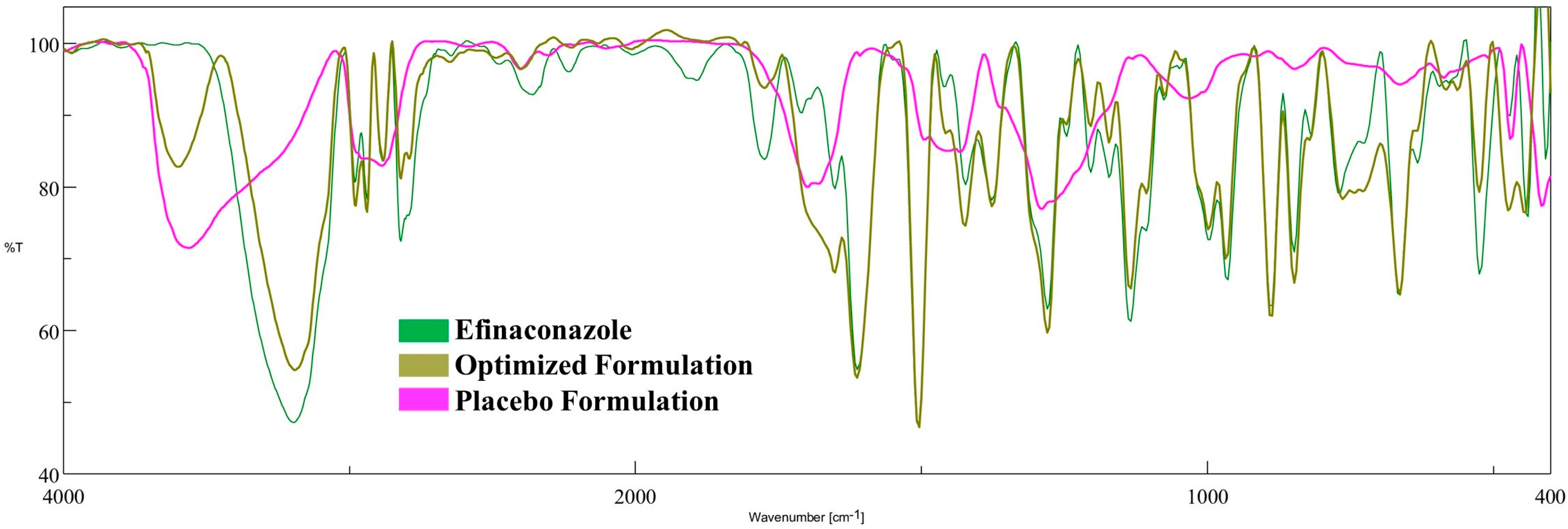
3.5.2. FTIR Spectroscopy
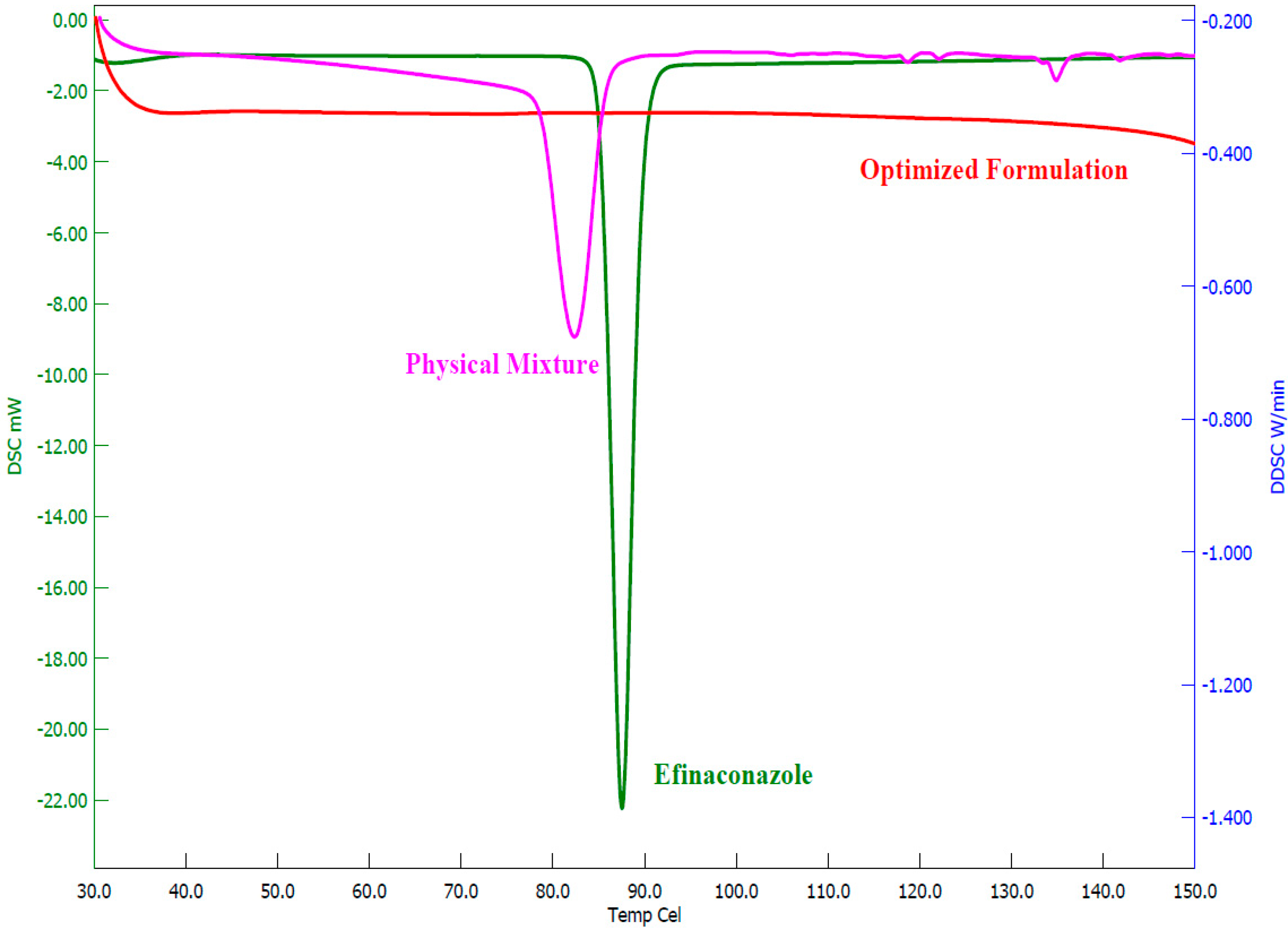
3.5.3. DSC Analysis
3.5.4. Field Emission SEM
3.6. Release of Efinaconazole from Loaded Nails
3.7. Antifungal Study
3.8. Formulation Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghannoum, M.; Isham, N. Fungal nail infections (onychomycosis): A never-ending story? PLoS Pathog. 2014, 10, e1004105. [Google Scholar] [CrossRef]
- Kushwaha, A.S.; Sharma, P.; Shivakumar, H.N.; Rappleye, C.; Zukiwski, A.; Proniuk, S.; Murthy, S.N. Trans-ungual Delivery of AR-12, a Novel Antifungal Drug. AAPS PharmSciTech. 2017, 18, 2702–2705. [Google Scholar] [CrossRef] [PubMed]
- Elewski, B.E.; Tosti, A. Risk Factors and Comorbidities for Onychomycosis: Implications for Treatment with Topical Therapy. J. Clin. Aesthetic Dermatol. 2015, 8, 38–42. [Google Scholar]
- Nair, A.B.; Vaka, S.R.; Murthy, S.N. Transungual delivery of terbinafine by iontophoresis in onychomycotic nails. Drug Dev. Ind. Pharm. 2011, 37, 1253–1258. [Google Scholar] [CrossRef]
- Kawa, N.; Lee, K.C.; Anderson, R.R.; Garibyan, L. ONYCHOMYCOSIS: A Review of New and Emerging Topical and Device-based Treatments. J. Clin. Aesthetic Dermatol. 2019, 12, 29–34. [Google Scholar]
- Elkeeb, R.; Hui, X.; Murthy, N.; Maibach, H.I. Emerging topical onychomycosis therapies-quo vadis? Expert Opin. Emerg. Drugs 2014, 19, 489–495. [Google Scholar] [CrossRef]
- Dias, M.F.; Quaresma-Santos, M.V.; Bernardes-Filho, F.; Amorim, A.G.; Schechtman, R.C.; Azulay, D.R. Update on therapy for superficial mycoses: Review article part I. An. Bras. Dermatol. 2013, 88, 764–774. [Google Scholar] [CrossRef]
- Gupta, A.K.; Stec, N.; Summerbell, R.C.; Shear, N.H.; Piguet, V.; Tosti, A.; Piraccini, B.M. Onychomycosis: A review. J. Eur. Acad. Dermatol. Venereol. JEADV 2020, 34, 1972–1990. [Google Scholar] [CrossRef]
- Del Rosso, J.Q. The role of topical antifungal therapy for onychomycosis and the emergence of newer agents. J. Clin. Aesthetic Dermatol. 2014, 7, 10–18. [Google Scholar]
- Ghannoum, M.; Isham, N.; Catalano, V. A second look at efficacy criteria for onychomycosis: Clinical and mycological cure. Br. J. Dermatol. 2014, 170, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Sharma, H.; Pathak, K. Onychomycosis: Potential of Nail Lacquers in Transungual Delivery of Antifungals. Scientifica 2016, 2016, 1387936. [Google Scholar] [CrossRef]
- Shanbhag, P.P.; Jani, U. Drug delivery through nails: Present and future. New Horiz. Transl. Med. 2017, 3, 252–263. [Google Scholar]
- Murdan, S. Enhancing the nail permeability of topically applied drugs. Expert Opin. Drug Deliv. 2008, 5, 1267–1282. [Google Scholar] [CrossRef]
