Photodynamic Therapy for Atherosclerosis: Past, Present, and Future
Abstract
1. Introduction
2. Atherosclerosis: An Overview
3. Photodynamic Therapy in Atherosclerosis
3.1. Early Discoveries in PDT for Atherosclerosis
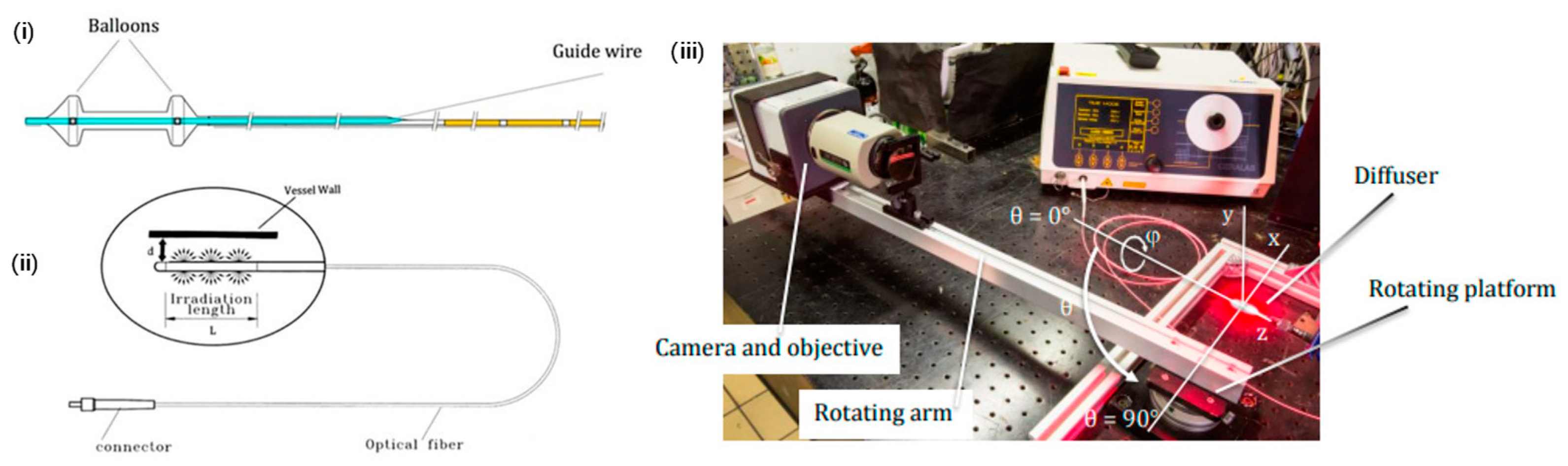
3.2. Innovative Optical Delivery Systems
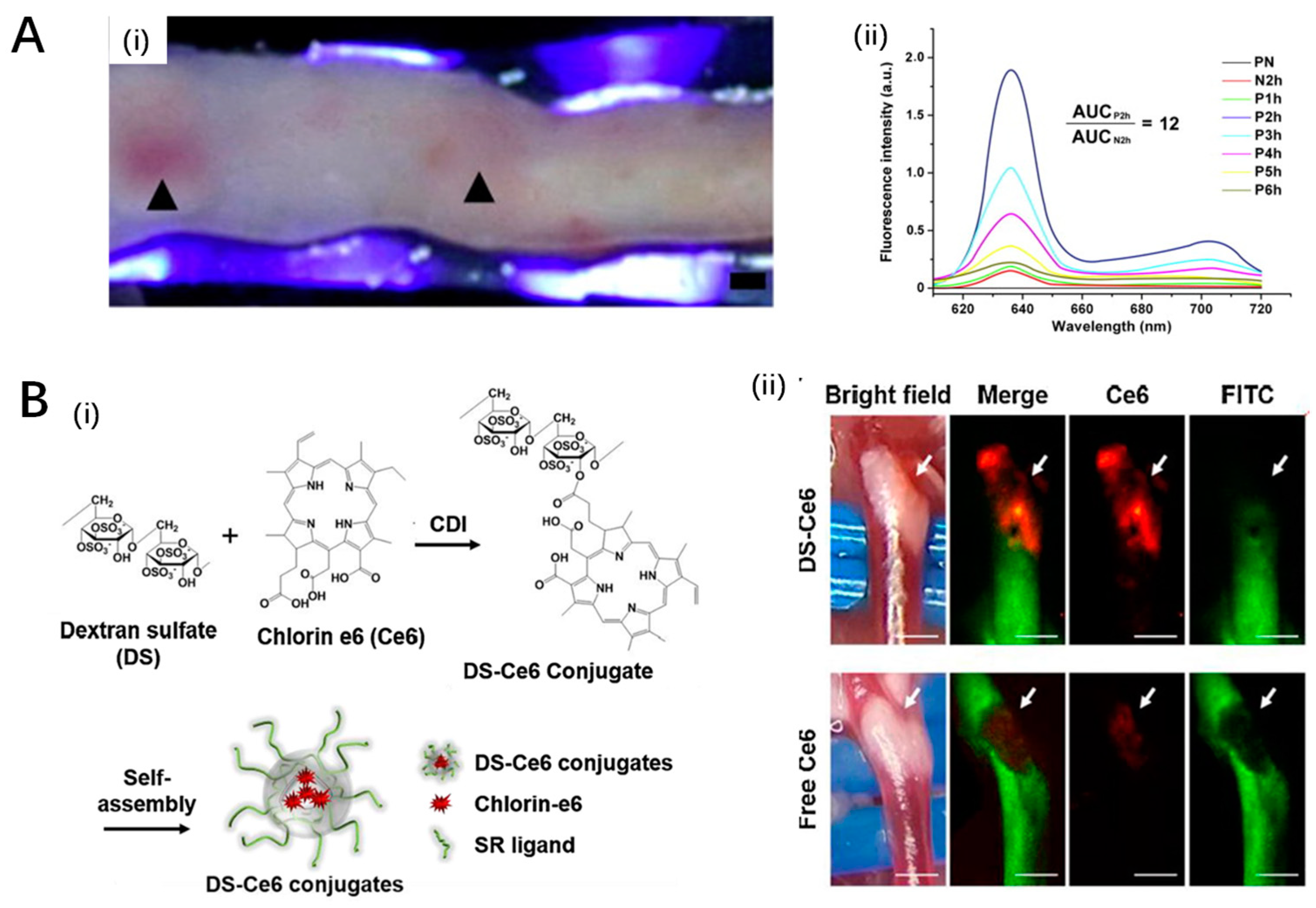
3.3. Innovative Photosensitizers
4. Nanoparticle-Based PDT Approaches
4.1. Polymeric Nanoparticles
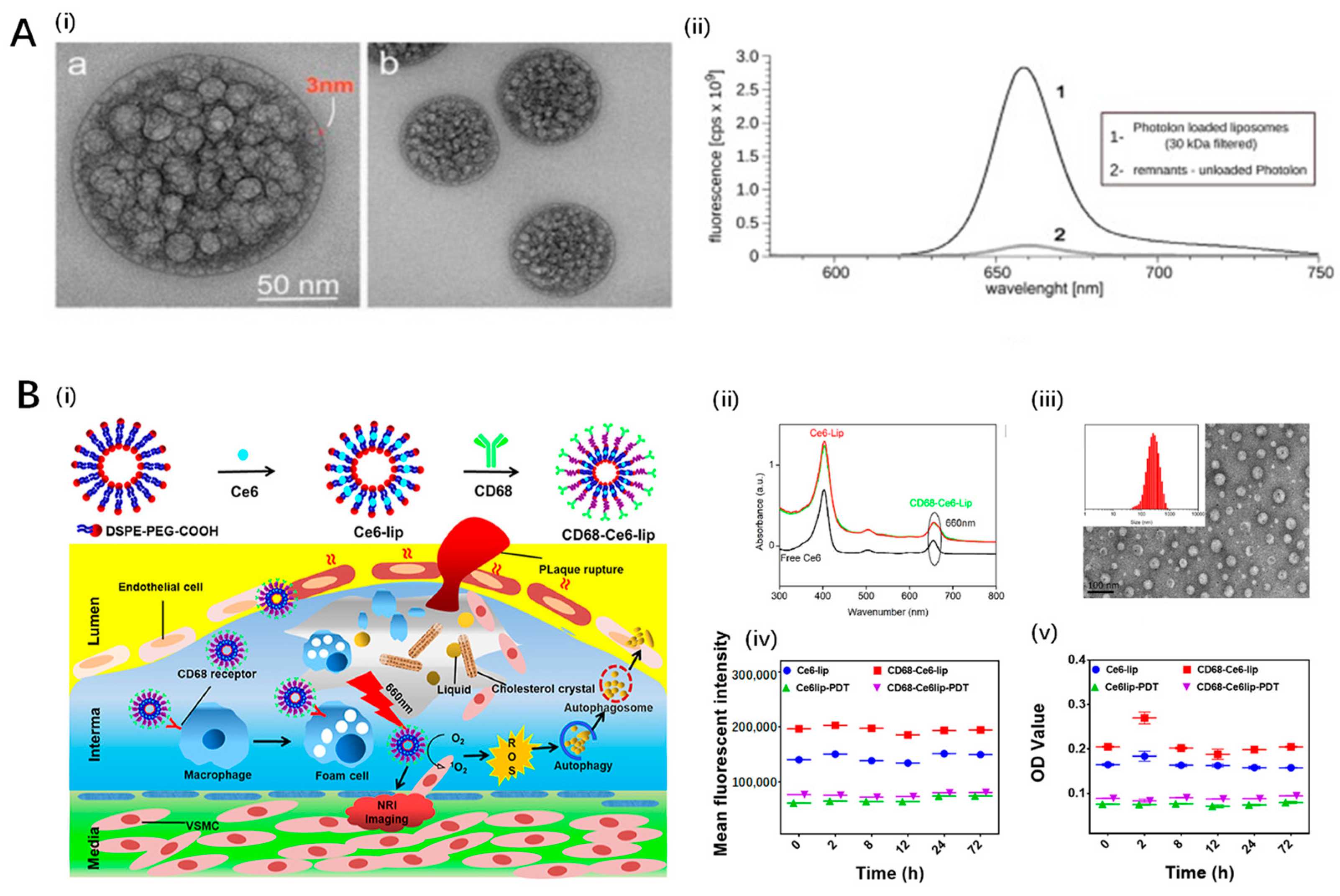
4.2. Liposomal Nanoparticles
4.3. Upconversion Nanoparticles
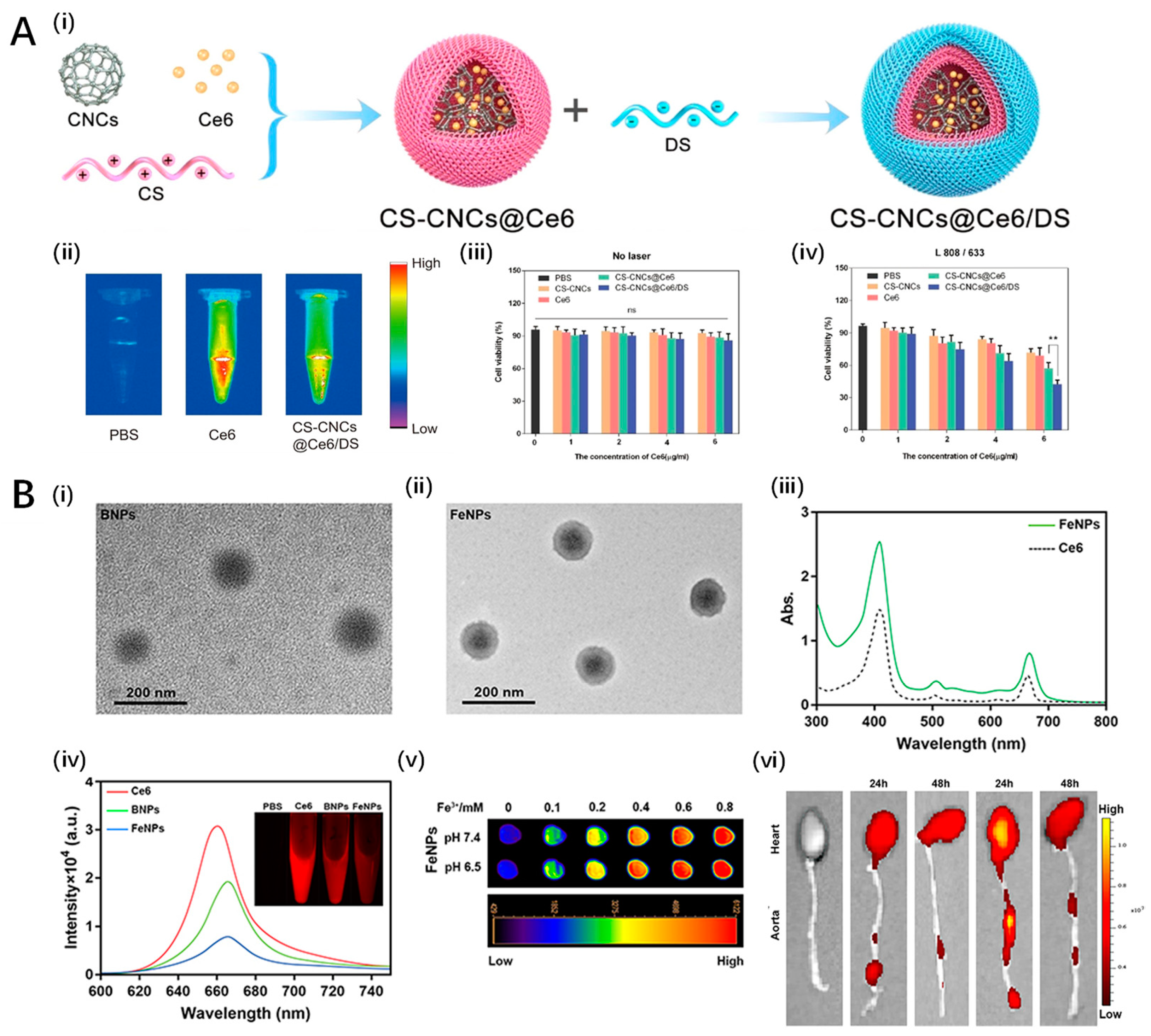
4.4. Other Inorganic Nanoparticles
4.5. Protein-Based Nanoparticles
| Study | Nanoparticle Approach | Brief Description |
|---|---|---|
| Spyropoulos-Antonakakis et al. 2015 [111] | Polymeric Nanoparticles | PAMAM dendrimers or PAMAM/zinc phthalocyanine (ZnPc) conjugates |
| Wennink et al. 2017 [112] | Polymeric Nanoparticles | m-tetra(hydroxyphenyl)chlorin (mTHPC)-loaded polymeric micelles based on benzyl-poly(ε-caprolactone)-b-methoxy poly(ethylene glycol) (Ben-PCL-mPEG) |
| Jain et al. 2016 [116] | Liposomal Nanoparticles | Visudyne® (Liposomal Verteporfin) |
| Kałas et al. 2019 [117] | Liposomal Nanoparticles | Novel liposomal formulation of PVP-conjugated chlorin e6 (Photolon) |
| Zou et al. 2023 [118] | Liposomal Nanoparticles | Chlorin e6 (Ce6)-loaded, CD68-modified liposomes |
| Han et al. 2017 [123] | Upconversion Nanoparticles | Upconversion fluorescent nanoparticles encapsulating chlorin e6 (UCNPs-Ce6) |
| Ma et al. 2021 [125] | Upconversion Nanoparticles | Platelet-mimicking photodynamic therapeutic system using photosensitizer-loaded upconversion nanoparticle cores coated with platelet membrane |
| Huang et al. 2023 [126] | Upconversion Nanoparticles | Protein-polysaccharide nanoemulsion co-loaded with chlorin e6 and upconversion nanoparticles (UCNPs-Ce6@DB) |
| Gerasymchuk et al. 2021 [127] | Other Inorganic Nanoparticles | Gallato zirconium (IV) phthalocyanine complex (PcZrGallate) bound to SiO2 nanocarriers |
| Liu et al. 2021 [128] | Other Inorganic Nanoparticles | Highly targeted nanoplatform CS-CNCs@Ce6/DS using chitosan-coated carbon nanocages (CS-CNCs) loaded with Chlorin e6 (Ce6) and dextran sulfate (DS) |
| Cao et al. 2022 [129] | Other Inorganic Nanoparticles | Copper sulfide/titanium oxide heterostructure nanosheets modified with hyaluronic acid and PEG (HA-HNSs) |
| Mu et al. 2022 [130] | Other Inorganic Nanoparticles | Chemiexcited PDT using polymeric nanoparticles integrated with magnetic resonance imaging (MRI) capabilities (FeCNPs) |
| Banerjee et al. 2019 [131] | Protein-based Nanoparticles | Zinc phthalocyanine-loaded human serum albumin nanoparticles (HSA NPs) |
5. Clinical Trials
6. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Nedkoff, L.; Briffa, T.; Zemedikun, D.; Herrington, S.; Wright, F.L. Global Trends in Atherosclerotic Cardiovascular Disease. Clin. Ther. 2023, 45, 1087–1091. [Google Scholar] [CrossRef]
- Libby, P. The Changing Landscape of Atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 1–18. [Google Scholar] [CrossRef]
- Libby, P. Inflammation during the Life Cycle of the Atherosclerotic Plaque. Cardiovasc. Res. 2021, 117, 2525–2536. [Google Scholar] [CrossRef]
- Fan, J.; Watanabe, T. Atherosclerosis: Known and Unknown. Pathol. Int. 2022, 72, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Sharif, H.; Akash, M.S.H.; Rehman, K.; Irshad, K.; Imran, I. Pathophysiology of Atherosclerosis: Association of Risk Factors and Treatment Strategies Using Plant-Based Bioactive Compounds. J. Food Biochem. 2020, 44, e13449. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Antithrombotic Trialists’ Collaboration. Collaborative Meta-Analysis of Randomised Trials of Antiplatelet Therapy for Prevention of Death, Myocardial Infarction, and Stroke in High Risk Patients. BMJ 2002, 324, 71–86. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, e13–e115. [Google Scholar] [CrossRef]
