Use of Plant Extracts in Polymeric Scaffolds in the Regeneration of Mandibular Injuries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Process for Obtaining Scaffolds
2.1.1. Materials
2.1.2. Scaffold Preparation
2.2. Characterization Process of the Scaffolds
2.2.1. Differential Scanning Calorimetry (DSC)
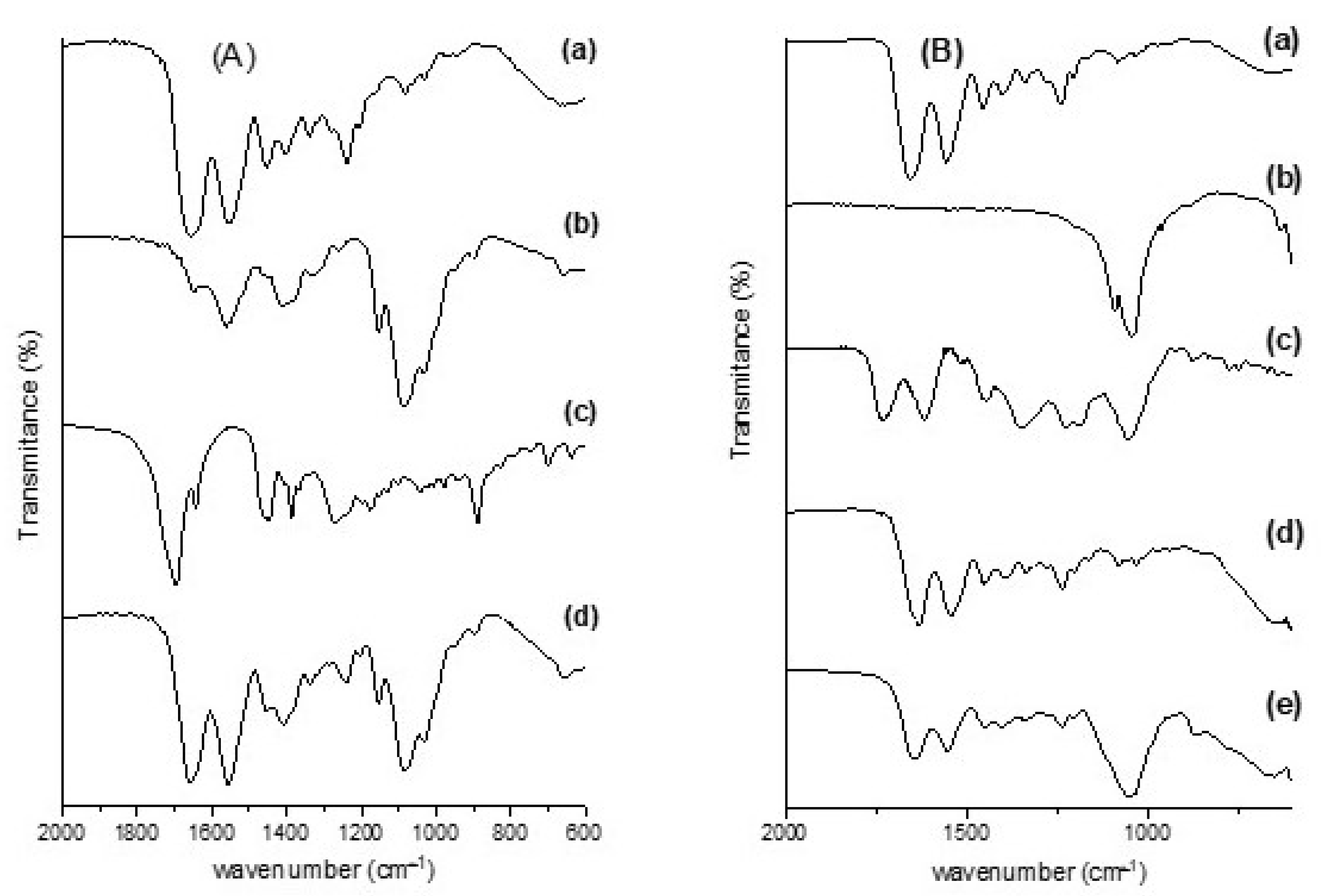
2.2.2. Fourier Transform Infrared Spectroscopy (FT-IR)
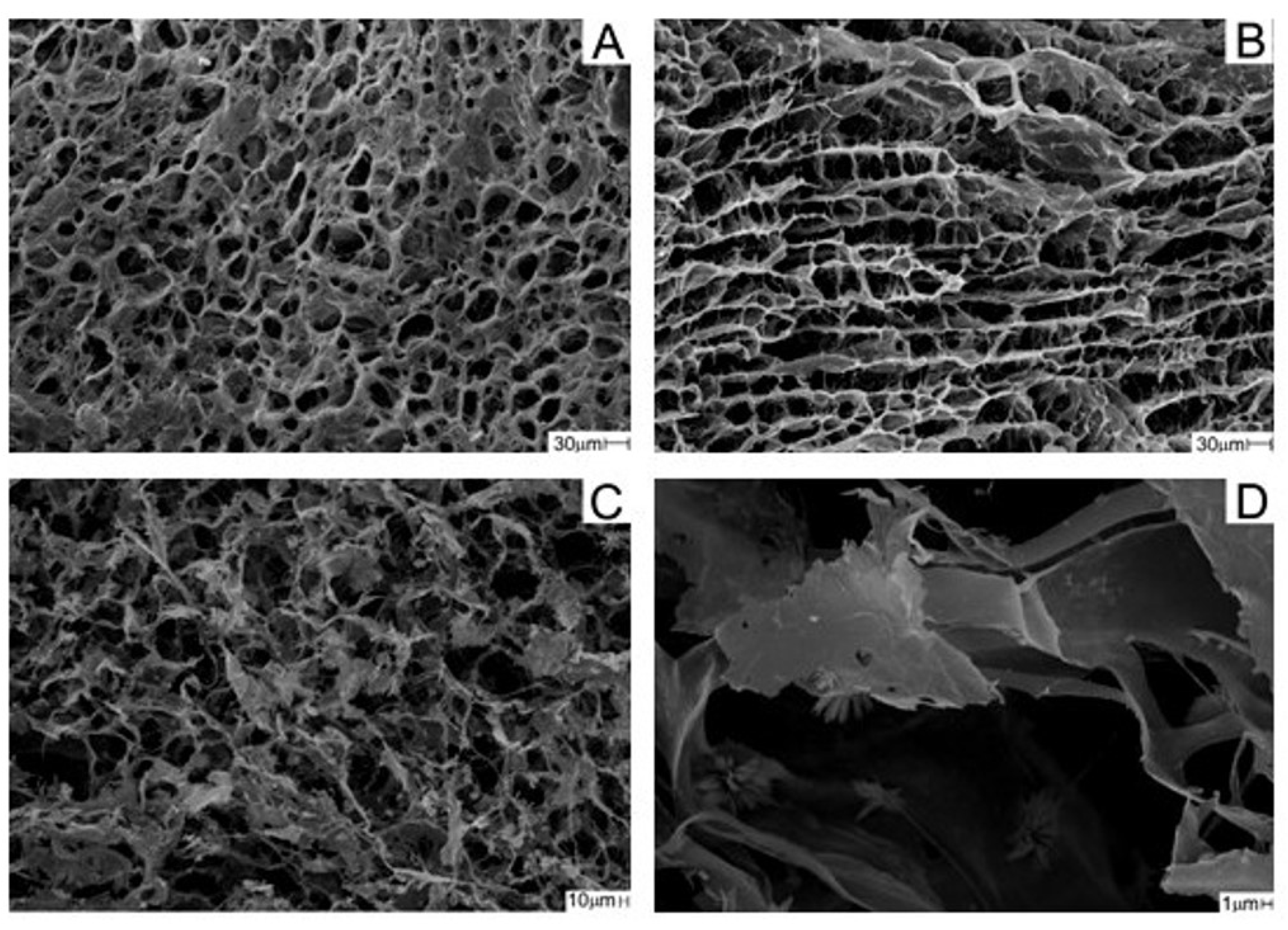
2.2.3. Analysis of Scaffolds with Scanning Electron Microscopy (SEM)
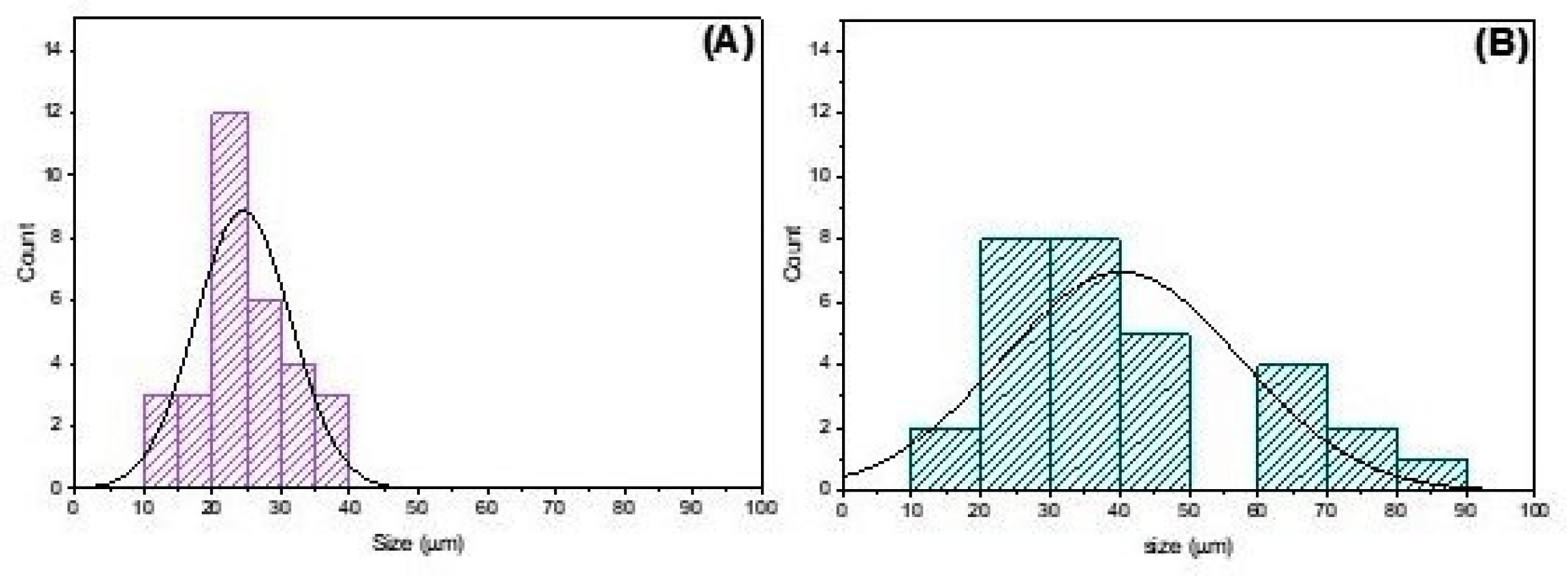
2.2.4. Porosity
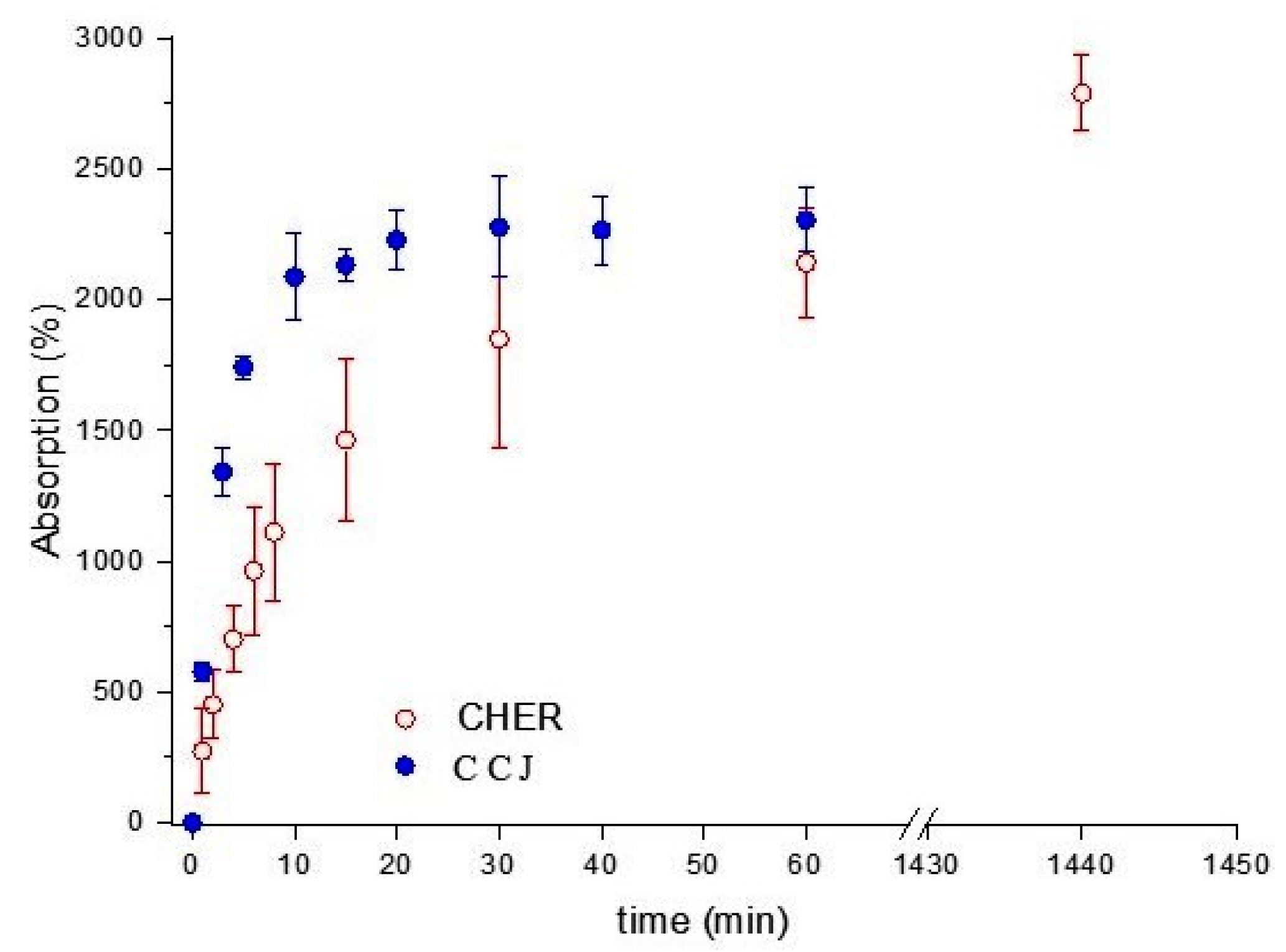
2.2.5. Absorption in Phosphate Buffered Saline (PBS)
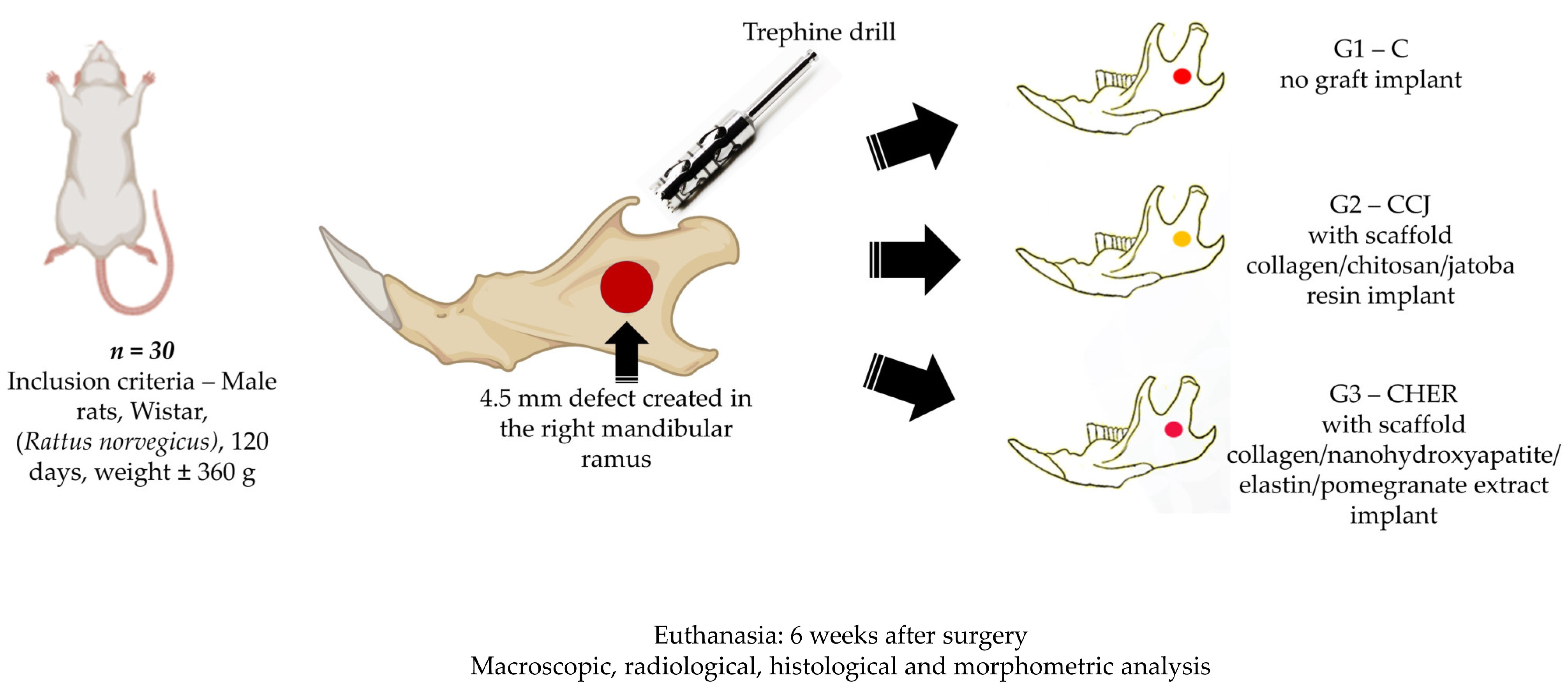
2.3. Experimental Design
2.4. Surgical Technique for Creating the Mandibular Defect
3. Results and Discussion
3.1. Physical–Chemical Analysis of the Scaffolds Used in the Experiment
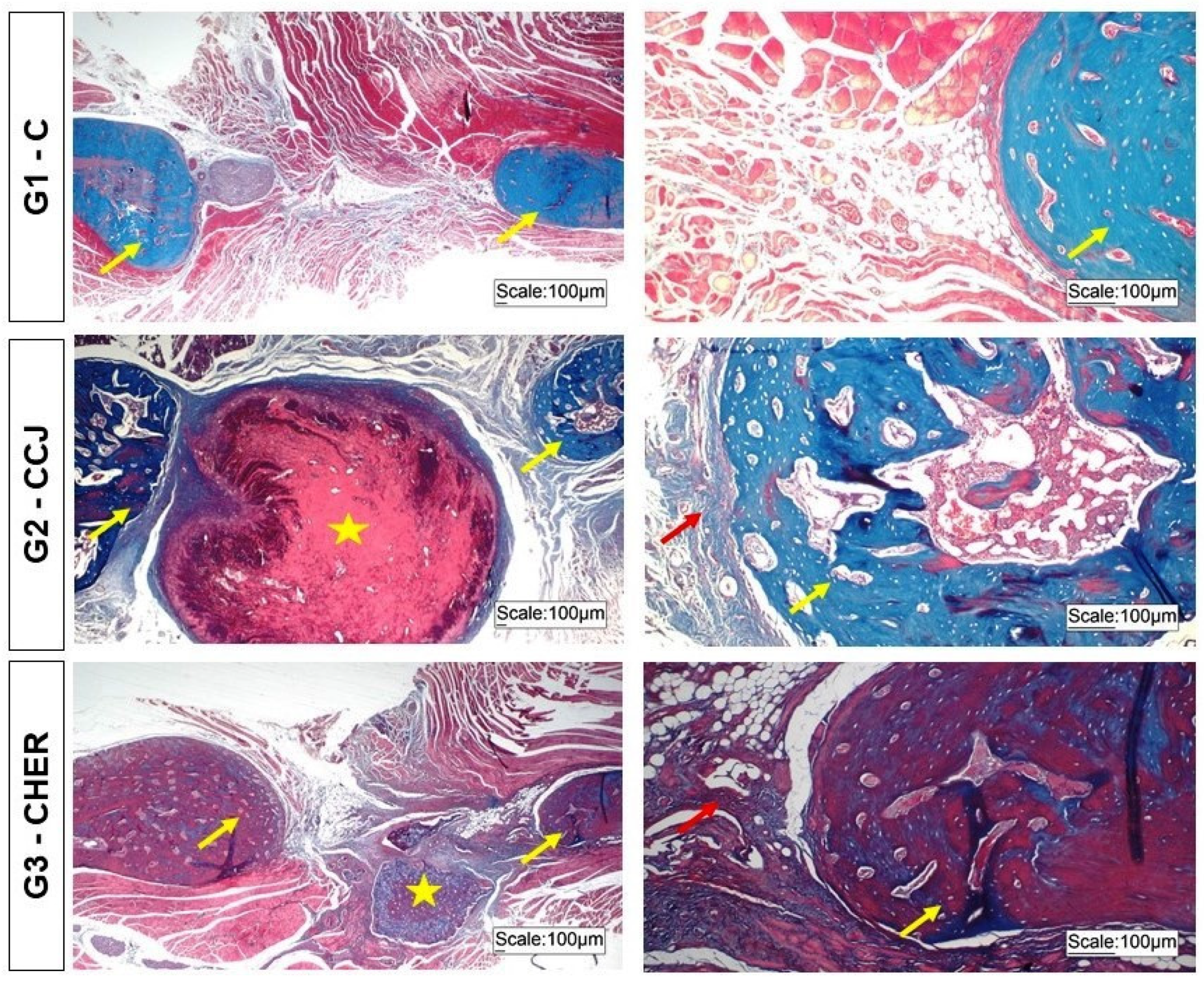
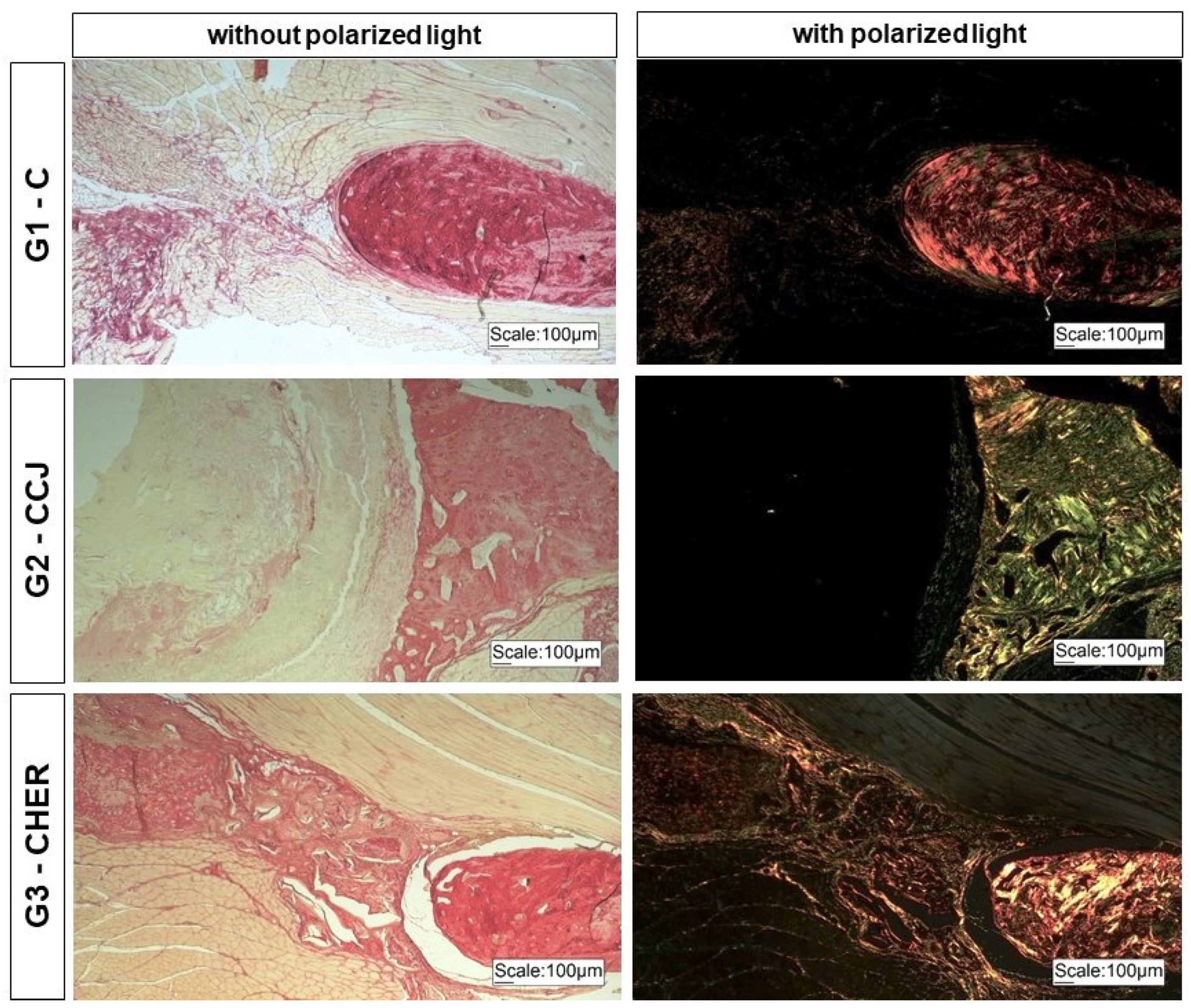
3.2. Clinical Analysis of Scaffold Grafting in the Repair of Mandibular Lesions in Rats
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erdmann, D.; Giessler, G.A.; Bergquist, G.E.O.; Bruno, W.; Young, H.; Heitmann, C.; Levin, L.S. Freier fibulatransfer: Analyse einer serie von 76 konsekutiven mikrochirugischen operationen und literaturübersicht. Chirurg 2004, 75, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Al-Sabahi, M.E.; Jamali, O.M.; Shindy, M.I.; Moussa, B.G.; Amin, A.A.W.; Zedan, M.H. Aesthetic Reconstruction of Onco-surgical Mandibular Defects Using Free Fibular Flap with and without CAD/CAM Customized Osteotomy Guide: A Randomized Controlled Clinical Trial. BMC Cancer 2022, 22, 1252. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.P.; Yetzer, J.G. Reconstruction of Acquired Oromandibular Defects. Oral Maxillofac. Surg. Clin. N. Am. 2013, 25, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Bevans, S.; Hammer, D. Tenants of Mandibular Reconstruction in Segmental Defects. Otolaryngol. Clin. N. Am. 2023, 56, 653–670. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Z.; Tian, L.; Li, D.; Lu, B.; Shi, C.; Niu, Q.; Liu, F.; Kong, L.; Zhang, J. Clinical application of 3D-printed PEEK implants for repairing mandibular defects. J. Cranio-Maxillofac. Surg. 2022, 50, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.F.M.B.; Maricevich, J.P.B.R.; Zacchê De Sá, J.; Kawamura, K.; Anlicoara, R. Mandibular reconstruction with fibula free flap: Case series. Rev. Bras. Cir. Plast. 2020, 35, 23–27. [Google Scholar] [CrossRef]
- Lin, C.H.; Kudva, A. Simultaneous Reconstruction of Mandibular and Maxillary Defects Using the Single Free Fibular Osseocutaneous Flap: Case Series and Review of the Literature. Ann. Plast. Surg. 2021, 86, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Marchiano, E.; Stevens, J.R.; Liao, E.; Rosko, A.J.; Powell, A.R.; Chinn, S.B.; Stucken, C.L.; Spector, M.E. Three-dimensional modeling of the scapular tip for anterolateral and lateral mandibular defects. Oral Oncol. 2020, 107, 104718. [Google Scholar] [CrossRef]
- Yang, H.J.; Oh, J.H. Reconstruction of Mandibular Contour Defect Using Patient-Specific Titanium Implant Manufactured by Selective Laser Melting Method. J. Craniofac. Surg. 2022, 33, 2055–2058. [Google Scholar] [CrossRef] [PubMed]
