Mechanism, Formulation, and Efficacy Evaluation of Natural Products for Skin Pigmentation Treatment
Abstract
1. Introduction
2. Pathogenesis and Therapeutic Approaches
2.1. Pathogenesis
2.2. Therapeutic Approaches
3. Natural Products for Treating Skin Pigmentation
3.1. Natural Medicinal Ingredients
3.1.1. Multi-Pathway Therapeutic Agents
- 1.
- Arbutin
- 2.
- Azelaic Acid
- 3.
- Aloesin
- 4.
- Glabridin
- 5.
- Auraptene
- 6.
- Resveratrol
3.1.2. Tyrosinase Inhibitors
- 1.
- Gedunin
- 2.
- Calycosin
- 3.
- Patuletin
- 4.
- Curcumin
- 5.
- Pulsae
- 6.
- Sour jujube kernel
3.1.3. Antioxidants
- 1.
- Pterostilbene
- 2.
- Ferulic acid
- 3.
- Salidroside
- 4.
- Gallic acid
- 5.
- Thymoquinone
| Function | Ingredient | Mechanism | In Vitro/In Vivo Studies | References |
|---|---|---|---|---|
| Multi-pathway agents | Arbutin | Inhibition of tyrosinase activity; Enhancement of SOD enzyme activity | Melasma guinea pig model | [36,37,38] |
| Azelaic acid | Anti-inflammatory; Inhibition of tyrosinase activity | B16F10 cell line | [39,40] | |
| Aloesin | Antioxidant; Inhibition of tyrosinase activity | In vitro Mushroom tyrosinase assay; B16F10 cell line | [46] | |
| Glabridin | Antioxidant; Inhibition of tyrosinase activity | In vitro Mushroom tyrosinase assay; B16F10 cell line | [48,49] | |
| Resveratrol | Antioxidant; Inhibition of tyrosinase activity | Guinea pig model | [54] | |
| Auraptene | Inhibition of tyrosinase activity; Antioxidant | HFF cell line; B16F10 cell line | [51] | |
| Tyrosinase inhibitors | Gedunin | Inhibition of tyrosinase activity and protein amounts | B16F10 cell line; Zebrafish embryo model | [56] |
| Calycosin | Inhibition of tyrosinase activity | Molecular docking technology; Zebrafish embryo model | [58] | |
| Patuletin | Reduced tyrosinase expression | B16F10 cell line; Zebrafish embryo model | [60] | |
| Curcumin | Inhibition of tyrosinase-related gene expression | B16F10 cell line; Zebrafish embryo model | [62] | |
| Pulsae | Inhibition of tyrosinase activity | In vitro Mushroom tyrosinase assay; B16F10 cell line | [65] | |
| Jujube flavonoids | Inhibition of MITF and tyrosinase | Mushroom tyrosinase assay; B16F10 cell line; Zebrafish embryo model | [65] | |
| Antioxidants | Pterostilbene | Antioxidant; Inhibition of NRF2/ARE signaling pathway | B16F10 cell line; Keratin-forming cell line; Zebrafish embryo model | [68,69] |
| Ferulic acid | Antioxidant | B16F10 cell line | [70,71] | |
| Salidroside | Antioxidant | B16F10 cell line; Guinea pig model | [73] | |
| Gallic acid | Antioxidant | B16F10 cell line | [75] | |
| Thymoquinone | Antioxidant | Swiss albino mice | [78] |
3.2. Natural Extracts
| Extract | Source | In Vitro/In Vivo Studies | Mechanism | References |
|---|---|---|---|---|
| Polysaccharides | Ganoderma | Zebrafish embryo model; Guinea pig model | Antagonism of cAMP/PKA and ROS/MAPK signaling pathways | [85] |
| Morchella esculenta | B16F10 cell line; Zebrafish embryo model | Dose-dependent inhibition of tyrosinase activity and reduction of MITF and TRPs protein expression | [86] | |
| Bletilla striata | In vitro free radical scavenging ability test | Antioxidant | [83] | |
| Poria | Mushroom tyrosinase assay | Inhibition of tyrosinase activity | [84] | |
| Esenticosus | In vitro free radical scavenging ability test | Antioxidant | [86] | |
| Flavonoids | Selaginella | Mushroom tyrosinase assay; B16F10 cell line, Zebrafish embryo model | Antioxidant; Inhibition of tyrosinase, MAPK, and MITF pathway expression | [93] |
| Theaflavin | Spectral analysis; Molecular docking; Zebrafish embryo model | Antioxidant; Inhibition of tyrosinase activity | [92] | |
| Hesperidin | Mushroom tyrosinase assay; B16F10 cell line | Activation of the MEK/ErK1/2 pathway | [94,95] | |
| Tanshinone | HEM cell line | Activation of Nrf2 antioxidant pathway | [94,96] | |
| Ginkgo leaves | In vitro free radical scavenging ability test | Antioxidant | [97] | |
| Polyphenols | Tea polyphenols | B16F10 cell line; Zebrafish embryo model | Inhibition of tyrosinase activity | [98] |
| Ginseng phenolic acid | B16F10 cell line; Zebrafish embryo model | Inhibition of melanin synthase through the cAMP/PKA signaling pathway | [91] | |
| Brown algae | B16F10 cell line; Zebrafish embryo model | Inhibition of tyrosinase activity and regulation of protein expression of the MITF/CREB signaling pathway | [99] | |
| Pomegranate | In vitro free radical scavenging ability test | Antioxidant | [100] | |
| Orange peel | In vitro free radical scavenging ability test | Antioxidant | [101] | |
| Other natural product extracts | CalendulaofficinalisL | Mushroom tyrosinase assay | Inhibition of tyrosinase activity | [102] |