- Nair, A.B.; Sammeta, S.M.; Vaka, S.R.; Narasimha Murthy, S. A study on the effect of inorganic salts in transungual drug delivery of terbinafine. J. Pharm. Pharmacol. 2009, 61, 431–437. [Google Scholar] [CrossRef]
- Nair, A.B.; Sammeta, S.M.; Kim, H.D.; Chakraborty, B.; Friden, P.M.; Murthy, S.N. Alteration of the diffusional barrier property of the nail leads to greater terbinafine drug loading and permeation. Int. J. Pharm. 2009, 375, 22–27. [Google Scholar] [CrossRef]
- Murthy, S.N.; Vaka, S.R.K.; Sammeta, S.M.; Nair, A.B. TranScreen-N™: Method for rapid screening of trans-ungual drug delivery enhancers. J. Pharm. Sci. 2009, 98, 4264–4271. [Google Scholar] [CrossRef] [PubMed]
- Saner, M.V.; Kulkarni, A.D.; Pardeshi, C.V. Insights into drug delivery across the nail plate barrier. J. Drug Target. 2014, 22, 769–789. [Google Scholar] [CrossRef] [PubMed]
- Šveikauskaitė, I.; Pockevičius, A.; Briedis, V. Potential of Chemical and Physical Enhancers for Transungual Delivery of Amorolfine Hydrochloride. Materials 2019, 12, 1028. [Google Scholar] [CrossRef]
- Shivakumar, H.N.; Juluri, A.; Desai, B.G.; Murthy, S.N. Ungual and transungual drug delivery. Drug Dev. Ind. Pharm. 2012, 38, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Albarahmieh, E.; AbuAmmouneh, L.; Kaddoura, Z.; AbuHantash, F.; Alkhalidi, B.A.; Al-Halhouli, A. Fabrication of Dissolvable Microneedle Patches Using an Innovative Laser-Cut Mould Design to Shortlist Potentially Transungual Delivery Systems: In Vitro Evaluation. AAPS PharmSciTech 2019, 20, 215. [Google Scholar] [CrossRef]
- Elsherif, N.I.; Shamma, R.N.; Abdelbary, G. Terbinafine Hydrochloride Trans-ungual Delivery via Nanovesicular Systems: In Vitro Characterization and Ex Vivo Evaluation. AAPS PharmSciTech 2017, 18, 551–562. [Google Scholar] [CrossRef]
- El-sherif, N.I.; Shamma, R.N.; Abdelbary, G. In-situ gels and nail lacquers as potential delivery systems for treatment of onychomycosis. A comparative study. J. Drug Deliv. Sci. Technol. 2018, 43, 253–261. [Google Scholar] [CrossRef]
- Tanrıverdi, S.T.; Özer, Ö. Novel topical formulations of Terbinafine-HCl for treatment of onychomycosis. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2013, 48, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Bhuptani, R.S.; Deshpande, K.M.; Patravale, V.B. Transungual permeation: Current insights. Drug Deliv. Transl. Res. 2016, 6, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Cordoba Díaz, D.; Losa Iglesias, M.E.; Becerro de Bengoa Vallejo, R.; Cordoba Diaz, M. Transungual Delivery of Ciclopirox Is Increased 3⁻4-Fold by Mechanical Fenestration of Human Nail Plate in an In Vitro Model. Pharmaceutics 2019, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Smith, K.A.; Li, S.K. Iontophoretically enhanced ciclopirox delivery into and across human nail plate. J. Pharm. Sci. 2009, 98, 3608–3616. [Google Scholar] [CrossRef]