- Moura, L.B.; Carvalho, P.H.d.A.; Xavier, C.B.; Post, L.K.; Torriani, M.A.; Santagata, M.; Chagas Júnior, O.L. Autogenous non-vascularized bone graft in segmental mandibular reconstruction: A systematic review. Int. J. Oral Maxillofac. Surg. 2016, 45, 1388–1394. [Google Scholar] [CrossRef]
- Kuriakose, M.A.; Shnayder, Y.; DeLacure, M.D. Reconstruction of segmental mandibular defects by distraction osteogenesis for mandibular reconstruction. Head Neck 2003, 25, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.A. A comparison between sternum and rib in osteomyocutaneous reconstruction of major mandibular defects. Ann. Plast. Surg. 1986, 17, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Penfold, C.N.; Davies, H.T.; Cole, R.P.; Evans, B.T.; Hobby, J.A.E. Combined latissimus dorsiserratus anterior/rib composite free flap in mandibular reconstruction. Int. J. Oral Maxillofac. Surg. 1992, 21, 92–96. [Google Scholar] [CrossRef]
- Cucchi, A.; Vignudelli, E.; Napolitano, A.; Marchetti, C.; Corinaldesi, G. Evaluation of complication rates and vertical bone gain after guided bone regeneration with non-resorbable membranes versus titanium meshes and resorbable membranes. A randomized clinical trial. Clin. Implant Dent. Relat. Res. 2017, 19, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, G.; Lieber, R.; Gazit, D.; Pelled, G. Recent Advances and Future of Gene Therapy for Bone Regeneration. Curr. Osteoporos. Rep. 2018, 16, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Huang, W.; Wang, F.; Wang, S.; Zhang, Z.; Zhang, C.; Kaigler, D.; Wu, Y. Autologous Ilium Grafts: Long-Term Results on Immediate or Staged Functional Rehabilitation of Mandibular Segmental Defects Using Dental Implants after Tumor Resection. Clin. Implant Dent. Relat. Res. 2015, 17, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Hanga-Farcaș, A.; Miere, F.; Filip, G.A.; Clichici, S.; Fritea, L.; Vicaș, L.G.; Marian, E.; Pallag, A.; Jurca, T.; Filip, S.M.; et al. Phytochemical Compounds Involved in the Bone Regeneration Process and Their Innovative Administration: A Systematic Review. Plants 2023, 12, 2055. [Google Scholar] [CrossRef] [PubMed]
- Zandi, M.; Dehghan, A.; Gheysari, F.; Rezaeian, L.; Mohammad Gholi Mezerji, N. Evaluation of teriparatide effect on healing of autografted mandibular defects in rats. J. Cranio-Maxillofac. Surg. 2019, 47, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.J.; Sittitavornwong, S. Managing Bone Grafts for the Mandible. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 317–330. [Google Scholar] [CrossRef]
- Fan, J.; Park, H.; Lee, M.K.; Bezouglaia, O.; Fartash, A.; Kim, J.; Aghaloo, T.; Lee, M. Adipose-derived stem cells and BMP-2 delivery in chitosan-based 3D constructs to enhance bone regeneration in a rat mandibular defect model. Tissue Eng.—Part A 2014, 20, 2169–2179. [Google Scholar] [CrossRef]
- Pandini, F.E.; Miyauchi Kubo, F.M.; De Guzzi Plepis, A.M.; Da Conceição Amaro Martins, V.; Da Cunha, M.R.; Silva, V.R.; Hirota, V.B.; Lopes, E.; Menezes, M.A.; Pelegrine, A.A.; et al. In Vivo Study of Nasal Bone Reconstruction with Collagen, Elastin and ChitosanMembranes in Abstainer and Alcoholic Rats. Polymers 2022, 14, 188. [Google Scholar] [CrossRef]