| Kava pepper | B16F10 cell line | Regulation of tyrosinase and MITF activity | [103] | |
| Edible mushrooms | Zebrafish embryo model | Dose-dependent inhibition of melanogenesis | [104] | |
| Olive leaves | Zebrafish embryo model | Inhibition of tyrosinase activity | [105] | |
| Coix seed bran oil | B16F10 cell line; Zebrafish embryo model | Inhibition of tyrosinase activity | [106] | |
| Rice extracts | B16 cell line; Zebrafish embryo model | Antioxidant; Regulation of tyrosinase; Regulation MITF activity | [107] |
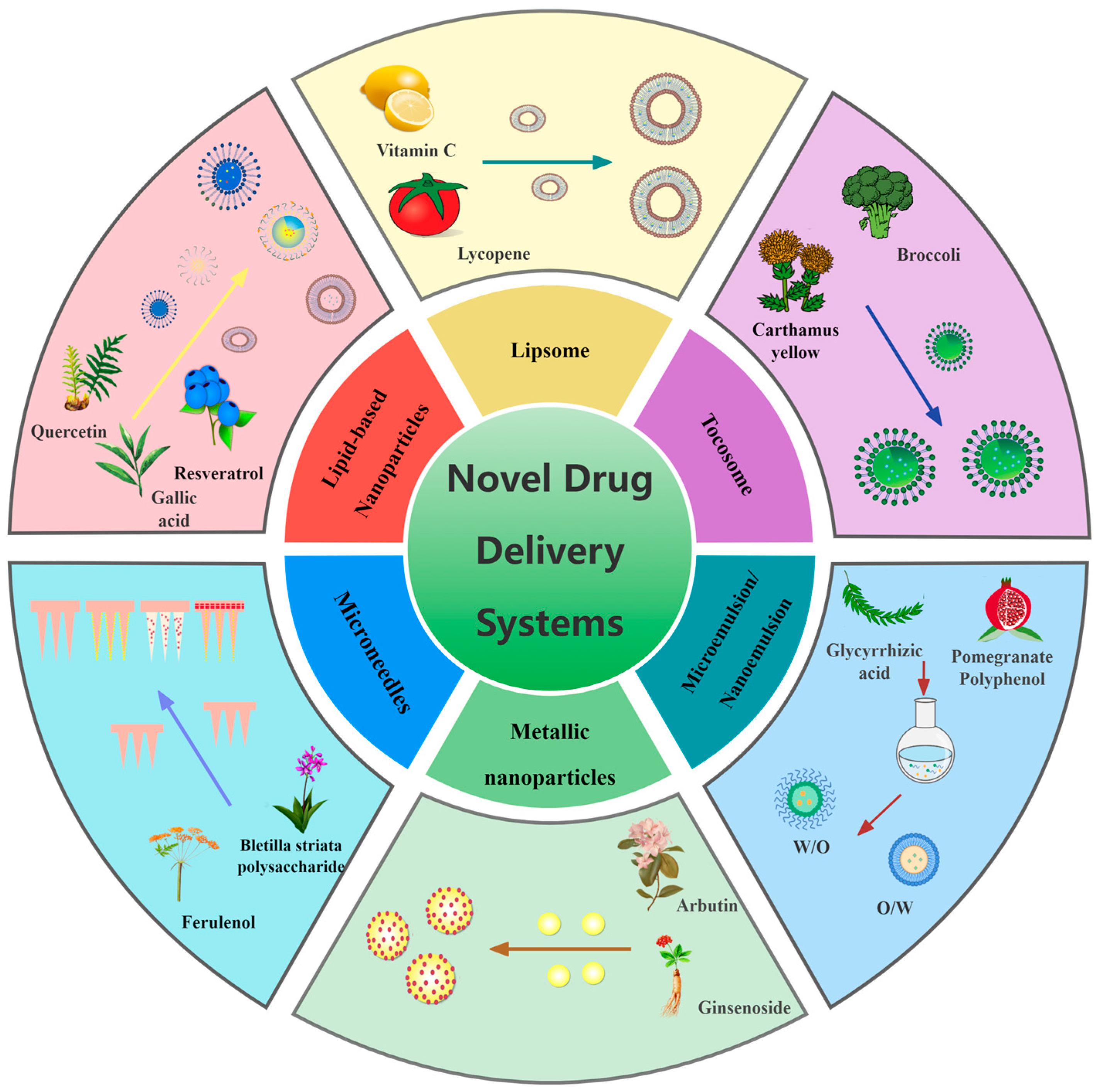
4. Novel Drug Delivery System for Skin Pigmentation Treatment
4.1. Lipsome
4.2. Lipid-Based Nanoparticles
4.2.1. Solid Lipid Nanoparticles
4.2.2. Nanostructured Lipid Carriers
4.2.3. Transferosomes
4.3. Microemulsions/Nanoemulsions
4.4. Metallic Nanoparticles
4.5. Microneedles
4.6. Tocosome
| Formulation | Ingredient | Method/Material | Enhancement | Reference |
|---|---|---|---|---|
| Lipsome | Glutathione | Liposome extruder purification | Increase medication uptake | [114] |
| Lycopene | Thin film hydration method | Skin permeability and antioxidation | [146] | |
| Solid lipid nanoparticles | Lignin | High-pressure homogenization | UV shielding effect and antioxidation | [119] |
| Quercetin | Nanoprecipitation | Hydrophilicity | [121] | |
| Auraptene | Hot homogenization and ultrasonication | Skin permeability | [51] | |
| Nanostructured lipid carrier | Resveratrol | Ultrasonication | Targeting and antioxidant activity | [125] |
| Arbutin | Ultrasonication | Skin permeability and stability | [126] | |
| Transferosomes | Epigallocatechin-3-gallate | High-pressure homogenization | Skin permeability and antioxidation | [129] |
| Microemulsions | Glycyrrhizic acid | Ionic liquid microemulsion | Solubility and permeability | [14] |
| Nanoemulsions | Pomegranate peel | Pomegranate seed oil | Skin permeation | [147] |
| Metallic nanoparticles | Arbutin | Eco-friendly synthesis | Antimelanogenic activity | [136] |
| Ginseng berry | Eco-friendly synthesis | Antibacterial and antioxidant activity | [138] | |
| Microneedles | Ferulic acid | Solid microneedles | Skin permeability | [142] |
| Tranexamic acid and licorice extract | PVA and PVP | Bioavailability | [15] | |
| Resveratrol | Acrylic resin E100/PVP-K90 | Stability | [148] | |
| Glabridin | Cyclodextrin | Transdermal penetration and retention time | [149] | |
| Arbutin | HPMC and PVP | Skin permeability | [150] | |
| Tocosome | vitamin C/glutathione | Mozafari method | Improve transshipment efficiency | [145] |
5. Evaluation Methods for Pigmentation Treatment
5.1. Tyrosinase Activity
5.2. Antioxidant Capacity
5.3. Cell Model
5.4. Zebrafish Model
5.5. Mouse Model
| Evaluation Methods | Type/Strain | Advantage | Disadvantage | Reference |
|---|---|---|---|---|
| In vitro tyrosinase assay | mTYR/hTYR | Lower cost and shorter experimental period | Poor enzyme homology and large differences in active sites | [155,158] |
| In vitro free radical scavenging ability assay | DPPH/ABTS/SRSA/FRAP | Inexpensive, fast detection, simple operation | Differences in free radical scavenging effects between in vivo and vitro | [162,163] |
| In vitro cell culture assay | B16/A375 cell line | A shorter experimental period and the possibility to study intracellular mechanisms | Specific culture conditions | [9,151] |
| In vitro zebrafish embryo model testing | Wild type zebrafish | Easy to observe and capable of specific mechanistic studies | Specific culture conditions | [10,168,169] |
| In vivo melasma mouse model experiments | Brown female guinea pig | Continuum of lifeforms from molecules to genetics, high homology, and the possibility of specific mechanism studies | The long incubation period, following 3R principles and experimental ethics required | [11,171,173] |
6. Conclusions and Future Discussions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TYR | tyrosinase |
| TRP-2 | tyrosinase-related protein-2 |
| TRP-1 | tyrosinase-related protein-1 |
| α-MSH | α-melanocyte stimulating hormone |
| MITF | microphthalmic aberrant transcription factor |
| ROS | reactive oxygen species |
| ARB | Arbutin |
| AZA | Azelaic Acid |
| DOPA | Dihydroxyphenylalanine |
| GLA | Glabridin |
| AUR | Auraptene |
| HFF | human foreskin fibroblasts |
| RES | Resveratrol |
| GED | Gedunin |
| CA | Calycosin |
| PN | Patuletin |
| CUR | Curcumin |
| PS | Pulsae |
| PTS | Paektanshim |
| SJK | Sour jujube kernel |
| JUB | Jujubesaponin B |
| PT | Pterostilbene |
| FA | Ferulic acid |
| TQ | Thymoquinone |
| GSH | Glutathione |
| SLNs | solid lipid nanoparticles |
| NLCs | nanostructured lipid carriers |