- Monti, D.; Mazzantini, D.; Tampucci, S.; Vecchione, A.; Celandroni, F.; Burgalassi, S.; Ghelardi, E. Ciclopirox and Efinaconazole Transungual Permeation, Antifungal Activity, and Proficiency To Induce Resistance in Trichophyton rubrum. Antimicrob. Agents Chemother. 2019, 63, e00442-19. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Kim, H.D.; Davis, S.P.; Etheredge, R.; Barsness, M.; Friden, P.M.; Murthy, S.N. An ex vivo toe model used to assess applicators for the iontophoretic ungual delivery of terbinafine. Pharm. Res. 2009, 26, 2194–2201. [Google Scholar] [CrossRef]
- Uchida, N.; Yanagi, M.; Hamada, H. Physical Enhancement? Nanocarrier? Current Progress in Transdermal Drug Delivery. Nanomaterials 2021, 11, 335. [Google Scholar] [CrossRef]
- Mahmud, M.; Rahman, A.; Salem, K.S.; Bari, M.L.; Qiu, H. Architecting Ultrathin Graphitic C(3)N(4) Nanosheets Incorporated PVA/Gelatin Bionanocomposite for Potential Biomedical Application: Effect on Drug Delivery, Release Kinetics, and Antibacterial Activity. ACS Appl. Bio. Mater. 2022, 5, 5126–5139. [Google Scholar] [CrossRef]
- Suh, T.C.; Twiddy, J.; Mahmood, N.; Ali, K.M.; Lubna, M.M.; Bradford, P.D.; Daniele, M.A.; Gluck, J.M. Electrospun Carbon Nanotube-Based Scaffolds Exhibit High Conductivity and Cytocompatibility for Tissue Engineering Applications. ACS Omega 2022, 7, 20006–20019. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Kim, H.D.; Chakraborty, B.; Singh, J.; Zaman, M.; Gupta, A.; Friden, P.M.; Murthy, S.N. Ungual and trans-ungual iontophoretic delivery of terbinafine for the treatment of onychomycosis. J. Pharm. Sci. 2009, 98, 4130–4140. [Google Scholar] [CrossRef]
- Nair, A.B.; Vaka, S.R.; Sammeta, S.M.; Kim, H.D.; Friden, P.M.; Chakraborty, B.; Murthy, S.N. Trans-ungual iontophoretic delivery of terbinafine. J. Pharm. Sci. 2009, 98, 1788–1796. [Google Scholar] [CrossRef]
- Nair, A.B.; Al-Dhubiab, B.E.; Shah, J.; Gorain, B.; Jacob, S.; Attimarad, M.; Sreeharsha, N.; Venugopala, K.N.; Morsy, M.A. Constant Voltage Iontophoresis Technique to Deliver Terbinafine via Transungual Delivery System: Formulation Optimization Using Box–Behnken Design and In Vitro Evaluation. Pharmaceutics 2021, 13, 1692. [Google Scholar] [CrossRef]
- Bhatt, V.; Pillai, R. Efinaconazole topical solution, 10%: Formulation development program of a new topical treatment of toenail onychomycosis. J. Pharm. Sci. 2015, 104, 2177–2182. [Google Scholar] [CrossRef]
