The Effect of High-Pressure Homogenization Conditions on the Physicochemical Properties and Stability of Designed Fluconazole-Loaded Ocular Nanoemulsions
Abstract
:1. Introduction
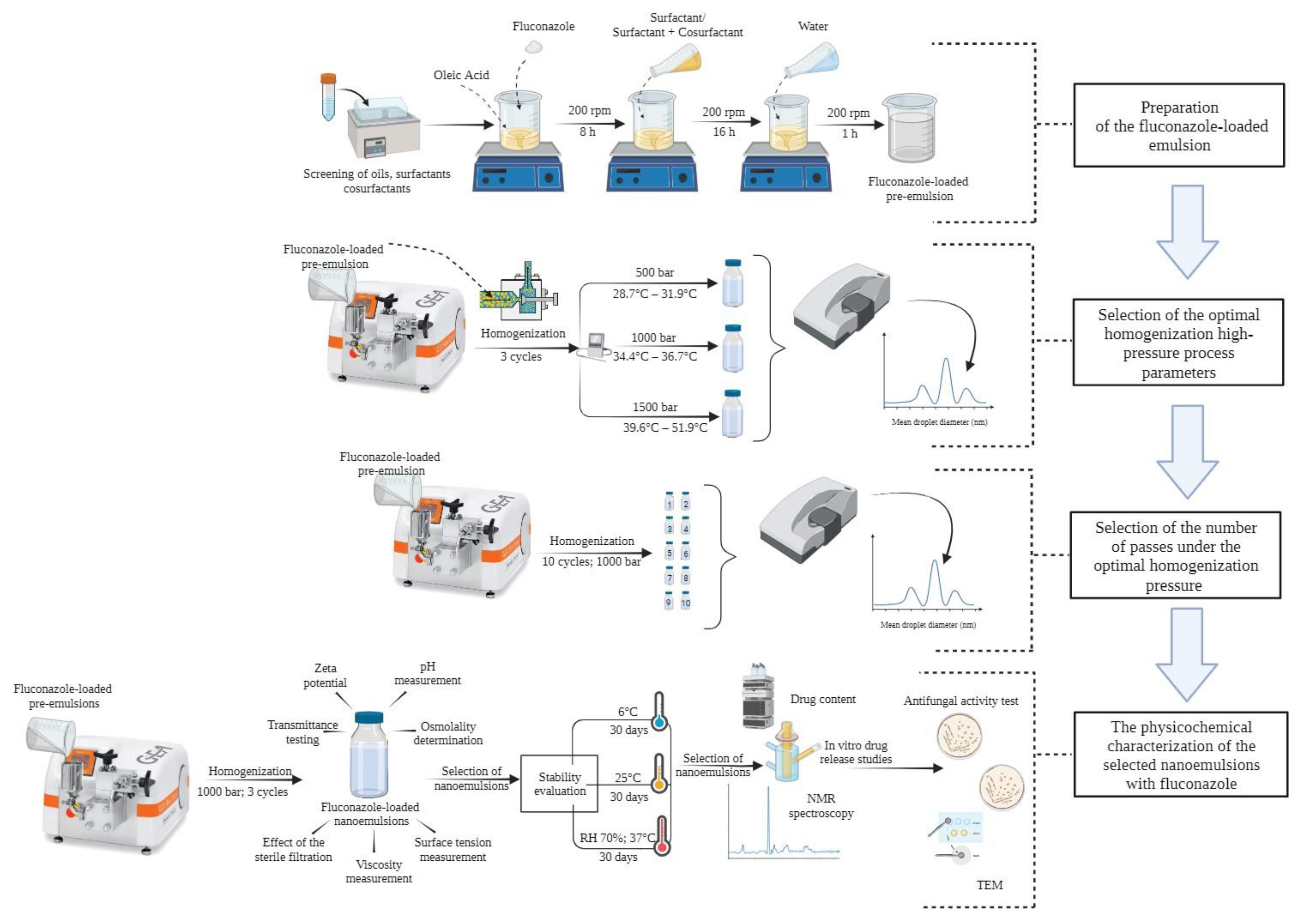
2. Materials and Methods
2.1. Materials
2.2. Screening of Oils, Surfactants, and Cosurfactants as the Components of the Fluconazole-Loaded Nanoemulsion
2.3. The Quantitative Determination of the FLZ Content via High-Performance Liquid Chromatography (HPLC)
2.4. Optimization of the High-Pressure Homogenization Parameters in the Preparation of the Fluconazole-Loaded Nanoemulsion
2.4.1. Selection of the Optimal Homogenization Pressure
2.4.2. Selection of the Number of Passes under the Optimal Homogenization Pressure
2.4.3. Measurement of the Particle Size (PS) and Polydispersity Index (PDI)
2.5. Effect of Sterile Filtration on the PS and PDI Parameters of the Nanoemulsions
2.6. Physicochemical Characterization of the Nanoemulsions Prepared Using the Optimal Homogenization Parameters
2.6.1. Visual Examination and Transmittance (%T) Testing
2.6.2. Refractive Index Measurement
2.6.3. Measurement of the Zeta Potential (ZP)
2.6.4. pH Measurement
2.6.5. Osmolality Determination
2.6.6. Surface Tension Measurement
2.6.7. Viscosity Determination
2.7. Stability Evaluation
2.8. Evaluation of the Properties of the Nanoemulsions Selected in the Stability Test
2.8.1. Nuclear Magnetic Resonance (NMR) Spectroscopy Assay
2.8.2. Drug Content
2.8.3. In Vitro Release Studies
2.8.4. Surface Morphology—Transmission Electron Microscopy (TEM)
2.8.5. Antifungal Activity Test
2.9. Statistical Analysis
3. Results and Discussion
3.1. Screening of Oils, Surfactants, and Cosurfactants as the Components of the Fluconazole-Loaded Nanoemulsion
3.2. Optimization of the High-Pressure Homogenization Parameters in the Preparation of the Fluconazole-Loaded Nanoemulsion
3.3. Effect of Sterile Filtration on the Mean PS and PDI
3.4. Characterization of the Optimized Nanoemulsions after the Third Cycle of Homogenization under the Pressure of 1000 Bar
3.4.1. Visual Examination and Transmittance Measurement
3.4.2. Refractive Index (RI) Measurement
3.4.3. Zeta Potential (ZP) Determination
3.4.4. pH Measurement
3.4.5. Osmolality Determination
3.4.6. Surface Tension Measurement
3.4.7. Viscosity Determination
3.5. Stability Study
3.6. Evaluation of the Properties of the Nanoemulsions Selected in the Stability Test
3.6.1. The Stability Analysis via NMR Spectroscopy
3.6.2. Drug Content
3.6.3. In Vitro Release Studies
3.6.4. Antifungal Activity Test
3.6.5. Surface Morphology—Transmission Electron Microscopy (TEM)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sutradhar, K.B.; Amin, L. Nanoemulsions: Increasing possibilities in drug delivery. Eur. J. Nanomed. 2013, 5, 97–110. [Google Scholar] [CrossRef]
- Chime, S.A.; Kenechukwu, F.C.; Attama, A.A. Nanoemulsions—Advances in Formulation, Characterization and Applications in Drug Delivery. Appl. Nanotechnol. Drug Deliv. 2014, 3, 77–126. [Google Scholar] [CrossRef]
- Youssef, A.A.A.; Cai, C.; Dudhipala, N.; Majumdar, S. Design of topical ocular ciprofloxacin nanoemulsion for the management of bacterial keratitis. Pharmaceuticals 2021, 14, 210. [Google Scholar] [CrossRef] [PubMed]
- Dukovski, B.J.; Ljubica, J.; Kocbek, P.; Kučuk, M.S.; Krtalić, I.; Hafner, A.; Pepić, I.; Lovrić, J. Towards the development of a biorelevant in vitro method for the prediction of nanoemulsion stability on the ocular surface. Int. J. Pharm. 2023, 633, 122622. [Google Scholar] [CrossRef] [PubMed]
- Yukuyama, M.N.; Kato, E.T.M.; Löbenberg, R.; Bou-Chacra, N.A. Challenges and Future Prospects of Nanoemulsion as a Drug Delivery System. Curr. Pharm. Des. 2017, 23, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Gawin-Mikołajewicz, A.; Nartowski, K.P.; Dyba, A.J.; Gołkowska, A.M.; Malec, K.; Karolewicz, B. Ophthalmic Nanoemulsions: From Composition to Technological Processes and Quality Control. Mol. Pharm. 2021, 18, 3719–3740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Z.; Tao, C.; Lin, X.; Zhang, M.; Zeng, L.; Chen, X.; Song, H. Cationic nanoemulsions with prolonged retention time as promising carriers for ophthalmic delivery of tacrolimus. Eur. J. Pharm. Sci. 2020, 144, 105229. [Google Scholar] [CrossRef] [PubMed]