- Fihn, S.D.; Blankenship, J.C.; Alexander, K.P.; Bittl, J.A.; Byrne, J.G.; Fletcher, B.J.; Fonarow, G.C.; Lange, R.A.; Levine, G.N.; Maddox, T.M.; et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS Focused Update of the Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease. Circulation 2014, 130, 1749–1767. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.J.; Thanassoulis, G.; Anderson, T.J.; Barry, A.R.; Couture, P.; Dayan, N.; Francis, G.A.; Genest, J.; Grégoire, J.; Grover, S.A.; et al. 2021 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in Adults. Can. J. Cardiol. 2021, 37, 1129–1150. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Overchuk, M.; Weersink, R.A.; Wilson, B.C.; Zheng, G. Photodynamic and Photothermal Therapies: Synergy Opportunities for Nanomedicine. ACS Nano 2023, 17, 7979–8003. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Rehman, E.; Mumtaz, A.; Jianglin, Z. Cardiovascular Disease Mortality and Potential Risk Factor in China: A Multi-Dimensional Assessment by a Grey Relational Approach. Int. J. Public Health 2022, 67, 1604599. [Google Scholar] [CrossRef]
- Chen, W.; Li, Z.; Zhao, Y.; Chen, Y.; Huang, R. Global and National Burden of Atherosclerosis from 1990 to 2019: Trend Analysis Based on the Global Burden of Disease Study 2019. Chin. Med. J. 2023, 136, 2442–2450. [Google Scholar] [CrossRef] [PubMed]
- Lorey, M.B.; Öörni, K.; Kovanen, P.T. Modified Lipoproteins Induce Arterial Wall Inflammation During Atherogenesis. Front. Cardiovasc. Med. 2022, 9, 841545. [Google Scholar] [CrossRef] [PubMed]
- Malekmohammad, K.; Bezsonov, E.E.; Rafieian-Kopaei, M. Role of Lipid Accumulation and Inflammation in Atherosclerosis: Focus on Molecular and Cellular Mechanisms. Front. Cardiovasc. Med. 2021, 8, 707529. [Google Scholar] [CrossRef]
- Mytych, W.; Bartusik-Aebisher, D.; Łoś, A.; Dynarowicz, K.; Myśliwiec, A.; Aebisher, D. Photodynamic Therapy for Atherosclerosis. Int. J. Mol. Sci. 2024, 25, 1958. [Google Scholar] [CrossRef]
- deGoma, E.M.; Knowles, J.W.; Angeli, F.; Budoff, M.J.; Rader, D.J. The Evolution and Refinement of Traditional Risk Factors for Cardiovascular Disease. Cardiol. Rev. 2012, 20, 118–129. [Google Scholar] [CrossRef]
- Wong, N.D.; Budoff, M.J.; Ferdinand, K.; Graham, I.M.; Michos, E.D.; Reddy, T.; Shapiro, M.D.; Toth, P.P. Atherosclerotic Cardiovascular Disease Risk Assessment: An American Society for Preventive Cardiology Clinical Practice Statement. Am. J. Prev. Cardiol. 2022, 10, 100335. [Google Scholar] [CrossRef]
- Rothman, A.M.; MacFadyen, J.; Thuren, T.; Webb, A.; Harrison, D.G.; Guzik, T.J.; Libby, P.; Glynn, R.J.; Ridker, P.M. Effects of Interleukin-1β Inhibition on Blood Pressure, Incident Hypertension, and Residual Inflammatory Risk: A Secondary Analysis of CANTOS. Hypertension 2020, 75, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dubland, J.A.; Allahverdian, S.; Asonye, E.; Sahin, B.; Jaw, J.E.; Sin, D.D.; Seidman, M.A.; Leeper, N.J.; Francis, G.A. Smooth Muscle Cells Contribute the Majority of Foam Cells in ApoE (Apolipoprotein E)-Deficient Mouse Atherosclerosis. Arter. Thromb. Vasc. Biol. 2019, 39, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Basatemur, G.L.; Jørgensen, H.F.; Clarke, M.C.H.; Bennett, M.R.; Mallat, Z. Vascular Smooth Muscle Cells in Atherosclerosis. Nat. Rev. Cardiol. 2019, 16, 727–744. [Google Scholar] [CrossRef] [PubMed]
- Doran, A.C.; Meller, N.; McNamara, C.A. Role of Smooth Muscle Cells in the Initiation and Early Progression of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 812–819. [Google Scholar] [CrossRef]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in Atherosclerosis: A Dynamic Balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Bobryshev, Y.V.; Ivanova, E.A.; Chistiakov, D.A.; Nikiforov, N.G.; Orekhov, A.N. Macrophages and Their Role in Atherosclerosis: Pathophysiology and Transcriptome Analysis. Biomed. Res. Int. 2016, 2016, 9582430. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Fang, J.; Liu, Z.; Shi, Y.; Agostini, M.; Bernassola, F.; Bove, P.; Candi, E.; Rovella, V.; Sica, G.; et al. Macrophage Polarization and Metabolism in Atherosclerosis. Cell Death Dis. 2023, 14, 691. [Google Scholar] [CrossRef]
- Barrett, T.J. Macrophages in Atherosclerosis Regression. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 20–33. [Google Scholar] [CrossRef]
- Zhang, X.; Kang, Z.; Yin, D.; Gao, J. Role of Neutrophils in Different Stages of Atherosclerosis. Innate Immun. 2023, 29, 97–109. [Google Scholar] [CrossRef]
- Döring, Y.; Libby, P.; Soehnlein, O. Neutrophil Extracellular Traps Participate in Cardiovascular Diseases: Recent Experimental and Clinical Insights. Circ. Res. 2020, 126, 1228–1241. [Google Scholar] [CrossRef]
- Soehnlein, O. Multiple Roles for Neutrophils in Atherosclerosis. Circ. Res. 2012, 110, 875–888. [Google Scholar] [CrossRef]
- Lin, A.; Brittan, M.; Baker, A.H.; Dimmeler, S.; Fisher, E.A.; Sluimer, J.C.; Misra, A. Clonal Expansion in Cardiovascular Pathology. JACC Basic Transl. Sci. 2024, 9, 120–144. [Google Scholar] [CrossRef]