- Silva, S.K.; Plepis, A.M.G.; da Martins, V.C.A.; Horn, M.M.; Buchaim, D.V.; Buchaim, R.L.; Pelegrine, A.A.; Silva, V.R.; Kudo, M.H.M.; Fernandes, J.F.R.; et al. Suitability of Chitosan Scaffolds with Carbon Nanotubes for Bone Defects Treated with Photobiomodulation. Int. J. Mol. Sci. 2022, 23, 6503. [Google Scholar] [CrossRef]
- Chacon, E.L.; Bertolo, M.R.V.; de Guzzi Plepis, A.M.; da Conceição Amaro Martins, V.; dos Santos, G.R.; Pinto, C.A.L.; Pelegrine, A.A.; Teixeira, M.L.; Buchaim, D.V.; Nazari, F.M.; et al. Collagen-chitosan-hydroxyapatite composite scaffolds for bone repair in ovariectomized rats. Sci. Rep. 2023, 13, 28. [Google Scholar] [CrossRef]
- de Moraes, R.; de Plepis, A.M.G.; da Martins, V.C.A.; Garcia, C.F.; Galdeano, E.A.; Maia, F.L.M.; Machado, E.G.; de Munhoz, M.A.; Buchaim, D.V.; Fernandes, V.A.R.; et al. Viability of Collagen Matrix Grafts Associated with Nanohydroxyapatite and Elastin in Bone Repair in the Experimental Condition of Ovariectomy. Int. J. Mol. Sci. 2023, 24, 15727. [Google Scholar] [CrossRef]
- Garcia, C.F.; Marangon, C.A.; Massimino, L.C.; Klingbeil, M.F.G.; Martins, V.C.A.; Plepis, A.M. de G. Development of collagen/nanohydroxyapatite scaffolds containing plant extract intended for bone regeneration. Mater. Sci. Eng. C 2021, 123, 111955. [Google Scholar] [CrossRef]
- Dziadek, M.; Dziadek, K.; Checinska, K.; Zagrajczuk, B.; Golda-Cepa, M.; Brzychczy-Wloch, M.; Menaszek, E.; Kopec, A.; Cholewa-Kowalska, K. PCL and PCL/bioactive glass biomaterials as carriers for biologically active polyphenolic compounds: Comprehensive physicochemical and biological evaluation. Bioact. Mater. 2021, 6, 1811–1826. [Google Scholar] [CrossRef]
- Nowak, B.; Matuszewska, A.; Szeląg, A.; Danielewski, M.; Dziewiszek, W.; Nikodem, A.; Filipiak, J.; Jędrzejuk, D.; Bolanowski, M.; Kucharska, A.Z.; et al. Cornelian cherry (Cornus mas L.) extract reduces cardiovascular risk and prevents bone loss in ovariectomized Wistar rats. J. Funct. Foods 2022, 90, 104974. [Google Scholar] [CrossRef]
- Massimino, L.C.; da Conceição Amaro Martins, V.; Vulcani, V.A.S.; de Oliveira, É.L.; Andreeta, M.B.; Bonagamba, T.J.; Klingbeil, M.F.G.; Mathor, M.B.; de Guzzi Plepis, A.M. Use of collagen and auricular cartilage in bioengineering: Scaffolds for tissue regeneration. Cell Tissue Bank. 2020, 25, 111–122. [Google Scholar] [CrossRef]
- Massimino, L.C. Biopolymers scaffolds and Jatobá resin for use in tissue engineering. 2020. [Google Scholar]
- Erickson, A.E.; Sun, J.; Lan Levengood, S.K.; Swanson, S.; Chang, F.C.; Tsao, C.T.; Zhang, M. Chitosan-based composite bilayer scaffold as an in vitro osteochondral defect regeneration model. Biomed. Microdevices 2019, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [PubMed]
- Iaquinta, M.R.; Mazzoni, E.; Manfrini, M.; D’Agostino, A.; Trevisiol, L.; Nocini, R.; Trombelli, L.; Barbanti-Brodano, G.; Martini, F.; Tognon, M. Innovative biomaterials for bone regrowth. Int. J. Mol. Sci. 2019, 20, 618. [Google Scholar] [CrossRef] [PubMed]