- Jurišić Dukovski, B.; Juretić, M.; Bračko, D.; Randjelović, D.; Savić, S.; Crespo Moral, M.; Diebold, Y.; Filipović-Grčić, J.; Pepić, I.; Lovrić, J. Functional ibuprofen-loaded cationic nanoemulsion: Development and optimization for dry eye disease treatment. Int. J. Pharm. 2020, 576, 118979. [Google Scholar] [CrossRef]
- Silva, H.D.; Cerqueira, M.A.; Vicente, A.A. Influence of surfactant and processing conditions in the stability of oil-in-water nanoemulsions. J. Food Eng. 2015, 167, 89–98. [Google Scholar] [CrossRef]
- Håkensson, A.; Rayner, M. Champter 5: General Principles of Nanoemulsion Formation by High-Energy Mechanical Methods. In Nanoemulsions: Formulation, Applications, and Characterization; Jafari, S.M., McClements, D.J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 104–139. [Google Scholar]
- Galvão, K.C.S.; Vicente, A.A.; Sobral, P.J.A. Development, Characterization, and Stability of O/W Pepper Nanoemulsions Produced by High-Pressure Homogenization. Food Bioprocess Technol. 2018, 11, 355–367. [Google Scholar] [CrossRef]
- Besseling, R.; Arribas-Bueno, R.; van Tuijn, R.; Gerich, A. Realtime droplet size monitoring of nano-emulsions during high pressure homogenization. Inprocess-LSP 2021, 1–11. [Google Scholar] [CrossRef]
- Patel, J.K.; Pathak, Y.V. Emerging Technologies for Nanoparticle Manufacturing; Springer Nature: Cham, Switzerland, 2021; ISBN 9783030507039. [Google Scholar]
- Yong, A.P.; Islam, M.A.; Hasan, N. A Review: Effect of Pressure on Homogenization. Sigma J. Eng. Nat. Sci. 2017, 35, 1–22. [Google Scholar]
- Yadav, K.S.; Kale, K. High Pressure Homogenizer in Pharmaceuticals: Understanding Its Critical Processing Parameters and Applications. J. Pharm. Innov. 2020, 15, 690–701. [Google Scholar] [CrossRef]
- Ruiz-Montañez, G.; Ragazzo-Sanchez, J.A.; Picart-Palmade, L.; Calderón-Santoyo, M.; Chevalier-Lucia, D. Optimization of nanoemulsions processed by high-pressure homogenization to protect a bioactive extract of jackfruit (Artocarpus heterophyllus Lam). Innov. Food Sci. Emerg. Technol. 2017, 40, 35–41. [Google Scholar] [CrossRef]
- Li, Y.; Wu, C.L.; Liu, J.; Zhu, Y.; Zhang, X.Y.; Jiang, L.Z.; Qi, B.K.; Zhang, X.N.; Wang, Z.J.; Teng, F. Soy protein isolate-phosphatidylcholine nanoemulsions prepared using high-pressure homogenization. Nanomaterials 2018, 8, 307. [Google Scholar] [CrossRef]
- Yuan, Y.; Gao, Y.; Zhao, J.; Mao, L. Characterization and stability evaluation of β-carotene nanoemulsions prepared by high pressure homogenization under various emulsifying conditions. Food Res. Int. 2008, 41, 61–68. [Google Scholar] [CrossRef]
- Chong, W.T.; Tan, C.P.; Cheah, Y.K.; Lajis, A.F.B.; Dian, N.L.H.M.; Kanagaratnam, S.; Lai, O.M. Optimization of process parameters in preparation of tocotrienol-rich red palm oil-based nanoemulsion stabilized by Tween80-Span 80 using response surface methodology. PLoS ONE 2018, 13, e0202771. [Google Scholar] [CrossRef]
- Håkensson, A. Champter 7: Fabrication of Nanoemulsions by High-Pressure Valve Homogenization. In Nanoemulsions: Formulation, Applications, and Characterization; Jafari, S.M., McClements, D.J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 176–206. [Google Scholar]
- Gallarate, M.; Chirio, D.; Bussano, R.; Peira, E.; Battaglia, L.; Baratta, F.; Trotta, M. Development of O/W nanoemulsions for ophthalmic administration of timolol. Int. J. Pharm. 2013, 440, 126–134. [Google Scholar] [CrossRef]
- Lallemand, F.; Daull, P.; Benita, S.; Buggage, R.; Garrigue, J.-S. Successfully Improving Ocular Drug Delivery Using the Cationic Nanoemulsion, Novasorb. J. Drug Deliv. 2012, 2012, 604204. [Google Scholar] [CrossRef]