- Vartak, T.; Kumaresan, S.; Brennan, E. Decoding microRNA Drivers in Atherosclerosis. Biosci. Rep. 2022, 42, BSR20212355. [Google Scholar] [CrossRef]
- Henning, R.J. Obesity and Obesity-Induced Inflammatory Disease Contribute to Atherosclerosis: A Review of the Pathophysiology and Treatment of Obesity. Am. J. Cardiovasc. Dis. 2021, 11, 504–529. [Google Scholar]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus–Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Sadykhov, N.K.; Kartuesov, A.G.; Borisov, E.E.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. Hypertension as a Risk Factor for Atherosclerosis: Cardiovascular Risk Assessment. Front. Cardiovasc. Med. 2022, 9, 959285. [Google Scholar] [CrossRef]
- Tada, H.; Nohara, A.; Usui, S.; Sakata, K.; Kawashiri, M.; Takamura, M. Impact of the Severe Familial Hypercholesterolemia Status on Atherosclerotic Risks. Sci. Rep. 2023, 13, 19782. [Google Scholar] [CrossRef]
- Palasubramaniam, J.; Wang, X.; Peter, K. Myocardial Infarction—From Atherosclerosis to Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e176–e185. [Google Scholar] [CrossRef]
- Banerjee, C.; Chimowitz, M.I. Stroke Caused by Atherosclerosis of the Major Intracranial Arteries. Circ. Res. 2017, 120, 502–513. [Google Scholar] [CrossRef]
- Yaghi, S.; Prabhakaran, S.; Khatri, P.; Liebeskind, D.S. Intracranial Atherosclerotic Disease. Stroke 2019, 50, 1286–1293. [Google Scholar] [CrossRef]
- Hiatt, W.R.; Goldstone, J.; Smith, S.C.; McDermott, M.; Moneta, G.; Oka, R.; Newman, A.B.; Pearce, W.H. Atherosclerotic Peripheral Vascular Disease Symposium II. Circulation 2008, 118, 2826–2829. [Google Scholar] [CrossRef]
- Kong, P.; Cui, Z.-Y.; Huang, X.-F.; Zhang, D.-D.; Guo, R.-J.; Han, M. Inflammation and Atherosclerosis: Signaling Pathways and Therapeutic Intervention. Signal Transduct. Target. Ther. 2022, 7, 131. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Fernando, S.; Schwarz, N.; Tan, J.T.; Bursill, C.A.; Psaltis, P.J. Inflammation as a Therapeutic Target in Atherosclerosis. J. Clin. Med. 2019, 8, 1109. [Google Scholar] [CrossRef]
- Engelen, S.E.; Robinson, A.J.B.; Zurke, Y.-X.; Monaco, C. Therapeutic Strategies Targeting Inflammation and Immunity in Atherosclerosis: How to Proceed? Nat. Rev. Cardiol. 2022, 19, 522–542. [Google Scholar] [CrossRef]
- Rocha, V.Z.; Rached, F.H.; Miname, M.H. Insights into the Role of Inflammation in the Management of Atherosclerosis. J. Inflamm. Res. 2023, 16, 2223–2239. [Google Scholar] [CrossRef]
- Moriya, J. Critical Roles of Inflammation in Atherosclerosis. J. Cardiol. 2019, 73, 22–27. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Sukhorukov, V.N.; Eremin, I.I.; Nadelyaeva, I.I.; Orekhov, A.N. Diagnostics of Atherosclerosis: Overview of the Existing Methods. Front. Cardiovasc. Med. 2023, 10, 1134097. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.C.; Newby, D.E.; Nicol, E.D. Coronary Atherosclerosis Imaging by CT to Improve Clinical Outcomes. J. Cardiovasc. Comput. Tomogr. 2019, 13, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Varga-Szemes, A.; Maurovich-Horvat, P.; Schoepf, U.J.; Zsarnoczay, E.; Pelberg, R.; Stone, G.W.; Budoff, M.J. Computed Tomography Assessment of Coronary Atherosclerosis. J. Thorac. Imaging 2023, 38, 226–234. [Google Scholar] [CrossRef]
- Eckert, J.; Schmidt, M.; Magedanz, A.; Voigtländer, T.; Schmermund, A. Coronary CT Angiography in Managing Atherosclerosis. Int. J. Mol. Sci. 2015, 16, 3740–3756. [Google Scholar] [CrossRef]
- Anderson, J.D.; Kramer, C.M. MRI of Atherosclerosis: Diagnosis and Monitoring Therapy. Expert. Rev. Cardiovasc. Ther. 2007, 5, 69–80. [Google Scholar] [CrossRef]
- Mézquita, A.J.V.; Biavati, F.; Falk, V.; Alkadhi, H.; Hajhosseiny, R.; Maurovich-Horvat, P.; Manka, R.; Kozerke, S.; Stuber, M.; Derlin, T.; et al. Clinical Quantitative Coronary Artery Stenosis and Coronary Atherosclerosis Imaging: A Consensus Statement from the Quantitative Cardiovascular Imaging Study Group. Nat. Rev. Cardiol. 2023, 20, 696–714. [Google Scholar] [CrossRef]
- Gaggini, M.; Gorini, F.; Vassalle, C. Lipids in Atherosclerosis: Pathophysiology and the Role of Calculated Lipid Indices in Assessing Cardiovascular Risk in Patients with Hyperlipidemia. Int. J. Mol. Sci. 2022, 24, 75. [Google Scholar] [CrossRef]
- Chiorescu, R.M.; Mocan, M.; Inceu, A.I.; Buda, A.P.; Blendea, D.; Vlaicu, S.I. Vulnerable Atherosclerotic Plaque: Is There a Molecular Signature? Int. J. Mol. Sci. 2022, 23, 13638. [Google Scholar] [CrossRef]