- Gupta, A.K.; Venkataraman, M.; Renaud, H.J.; Summerbell, R.; Shear, N.H.; Piguet, V. A Paradigm Shift in the Treatment and Management of Onychomycosis. Skin Appendage Disord. 2021, 7, 351–358. [Google Scholar] [CrossRef]
- Hur, M.S.; Park, M.; Jung, W.H.; Lee, Y.W. Evaluation of drug susceptibility test for Efinaconazole compared with conventional antifungal agents. Mycoses 2019, 62, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Kircik, L.H. Enhancing transungual delivery and spreading of efinaconazole under the nail plate through a unique formulation approach. J. Drugs Dermatol. JDD 2014, 13, 1457–1461. [Google Scholar]
- Lee, B.C.; Pangeni, R.; Na, J.; Koo, K.T.; Park, J.W. Preparation and in vivo evaluation of a highly skin- and nail-permeable efinaconazole topical formulation for enhanced treatment of onychomycosis. Drug Deliv. 2019, 26, 1167–1177. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, J.S.; Ho, M.J.; Park, D.W.; Kim, E.A.; Choi, Y.S.; Jang, S.W.; Kang, M.J. Effect of Penetration Enhancers on Toenail Delivery of Efinaconazole from Hydroalcoholic Preparations. Molecules 2021, 26, 1650. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Targhotra, M.; Sahoo, P.; Chauhan, M.K. Efinaconazole nail lacquer for the transungual drug delivery: Formulation, optimization, characterization and in vitro evaluation. J. Drug Deliv. Sci. Technol. 2020, 60, 101998. [Google Scholar] [CrossRef]
- Almuqbil, R.M.; Sreeharsha, N.; Nair, A.B. Formulation-by-Design of Efinaconazole Spanlastic Nanovesicles for Transungual Delivery Using Statistical Risk Management and Multivariate Analytical Techniques. Pharmaceutics 2022, 14, 1419. [Google Scholar] [CrossRef] [PubMed]
- Anroop, B.; Ghosh, B.; Parcha, V.; Kumar, A.; Khanam, J. Synthesis and comparative skin permeability of atenolol and propranolol esters. J. Drug Deliv. Sci. Technol. 2005, 15, 187–190. [Google Scholar] [CrossRef]
- Shah, H.; Nair, A.B.; Shah, J.; Bharadia, P.; Al-Dhubiab, B.E. Proniosomal gel for transdermal delivery of lornoxicam: Optimization using factorial design and in vivo evaluation in rats. DARU J. Pharm. Sci. 2019, 27, 59–70. [Google Scholar] [CrossRef]
- Vörös-Horváth, B.; Das, S.; Salem, A.; Nagy, S.; Böszörményi, A.; Kőszegi, T.; Pál, S.; Széchenyi, A. Formulation of Tioconazole and Melaleuca alternifolia Essential Oil Pickering Emulsions for Onychomycosis Topical Treatment. Molecules 2020, 25, 5544. [Google Scholar] [CrossRef] [PubMed]
- Mady, O.Y.; Al-Madboly, L.A.; Donia, A.A. Preparation, and Assessment of Antidermatophyte Activity of Miconazole-Urea Water-Soluble Film. Front. Microbiol. 2020, 11, 385. [Google Scholar] [CrossRef]