| LNPs | lignin nanoparticles |
| PLGA-TPGSNPs | PLGA-TPGS nanoparticles |
| AUR-SLNs | AUR-loaded solid lipid nanoparticles |
| HA | hyaluronic acid |
| EGCG | epigallocatechin-3-gallate |
| GA | glycyrrhizic acid |
| IL-ME | ionic liquid microemulsion |
| O/W | oil-in-water |
| AuNPs | gold nanoparticles |
| AgNPs | silver nanoparticles |
| PVA | polyvinyl alcohol |
| PVP | polyvinylpyrrolidone |
| TP | a-tocopheryl phosphate |
| T2P | di-a-tocopheryl phosphate |
| HPMC | hydroxypropyl methyl cellulose |
| mTYR | Mushroom tyrosinase |
| hTYR | human tyrosinase |
| L-DOPA | Levodopa |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| ABTS | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| SRSA | superoxide anion radical scavenging |
| FRAP | ferric ion reducing/antioxidant power |
| B16 | mouse melanoma cell line |
| A375 | human melanoma cell line |
| WT | wild-type |
References
- Del Bino, S.; Duval, C.; Bernerd, F. Clinical and Biological Characterization of Skin Pigmentation Diversity and Its Consequences on UV Impact. Int. J. Mol. Sci. 2018, 19, 2668. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, A.; Wairkar, S. Management of Hyperpigmentation: Current Treatments and Emerging Therapies. Pigment. Cell Melanoma Res. 2021, 34, 1000–1014. [Google Scholar] [CrossRef] [PubMed]
- Mpofana, N.; Abrahamse, H. Natural Options for Management of Melasma, a Review. J. Cosmet. Laser Ther. 2018, 20, 470–481. [Google Scholar]
- Espósito, A.C.C.; Cassiano, D.P.; da Silva, C.N.; Lima, P.B.; Dias, J.A.F.; Hassun, K.; Bagatin, E.; Miot, L.D.B.; Miot, H.A. Update on Melasma—Part I: Pathogenesis. Dermatol. Ther. 2022, 12, 1967–1988. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Namasivayam, V.; Manickam, M.; Jung, S.H. Inhibitors of Melanogenesis: An Updated Review. J. Med. Chem. 2018, 61, 7395–7418. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Fang, Z.; Zhang, P. The Melanin Inhibitory Effect of Plants and Phytochemicals: A Systematic Review. Phytomedicine 2022, 107, 154449. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, L.; Li Ting, Z.; Li, B. Screening and identification of natural inhibitors of tyrosinase from Ampelopsis grossedentata using ultra-filtration afinity-liquid chromatograpny-mass spectrometry. Chin. Herb. Med. 2021, 52, 6796–6805. [Google Scholar]
- Wang, Y.; Yuan, M.; Zhang, L.; Zhang, Y.; Liao, P.; Yao, Z. Research progress on antioxidant actions and related mechanisms of quercetin. Acta Nutr. Sin. 2022, 44, 204–208. [Google Scholar]
- Liu, S.; Jin, L.; Meng, X. Study on the effect and mechanism of resveratrol on melanogenesis in mouse melanoma cells B16. Mod. Prev. Med. 2017, 44, 4147–4150. [Google Scholar]
- Ferreira, A.M.; de Souza, A.A.; Koga, R.d.C.R.; Sena, I.d.S.; Matos, M.d.J.S.; Tomazi, R.; Ferreira, I.M.; Carvalho, J.C.T. Anti-Melanogenic Potential of Natural and Synthetic Substances: Application in Zebrafish Model. Molecules 2023, 28, 1053. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; Zhu, P.; Hu, Y.; Liu, S.; Zhao, R.; Fan, Q.; Su, Z. Research status on animal models for melasma. Chin. J. Exp. Tradit. Med. Formulae 2020, 200–208. [Google Scholar] [CrossRef]
- D’Souza, A.; Shegokar, R. Nanostructured Lipid Carriers (NLCs) for Drug Delivery: Role of Liquid Lipid (Oil). Curr. Drug Deliv. 2021, 18, 249–270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liao, Y.; Liao, W.; Li, R. Curcumin solid lipid nanoparticles and microcapsules: Comparative study on preparation, characterization and pharmacokinetics. Chin. Herb. Med. 2023, 54, 1386–1396. [Google Scholar]
- Yan, Q.; Xue, Y.; Li, F.; Qin, Z.; Dai, B.; Yuan, H. Preparation and in vitro evaluation of a novel ionic liquid microemulsion of glycyrrhizic acid. Chin. Herb. Med. 2023, 54, 62–71. [Google Scholar]
- Xing, M.; Wang, X.; Zhao, L.; Zhou, Z.; Liu, H.; Wang, B.; Cheng, A.; Zhang, S.; Gao, Y. Novel Dissolving Microneedles Preparation for Synergistic Melasma Therapy: Combined Effects of Tranexamic Acid and Licorice Extract. Int. J. Pharm. 2021, 600, 120406. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wolfe, S.; Laughter, M.R.; Sadeghpour, M. The Use of Botanical Extracts in East Asia for Treatment of Hyperpigmentation: An Evidenced-Based Review. J. Drugs Dermatol. 2020, 19, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Ebanks, J.P.; Wickett, R.R.; Boissy, R.E. Mechanisms Regulating Skin Pigmentation: The Rise and Fall of Complexion Coloration. Int. J. Mol. Sci. 2009, 10, 4066–4087. [Google Scholar] [CrossRef] [PubMed]