- Jin, Y.; Fu, J. Novel Insights Into the NLRP3 Inflammasome in Atherosclerosis. J. Am. Heart Assoc. 2019, 8, e012219. [Google Scholar] [CrossRef]
- Grailer, J.J.; Canning, B.A.; Kalbitz, M.; Haggadone, M.D.; Dhond, R.M.; Andjelkovic, A.V.; Zetoune, F.S.; Ward, P.A. Critical Role for the NLRP3 Inflammasome during Acute Lung Injury. J. Immunol. 2014, 192, 5974–5983. [Google Scholar] [CrossRef]
- Karasawa, T.; Takahashi, M. Role of NLRP3 Inflammasomes in Atherosclerosis. J. Atheroscler. Thromb. 2017, 24, 443–451. [Google Scholar] [CrossRef]
- Lu, N.; Cheng, W.; Liu, D.; Liu, G.; Cui, C.; Feng, C.; Wang, X. NLRP3-Mediated Inflammation in Atherosclerosis and Associated Therapeutics. Front. Cell Dev. Biol. 2022, 10, 823387. [Google Scholar] [CrossRef]
- Grebe, A.; Hoss, F.; Latz, E. NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ. Res. 2018, 122, 1722–1740. [Google Scholar] [CrossRef]
- Abbate, A.; Toldo, S.; Marchetti, C.; Kron, J.; Van Tassell, B.W.; Dinarello, C.A. Interleukin-1 and the Inflammasome as Therapeutic Targets in Cardiovascular Disease. Circ. Res. 2020, 126, 1260–1280. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Dhorepatil, A.; Ball, S.; Ghosh, R.K.; Kondapaneni, M.; Lavie, C.J. Canakinumab: Promises and Future in Cardiometabolic Diseases and Malignancy. Am. J. Med. 2019, 132, 312–324. [Google Scholar] [CrossRef]
- Cheng, X.; Xie, Q.; Sun, Y. Advances in Nanomaterial-Based Targeted Drug Delivery Systems. Front. Bioeng. Biotechnol. 2023, 11, 1177151. [Google Scholar] [CrossRef] [PubMed]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in Drug Delivery Systems, Challenges and Future Directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, K.; Srinivasan, S.; Shanmugam, A. Review of the Efficacy of Nanoparticle-Based Drug Delivery Systems for Cancer Treatment. Biomed. Technol. 2024, 5, 109–122. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy—Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, C.Y.; Gao, J.; Wang, Z. Recent Advances in Photodynamic Therapy for Cancer and Infectious Diseases. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1560. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical Development and Potential of Photothermal and Photodynamic Therapies for Cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic Therapy for Cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New Photosensitizers for Photodynamic Therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Tsubone, T.M.; Martins, W.K.; Pavani, C.; Junqueira, H.C.; Itri, R.; Baptista, M.S. Enhanced Efficiency of Cell Death by Lysosome-Specific Photodamage. Sci. Rep. 2017, 7, 6734. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, S.M.; Ordaz, J.D.; Low, P.S. Targeting of a Photosensitizer to the Mitochondrion Enhances the Potency of Photodynamic Therapy. ACS Omega 2018, 3, 6066–6074. [Google Scholar] [CrossRef]
- Satrialdi; Takano, Y.; Hirata, E.; Ushijima, N.; Harashima, H.; Yamada, Y. An Effective in Vivo Mitochondria-Targeting Nanocarrier Combined with a π-Extended Porphyrin-Type Photosensitizer. Nanoscale Adv. 2021, 3, 5919–5927. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Inai, M.; Saito, S.; Honda, N.; Hazama, H.; Nishikawa, T.; Kaneda, Y.; Awazu, K. Photodynamic Therapy by Lysosomal-Targeted Drug Delivery Using Talaporfin Sodium Incorporated into Inactivated Virus Particles. Laser Ther. 2019, 28, 245–256. [Google Scholar] [CrossRef]
- Sai, D.L.; Lee, J.; Nguyen, D.L.; Kim, Y.-P. Tailoring Photosensitive ROS for Advanced Photodynamic Therapy. Exp. Mol. Med. 2021, 53, 495–504. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive Oxygen Species Generating Systems Meeting Challenges of Photodynamic Cancer Therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Wang, H.; Xu, J.-F.; Zhang, X. Activatable Photosensitizer for Smart Photodynamic Therapy Triggered by Reactive Oxygen Species in Tumor Cells. ACS Appl. Mater. Interfaces 2020, 12, 26982–26990. [Google Scholar] [CrossRef]
- Przygoda, M.; Bartusik-Aebisher, D.; Dynarowicz, K.; Cieślar, G.; Kawczyk-Krupka, A.; Aebisher, D. Cellular Mechanisms of Singlet Oxygen in Photodynamic Therapy. Int. J. Mol. Sci. 2023, 24, 16890. [Google Scholar] [CrossRef]