- Munhoz, M.A.S.; Hirata, H.H.; Plepis, A.M.G.; Martins, V.C.A.; Cunha, M.R. Use of collagen/chitosan sponges mineralized with hydroxyapatite for the repair of cranial defects in rats. Injury 2018, 49, 2154–2160. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, R.; de Guzzi Plepis, A.M.; da Conceição Amaro Martins, V.; Duarte, M.A.H.; Alcalde, M.P.; Buchaim, R.L.; Pomini, K.T.; Machado, E.G.; de Azevedo E Sousa Munhoz, M.; Cunha, F.B.; et al. Suitability of the use of an elastin matrix combined with bone morphogenetic protein for the repair of cranial defects. Am. J. Transl. Res. 2019, 11, 5261–5271. [Google Scholar] [PubMed]
- de Azevedo e Sousa Munhoz, M.; Pomini, K.T.; de Guzzi Plepis, A.M.; da Conceição Amaro Martins, V.; Machado, E.G.; de Moraes, R.; Cunha, F.B.; Santos, A.R.; Cardoso, G.B.C.; Duarte, M.A.H.; et al. Elastin-derived scaffolding associated or not with bone morphogenetic protein (BMP) or hydroxyapatite (HA) in the repair process of metaphyseal bone defects. PLoS ONE 2020, 15, e0231112. [Google Scholar] [CrossRef]
- Cunha, F.B.; Pomini, K.T.; de Plepis, A.M.G.; da Martins, V.C.A.; Machado, E.G.; de Moraes, R.; de Munhoz, M.A.E.S.; Machado, M.V.R.; Duarte, M.A.H.; Alcalde, M.P.; et al. In Vivo Biological Behavior of Polymer Scaffolds of Natural Origin in the Bone Repair Process. Molecules 2021, 26, 1598. [Google Scholar] [CrossRef] [PubMed]
- Logithkumar, R.; Keshavnarayan, A.; Dhivya, S.; Chawla, A.; Saravanan, S.; Selvamurugan, N. A review of chitosan and its derivatives in bone tissue engineering. Carbohydr. Polym. 2016, 151, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Kaczmarek, B.; Lewandowska, K.; Grabska, S.; Pokrywczyńska, M.; Kloskowski, T.; Drewa, T. 3D composites based on the blends of chitosan and collagen with the addition of hyaluronic acid. Int. J. Biol. Macromol. 2016, 89, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Grabska-Zielińska, S.; Pin, J.M.; Kaczmarek-Szczepańska, B.; Olewnik-Kruszkowska, E.; Sionkowska, A.; Monteiro, F.J.; Steinbrink, K.; Kleszczyński, K. Scaffolds Loaded with Dialdehyde Chitosan and Collagen—Their Physico-Chemical Properties and Biological Assessment. Polymers 2022, 14, 1818. [Google Scholar] [CrossRef] [PubMed]
- de Scaramussa, S.A.L.; de Soares, L.A.; de Santana, L.C.L.A. Extracts from jatobá (Hymenaea courbaril L.) peel and seeds: Antioxidant and antimicrobial activities and synergistic effect of extract combinations. Food Sci. Technol. Int. 2024, 30, 128–136. [Google Scholar] [CrossRef]
- Veggi, P.C.; Prado, J.M.; Bataglion, G.A.; Eberlin, M.N.; Meireles, M.A.A. Obtaining phenolic compounds from jatoba (Hymenaea courbaril L.) bark by supercritical fluid extraction. J. Supercrit. Fluids 2014, 89, 68–77. [Google Scholar] [CrossRef]
- Veggi, P.C.; Santos, D.T.; Fabiano-Tixier, A.-S.; Le Bourvellec, C.; Angela, A.; Meireles, M.; Chemat, F. Extraction of Polyphenols and Anthocyanins from the Jambul (Syzygium cumini) Fruit Peels. Food Public Health 2013, 3, 119–129. [Google Scholar] [CrossRef]
- Viswanath, M.; Sridevi, P.; Ravindra Kumar, K.; Pradesh, A.; Subbaramamma, I.P.; Bhagavan, B.; Kumar, R.; Subbaramamma, P. Toxicological, Pharmacological and Cellular properties of Pomegranate (Punica granatum L.): A Review. J. Pharmacogn. Phytochem. 2019, 8, 172–176. [Google Scholar]