- Duval, C.; Cohen, C.; Chagnoleau, C.; Flouret, V.; Bourreau, E.; Bernerd, F. Key Regulatory Role of Dermal Fibroblasts in Pigmentation as Demonstrated Using a Reconstructed Skin Model: Impact of Photo-Aging. PLoS ONE 2014, 9, e114182. [Google Scholar] [CrossRef] [PubMed]
- Handel, A.C.; Lima, P.B.; Tonolli, V.M.; Miot, L.D.B.; Miot, H.A. Risk Factors for Facial Melasma in Women: A Case–Control Study. Br. J. Dermatol. 2014, 171, 588–594. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Hearing, V.J. Physiological Factors That Regulate Skin Pigmentation. BioFactors 2009, 35, 193–199. [Google Scholar] [CrossRef]
- Le, L.; Sirés-Campos, J.; Raposo, G.; Delevoye, C.; Marks, M.S. Melanosome Biogenesis in the Pigmentation of Mammalian Skin. Integr. Comp. Biol. 2021, 61, 1517–1545. [Google Scholar] [CrossRef]
- Seiberg, M. Keratinocyte-Melanocyte Interactions During Melanosome Transfer: Melanosome Transfer. Pigment Cell Res. 2001, 14, 236–242. [Google Scholar] [CrossRef]
- Seiberg, M.; Paine, C.; Sharlow, E.; Eisinger, M.; Shapiro, S.S.; Andrade-Gordon, P.; Costanzo, M. Inhibition of Melanosome Transfer Results in Skin Lightening1. J. Investig. Dermatol. 2000, 115, 162–167. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K. Chemistry of Mixed Melanogenesis—Pivotal Roles of Dopaquinone. Photochem. Photobiol. 2008, 84, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, M.V. Signaling Pathways in Melanosome Biogenesis and Pathology. Int. J. Biochem. Cell Biol. 2010, 42, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Cassiano, D.P.; Espósito, A.C.C.; da Silva, C.N.; Lima, P.B.; Dias, J.A.F.; Hassun, K.; Miot, L.D.B.; Miot, H.A.; Bagatin, E. Update on Melasma—Part II: Treatment. Dermatol. Ther. 2022, 12, 1989–2012. [Google Scholar] [CrossRef]
- He, L. The Guide for Topical Use of Skin Lightening Cosmetics in the Treatment of Melasma. Chin. J. Dermatol. Venereol. 2022, 36, 123–127. [Google Scholar]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A Comprehensive Review on Tyrosinase Inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Bickers, D.R.; Athar, M. Oxidative Stress in the Pathogenesis of Skin Disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef]
- Qi, J.; Dong, F. The Relevant Targets of Anti-Oxidative Stress: A Review. J. Drug Target. 2021, 29, 677–686. [Google Scholar] [CrossRef]
- Choubey, V.; Sarkar, R.; Garg, V.; Kaushik, S.; Ghunawat, S.; Sonthalia, S. Role of Oxidative Stress in Melasma: A Prospective Study on Serum and Blood Markers of Oxidative Stress in Melasma Patients. Int. J. Dermatol. 2017, 56, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Devadasan, S.; Choubey, V.; Goswami, B. Melatonin and Oxidative Stress in Melasma—An Unexplored Territory; A Prospective Study. Int. J. Dermatol. 2020, 59, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Qu, L.; Li, H.; He, J.; Wang, L.; Fang, Y.; Yan, X.; Yang, Q.; Peng, B.; Wu, W. Advances in Biomedical Functions of Natural Whitening Substances in the Treatment of Skin Pigmentation Diseases. Pharmaceutics 2022, 14, 2308. [Google Scholar] [CrossRef]
- Nahar, L.; Al-Groshi, A.; Kumar, A.; Sarker, S.D. Arbutin: Occurrence in Plants, and Its Potential as an Anticancer Agent. Molecules 2022, 27, 8786. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, P.; Liang, G.; Luo, X.; Zhou, D. Progress in development of three kinds of arbutin product. China Surfactant Deterg. Cosmet. 2015, 45, 529–532. [Google Scholar]
- He, D.; Wu, F.; Xu, X.; Liao, Y.; Feng, H. Research of effect of arbutin on chloasma in guinea pigs and mechanism. Chin. J. Mod. Med. 2018, 28, 6–10. [Google Scholar]
- Zhang, F.; Wu, J.; Wang, G.; Xing, S. Whitening effect of a-arbutin and deoxyarbutin and safety evaluation: A review of recent studies. J. Environ. Health 2018, 35, 370–375. [Google Scholar]
- Sugimoto, K.; Nishimura, T.; Nomura, K.; Sugimoto, K.; Kuriki, T. Inhibitory Effects of A-Arbutin on Melanin Synthesis in Cultured Human Melanoma Cells and a Three-Dimensional Human Skin Model. Biol. Pharm. Bull. 2004, 27, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Shen, J.; Zhang, R. Effects of azela ic acida on melan in synthesis of human melanocyte and mouse B16 melanoma cells in vitrio. Acta Univ. Med. Anhui 2006, 41, 503–505. [Google Scholar]
- Fitton, A.; Goa, K.L. Azelaic Acid: A Review of Its Pharmacological Properties and Therapeutic Efficacy in Acne and Hyperpigmentary Skin Disorders. Drugs 1991, 41, 780–798. [Google Scholar] [CrossRef]
- Mayer-da-Silva, A.; Gollnick, H.; Detmar, M.; Gassmüller, J.; Parry, A.; Müller, R.; Orfanos, C.E. Effects of Azelaic Acid on Sebaceous Gland, Sebum Excretion Rate and Keratinization Pattern in Human Skin. An in Vivo and in Vitro Study. Acta Derm. Venereol. Suppl. 1989, 143, 20–30. [Google Scholar]