- Daull, P.; Lallemand, F.; Philips, B.; Lambert, G.; Buggage, R.; Garrigue, J.S. Distribution of cyclosporine A in ocular tissues after topical administration of cyclosporine A cationic emulsions to pigmented rabbits. Cornea 2013, 32, 345–354. [Google Scholar] [CrossRef]
- Henostroza, M.A.B.; Melo, K.J.C.; Yukuyama, M.N.; Löbenberg, R.; Bou-Chacra, N.A. Cationic rifampicin nanoemulsion for the treatment of ocular tuberculosis. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 597, 124755. [Google Scholar] [CrossRef]
- Pathak, M.K.; Chhabra, G.; Pathak, K. Design and development of a novel pH triggered nanoemulsified in-situ ophthalmic gel of fluconazole: Ex-vivo transcorneal permeation, corneal toxicity and irritation testing. Drug Dev. Ind. Pharm. 2013, 39, 780–790. [Google Scholar] [CrossRef]
- Soliman, O.A.E.A.; Mohamed, E.A.; Khatera, N.A.A. Enhanced ocular bioavailability of fluconazole from niosomal gels and microemulsions: Formulation, optimization, and in vitro–in vivo evaluation. Pharm. Dev. Technol. 2017, 24, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Kassaee, S.N.; Mahboobian, M.M. Besifloxacin-loaded ocular nanoemulsions: Design, formulation and efficacy evaluation. Drug Deliv. Transl. Res. 2022, 12, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Muzzalupo, R.; Tavano, L. Niosomal drug delivery for transdermal targeting: Recent advances. Res. Rep. Transdermal Drug Deliv. 2015, 4, 23–33. [Google Scholar] [CrossRef]
- Durak, S.; Rad, M.E.; Yetisgin, A.A.; Sutova, H.E.; Kutlu, O.; Cetinel, S.; Zarrabi, A. Niosomal drug delivery systems for ocular disease—Recent advances and future prospects. Nanomaterials 2020, 10, 1191. [Google Scholar] [CrossRef]
- Moreira, M.P.; Budel, R.G.; Wergutz, J.; Machado, M.L.; Guerino, B.C.; Soares, F.A.A.; Krause, L.M.F.; Ourique, A.F.; Boeck, C.R. Simvastatin-loaded nanoemulsions: Development, characterization, stability study and toxicity assays. Ther. Deliv. 2020, 11, 497–505. [Google Scholar] [CrossRef]
- Zhang, W.; Qin, Y.; Gao, Z.; Ou, W.; Zhu, H.; Zhang, Q. Phase behavior and stability of nano-emulsions prepared by D phase emulsification method. J. Mol. Liq. 2019, 285, 424–429. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, M.; Chen, K.; Wang, M. Nano-emulsion prepared by high pressure homogenization method as a good carrier for Sichuan pepper essential oil: Preparation, stability, and bioactivity. LWT 2022, 154, 112779. [Google Scholar] [CrossRef]
- Komoroski, E.M.; Komoroski, R.A.; Valentine, J.L.; Pearce, J.M.; Kearns, G.L. The Use of Nuclear Magnetic Resonance Spectroscopy in the Detection of Drug Intoxication. J. Anal. Toxicol. 2000, 24, 180–187. [Google Scholar] [CrossRef]
- Dust, J.M.; Fang, Z.; Harris, J.M. Proton NMR Characterization of Poly (ethylene glycols) and Derivatives. Macromolecules 1990, 23, 3742–3746. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, A.; Meng, Y.; Ning, T.; Yang, H.; Ding, L.; Xiao, X.; Li, X. NMR Method for Accurate Quantification of Polysorbate 80 Copolymer Composition. Anal. Chem. 2015, 87, 9810–9816. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Eisner, R.; Young, N.; Gautam, B.; Hau, D.D.; Psychogios, N.; Dong, E.; Bouatra, S.; et al. HMDB: A knowledgebase for the human metabolome. Nucleic Acids Res. 2009, 37, D603–D610. [Google Scholar] [CrossRef] [PubMed]
- Cyr, T.D.; Dawson, B.A.; Neville, G.A.; Shurvell, H.F. Spectral characterization of fluconazole. J. Pharm. Biomed. Anal. 1996, 14, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Malec, K.; Monaco, S.; Delso, I.; Nestorowicz, J.; Kozakiewicz-Latała, M.; Karolewicz, B.; Khimyak, Y.Z.; Angulo, J.; Nartowski, K.P. Unravelling the mechanisms of drugs partitioning phenomena in micellar systems via NMR spectroscopy. J. Colloid Interface Sci. 2023, 638, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Espinel-Ingroff, A. Standardized disk diffusion method for yeasts. Clin. Microbiol. Newsl. 2007, 29, 97–100. [Google Scholar] [CrossRef]
- Sharma, S.; Sahni, J.K.; Ali, J.; Baboota, S. Effect of high-pressure homogenization on formulation of TPGS loaded nanoemulsion of rutin—Pharmacodynamic and antioxidant studies. Drug Deliv. 2015, 22, 541–551. [Google Scholar] [CrossRef]