- Plaetzer, K.; Kiesslich, T.; Verwanger, T.; Krammer, B. The Modes of Cell Death Induced by PDT: An Overview. Med. Laser Appl. 2003, 18, 7–19. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Tanaka, M.; Vecchio, D.; Garcia-Diaz, M.; Chang, J.; Morimoto, Y.; Hamblin, M.R. Photodynamic Therapy Induces an Immune Response against a Bacterial Pathogen. Expert. Rev. Clin. Immunol. 2012, 8, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Bte Rahmat, J.N.; Zhang, Y. Advanced Techniques for Performing Photodynamic Therapy in Deep-Seated Tissues. Biomaterials 2022, 291, 121875. [Google Scholar] [CrossRef] [PubMed]
- Reginato, E.; Wolf, P.; Hamblin, M.R. Immune Response after Photodynamic Therapy Increases Anti-Cancer and Anti-Bacterial Effects. World J. Immunol. 2014, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Stringer, M.R.; Collins, P.; Robinson, D.J.; Stables, G.I.; Sheehan-Dare, R.A. The Accumulation of Protoporphyrin IX in Plaque Psoriasis After Topical Application of 5-Aminolevulinic Acid Indicates a Potential for Superficial Photodynamic Therapy. J. Investig. Dermatol. 1996, 107, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Di Fusco, S.A.; Maggioni, A.P.; Scicchitano, P.; Zuin, M.; D’Elia, E.; Colivicchi, F. Lipoprotein (a), Inflammation, and Atherosclerosis. J. Clin. Med. 2023, 12, 2529. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, H.; Mao, Y.; Zhang, Y.; Gu, N. Advances in Atherosclerosis Theranostics Harnessing Iron Oxide-Based Nanoparticles. Adv. Sci. 2024, 11, 2308298. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Moghissi, K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Depuydt, M.A.C.; Prange, K.H.M.; Slenders, L.; Örd, T.; Elbersen, D.; Boltjes, A.; de Jager, S.C.A.; Asselbergs, F.W.; de Borst, G.J.; Aavik, E.; et al. Microanatomy of the Human Atherosclerotic Plaque by Single-Cell Transcriptomics. Circ. Res. 2020, 127, 1437–1455. [Google Scholar] [CrossRef]
- Hayase, M.; Woodbum, K.W.; Perlroth, J.; Miller, R.A.; Baumgardner, W.; Yock, P.G.; Yeung, A. Photoangioplasty with Local Motexafin Lutetium Delivery Reduces Macrophages in a Rabbit Post-Balloon Injury Model. Cardiovasc. Res. 2001, 49, 449–455. [Google Scholar] [CrossRef]
- Tang, G.; Hyman, S.; Schneider, J.H.J.; Giannotta, S.L. Application of Photodynamic Therapy to the Treatment of Atherosclerotic Plaques. Neurosurgery 1993, 32, 438. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.T.; Neves, M.G.; Cavaleiro, J.A. Cancer, Photodynamic Therapy and Porphyrin-Type Derivatives. An. Acad. Bras. Ciênc. 2018, 90, 993–1026. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, Q.; Yu, L.; Bai, D. Pyropheophorbide-α Methyl Ester-Mediated Photodynamic Therapy Induces Apoptosis and Inhibits LPS-Induced Inflammation in RAW264.7 Macrophages. Photodiagn. Photodyn. Ther. 2019, 25, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Hafiane, A. Vulnerable Plaque, Characteristics, Detection, and Potential Therapies. J. Cardiovasc. Dev. Dis. 2019, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yin, G.; Le, V.; Zhang, A.; Chen, S.; Liang, X.; Liu, J. Photodynamic-Therapy Activates Immune Response by Disrupting Immunity Homeostasis of Tumor Cells, Which Generates Vaccine for Cancer Therapy. Int. J. Biol. Sci. 2016, 12, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, T.; Nakajima, H.; Katoh, T.; Rakue, H.; Miyagi, M.; Ibukiyama, C. Photodynamic Therapy of Atherosclerosis Using YAG-OPO Laser and Porfimer Sodium, and Comparison With Using Argon-Dye Laser. Jpn. Circ. J. 1999, 63, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, P.J.; White, W.M.; Fabian, R.L.; Anderson, R.R. Optical Integrating Balloon Device for Photodynamic Therapy. Lasers Surg. Med. 2000, 26, 58–66. [Google Scholar] [CrossRef]
- Waksman, R.; McEwan, P.E.; Moore, T.I.; Pakala, R.; Kolodgie, F.D.; Hellinga, D.G.; Seabron, R.C.; Rychnovsky, S.J.; Vasek, J.; Scott, R.W.; et al. PhotoPoint Photodynamic Therapy Promotes Stabilization of Atherosclerotic Plaques and Inhibits Plaque Progression. J. Am. Coll. Cardiol. 2008, 52, 1024–1032. [Google Scholar] [CrossRef]
- Zellweger, M.; Xiao, Y.; Jain, M.; Giraud, M.-N.; Pitzschke, A.; de Kalbermatten, M.; Berger, E.; van den Bergh, H.; Cook, S.; Wagnières, G. Optical Characterization of an Intra-Arterial Light and Drug Delivery System for Photodynamic Therapy of Atherosclerotic Plaque. Appl. Sci. 2020, 10, 4304. [Google Scholar] [CrossRef]
- Pai, M.; Jamal, W.; Mosse, A.; Bishop, C.; Bown, S.; McEwan, J. Inhibition of In-Stent Restenosis in Rabbit Iliac Arteries with Photodynamic Therapy. Eur. J. Vasc. Endovasc. Surg. 2005, 30, 573–581. [Google Scholar] [CrossRef]