- Guideline, I.H.T. Stability testing of new drug substances and products. Q1A (R2) Curr. Step 2003, 4, 1–24. [Google Scholar]
- Wang, Y.; Zeng, L.; Song, W.; Liu, J. Influencing factors and drug application of iontophoresis in transdermal drug delivery: An overview of recent progress. Drug Deliv. Transl. Res. 2022, 12, 15–26. [Google Scholar] [CrossRef]
- Anroop, B.; Ghosh, B.; Parcha, V.; Khanam, J. Transdermal delivery of atenolol: Effect of prodrugs and iontophoresis. Curr. Drug Deliv. 2009, 6, 280–290. [Google Scholar] [CrossRef]
- Nair, A.; Vyas, H.; Shah, J.; Kumar, A. Effect of permeation enhancers on the iontophoretic transport of metoprolol tartrate and the drug retention in skin. Drug Deliv. 2011, 18, 19–25. [Google Scholar] [CrossRef]
- Nair, A.; Reddy, C.; Jacob, S. Delivery of a classical antihypertensive agent through the skin by chemical enhancers and iontophoresis. Skin Res. Technol. 2009, 15, 187–194. [Google Scholar] [CrossRef]
- Kreutz, T.; de Matos, S.P.; Koester, L.S. Recent Patents on Permeation Enhancers for Drug Delivery Through Nails. Recent Pat. Drug Deliv. Formul. 2019, 13, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Chakraborty, B.; Murthy, S.N. Effect of polyethylene glycols on the trans-ungual delivery of terbinafine. Curr. Drug Deliv. 2010, 7, 407–414. [Google Scholar] [CrossRef]
- Myoung, Y.; Choi, H.K. Permeation of ciclopirox across porcine hoof membrane: Effect of pressure sensitive adhesives and vehicles. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2003, 20, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Cutrín-Gómez, E.; Anguiano-Igea, S.; Delgado-Charro, M.B.; Gómez-Amoza, J.L.; Otero-Espinar, F.J. Effect of Penetration Enhancers on Drug Nail Permeability from Cyclodextrin/Poloxamer-Soluble Polypseudorotaxane-Based Nail Lacquers. Pharmaceutics 2018, 10, 273. [Google Scholar] [CrossRef]
- Alqahtani, A.; Raut, B.; Khan, S.; Mohamed, J.M.M.; Fatease, A.A.; Alqahtani, T.; Alamri, A.; Ahmad, F.; Krishnaraju, V. The Unique Carboxymethyl Fenugreek Gum Gel Loaded Itraconazole Self-Emulsifying Nanovesicles for Topical Onychomycosis Treatment. Polymers 2022, 14, 325. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Patel, V.; Shah, J. 3D Printing Technologies: Recent Development and Emerging Applications in Various Drug Delivery Systems. AAPS PharmSciTech 2020, 21, 220. [Google Scholar] [CrossRef]
- Kurakula, M.; Rao, G. Pharmaceutical assessment of polyvinylpyrrolidone (PVP): As excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef]
- Pal, P.; Thakur, R.S.; Ray, S.; Mazumder, B. Design and development of a safer non-invasive transungual drug delivery system for topical treatment of onychomycosis. Drug Dev. Ind. Pharm. 2015, 41, 1095–1099. [Google Scholar] [CrossRef]