- Karami, Z.; Zanjani, M.R.S.; Hamidi, M. Nanoemulsions in CNS drug delivery: Recent developments, impacts and challenges. Drug Discov. Today 2019, 24, 1104–1115. [Google Scholar] [CrossRef]
- Shafiq, S.; Shakeel, F.; Talegaonkar, S.; Ahmad, F.J.; Khar, R.K.; Ali, M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur. J. Pharm. Biopharm. 2007, 66, 227–243. [Google Scholar] [CrossRef]
- Ahuja, M.; Dhake, A.S.; Sharma, S.K.; Majumdar, D.K. Stability studies on aqueous and oily ophthalmic solutions of diclofenac. Yakugaku Zasshi 2009, 129, 495–502. [Google Scholar] [CrossRef]
- Morsi, N.M.; Mohamed, M.I.; Refai, H.; El Sorogy, H.M. Nanoemulsion as a novel ophthalmic delivery system for acetazolamide. Int. J. Pharm. Pharm. Sci. 2014, 6, 227–236. [Google Scholar]
- Lee, J.Y. Patent Application Publication Pub. U.S. Patent US 2016/0101050 A1, 14 April 2016. [Google Scholar]
- Rasoanirina, B.N.V.; Lassoued, M.A.; Kamoun, A.; Bahloul, B.; Miladi, K.; Sfar, S. Voriconazole-loaded self-nanoemulsifying drug delivery system (SNEDDS) to improve transcorneal permeability. Pharm. Dev. Technol. 2020, 25, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Akhter, S. Design and Development of Nano Sized Ocular Drug Delivery System. Ph.D. Thesis, Jamia Hamdard University, New Delhi, India, 2012. [Google Scholar]
- Gao, X.C.; Qi, H.P.; Bai, J.H.; Huang, L.; Cui, H. Effects of Oleic Acid on the Corneal Permeability of Compounds and Evaluation of its Ocular Irritation of Rabbit Eyes. Curr. Eye Res. 2014, 39, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Moghimipour, E.; Salimi, A.; Yousefvand, T. Preparation and Evaluation of Celecoxib Nanoemulsion for Ocular Drug Delivery. Asian J. Pharm. AJP 2017, 11, 543–550. [Google Scholar]
- Nagariya, K.; Sharma, P.K.; Sarangdevot, Y.S. Nanoemulsion: Safe, Stable and Effective Formulation System for Ophthalmology. Am. J. Pharm. Tech. Res. 2013, 3, 252–267. [Google Scholar]
- Kommuru, T.R.; Gurley, B.; Khan, M.A.; Reddy, I.K. Self-emulsifying drug delivery systems (SEDDS) of coenzyme Q 10: Formulation development and bioavailability assessment. Int. J. Pharm. 2001, 212, 233–246. [Google Scholar] [CrossRef]
- Ammar, H.O.; Salama, H.A.; Ghorab, M.; Mahmoud, A.A. Nanoemulsion as a Potential Ophthalmic Delivery System for Dorzolamide Hydrochloride. AAPS PharmSciTech 2009, 10, 808–819. [Google Scholar] [CrossRef]
- Jiao, J. Polyoxyethylated nonionic surfactants and their applications in topical ocular drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1663–1673. [Google Scholar] [CrossRef]
- Liebert, M.A. Final Report on the Safty Assessment of Polysorbates 20,,21, 40, 60, 61, 65, 80, 81, and 85. J. Am. Coll. Toxicol. 1984, 3, 1–82. [Google Scholar]
- Solomon, R.; Perry, H.D.; Donnenfeld, E.D.; Greenman, H.E. Slitlamp biomicroscopy of the tear film of patients using topical Restasis and Refresh Endura. J. Cataract. Refract. Surg. 2005, 31, 661–663. [Google Scholar] [CrossRef]
- Ravichandran, V.; Lee, M.; Cao, T.G.N.; Shim, M.S. Polysorbate-based drug formulations for brain-targeted drug delivery and anticancer therapy. Appl. Sci. 2021, 11, 9336. [Google Scholar] [CrossRef]
- Lv, F.F.; Zheng, L.Q.; Tung, C.H. Phase behavior of the microemulsions and the stability of the chloramphenicol in the microemulsion-based ocular drug delivery system. Int. J. Pharm. 2005, 301, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Nair, A.B.; Jacob, S.; Patel, R.K.; Shah, H. Nanoemulsion Based Vehicle for Effective Ocular Delivery of Moxifloxacin Using Experimental Design and Pharmacokinetic Study in Rabbits. Pharmaceutics 2019, 11, 230. [Google Scholar] [CrossRef] [PubMed]