- Peng, C.; Li, Y.; Liang, H.; Cheng, J.; Li, Q.; Sun, X.; Li, Z.; Wang, F.; Guo, Y.; Tian, Z.; et al. Detection and Photodynamic Therapy of Inflamed Atherosclerotic Plaques in the Carotid Artery of Rabbits. J. Photochem. Photobiol. B Biol. 2011, 102, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhu, Y.; Li, K.; Liao, B.; Wang, F.; Shao, L.; Huang, L.; Bai, D. Curcumin-Mediated Photodynamic Therapy Inhibits the Phenotypic Transformation, Migration, and Foaming of Oxidized Low-Density Lipoprotein-Treated Vascular Smooth Muscle Cells by Promoting Autophagy. J. Cardiovasc. Pharmacol. 2021, 78, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Ahn, J.W.; Lee, M.W.; Kim, H.J.; Kang, D.O.; Kim, R.H.; Kang, U.G.; Kim, Y.H.; Han, J.; Park, Y.H.; et al. Targeted Theranostic Photoactivation on Atherosclerosis. J. Nanobiotechnol. 2021, 19, 338. [Google Scholar] [CrossRef] [PubMed]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Zhou, X.; Zhang, J.; Shi, Y.; Zhong, L. Metal Nanoparticles for Photodynamic Therapy: A Potential Treatment for Breast Cancer. Molecules 2021, 26, 6532. [Google Scholar] [CrossRef]
- Pashootan, P.; Saadati, F.; Fahimi, H.; Rahmati, M.; Strippoli, R.; Zarrabi, A.; Cordani, M.; Moosavi, M.A. Metal-Based Nanoparticles in Cancer Therapy: Exploring Photodynamic Therapy and Its Interplay with Regulated Cell Death Pathways. Int. J. Pharm. 2024, 649, 123622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Han, T.; Xia, H.; Dong, L.; Chen, L.; Lei, L. Advances in Photodynamic Therapy Based on Nanotechnology and Its Application in Skin Cancer. Front. Oncol. 2022, 12, 836397. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, N.; Sevilla, A. Current Advances in Photodynamic Therapy (PDT) and the Future Potential of PDT-Combinatorial Cancer Therapies. Int. J. Mol. Sci. 2024, 25, 1023. [Google Scholar] [CrossRef] [PubMed]
- Alsaab, H.O.; Alharbi, F.D.; Alhibs, A.S.; Alanazi, N.B.; Alshehri, B.Y.; Saleh, M.A.; Alshehri, F.S.; Algarni, M.A.; Almugaiteeb, T.; Uddin, M.N.; et al. PLGA-Based Nanomedicine: History of Advancement and Development in Clinical Applications of Multiple Diseases. Pharmaceutics 2022, 14, 2728. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Spyropoulos-Antonakakis, N.; Sarantopoulou, E.; Trohopoulos, P.N.; Stefi, A.L.; Kollia, Z.; Gavriil, V.E.; Bourkoula, A.; Petrou, P.S.; Kakabakos, S.; Semashko, V.V.; et al. Selective Aggregation of PAMAM Dendrimer Nanocarriers and PAMAM/ZnPc Nanodrugs on Human Atheromatous Carotid Tissues: A Photodynamic Therapy for Atherosclerosis. Nanoscale Res. Lett. 2015, 10, 210. [Google Scholar] [CrossRef] [PubMed]
- Wennink, J.W.H.; Liu, Y.; Mäkinen, P.I.; Setaro, F.; de la Escosura, A.; Bourajjaj, M.; Lappalainen, J.P.; Holappa, L.P.; van den Dikkenberg, J.B.; al Fartousi, M.; et al. Macrophage Selective Photodynamic Therapy by Meta-Tetra(Hydroxyphenyl)Chlorin Loaded Polymeric Micelles: A Possible Treatment for Cardiovascular Diseases. Eur. J. Pharm. Sci. 2017, 107, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Dhilip Kumar, S.S.; Abrahamse, H. Biocompatible Nanocarriers for Enhanced Cancer Photodynamic Therapy Applications. Pharmaceutics 2021, 13, 1933. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.J.; Choi, D.G.; Shim, M.S. Targeted and Effective Photodynamic Therapy for Cancer Using Functionalized Nanomaterials. Acta Pharm. Sin. B 2016, 6, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Moghassemi, S.; Dadashzadeh, A.; Azevedo, R.; Feron, O.; Amorim, C. Photodynamic Cancer Therapy Using Liposomes as an Advanced Vesicular Photosensitizer Delivery System. J. Control. Release 2021, 339, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Zellweger, M.; Frobert, A.; Valentin, J.; van den Bergh, H.; Wagnières, G.; Cook, S.; Giraud, M.-N. Intra-Arterial Drug and Light Delivery for Photodynamic Therapy Using Visudyne®: Implication for Atherosclerotic Plaque Treatment. Front. Physiol. 2016, 7, 400. [Google Scholar] [CrossRef] [PubMed]
- Kałas, W.; Wysokińska, E.; Przybyło, M.; Langner, M.; Ulatowska-Jarża, A.; Biały, D.; Wawrzyńska, M.; Zioło, E.; Gil, W.; Trzeciak, A.M.; et al. Photoactive Liposomal Formulation of PVP-Conjugated Chlorin E6 for Photodynamic Reduction of Atherosclerotic Plaque. Int. J. Mol. Sci. 2019, 20, 3852. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Zhang, Y.; Cheraga, N.; Abodunrin, O.D.; Qu, K.-Y.; Qiao, L.; Ma, Y.-Q.; Chen, L.-J.; Huang, N.-P. Chlorin E6 (Ce6)-Loaded Plaque-Specific Liposome with Enhanced Photodynamic Therapy Effect for Atherosclerosis Treatment. Talanta 2023, 265, 124772. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cheng, L.; Liu, Z. Upconversion Nanoparticles for Photodynamic Therapy and Other Cancer Therapeutics. Theranostics 2013, 3, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; La, H.; Zhu, R.; El-Banna, G.; Wei, Y.; Han, G. Upconverting NIR Photons for Bioimaging. Nanomaterials 2015, 5, 2148–2168. [Google Scholar] [CrossRef]