- Vejnovic, I.; Simmler, L.; Betz, G. Investigation of different formulations for drug delivery through the nail plate. Int. J. Pharm. 2010, 386, 185–194. [Google Scholar] [CrossRef]
- Smith, K.A.; Hao, J.; Li, S.K. Effects of organic solvents on the barrier properties of human nail. J. Pharm. Sci. 2011, 100, 4244–4257. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, V.; Patel, R.; Patel, M.; Thanki, K.; Mishra, S. Design and evaluation of microemulsion-based efinaconazole formulations for targeted treatment of onychomycosis through transungual route: Ex vivo and nail clipping studies. Colloids Surf. B Biointerfaces 2021, 201, 111652. [Google Scholar] [CrossRef]
- McCartney, F.; Jannin, V.; Chevrier, S.; Boulghobra, H.; Hristov, D.R.; Ritter, N.; Miolane, C.; Chavant, Y.; Demarne, F.; Brayden, D.J. Labrasol® is an efficacious intestinal permeation enhancer across rat intestine: Ex vivo and in vivo rat studies. J. Control. Release Off. J. Control. Release Soc. 2019, 310, 115–126. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Li, J.; Liu, R.; Shu, L.; Jin, J. Effects of Labrasol on the corneal drug delivery of baicalin. Drug Deliv. 2009, 16, 399–404. [Google Scholar] [CrossRef]
- Panapisal, V.; Charoensri, S.; Tantituvanont, A. Formulation of microemulsion systems for dermal delivery of silymarin. AAPS PharmSciTech 2012, 13, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jia, X.; Pang, D.; Jiang, S.; Zhu, M.; Lu, G.; Tian, Y.; Wang, C.; Chao, D.; Wallace, G. An integrated Mg battery-powered iontophoresis patch for efficient and controllable transdermal drug delivery. Nat. Commun. 2023, 14, 297. [Google Scholar] [CrossRef]
- Chen, K.; Puri, V.; Michniak-Kohn, B. Iontophoresis to Overcome the Challenge of Nail Permeation: Considerations and Optimizations for Successful Ungual Drug Delivery. The AAPS J. 2021, 23, 25. [Google Scholar] [CrossRef]
- Teaima, M.H.; Mohamed, M.A.A.; Abd El Rehem, R.T.; Tayel, S.A.; El-Nabarawi, M.A.; Fouad, S.A. Enhanced Transdermal Delivery of Bisoprolol Hemifumarate via Combined Effect of Iontophoresis and Chemical Enhancers: Ex Vivo Permeation/In Vivo Pharmacokinetic Studies. Pharmaceutics 2021, 13, 682. [Google Scholar] [CrossRef]
- Town, A.R.; Taylor, J.; Dawson, K.; Niezabitowska, E.; Elbaz, N.M.; Corker, A.; Garcia-Tuñón, E.; McDonald, T.O. Tuning HIV drug release from a nanogel-based in situ forming implant by changing nanogel size. J. Mater. Chem. B 2019, 7, 373–383. [Google Scholar] [CrossRef]