- Tayel, S.A.; El-Nabarawi, M.A.; Tadros, M.I.; Abd-Elsalam, W.H. Promising ion-sensitive in situ ocular nanoemulsion gels of terbinafine hydrochloride: Design, in vitro characterization and in vivo estimation of the ocular irritation and drug pharmacokinetics in the aqueous humor of rabbits. Int. J. Pharm. 2013, 443, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Al Khateb, K.; Ozhmukhametova, E.K.; Mussin, M.N.; Seilkhanov, S.K.; Rakhypbekov, T.K.; Lau, W.M.; Khutoryanskiy, V.V. In situ gelling systems based on Pluronic F127/Pluronic F68 formulations for ocular drug delivery. Int. J. Pharm. 2016, 502, 70–79. [Google Scholar] [CrossRef] [PubMed]
- El-Kamel, A.H. In vitro and in vivo evaluation of Pluronic F127-based ocular delivery system for timolol maleate. Int. J. Pharm. 2002, 241, 47–55. [Google Scholar] [CrossRef]
- Setya, S.; Talegaonkar, S.; Razdan, B.K. Nanoemulsions: Formulation Methods and Stability Aspects. World J. Pharm. Pharm. Sci. 2014, 3, 2214–2228. [Google Scholar]
- Gupta, A.; Badruddoza, A.Z.M.; Doyle, P.S. A General Route for Nanoemulsion Synthesis Using Low-Energy Methods at Constant Temperature. Langmuir 2017, 33, 7118–7123. [Google Scholar] [CrossRef]
- Gallarate, M.; Gasco, M.R.; Trotta, M.; Chetoni, P.; Saettone, M.F. Preparation and evaluation in vitro of solutions and o/w microemulsions containing levobunolol as ion-pair. Int. J. Pharm. 1993, 100, 219–225. [Google Scholar] [CrossRef]
- Lee, L.; Norton, I.T. Comparing droplet breakup for a high-pressure valve homogeniser and a Microfluidizer for the potential production of food-grade nanoemulsions. J. Food Eng. 2013, 114, 158–163. [Google Scholar] [CrossRef]
- Hidajat, M.J.; Jo, W.; Kim, H.; Noh, J. Effective droplet size reduction and excellent stability of limonene nanoemulsion formed by high-pressure homogenizer. Colloids Interfaces 2020, 4, 5. [Google Scholar] [CrossRef]
- Klang, V.; Matsko, N.; Zimmermann, A.M.; Vojnikovic, E.; Valenta, C. Enhancement of stability and skin permeation by sucrose stearate and cyclodextrins in progesterone nanoemulsions. Int. J. Pharm. 2010, 393, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Çinar, K. A Review on Nanoemulsions: Preparation Methods and Stability. Trak. Univ. J. Eng. Sci. 2017, 18, 73–83. [Google Scholar]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Re-coalescence of emulsion droplets during high-energy emulsification. Food Hydrocoll. 2008, 22, 1191–1202. [Google Scholar] [CrossRef]
- Lee, L.; Hancocks, R.; Noble, I.; Norton, I.T. Production of water-in-oil nanoemulsions using high pressure homogenisation: A study on droplet break-up. J. Food Eng. 2014, 131, 33–37. [Google Scholar] [CrossRef]
- Gué, E.; Since, M.; Ropars, S.; Herbinet, R.; Le Pluart, L.; Malzert-Fréon, A. Evaluation of the versatile character of a nanoemulsion formulation. Int. J. Pharm. 2016, 498, 49–65. [Google Scholar] [CrossRef]
- Singh, M.; Bharadwaj, S.; Lee, K.E.; Kang, S.G. Therapeutic nanoemulsions in ophthalmic drug administration: Concept in formulations and characterization techniques for ocular drug delivery. J. Control. Release 2020, 328, 895–916. [Google Scholar] [CrossRef]
- Cappellani, M.R.; Perinelli, D.R.; Pescosolido, L.; Schoubben, A.; Cespi, M.; Cossi, R.; Blasi, P. Injectable nanoemulsions prepared by high pressure homogenization: Processing, sterilization, and size evolution. Appl. Nanosci. 2018, 8, 1483–1491. [Google Scholar] [CrossRef]
- Ismail, A.; Nasr, M.; Sammour, O. Nanoemulsion as a feasible and biocompatible carrier for ocular delivery of travoprost: Improved pharmacokinetic/pharmacodynamic properties. Int. J. Pharm. 2020, 583, 119402. [Google Scholar] [CrossRef]
- Mason, T.G.; Wilking, J.N.; Meleson, K.; Chang, C.B.; Graves, S.M. Nanoemulsions: Formation, structure, and physical properties. J. Phys. Condens. Matter 2006, 18, 635–666. [Google Scholar] [CrossRef]
- Gurpreet, K.; Singh, S.K. Review of Nanoemulsion Formulation and Characterization Techniques. Indian J. Pharm. Sci. 2018, 80, 781–789. [Google Scholar] [CrossRef]