- Liang, G.; Wang, H.; Shi, H.; Wang, H.; Zhu, M.; Jing, A.; Li, J.; Li, G. Recent Progress in the Development of Upconversion Nanomaterials in Bioimaging and Disease Treatment. J. Nanobiotechnol. 2020, 18, 154. [Google Scholar] [CrossRef] [PubMed]
- Yaguchi, M.; Jia, X.; Schlesinger, R.; Jiang, X.; Ataka, K.; Heberle, J. Near-Infrared Activation of Sensory Rhodopsin II Mediated by NIR-to-Blue Upconversion Nanoparticles. Front. Mol. Biosci. 2022, 8, 782688. [Google Scholar] [CrossRef]
- Han, X.B.; Li, H.X.; Jiang, Y.Q.; Wang, H.; Li, X.S.; Kou, J.Y.; Zheng, Y.H.; Liu, Z.N.; Li, H.; Li, J.; et al. Upconversion Nanoparticle-Mediated Photodynamic Therapy Induces Autophagy and Cholesterol Efflux of Macrophage-Derived Foam Cells via ROS Generation. Cell Death Dis. 2017, 8, e2864. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Han, R.; Yang, L.; Shi, J.; Liu, Z.; Hu, Y.; Wang, Y.; Liu, S.; Gan, Y. Design and Synthesis of Core–Shell–Shell Upconversion Nanoparticles for NIR-Induced Drug Release, Photodynamic Therapy, and Cell Imaging. ACS Appl. Mater. Interfaces 2016, 8, 4416–4423. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ma, Y.; Gao, M.; Han, Z.; Jiang, W.; Gu, Y.; Liu, Y. Platelet-Mimicking Therapeutic System for Noninvasive Mitigation of the Progression of Atherosclerotic Plaques. Adv. Sci. 2021, 8, 2004128. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xu, S.; Liu, L.; Zhang, J.; Xu, J.; Zhang, L.; Zhou, X.; Huang, L.; Peng, J.; Wang, J.; et al. Targeted Treatment of Atherosclerosis with Protein–Polysaccharide Nanoemulsion Co-Loaded with Photosensitiser and Upconversion Nanoparticles. J. Drug Target. 2023, 31, 1111–1127. [Google Scholar] [CrossRef] [PubMed]
- Gerasymchuk, Y.; Kałas, W.; Arkowski, J.; Marciniak, Ł.; Hreniak, D.; Wysokińska, E.; Strządała, L.; Obremska, M.; Tomachynski, L.; Chernii, V.; et al. Gallato Zirconium (IV) Phtalocyanine Complex Conjugated with SiO2 Nanocarrier as a Photoactive Drug for Photodynamic Therapy of Atheromatic Plaque. Molecules 2021, 26, 260. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, B.; Guo, Y.; Zhang, A.; Yang, K.; He, Y.; Wang, J.; Cheng, Y.; Cui, D. SR-A-Targeted Nanoplatform for Sequential Photothermal/Photodynamic Ablation of Activated Macrophages to Alleviate Atherosclerosis. ACS Appl. Mater. Interfaces 2021, 13, 29349–29362. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Yuan, G.; Zeng, L.; Bai, L.; Liu, X.; Wu, M.; Sun, R.; Chen, Z.; Jiang, Y.; Gao, Q.; et al. Macrophage-Targeted Sonodynamic/Photothermal Synergistic Therapy for Preventing Atherosclerotic Plaque Progression Using CuS/TiO2 Heterostructured Nanosheets. ACS Nano 2022, 16, 10608–10622. [Google Scholar] [CrossRef]
- Mu, D.; Wang, X.; Wang, H.; Sun, X.; Dai, Q.; Lv, P.; Liu, R.; Qi, Y.; Xie, J.; Xu, B.; et al. Chemiexcited Photodynamic Therapy Integrated in Polymeric Nanoparticles Capable of MRI Against Atherosclerosis. Int. J. Nanomed. 2022, 17, 2353–2366. [Google Scholar] [CrossRef]
- Banerjee, S.; Sengupta, J.; Aljarilla, A.I.; Setaro, F.; Makinen, P.; Wu, L.; Holappa, L.; De La Escosura, A.; Martinelli, C.; Trohopoulos, P.; et al. Human Serum Albumin Nanoparticles Loaded with Phthalocyanine Dyes for Potential Use in Photodynamic Therapy for Atherosclerotic Plaques. PRNANO 2019, 2, 279–302. [Google Scholar] [CrossRef]
- Erlinge, D.; Maehara, A.; Ben-Yehuda, O.; Bøtker, H.E.; Maeng, M.; Kjøller-Hansen, L.; Engstrøm, T.; Matsumura, M.; Crowley, A.; Dressler, O.; et al. Identification of Vulnerable Plaques and Patients by Intracoronary Near-Infrared Spectroscopy and Ultrasound (PROSPECT II): A Prospective Natural History Study. Lancet 2021, 397, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.; Todd, M.; Turnbull, R.; Allison, B.; Mornin, L.; Carvalho, A.-M.; Spurr, G.; Hsiang, Y. Longer Term Assessment of Photodynamic Therapy for Intimal Hyperplasia: A Pilot Study. J. Photochem. Photobiol. B Biol. 2004, 73, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Hsiang, Y.N.; Fragoso, M.; Tsang, V.; Schreiber, W.E. Determining the Optimal Dose of Photofrin® in Miniswine Atherosclerotic Plaque. Photochem. Photobiol. 1993, 57, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Hsiang, Y.N.; Todd, M.E.; Bower, R.D. Determining Light Dose for Photodynamic Therapy of Atherosclerotic Lesions in the Yucatan Miniswine. J. Endovasc. Surg. 1995, 2, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Usui, M.; Asahara, T.; Naitoh, Y.; Katoh, T.; Ibukiyama, C. Photodynamic Therapy for the Prevention of Intimal Hyperplasia in Balloon-Injured Rabbit Arteries. Jpn. Circ. J. 1999, 63, 387–393. [Google Scholar] [CrossRef]