- Baraldi, A.; Jones, S.A.; Guesné, S.; Traynor, M.J.; McAuley, W.J.; Brown, M.B.; Murdan, S. Human nail plate modifications induced by onychomycosis: Implications for topical therapy. Pharm. Res. 2015, 32, 1626–1633. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, K.; Sugimoto, N.; Hosaka, S.; Katafuchi-Nagashima, M.; Arakawa, Y.; Tatsumi, Y.; Jo Siu, W.; Pillai, R. The low keratin affinity of efinaconazole contributes to its nail penetration and fungicidal activity in topical onychomycosis treatment. Antimicrob. Agents Chemother. 2014, 58, 3837–3842. [Google Scholar] [CrossRef] [PubMed]

| Ingredients (% w/w) | Batch Code | ||||||
|---|---|---|---|---|---|---|---|
| E1 | E2 | E3 | E4 | E5 | E6 | E7 | |
| Efinaconazole | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 |
| Ethyl alcohol | 5 | 10 | 15 | 20 | 15 | 25 | 25 |
| Labrasol® | 5 | 5 | 5 | 5 | 10 | 10 | 15 |
| Tween 80 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Polyethylene glycol 400 | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
| Polyvinylpyrrolidone K30 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Disodium ethylenediaminetetraacetic acid | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Butylated hydroxytoluene | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Water (up to) | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Parameters | Batch Code | ||||||
|---|---|---|---|---|---|---|---|
| E1 | E2 | E3 | E4 | E5 | E6 | E7 | |
| pH | 3.16 ± 0.12 | 3.05 ± 0.08 | 3.11 ± 0.05 | 3.20 ± 0.14 | 3.15 ± 0.06 | 3.08 ± 0.13 | 3.12 ± 0.14 |
| Loading efficacy (%) | 98.20 ± 1.97 | 97.02 ± 2.31 | 98.44 ± 1.55 | 98.30 ± 1.55 | 97.84 ± 2.11 | 98.61 ± 1.08 | 97.92 ± 1.95 |
| Drug loading (%) | 7.45 ± 0.22 | 7.40 ± 0.16 | 7.48 ± 0.18 | 7.52 ± 0.05 | 7.41 ± 0.21 | 7.45 ± 0.16 | 7.47 ± 0.15 |
| Viscosity (cP) at 50 rpm | 56.92 ± 1.85 | 59.00 ± 2.31 | 57.76 ± 2.22 | 61.11 ± 2.61 | 68.42 ± 2.37 | 67.22 ± 2.01 | 72.87 ± 3.54 |
| Formulation | Voltage Range (V) | Solvent:Cosolvent | Enhancer-PEG 400 (%) | Conductivity (mS) | Permeation (µg/cm2) | Drug Loaded in the Nail (µg/mg) |
|---|---|---|---|---|---|---|
| F8 | 4.5 | 1:0.5 | 30 | 4.16 ± 0.13 | 28.65 ± 3.05 | 1.51 ± 0.16 |
| F10 | 4.5 | 1:1 | 20 | 4.66 ± 0.11 | 24.80 ± 2.64 | 1.46 ± 0.26 |
| F15 | 4.5 | 1.5:1 | 25 | 4.02 ± 0.06 | 27.78 ± 3.56 | 1.50 ± 0.17 |
| F12 | 4.5 | 1.5:1 | 30 | 3.51 ± 0.10 | 31.03 ± 2.92 | 1.54 ± 0.15 |
| F7 | 7.5 | 1:0.5 | 25 | 4.52 ± 0.12 | 47.21 ± 2.89 | 1.90 ± 0.24 |
| F4 | 7.5 | 1:0.5 | 30 | 4.16 ± 0.13 | 55.69 ± 4.05 | 2.28 ± 0.31 |
| F3 | 7.5 | 1:1 | 25 | 4.28 ± 0.08 | 51.98 ± 3.84 | 2.07 ± 0.25 |
| F9 | 7.5 | 1:1 | 30 | 3.82 ± 0.05 | 55.03 ± 4.63 | 2.17 ± 0.29 |
| F1 | 7.5 | 1.5:1 | 20 | 4.38 ± 0.15 | 43.94 ± 3.61 | 1.80 ± 0.26 |
| F11 | 7.5 | 1.5:1 | 20 | 4.38 ± 0.15 | 43.94 ± 3.60 | 1.80 ± 0.21 |