- Bali, V.; Ali, M.; Ali, J. Study of surfactant combinations and development of a novel nanoemulsion for minimising variations in bioavailability of ezetimibe. Colloids Surf. B Biointerfaces 2010, 76, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, M.; Pawar, P.K. Preparation and in Vitro/Ex Vivo evaluation of moxifloxacin-loaded PLGA nanosuspensions for ophthalmic application. Sci. Pharm. 2013, 81, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Bloemen, M.; Brullot, W.; Luong, T.T.; Geukens, N.; Gils, A.; Verbiest, T. Improved functionalization of oleic acid-coated iron oxide nanoparticles for biomedical applications. J. Nanoparticle Res. 2012, 14, 1100. [Google Scholar] [CrossRef] [PubMed]
- Maruno, M.; da Rocha-Filho, P.A. O/W Nanoemulsion After 15 Years of Preparation: A Suitable Vehicle for Pharmaceutical and Cosmetic Applications. J. Dispers. Sci. Technol. 2009, 31, 17–22. [Google Scholar] [CrossRef]
- De Morais, J.M.; dos Santos, O.D.H.; Delicato, T.; Gonçalves, R.A.; da Rocha-Filho, P.A. Physicochemical Characterization of Canola Oil/Water Nano—Emulsions Obtained by Determination of Required HLB Number and Emulsion Phase Inversion Methods. J. Dispers. Sci. Technol. 2007, 27, 109–115. [Google Scholar] [CrossRef]
- Naveh, N.; Weissman, C.; Muchtar, S.; Benita, S.; Mechoulam, R. A submicron emulsion of HU-211, a synthetic cannabinoid, reduces intraocular pressure in rabbits. Graefe’s Arch. Clin. Exp. Ophthalmol. 2000, 238, 334–338. [Google Scholar] [CrossRef]
- Tamilvanan, S.; Benita, S. The potential of lipid emulsion for ocular delivery of lipophilic drugs. Eur. J. Pharm. Biopharm. 2004, 58, 357–368. [Google Scholar] [CrossRef]
- Kumar, R.; Sinha, V.R. Preparation and optimization of voriconazole microemulsion for ocular delivery. Colloids Surfaces B Biointerfaces 2014, 117, 82–88. [Google Scholar] [CrossRef]
- Haße, A.; Keipert, S. Development and characterization of microemulsions for ocular application. Eur. J. Pharm. Biopharm. 1997, 43, 179–183. [Google Scholar] [CrossRef]
- Fialho, S.L.; da Silva-Cunha, A. New vehicle based on a microemulsion for topical ocular administration of dexamethasone. Clin. Exp. Ophthalmol. 2004, 32, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Dhahir, R.K.; Al-Nima, A.M.; Al-Bazzaz, F.Y. Nanoemulsions as Ophthalmic Drug Delivery Systems. Turk. J. Pharm. Sci. 2020, 18, 652–664. [Google Scholar] [CrossRef]
- Li, X.; Muller, R.H.; Keck, C.M.; Bou-Chacra, N.A. Mucoadhesive dexamethasone acetate-polymyxin B sulfate cationic ocular nanoemulsion—Novel combinatorial formulation concept. Pharmazie 2016, 71, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Choradiya, B.R.; Patil, S.B. A comprehensive review on nanoemulsion as an ophthalmic drug delivery system. J. Mol. Liq. 2021, 339, 116751. [Google Scholar] [CrossRef]
- Fernandes, A.R.; Sanchez-Lopez, E.; Dos Santos, T.; Garcia, M.L.; Silva, A.M.; Souto, E.B. Development and characterization of nanoemulsions for ophthalmic applications: Role of cationic surfactants. Materials 2021, 14, 7541. [Google Scholar] [CrossRef] [PubMed]
- Delmas, T.; Piraux, H.; Couffin, A.C.; Texier, I.; Vinet, F.; Poulin, P.; Cates, M.E.; Bibette, J. How to prepare and stabilize very small nanoemulsions. Langmuir 2011, 27, 1683–1692. [Google Scholar] [CrossRef]
- Qian, C.; Mcclements, D.J. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: Factors affecting particle size. Food Hydrocoll. 2011, 25, 1000–1008. [Google Scholar] [CrossRef]
- Wooster, T.J.; Golding, M.; Sanguansri, P. Impact of Oil Type on Nanoemulsion Formation and Ostwald Ripening Stability. Langmuir 2008, 24, 12758–12765. [Google Scholar] [CrossRef]
- Gorantla, S.; Rapalli, V.K.; Waghule, T.; Singh, P.P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Nanocarriers for ocular drug delivery: Current status and translational opportunity. RSC Adv. 2020, 10, 27835–27855. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Emara, M.M.; El-Din, M.R.N.; Aly, W.R. Formation of water-in-diesel oil nano-emulsions using high energy method and studying some of their surface active properties. Egypt. J. Pet. 2011, 20, 17–23. [Google Scholar] [CrossRef]
- Chempur. Kwas Oleinowy. 2019. Available online: http://chempur.pl/katalog/karty-charakterystyk/ (accessed on 20 May 2022).