- Peng, G.; Li, Y.; He, S.; Wang, Y.; Zhao, H. Research on whitening effect of supramolecular azelaic acid on zebrafish model. Dly. Chem. Sci. 2022, 45, 36–39. [Google Scholar]
- Berlitz, S.J.; De Villa, D.; Inácio, L.A.M.; Davies, S.; Zatta, K.C.; Guterres, S.S.; Külkamp-Guerreiro, I.C. Azelaic Acid-Loaded Nanoemulsion with Hyaluronic Acid—A New Strategy to Treat Hyperpigmentary Skin Disorders. Drug Dev. Ind. Pharm. 2019, 45, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Radha, M.H.; Laxmipriya, N.P. Evaluation of Biological Properties and Clinical Effectiveness of Aloe Vera: A Systematic Review. J. Tradit. Complement. Med. 2015, 5, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Gómez-Serranillos, M.P. Pharmacological Update Properties of Aloe Vera and Its Major Active Constituents. Molecules 2020, 25, 1324. [Google Scholar] [CrossRef] [PubMed]
- Mikayoulou, M.; Mayr, F.; Temml, V.; Pandian, A.; Vermaak, I.; Chen, W.; Komane, B.; Stuppner, H.; Viljoen, A. Anti-Tyrosinase Activity of South African Aloe Species and Isolated Compounds Plicataloside and Aloesin. Fitoterapia 2021, 150, 104828. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Liu, D.; Wu, X.; Ding, W.; Wan, J. Effects of Seven Compounds from Aloe barbadensis Mill on Activities of Mushroom Tyrosinase. New Chin. Med. Clin. Pharmacol. 2013, 24, 114–117. [Google Scholar]
- Wahab, S.; Annadurai, S.; Abullais, S.S.; Das, G.; Ahmad, W.; Ahmad, M.F.; Kandasamy, G.; Vasudevan, R.; Ali, M.S.; Amir, M. Glycyrrhiza Glabra (Licorice): A Comprehensive Review on Its Phytochemistry, Biological Activities, Clinical Evidence and Toxicology. Plants 2021, 10, 2751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, X.; Zhong, B.; Liao, Q.; Wang, X.; Xie, Y.; He, X. Review on the Diverse Biological Effects of Glabridin. Drug Des. Dev. Ther. 2023, 17, 15–37. [Google Scholar] [CrossRef]
- Ding, L.; Cui, B.; Wang, Y.; Li, D.; Zhang, L.; Liu, J.; Bai, W. Evaluation of synergistic treatment of chloasma with glabridin and oxyresveratrol. Fine Chem. Ind. 2020, 37, 2035–2040. [Google Scholar]
- Daneshmand, S.; Jaafari, M.R.; Movaffagh, J.; Malaekeh-Nikouei, B.; Iranshahi, M.; Seyedian Moghaddam, A.; Tayarani Najaran, Z.; Golmohammadzadeh, S. Preparation, Characterization, and Optimization of Auraptene-Loaded Solid Lipid Nanoparticles as a Natural Anti-Inflammatory Agent: In Vivo and in Vitro Evaluations. Colloids Surf. B Biointerfaces 2018, 164, 332–339. [Google Scholar] [CrossRef]
- Charmforoshan, E.; Karimi, E.; Oskoueian, E.; Iranshahi, M. Antibacterial, Antioxidant and Melanogenesis Inhibitory Activity of Auraptene, a Coumarin from Ferula Szowitsiana Root. Nutr. Cancer 2022, 74, 1829–1836. [Google Scholar] [CrossRef]
- Ratz-Łyko, A.; Arct, J. Resveratrol as an Active Ingredient for Cosmetic and Dermatological Applications: A Review. J. Cosmet. Laser Ther. 2019, 21, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Seo, J.O.; Baek, S.-H.; Kim, S.Y. Inhibitory Effects of Resveratrol on Melanin Synthesis in Ultraviolet B-Induced Pigmentation in Guinea Pig Skin. Biomol. Ther. 2014, 22, 35–40. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, J.; Wang, X.; Xia, X.; Zhu, L.; Feng, T. Research Progress of Bioactive Compounds in Pomelo Peel. Chin. Fruit Veg. 2023, 43, 42–48. [Google Scholar]
- Jeon, H.-J.; Kim, K.; Kim, C.; Kim, M.-J.; Kim, T.-O.; Lee, S.-E. Molecular Mechanisms of Anti-Melanogenic Gedunin Derived from Neem Tree (Azadirachta Indica) Using B16F10 Mouse Melanoma Cells and Early-Stage Zebrafish. Plants 2021, 10, 330. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, J.; Liu, L.; Shi, Y.; Sha, T.; Wang, C.; Wang, B.; Abdulmalik, K.; Liu, J. The pharmacological effects and current situation of clinical application of Astragalus and its effective components. Shaanxi Tradit. Chin. Med. 2021, 42, 1138–1141+1146. [Google Scholar]
- Tayier, N.; Qin, N.-Y.; Zhao, L.-N.; Zeng, Y.; Wang, Y.; Hu, G.; Wang, Y.-Q. Theoretical Exploring of a Molecular Mechanism for Melanin Inhibitory Activity of Calycosin in Zebrafish. Molecules 2021, 26, 6998. [Google Scholar] [CrossRef]
- Niu, Z.; Ma, L.; Yao, T.; Ding, L.; Qiu, F.; Zhang, D. Research progress on chemical compositions and pharmacological effects of Inulae Flos. Drug Eval. Res. 2022, 45, 2591–2601. [Google Scholar]
- Lee, I.S.; Kim, J.H. Antimelanogenic Activity of Patuletin from Inula Japonica Flowers in B16F10 Melanoma Cells and Zebrafish Embryos. Nat. Prod. Res. 2022, 36, 4451–4454. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Nanda, D.K. Potential of Curcumin nanoemulsion as antimicrobial and wound healing agent in burn wound infection. Burns 2023, 49, 1003–1016. [Google Scholar] [CrossRef]