- Anibal, P.C.; Peixoto, I.T.A.; Foglio, M.A.; Höfling, J.F. Antifungal activity of the ethanolic extracts of Punica granatum L. and evaluation of the morphological and structural modifications of its compounds upon the cells of Candida spp. Brazilian J. Microbiol. 2013, 44, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Bickforb, P.C.; Tan, J.; Douglas Shytle, R.; Sanberg, C.D.; El-Badri, N.; Sanberg, P.R. Nutraceuticals synergistically promote proliferation of human stem cells. Stem Cells Dev. 2006, 15, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Al-obaidi, M.M.J.; Al-bayaty, F.H.; Al, R.; Hassandarvish, P.; Rouhollahi, E. ScienceDirect Protective effect of ellagic acid on healing alveolar bone after tooth extraction in rat—A histological and immunohistochemical study. Arch. Oral Biol. 2014, 59, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Horn, M.M.; Martins, V.C.A.; de Guzzi Plepis, A.M. Influence of collagen addition on the thermal and morphological properties of chitosan/xanthan hydrogels. Int. J. Biol. Macromol. 2015, 80, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Miguel, F.B.; Barbosa Júnior, A.A.; de Paula, F.L.; Barreto, I.C.; Goissis, G.; Rosa, F.P. Regeneration of critical bone defects with anionic collagen matrix as scaffolds. J. Mater. Sci. Mater. Med. 2013, 24, 2567–2575. [Google Scholar] [CrossRef]
- Horn, M.M.; Martins, V.C.A.; de Guzzi Plepis, A.M. Interaction of anionic collagen with chitosan: Effect on thermal and morphological characteristics. Carbohydr. Polym. 2009, 77, 239–243. [Google Scholar] [CrossRef]
- Sarfraz, S.; Naseem, B.; Amin, S.; Mujahid, M. Synthesis and characterization of nano hydroxyapatite. Adv. Mater. Res. 2011, 264–265, 1370–1375. [Google Scholar] [CrossRef]
- de Lima, A.J.B.; Corrêa, A.D.; Saczk, A.A.; Martins, M.P.; Castilho, R.O. Anthocyanins, pigment stability and antioxidant activity in jabuticaba [Myrciaria cauliflora (Mart.) o. berg]. Rev. Bras. Frutic. 2011, 33, 877–887. [Google Scholar] [CrossRef]
- Zahedi, S.; Legrand, R.; Brunel, G.; Albert, A.; Dewé, W.; Coumans, B.; Bernard, J. Evaluation of a Diphenylphosphorylazide-Crosslinked Collagen Membrane for Guided Bone Regeneration in Mandibular Defects in Rats. J. Periodontol. 1998, 69, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, D.M.B.; de Figadoli, A.L.F.; Alcantara, P.L.; Pomini, K.T.; Santos German, I.J.; Reis, C.H.B.; Rosa Júnior, G.M.; de Rosso, M.P.O.; da Santos, P.S.S.; Zangrando, M.S.R.; et al. Biological Behavior of Xenogenic Scaffolds in Alcohol-Induced Rats: Histomorphometric and Picrosirius Red Staining Analysis. Polymers 2022, 14, 584. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.H.B.; Buchaim, R.L.; Pomini, K.T.; Hamzé, A.L.; Zattiti, I.V.; Duarte, M.A.H.; Alcalde, M.P.; Barraviera, B.; Ferreira Júnior, R.S.; Pontes, F.M.L.; et al. Effects of a Biocomplex Formed by Two Scaffold Biomaterials, Hydroxyapatite/Tricalcium Phosphate Ceramic and Fibrin Biopolymer, with Photobiomodulation, on Bone Repair. Polymers 2022, 14, 2075. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.D.; dos Prazeres Campos, J.S.; de Oliveira Carvalho Granado Santos, T. Nutritional status and food consumption of patients with Parkinson disease. Arq. Neuropsiquiatr. 2021, 79, 676–681. [Google Scholar] [CrossRef]
- Tran, S.D.; Bakkar, M.O.; Sumita, Y.; Kishimoto, N. Regenerative dentistry in periodontics. Saudi Dent. J. 2019, 31, 301–302. [Google Scholar] [CrossRef] [PubMed]