- Patravale, V.B.; Date, A.A. Microemulsions: Pharmaceutical Applications. In Microemulsions: Background, New Concepts, Applications, Perspectives; Wiley: Hoboken, NJ, USA, 2009; ISBN 9781405167826. [Google Scholar]
- Fu, Z.; Liu, M.; Xu, J.; Wang, Q.; Fan, Z. Stabilization of water-in-octane nano-emulsion. Part I: Stabilized by mixed surfactant systems. Fuel 2010, 89, 2838–2843. [Google Scholar] [CrossRef]
- Lv, F.F.; Li, N.; Zheng, L.Q.; Tung, C.H. Studies on the stability of the chloramphenicol in the microemulsion free of alcohols. Eur. J. Pharm. Biopharm. 2006, 62, 288–294. [Google Scholar] [CrossRef] [PubMed]
- British Pharmacopoeia. British Pharmacoeia; The Council of Europe: London, UK, 2010; Volume III. [Google Scholar]










| Formulation Code | Compositions (% w/w) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oleic Acid | Surfactants (S) | Cosurfactants (Cos) | Water | % Smix | S:Cos Ratio | HLB/ HLBmix* Value | |||||
| KOL | T20 | T80 | PLU | PEG 200 | PG | ||||||
| NE1_KOL | 20 | 10 | 69.7 | 13.50 | |||||||
| NE2_KOL_PEG10 | 20 | 10 | 10 | 59.7 | 20 | 1:1 | 15.80 | ||||
| NE3_KOL_PEG5 | 20 | 10 | 5 | 64.7 | 15 | 2:1 | 15.03 | ||||
| NE4_KOL_PG10 | 20 | 10 | 10 | 59.7 | 20 | 1:1 | 11.44 | ||||
| NE5_KOL_PG5 | 20 | 10 | 5 | 64.7 | 15 | 2:1 | 12.13 | ||||
| NE6_T20 | 20 | 10 | 69.7 | 16.70 | |||||||
| NE7_T20_PEG10 | 20 | 10 | 10 | 59.7 | 20 | 1:1 | 17.40 | ||||
| NE8_T20_PEG5 | 20 | 10 | 5 | 64.7 | 15 | 2:1 | 17.17 | ||||
| NE9_T20_PG10 | 20 | 10 | 10 | 59.7 | 20 | 1:1 | 13.04 | ||||
| NE10_T20_PG5 | 20 | 10 | 5 | 64.7 | 15 | 2:1 | 14.26 | ||||
| NE11_T80 | 20 | 10 | 69.7 | 15.00 | |||||||
| NE12_T80_PEG10 | 20 | 10 | 10 | 59.7 | 20 | 1:1 | 16.55 | ||||
| NE13_T80_PEG5 | 20 | 10 | 5 | 64.7 | 15 | 2:1 | 16.03 | ||||
| NE14_T80_PG10 | 20 | 10 | 10 | 59.7 | 20 | 1:1 | 12.19 | ||||
| NE15_T80_PG5 | 20 | 10 | 5 | 64.7 | 15 | 2:1 | 13.13 | ||||
| NE1_KOL | 20 | 10 | 69.7 | 22.00 | |||||||
| NE2_KOL_PEG10 | 20 | 10 | 10 | 59.7 | 20 | 1:1 | 20.05 | ||||
| NE3_KOL_PEG5 | 20 | 10 | 10 | 59.7 | 20 | 2:1 | 15.69 | ||||
| Formulation Code | Mean Droplet Diameter (nm) * | Polydispersity Index | Zeta Potential (mV) | Osmolality (mOsm/kg) | pH | Surface Tension (mN/m) | Refractive Index | T10 (%) | T500 (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | |
| NE1_KOL | 91.85 | 2.20 | 0.149 | 0.031 | −20.39 | 7.72 | 20.67 | 0.58 | 4.42 | 0.05 | 36.03 | 0.34 | 1.3623 | 0.0002 | 64.40 | 0.03 | 99.74 | 0.07 |
| NE2_KOL_PEG10 | 100.78 | 0.85 | 0.104 | 0.019 | −16.96 | 0.70 | 1026.33 | 6.81 | 4.78 | 0.05 | 35.45 | 0.45 | 1.3848 | 0.0006 | 48.87 | 0.14 | 92.18 | 0.11 |
| NE3_KOL_PEG5 | 99.39 | 1.07 | 0.084 | 0.011 | −17.76 | 0.91 | 426.33 | 8.62 | 3.86 | 0.08 | 37.43 | 0.30 | 1.3731 | 0.0002 | 52.33 | 0.04 | 97.10 | 0.27 |
| NE4_KOL_PG10 | 81.65 | 0.85 | 0.103 | 0.024 | −25.48 | 5.73 | >2000 | 4.62 | 0.22 | 35.03 | 0.46 | 1.3852 | 0.0006 | 53.46 | 0.16 | 98.66 | 0.13 | |