- Jeon, H.-J.; Kim, K.; Kim, C.; Lee, S.-E. Antimelanogenic Effects of Curcumin and Its Dimethoxy Derivatives: Mechanistic Investigation Using B16F10 Melanoma Cells and Zebrafish (Danio rerio) Embryos. Foods 2023, 12, 926. [Google Scholar] [CrossRef]
- Jin, Y.; Ying, T. Primary Study on the Biological Activities of Hibiscus Fower. Chin. J. Food Sci. 2008, 3, 37–41. [Google Scholar]
- Yang, J.-E.; Ngo, H.T.T.; Hwang, E.; Seo, S.A.; Park, S.W.; Yi, T.-H. Dietary Enzyme-Treated Hibiscus Syriacus L. Protects Skin against Chronic UVB-Induced Photoaging via Enhancement of Skin Hydration and Collagen Synthesis. Arch. Biochem. Biophys. 2019, 662, 190–200. [Google Scholar] [CrossRef]
- Karunarathne, W.A.H.M.; Molagoda, I.M.N.; Park, S.R.; Kim, J.W.; Lee, O.-K.; Kwon, H.Y.; Oren, M.; Choi, Y.H.; Ryu, H.W.; Oh, S.-R. Anthocyanins from Hibiscus Syriacus L. Inhibit Melanogenesis by Activating the ERK Signaling Pathway. Biomolecules 2019, 9, 645. [Google Scholar] [CrossRef]
- Dong, X.; Li, M.; Gu, H.; Zhu, Y.; Gu, X. Advances in pharmacological effects of jujuboside B. China J. Chin. Mater. Medica 2023, 48, 4295–4301. [Google Scholar]
- Molagoda, I.M.N.; Lee, K.-T.; Athapaththu, A.M.G.K.; Choi, Y.-H.; Hwang, J.; Sim, S.-J.; Kang, S.; Kim, G.-Y. Flavonoid Glycosides from Ziziphus Jujuba Var. Inermis (Bunge) Rehder Seeds Inhibit α-Melanocyte-Stimulating Hormone-Mediated Melanogenesis. Int. J. Mol. Sci. 2021, 22, 7701. [Google Scholar] [CrossRef]
- An, X.; Lv, J.; Wang, F. Pterostilbene Inhibits Melanogenesis, Melanocyte Dendricity and Melanosome Transport through cAMP/PKA/CREB Pathway. Eur. J. Pharmacol. 2022, 932, 175231. [Google Scholar] [CrossRef]
- Hseu, Y.-C.; Vudhya Gowrisankar, Y.; Wang, L.-W.; Zhang, Y.-Z.; Chen, X.-Z.; Huang, P.-J.; Yen, H.-R.; Yang, H.-L. The in Vitro and in Vivo Depigmenting Activity of Pterostilbene through Induction of Autophagy in Melanocytes and Inhibition of UVA-Irradiated α-MSH in Keratinocytes via Nrf2-Mediated Antioxidant Pathways. Redox Biol. 2021, 44, 102007. [Google Scholar] [CrossRef]
- Cavalcanti, G.R.; Duarte, F.I.C.; Converti, A.; De Lima, Á.A.N. Ferulic Acid Activity in Topical Formulations: Technological and Scientific Prospecting. Curr. Pharm. Des. 2021, 27, 2289–2298. [Google Scholar] [CrossRef]
- Stompor-Gorący, M.; Machaczka, M. Recent Advances in Biological Activity, New Formulations and Prodrugs of Ferulic Acid. Int. J. Mol. Sci. 2021, 22, 12889. [Google Scholar] [CrossRef]
- Ju, L.; Wen, X.; Wang, C.; Wei, Y.; Peng, Y.; Ding, Y.; Feng, L.; Shu, L.; Salidroside, A. Natural Antioxidant, Improves β-Cell Survival and Function via Activating AMPK Pathway. Front. Pharmacol. 2017, 8, 749. [Google Scholar] [CrossRef]
- Peng, L.-H.; Liu, S.; Xu, S.-Y.; Chen, L.; Shan, Y.-H.; Wei, W.; Liang, W.-Q.; Gao, J.-Q. Inhibitory Effects of Salidroside and Paeonol on Tyrosinase Activity and Melanin Synthesis in Mouse B16F10 Melanoma Cells and Ultraviolet B-Induced Pigmentation in Guinea Pig Skin. Phytomedicine 2013, 20, 1082–1087. [Google Scholar] [CrossRef]
- Kim, Y.-J. Antimelanogenic and Antioxidant Properties of Gallic Acid. Biol. Pharm. Bull. 2007, 30, 1052–1055. [Google Scholar] [CrossRef]
- Su, T.-R.; Lin, J.-J.; Tsai, C.-C.; Huang, T.-K.; Yang, Z.-Y.; Wu, M.-O.; Zheng, Y.-Q.; Su, C.-C.; Wu, Y.-J. Inhibition of Melanogenesis by Gallic Acid: Possible Involvement of the PI3K/Akt, MEK/ERK and Wnt/β-Catenin Signaling Pathways in B16F10 Cells. Int. J. Mol. Sci. 2013, 14, 20443–20458. [Google Scholar] [CrossRef]
- Mahmoud, Y.K.; Abdelrazek, H.M.A. Cancer: Thymoquinone Antioxidant/pro-Oxidant Effect as Potential Anticancer Remedy. Biomed. Pharmacother. 2019, 115, 108783. [Google Scholar] [CrossRef]
- Ince, S.; Kucukkurt, I.; Demirel, H.H.; Turkmen, R.; Zemheri, F.; Akbel, E. The role of thymoquinone as antioxidant protection on oxidative stress induced by imidacloprid in male and female Swiss albino mice. Toxicol. Environ. Chem. 2013, 95, 318–329. [Google Scholar] [CrossRef]
- Ince, S.; Kucukkurt, I.; Demirel, H.H.; Turkmen, R.; Sever, E. Thymoquinone attenuates cypermethrin induced oxidative stress in Swiss albino mice. Pestic. Biochem. Physiol. 2012, 104, 229–235. [Google Scholar] [CrossRef]
- An, S.M.; Koh, J.-S.; Boo, Y.C. p-Coumaric Acid Not Only Inhibits Human Tyrosinase Activity in Vitro but Also Melanogenesis in Cells Exposed to UVB. Phytother. Res. 2010, 24, 1175–1180. [Google Scholar] [CrossRef]