- Mishchenko, O.; Yanovska, A.; Kosinov, O.; Maksymov, D.; Moskalenko, R.; Ramanavicius, A.; Pogorielov, M. Synthetic Calcium–Phosphate Materials for Bone Grafting. Polymers 2023, 15, 3822. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Yu, J.; Chen, W. Plants and Their Bioactive Constituents in Mesenchymal Stem Cell-Based Periodontal Regeneration: A Novel Prospective. Biomed Res. Int. 2018, 2018, 7571363. [Google Scholar] [CrossRef] [PubMed]
- Odabas, S.; Derkuş, B.; Vargel, İ.; Vural, A.C. Surgical method for critical sized cranial defects in rat cranium. MethodsX 2023, 10, 102208. [Google Scholar] [CrossRef] [PubMed]
- Bilirgen, A.C.; Toker, M.; Odabas, S.; Yetisen, A.K.; Garipcan, B.; Tasoglu, S. Plant-Based Scaffolds in Tissue Engineering. ACS Biomater. Sci. Eng. 2021, 7, 926–938. [Google Scholar] [CrossRef]
- Chen, Z.; Mo, X.; Qing, F. Electrospinning of collagen-chitosan complex. Mater. Lett. 2007, 61, 3490–3494. [Google Scholar] [CrossRef]
- Pereda, M.; Ponce, A.G.; Marcovich, N.E.; Ruseckaite, R.A.; Martucci, J.F. Chitosan-gelatin composites and bi-layer films with potential antimicrobial activity. Food Hydrocoll. 2011, 25, 1372–1381. [Google Scholar] [CrossRef]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef]
- Bertolo, M.R.V.; Martins, V.C.A.; Plepis, A.M. de G. Effects of calcium phosphates incorporation on structural, thermal and drug-delivery properties of collagen:chitosan scaffolds. Int. J. Adv. Med. Biotechnol.—IJAMB 2020, 2, 25–35. [Google Scholar] [CrossRef]
- Tappert, R.; McKellar, R.C.; Wolfe, A.P.; Tappert, M.C.; Ortega-Blanco, J.; Muehlenbachs, K. Stable carbon isotopes of C3 plant resins and ambers record changes in atmospheric oxygen since the Triassic. Geochim. Cosmochim. Acta 2013, 121, 240–262. [Google Scholar] [CrossRef]
- Ragini, B.; Kandhasamy, S.; Jacob, J.P.; Vijayakumar, S. Synthesis and in vitro characteristics of biogenic-derived hydroxyapatite for bone remodeling applications. Bioprocess Biosyst. Eng. 2024, 47, 23–37. [Google Scholar] [CrossRef]
- Ślósarczyk, A.; Paszkiewicz, Z.; Paluszkiewicz, C. FTIR and XRD evaluation of carbonated hydroxyapatite powders synthesized by wet methods. J. Mol. Struct. 2005, 744–747, 657–661. [Google Scholar] [CrossRef]
- Lugo, R.; Karthik, T.V.K.; Anaya, M.; Rosas, R.; Ceron, V.; Valderama, R.; Rodrguez, S. Wet chemical synthesis of nanocrystalline hydroxyapatite flakes: Effect of pH and sintering temperature on structural and morphological properties. R. Soc. Open Sci. 2018, 5, 180962. [Google Scholar] [CrossRef]
- Cheheltani, R.; McGoverin, C.M.; Rao, J.; Vorp, D.A.; Kiani, M.F.; Pleshko, N. Fourier transform infrared spectroscopy to quantify collagen and elastin in an in vitro model of extracellular matrix degradation in aorta. Analyst 2014, 139, 3039–3047. [Google Scholar] [CrossRef]
- Yin, L.; Wang, J.; Shi, K.; Zhang, Y.; Xu, Y.; Kong, D.; Ni, L.; Li, S. Interactions between tannins allelochemicals and extracellular polymeric substance (EPS) of Microcystis aeruginosa. Environ. Sci. Pollut. Res. 2022, 29, 83211–83219. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.P.R. Quantificação dos Taninos dos Vinhos por Transformada de Fourier dos Espectros no Infravermelho Médio (FTIR). Master’s Thesis, Universidade Técnica de Lisboa, Lisboa, Portugal, 2011. [Google Scholar]