- Spokojny, A.M.; Serur, J.R.; Skillman, J.; Richard Spears, J. Uptake of Hematoporphyrin Derivative by Atheromatous Plaques: Studies in Human in Vitro and Rabbit in Vivo. J. Am. Coll. Cardiol. 1986, 8, 1387–1392. [Google Scholar] [CrossRef]
- Spears, J.R.; Serur, J.; Shropshire, D.; Paulin, S. Fluorescence of Experimental Atheromatous Plaques with Hematoporphyrin Derivative. J. Clin. Investig. 1983, 71, 395–399. [Google Scholar] [CrossRef]
- Eton, D.; Shim, V.; Maibenco, T.A.; Spero, K.; Cava, R.A.; Borhani, M.; Grossweiner, L.; Ahn, S.S. Cytotoxic Effect of Photodynamic Therapy with Photofrin II on Intimal Hyperplasia. Ann. Vasc. Surg. 1996, 10, 273–282. [Google Scholar] [CrossRef]
- Eton, D.; Colburn, M.D.; Shim, V.; Panek, W.; Lee, D.; Moore, W.S.; Ahn, S.S. Inhibition of Intimal Hyperplasia by Photodynamic Therapy Using Photofrin. J. Surg. Res. 1992, 53, 558–562. [Google Scholar] [CrossRef]
- Jenkins, M.P.; Buonaccorsi, G.A.; Raphael, M.; Nyamekye, I.; McEwan, J.R.; Bown, S.G.; Bishop, C.C.R. Clinical Study of Adjuvant Photodynamic Therapy to Reduce Restenosis Following Femoral Angioplasty. Br. J. Surg. 1999, 86, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.P.; Buonaccorsi, G.A.; Mansfield, R.; Bishop, C.C.R.; Bown, S.G.; McEwan, J.R. Reduction in the Response to Coronary and Iliac Artery Injury with Photodynamic Therapy Using 5-Aminolaevulinic Acid. Cardiovasc. Res. 2000, 45, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.P.; Buonaccorsi, G.; MacRobert, A.; Bishop, C.C.R.; Bown, S.G.; McEwan, J.R. Intra-Arterial Photodynamic Therapy Using 5-ALA in a Swine Model. Eur. J. Vasc. Endovasc. Surg. 1998, 16, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Nyamekye, I.; Buonaccorsi, G.; McEwan, J.; MacRobert, A.; Bown, S.; Bishop, C. Inhibition of Intimal Hyperplasia in Balloon Injured Arteries with Adjunctive Phthalocyanine Sensitised Photodynamic Therapy. Eur. J. Vasc. Endovasc. Surg. 1996, 11, 19–28. [Google Scholar] [CrossRef]
- Rockson, S.G.; Kramer, P.; Razavi, M.; Szuba, A.; Filardo, S.; Fitzgerald, P.; Cooke, J.P.; Yousuf, S.; DeVault, A.R.; Renschler, M.F.; et al. Photoangioplasty for Human Peripheral Atherosclerosis. Circulation 2000, 102, 2322–2324. [Google Scholar] [CrossRef]



| Imaging Technologies | Fibrous Cap | Lipid Core | Macrophages | Calcification | Thrombus | Lumen Integrity | Angiogenesis | Plaque Burden | |
|---|---|---|---|---|---|---|---|---|---|
| Non-invasive Technologies | Doppler ultrasound (carotid/femoral arteries) | − | − | − | − | − | − | + | + |
| Coronary CT Angiography (CCTA) | − | + | − | + | − | + | − | + | |
| Coronary artery calcium score (CAC) | − | − | − | + | − | − | − | − | |
| Invasive Technologies | Coronary Angiography (CAG) | − | − | − | − | − | + | − | − |
| Intravascular Ultrasound (IVUS) | + | + | − | + | + | + | − | + | |
| Virtual Histology-Intravascular Ultrasound (VH-IVUS) | − | + | − | + | − | + | − | + | |
| Angioscope | + | + | − | − | + | + | − | − | |
| Optical coherence tomography (OCT) | + | + | + | + | + | + | + | − | |
| Near infrared spectroscopy (NIRS) | + | + | + | + | − | − | − | − | |
| Emerging Technologies | Magnetic resonance angiography (MRA) | − | − | − | − | + | + | − | − |
| Positron emission computed tomography (PET) | − | − | + | + | − | − | − | − | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Xie, R.; Yu, T. Photodynamic Therapy for Atherosclerosis: Past, Present, and Future. Pharmaceutics 2024, 16, 729. https://doi.org/10.3390/pharmaceutics16060729
Lin Y, Xie R, Yu T. Photodynamic Therapy for Atherosclerosis: Past, Present, and Future. Pharmaceutics. 2024; 16(6):729. https://doi.org/10.3390/pharmaceutics16060729
Chicago/Turabian StyleLin, Yanqing, Ruosen Xie, and Tao Yu. 2024. "Photodynamic Therapy for Atherosclerosis: Past, Present, and Future" Pharmaceutics 16, no. 6: 729. https://doi.org/10.3390/pharmaceutics16060729
APA StyleLin, Y., Xie, R., & Yu, T. (2024). Photodynamic Therapy for Atherosclerosis: Past, Present, and Future. Pharmaceutics, 16(6), 729. https://doi.org/10.3390/pharmaceutics16060729