| NE5_KOL_PG5 | 82.07 | 0.88 | 0.079 | 0.021 | −18.47 | 0.74 | 1035.33 | 23.35 | 4.33 | 0.05 | 37.25 | 0.45 | 1.3757 | 0.0003 | 54.98 | 0.31 | 98.03 | 0.47 |
| NE6_T20 | 80.63 | 2.85 | 0.161 | 0.010 | −27.21 | 3.01 | 70.00 | 1.73 | 4.24 | 0.34 | 36.10 | 0.61 | 1.3662 | 0.0002 | 49.18 | 0.60 | 97.28 | 0.21 |
| NE7_T20_PEG10 | 129.68 | 0.99 | 0.094 | 0.019 | −32.36 | 3.80 | 1238.67 | 24.01 | 4.42 | 0.06 | 36.90 | 0.53 | 1.3854 | 0.0001 | 28.87 | 0.10 | 88.81 | 0.26 |
| NE8_T20_PEG5 | 90.25 | 0.91 | 0.130 | 0.015 | −23.21 | 0.52 | 546.00 | 1.73 | 3.79 | 0.11 | 35.50 | 0.42 | 1.3788 | 0.0001 | 31.96 | 0.93 | 94.23 | 0.35 |
| NE9_T20_PG10 | 98.69 | 0.32 | 0.106 | 0.012 | −26.14 | 0.76 | >2000 | 4.27 | 0.20 | 36.68 | 1.00 | 1.3812 | 0.0003 | 29.89 | 0.06 | 92.90 | 0.15 | |
| NE10_T20_PG5 | 87.32 | 0.64 | 0.136 | 0.010 | −34.81 | 2.87 | 893.33 | 4.93 | 4.04 | 0.05 | 34.38 | 0.92 | 1.3798 | 0.0001 | 53.60 | 0.70 | 97.21 | 0.06 |
| NE11_T80 | 95.67 | 1.13 | 0.132 | 0.013 | −27.90 | 4.42 | 31.67 | 1.53 | 3.86 | 0.06 | 35.80 | 0.94 | 1.3662 | 0.0002 | 35.69 | 0.16 | 97.64 | 0.20 |
| NE12_T80_PEG10 | 93.55 | 1.43 | 0.143 | 0.014 | −25.54 | 1.75 | 1029.67 | 33.65 | 4.40 | 0.11 | 35.13 | 0.39 | 1.3828 | 0.0003 | 27.82 | 0.03 | 94.83 | 0.34 |
| NE13_T80_PEG5 | 90.72 | 1.85 | 0.155 | 0.024 | −28.01 | 1.54 | 446.67 | 15.17 | 4.15 | 0.04 | 36.08 | 0.90 | 1.3795 | 0.0004 | 33.03 | 0.10 | 93.59 | 0.30 |
| NE14_T80_PG10 | 105.89 | 13.32 | 0.240 | 0.070 | −29.73 | 1.82 | >2000 | 4.22 | 0.12 | 35.93 | 0.47 | 1.3841 | 0.0003 | 31.08 | 0.05 | 92.55 | 0.42 | |
| NE15_T80_PG5 | 90.73 | 0.93 | 0.158 | 0.014 | −28.11 | 0.65 | 874.67 | 16.74 | 3.94 | 0.14 | 35.55 | 0.39 | 1.3714 | 0.0004 | 32.99 | 0.15 | 98.69 | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawin-Mikołajewicz, A.; Nawrot, U.; Malec, K.H.; Krajewska, K.; Nartowski, K.P.; Karolewicz, B.L. The Effect of High-Pressure Homogenization Conditions on the Physicochemical Properties and Stability of Designed Fluconazole-Loaded Ocular Nanoemulsions. Pharmaceutics 2024, 16, 11. https://doi.org/10.3390/pharmaceutics16010011
Gawin-Mikołajewicz A, Nawrot U, Malec KH, Krajewska K, Nartowski KP, Karolewicz BL. The Effect of High-Pressure Homogenization Conditions on the Physicochemical Properties and Stability of Designed Fluconazole-Loaded Ocular Nanoemulsions. Pharmaceutics. 2024; 16(1):11. https://doi.org/10.3390/pharmaceutics16010011
Chicago/Turabian StyleGawin-Mikołajewicz, Agnieszka, Urszula Nawrot, Katarzyna Hanna Malec, Karolina Krajewska, Karol Przemysław Nartowski, and Bożena Lucyna Karolewicz. 2024. "The Effect of High-Pressure Homogenization Conditions on the Physicochemical Properties and Stability of Designed Fluconazole-Loaded Ocular Nanoemulsions" Pharmaceutics 16, no. 1: 11. https://doi.org/10.3390/pharmaceutics16010011