- Goissis, G.; Suzigan, S.; Parreira, D.R.; Maniglia, J.V.; Braile, D.M.; Raymundo, S. Preparation and characterization of collagen-elastin matrices from blood vessels intended as small diameter vascular grafts. Artif. Organs 2000, 24, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Bet, M.R.; Goissis, G.; Lacerda, C.A. Characterization of polyanionic collagen prepared by selective hydrolysis of asparagine and glutamine carboxyamide side chains. Biomacromolecules 2001, 2, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Massimino, L.C. Scaffolds de Biopolímeros e Resina de Jatobá Para Utilização em Engenharia Tecidual. Ph.D. Thesis, Universidade de São Paulo, São Carlos, Brazil, 2020. [Google Scholar]
- Da Cunha, M.R.; Maia, F.L.M.; Iatecola, A.; Massimino, L.C.; de Plepis, A.M.G.; da Martins, V.C.A.; Da Rocha, D.N.; Mariano, E.D.; Hirata, M.C.; Ferreira, J.R.M.; et al. In Vivo Evaluation of Collagen and Chitosan Scaffold, Associated or Not with Stem Cells, in Bone Repair. J. Funct. Biomater. 2023, 14, 357. [Google Scholar] [CrossRef] [PubMed]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Daskalova, A.; Nathala, C.S.R.; Bliznakova, I.; Stoyanova, E.; Zhelyazkova, A.; Ganz, T.; Lueftenegger, S.; Husinsky, W. Controlling the porosity of collagen, gelatin and elastin biomaterials by ultrashort laser pulses. Appl. Surf. Sci. 2014, 292, 367–377. [Google Scholar] [CrossRef]
- Iacob, A.T.; Drăgan, M.; Gheţu, N.; Pieptu, D.; Vasile, C.; Buron, F.; Routier, S.; Giusca, S.E.; Caruntu, I.D.; Profire, L. Preparation, characterization and wound healing effects of new membranes based on chitosan, hyaluronic acid and arginine derivatives. Polymers 2018, 10, 607. [Google Scholar] [CrossRef] [PubMed]
- Grabska-Zielińska, S.; Sionkowska, A.; Coelho, C.C.; Monteiro, F.J. Silk fibroin/collagen/chitosan scaffolds cross-linked by a glyoxal solution as biomaterials toward bone tissue regeneration. Materials 2020, 13, 3433. [Google Scholar] [CrossRef] [PubMed]
- Grover, C.N.; Cameron, R.E.; Best, S.M. Investigating the morphological, mechanical and degradation properties of scaffolds comprising collagen, gelatin and elastin for use in soft tissue engineering. J. Mech. Behav. Biomed. Mater. 2012, 10, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.P.; Hsieh, C.Y.; Hsieh, C.Y.; Wang, D.M.; Huang, L.L.H.; Lai, J.Y.; Hsieh, H.J. Preparation and cell compatibility evaluation of chitosan/collagen composite scaffolds using amino acids as crosslinking bridges. J. Appl. Polym. Sci. 2007, 105, 1774–1785. [Google Scholar] [CrossRef]
- Della Coletta, B.B.; Jacob, T.B.; de Moreira, L.A.C.; Pomini, K.T.; Buchaim, D.V.; Eleutério, R.G.; de Pereira, E.S.B.M.; Roque, D.D.; de Rosso, M.P.O.; Shindo, J.V.T.C.; et al. Photobiomodulation Therapy on the Guided Bone Regeneration Process in Defects Filled by Biphasic Calcium Phosphate Associated with Fibrin Biopolymer. Molecules 2021, 26, 847. [Google Scholar] [CrossRef]
- Konig, G.; McAllister, T.N.; Dusserre, N.; Garrido, S.A.; Iyican, C.; Marini, A.; Fiorillo, A.; Avila, H.; Wystrychowski, W.; Zagalski, K.; et al. Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery. Biomaterials 2009, 30, 1542–1550. [Google Scholar] [CrossRef]