| F2 | 10.5 | 1:0.5 | 20 | 4.93 ± 0.09 | 68.56 ± 5.36 | 3.07 ± 0.31 |
| F6 | 10.5 | 1:0.5 | 25 | 4.52 ± 0.12 | 71.94 ± 5.20 | 3.21 ± 0.33 |
| F14 | 10.5 | 1:1 | 30 | 3.82 ± 0.05 | 78.66 ± 5.66 | 3.37 ± 0.35 |
| F13 | 10.5 | 1.5:1 | 25 | 4.02 ± 0.06 | 69.38 ± 5.08 | 3.14 ± 0.11 |
| F5 | 10.5 | 1.5:1 | 30 | 3.51 ± 0.10 | 73.39 ± 3.96 | 3.28 ± 0.31 |
| Source | Efinaconazole Diffusion | Efinaconazole Accumulation inside the Nail | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nparm | DF | Sum of Squares | F Ratio | Prob > F | Nparm | DF | Sum of Squares | F Ratio | Prob > F | |
| Voltage range | 2 | 2 | 4316.5128 | 685.9742 | <0.0001 | 2 | 2 | 7.0468285 | 549.7144 | <0.0001 |
| Solvent: cosolvent | 2 | 2 | 26.6675 | 4.2380 | 0.0556 | 2 | 2 | 0.0315546 | 2.4615 | 0.1469 |
| Enhancer-PEG 400 | 2 | 2 | 151.9646 | 24.1500 | 0.0004 | 2 | 2 | 0.1549844 | 12.0901 | 0.0038 |
| Source | LogWorth | p Value |
|---|---|---|
| Voltage range | 8.947 | 0.00000 |
| Enhancer-PEG 400 | 3.390 | 0.00041 |
| Solvent:cosolvent | 1.255 | 0.05559 |
| Optimized Transungual Iontophoretic Delivery | |||||
|---|---|---|---|---|---|
| Drug Permeation (µg/cm2) | % Difference | Drug Loading (µg/mg) | % Difference | ||
| Predicted | Observed | Predicted | Observed | ||
| 77.98 | 78.59 | 0.782 | 3.39 | 3.2 | −2.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nair, A.B.; Aldhubiab, B.; Shah, J.; Jacob, S.; Attimarad, M.; Sreeharsha, N.; Venugopala, K.N.; Joseph, A.; Morsy, M.A. Design, Development, and Evaluation of Constant Voltage Iontophoresis for the Transungual Delivery of Efinaconazole. Pharmaceutics 2023, 15, 1422. https://doi.org/10.3390/pharmaceutics15051422
Nair AB, Aldhubiab B, Shah J, Jacob S, Attimarad M, Sreeharsha N, Venugopala KN, Joseph A, Morsy MA. Design, Development, and Evaluation of Constant Voltage Iontophoresis for the Transungual Delivery of Efinaconazole. Pharmaceutics. 2023; 15(5):1422. https://doi.org/10.3390/pharmaceutics15051422
Chicago/Turabian StyleNair, Anroop B., Bandar Aldhubiab, Jigar Shah, Shery Jacob, Mahesh Attimarad, Nagaraja Sreeharsha, Katharigatta N. Venugopala, Alex Joseph, and Mohamed A. Morsy. 2023. "Design, Development, and Evaluation of Constant Voltage Iontophoresis for the Transungual Delivery of Efinaconazole" Pharmaceutics 15, no. 5: 1422. https://doi.org/10.3390/pharmaceutics15051422
APA StyleNair, A. B., Aldhubiab, B., Shah, J., Jacob, S., Attimarad, M., Sreeharsha, N., Venugopala, K. N., Joseph, A., & Morsy, M. A. (2023). Design, Development, and Evaluation of Constant Voltage Iontophoresis for the Transungual Delivery of Efinaconazole. Pharmaceutics, 15(5), 1422. https://doi.org/10.3390/pharmaceutics15051422