- Zaidi, K.U.; Ali, S.A.; Ali, A.; Naaz, I. Natural Tyrosinase Inhibitors: Role of Herbals in the Treatment of Hyperpigmentary Disorders. Mini Rev. Med. Chem. 2019, 19, 796–808. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; El-Din, M.I.G.; Hritcu, L.; Eldahshan, O.A. Insights on the Inhibitory Power of Flavonoids on Tyrosinase Activity: A Survey from 2016 to 2021. Molecules 2021, 26, 7546. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, C.; Gong, X.; Xiao, Z.; Shi, X.; Zheng, X.; Pan, Y.; Yi, C. Synthesis of transdermal aloesin loaded zinc oxide nanoparticles and its inhibitory effect on the activity of tyrosinase. J. Biomed. Eng. 2019, 36, 254–259. [Google Scholar]
- Wu, S.; Wang, J.; Kai, K.; Zhu, N.; Yang, N.; Ji, S.; Zhang, S.; Yang, A. Study on Ultrasonic Extraction Technology and Antioxidant Activity of Bletillastriata Polysaccharides Extraction Process. World Tradit. Chin. Med. 2020, 15, 2556–2560. [Google Scholar]
- Chen, H. Study on extraction technology and antioxidative activity of polysaccharides and triterpenoids from peel of Poria cocos. J. Biol. 2015, 32, 48–52. [Google Scholar]
- Hu, S.; Huang, J.; Pei, S.; Ouyang, Y.; Ding, Y.; Jiang, L.; Lu, J.; Kang, L.; Huang, L.; Xiang, H. Ganoderma Lucidum Polysaccharide Inhibits UVB-Induced Melanogenesis by Antagonizing cAMP/PKA and ROS/MAPK Signaling Pathways. J. Cell. Physiol. 2019, 234, 7330–7340. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.-N.; Li, W.; Mehmood, S.; Pan, W.-J.; Wu, Q.-X.; Chen, Y.; Lu, Y.-M. Effect of Polysaccharide FMP-1 from Morchella Esculenta on Melanogenesis in B16F10 Cells and Zebrafish. Food Funct. 2018, 9, 5007–5015. [Google Scholar] [CrossRef]
- Li, X.; Chen, C.; Leng, A.; Qu, J. Advances in the Extraction, Purification, Structural Characteristics and Biological Activities of Eleutherococcus Senticosus Polysaccharides: A Promising Medicinal and Edible Resource with Development Value. Front. Pharmacol. 2021, 12, 753007. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Liu, W.; Liang, L.; Yang, C.; Yan, J.; Gou, X.; He, J. Antioxidant and antiproliferative activities of total flavonoids extracted from Selaginella pulvinata (Hook, et Grev.) Maxim. Food Ind. Sci. Technol. 2017, 38, 37–41. [Google Scholar]
- Zhang, L.; Liu, J.; Li, C.; Zhang, Y.; Song, Y.; Cheng, G. Advance in research of total flavonoids from Selaginella tamariscina. Chin. Med. 2022, 17, 472–476. [Google Scholar]
- Qu, W.; Breksa, A.P., III; Pan, Z.; Ma, H. Quantitative Determination of Major Polyphenol Constituents in Pomegranate Products. Food Chem. 2012, 132, 1585–1591. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, R.; Zhou, J.; Xu, X.; Sun, Z.; Li, J.; Chen, X.; Li, Z.; Yan, X.; Zhao, D.; et al. Salicylic Acid in Ginseng Root Alleviates Skin Hyperpigmentation Disorders by Inhibiting Melanogenesis and Melanosome Transport. Eur. J. Pharmacol. 2021, 910, 174458. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ran, M.; Wang, M.; Liu, X.; Liu, S.; Ruan, Z.; Jin, N. Evaluation of Antityrosinase Activity and Mechanism, Antioxidation, and UV Filter Properties of Theaflavin. Biotechnol. Appl. Biochem. 2022, 69, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zeng, H.; Wen, X.; Jiang, L.; Fu, C.; Hu, Y.; Lei, X.; Zhang, L.; Yu, X.; Yang, S.; et al. Selaginellin Inhibits Melanogenesis via the MAPK Signaling Pathway. J. Nat. Prod. 2022, 85, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J. Pharmacological Properties Oft Anshinones, the Natural Products from Salviamiltiorrhiza. BMC Complement. Altern. Med. 2018. [Google Scholar]
- Rodrigues, C.V.; Pintado, M. Hesperidin from Orange Peel as a Promising Skincare Bioactive: An Overview. Int. J. Mol. Sci. 2024, 25, 1890. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yin, Z.; Yu, N.; Ou, S.; Wang, X.; Li, H.; Zhu, H. Tanshinone Alleviates UVA-Induced Melanogenesis in Melanocytes via the Nrf2-Regulated Antioxidant Defense Signaling Pathway. Curr. Mol. Med. 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, Q.; Liu, J.; Cheng, Y.; Yan, X.; Wang, J.; Hui, A.; Zhang, W. Comprehensive Extraction and Antioxidant Activity Research of Polysaccharides and Flavonoids from Ginkgo biloba Leaf Residue. J. Henan Agric. Sci. 2023, 52, 171. [Google Scholar]
- Kazi, M.A.; Sahito, R.; Abbas, Q.; Ullah, S.; Majid, A.; Phull, A.R.; Rahman, M.M.; Kim, S.J. The Inhibitory Effect of Polyphenon 60 from Green Tea on Melanin and Tyrosinase in Zebrafish and A375 Human Melanoma Cells. Evid. Based Complement. Altern. Med. 2022, 7739023. [Google Scholar] [CrossRef] [PubMed]
- Je, J.-G.; Jiang, Y.; Heo, J.-H.; Li, X.; Jeon, Y.-J.; Ryu, B.-M. Mitigative Effects of PFF-A Isolated from Ecklonia Cava on Pigmentation in a Zebrafish Model and Melanogenesis in B16F10 Cells. Mar. Drugs 2022, 20, 123. [Google Scholar] [CrossRef]