- Huang, E.E.; Zhang, N.; Shen, H.; Li, X.; Maruyama, M.; Utsunomiya, T.; Gao, Q.; Guzman, R.A.; Goodman, S.B. Novel Techniques and Future Perspective for Investigating Critical-Size Bone Defects. Bioengineering 2022, 9, 171. [Google Scholar] [CrossRef]
- Heitzer, M.; Modabber, A.; Zhang, X.; Winnand, P.; Zhao, Q.; Bläsius, F.M.; Buhl, E.M.; Wolf, M.; Neuss, S.; Hölzle, F.; et al. In vitro comparison of the osteogenic capability of human pulp stem cells on alloplastic, allogeneic, and xenogeneic bone scaffolds. BMC Oral Health 2023, 23, 56. [Google Scholar] [CrossRef]
- Boller, L.A.; Shiels, S.M.; Florian, D.C.; Peck, S.H.; Schoenecker, J.G.; Duvall, C.; Wenke, J.C.; Guelcher, S.A. Effects of nanocrystalline hydroxyapatite concentration and skeletal site on bone and cartilage formation in rats. Acta Biomater. 2021, 130, 485–496. [Google Scholar] [CrossRef]
- Vajgel, A.; Mardas, N.; Farias, B.C.; Petrie, A.; Cimões, R.; Donos, N. A systematic review on the critical size defect model. Clin. Oral Implants Res. 2014, 25, 879–893. [Google Scholar] [CrossRef]
- Yao, H.; Guo, J.; Zhu, W.; Su, Y.; Tong, W.; Zheng, L.; Chang, L.; Wang, X.; Lai, Y.; Qin, L.; et al. Controlled Release of Bone Morphogenetic Protein-2 Augments the Coupling of Angiogenesis and Osteogenesis for Accelerating Mandibular Defect Repair. Pharmaceutics 2022, 14, 2397. [Google Scholar] [CrossRef]
- Aoki, K.; Saito, N. Biodegradable polymers as drug delivery systems for bone regeneration. Pharmaceutics 2020, 12, 95. [Google Scholar] [CrossRef]



| G1-C | G2-CCJ | G3-CHER |
|---|---|---|
| 17.17 ± 2.68 a | 27.45 ± 1.65 b | 34.07 ± 0.64 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, B.E.G.; Maia, F.L.M.; Massimino, L.C.; Garcia, C.F.; Plepis, A.M.d.G.; Martins, V.d.C.A.; Reis, C.H.B.; Silva, V.R.; Bezerra, A.A.; Pauris, C.C.; et al. Use of Plant Extracts in Polymeric Scaffolds in the Regeneration of Mandibular Injuries. Pharmaceutics 2024, 16, 491. https://doi.org/10.3390/pharmaceutics16040491
de Oliveira BEG, Maia FLM, Massimino LC, Garcia CF, Plepis AMdG, Martins VdCA, Reis CHB, Silva VR, Bezerra AA, Pauris CC, et al. Use of Plant Extracts in Polymeric Scaffolds in the Regeneration of Mandibular Injuries. Pharmaceutics. 2024; 16(4):491. https://doi.org/10.3390/pharmaceutics16040491
Chicago/Turabian Stylede Oliveira, Bruna Eduarda Gandra, Fernanda Latorre Melgaço Maia, Lívia Contini Massimino, Claudio Fernandes Garcia, Ana Maria de Guzzi Plepis, Virgínia da Conceição Amaro Martins, Carlos Henrique Bertoni Reis, Vinícius Rodrigues Silva, Andre Alves Bezerra, Carolina Chen Pauris, and et al. 2024. "Use of Plant Extracts in Polymeric Scaffolds in the Regeneration of Mandibular Injuries" Pharmaceutics 16, no. 4: 491. https://doi.org/10.3390/pharmaceutics16040491
APA Stylede Oliveira, B. E. G., Maia, F. L. M., Massimino, L. C., Garcia, C. F., Plepis, A. M. d. G., Martins, V. d. C. A., Reis, C. H. B., Silva, V. R., Bezerra, A. A., Pauris, C. C., Buchaim, D. V., Silva, Y. B. e., Buchaim, R. L., & da Cunha, M. R. (2024). Use of Plant Extracts in Polymeric Scaffolds in the Regeneration of Mandibular Injuries. Pharmaceutics, 16(4), 491. https://doi.org/10.3390/pharmaceutics16040491