- Li, Z.; Yan, X.; Zhao, X.; Wang, X.; Zhang, Q.; Yin, Z. Optimization of Ultrasonic-Assisted Extraction of Polyphenols from Pomegranate Root Bark and Its Antioxidant Capacity. Food Ind. Sci. Technol. 2019, 40, 136–142. [Google Scholar]
- Bai, L.; Wu, Y.; Dong, H.; Shi, Y.; Wang, H.; Yu, L. Comparative study on flavonoids content and antioxidant activity of 23 kinds of Citrus reticulata Blanco peels. J. Food Saf. Qual. Insp. 2023, 14, 133–142. [Google Scholar]
- Wu, Y.; Wang, S.; Tang, W.; Wang, Q.L.J.; Zhang, B. Phenolic Ketone Contents and Antioxidant, Antibacterial Properties and Inhibitory Tyrosinase Activity of the Ethyl Acetate Extracted from Calendula officinalis L. Int. Syst. Agric. Sci. Andtechnol. 2023, 44, 347–355. [Google Scholar]
- Mohd Sakeh, N.; Md Razip, N.N.; Mohd Ma’in, F.I.; Abdul Bahari, M.N.; Latif, N.; Akhtar, M.N.; Balia Yusof, Z.N.; Ahmad, S. Melanogenic Inhibition and Toxicity Assessment of Flavokawain A and B on B16/F10 Melanoma Cells and Zebrafish (Danio Rerio). Molecules 2020, 25, 3403. [Google Scholar] [CrossRef] [PubMed]
- Pavic, A.; Ilic-Tomic, T.; Glamočlija, J. Melanogenic Inhibition and Toxicity Assessment of Flavokawain A and B on B16/F10 Melanoma Cells and Zebrafish (Danio Rerio). J. Fungi 2021, 7. [Google Scholar]
- Huang, C.-Y.; Liu, I.-H.; Huang, X.-Z.; Chen, H.-J.; Chang, S.-T.; Chang, M.-L.; Ho, Y.-T.; Chang, H.-T. Antimelanogenesis Effects of Leaf Extract and Phytochemicals from Ceylon Olive (Elaeocarpus serratus) in Zebrafish Model. Pharmaceutics 2021, 13, 1059. [Google Scholar] [CrossRef]
- Ting, Y.; Hu, Y.-T.; Hu, J.-Y.; Chang, W.-C.; Huang, Q.; Hsieh, S.-C. Nanoemulsified Adlay Bran Oil Reduces Tyrosinase Activity and Melanin Synthesis in B16F10 Cells and Zebrafish. Food Sci. Nutr. 2019, 7, 3216–3223. [Google Scholar] [CrossRef]
- Rodboon, T.; Sirilun, S.; Okada, S.; Kariya, R.; Chontananarth, T.; Suwannalert, P. Modified Riceberry Rice Extract Suppresses Melanogenesis-Associated Cell Differentiation through Tyrosinase-Mediated MITF Downregulation on B16 Cells and in Vivo Zebrafish Embryos. Res. Pharm. Sci. 2020, 15, 491–502. [Google Scholar]
- Jain, N.; Valli, K.; Devi, V. Importance of Novel Drug Delivery Systems in Herbal Medicines. Pharmacogn. Rev. 2010, 4, 27. [Google Scholar] [CrossRef]
- Rachmin, I.; Ostrowski, S.M.; Weng, Q.Y.; Fisher, D.E. Topical Treatment Strategies to Manipulate Human Skin Pigmentation. Adv. Drug Deliv. Rev. 2020, 153, 65–71. [Google Scholar] [CrossRef]
- Ganesan, P.; Choi, D.K. Current Application of Phytocompound-Based Nanocosmeceuticals for Beauty and Skin Therapy. Int. J. Nanomed. 2016, 11, 1987–2007. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Suys, E.J.A.; Lee, J.S.; Nguyen, D.H.; Park, K.D.; Truong, N.P. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines 2021, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Faghihi, H.; Mozafari, M.R.; Bumrungpert, A.; Parsaei, H.; Taheri, S.V.; Mardani, P.; Dehkharghani, F.M.; Pudza, M.Y.; Alavi, M. Prospects and Challenges of Synergistic Effect of Fluorescent Carbon Dots, Liposomes and Nanoliposomes for Theragnostic Applications. Photodiagn. Photodyn. Ther. 2023, 42, 103614. [Google Scholar] [CrossRef] [PubMed]
- Haddadzadegan, S.; Dorkoosh, F.; Bernkop-Schnürch, A. Oral Delivery of Therapeutic Peptides and Proteins: Technology Landscape of Lipid-Based Nanocarriers. Adv. Drug Deliv. Rev. 2022, 182, 114097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Hsiung, K.C.; Kern, C.C.; Wang, Y.; Girtle, A.L.; Xu, N.; Gems, D. Unraveling Effects of Anti-Aging Drugs on C. Elegans Using Liposomes. GeroScience 2023, 45, 1583–1603. [Google Scholar] [CrossRef]
- Mozafari, M.R. Liposomes: An overview of manufacturing techniques. Cell. Mol. Biol. Lett. 2005, 10, 711. [Google Scholar]
- Elsayed, M.M.A.; Abdallah, O.Y.; Naggar, V.F.; Khalafallah, N.M. Lipid Vesicles for Skin Delivery of Drugs: Reviewing Three Decades of Research. Int. J. Pharm. 2007, 332, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mehrdadi, S. Drug Delivery of Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs) to Target Brain Tumors. Adv. Pharm. Bull. 2023, 13, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Gorain, B.; Pandey, M.; Leng, N.H.; Yan, C.W.; Nie, K.W.; Kaur, S.J.; Marshall, V.; Sisinthy, S.P.; Panneerselvam, J.; Molugulu, N.; et al. Advanced Drug Delivery Systems Containing Herbal Components for Wound Healing. Int. J. Pharm. 2022, 617, 121617. [Google Scholar] [CrossRef] [PubMed]
- Piccinino, D.; Capecchi, E.; Tomaino, E.; Gabellone, S.; Gigli, V.; Avitabile, D.; Saladino, R. Nano-Structured Lignin as Green Antioxidant and UV Shielding Ingredient for Sunscreen Applications. Antioxidants 2021, 10, 274. [Google Scholar] [CrossRef]
- Hung, C.-F.; Fang, C.-L.; Al-Suwayeh, S.A.; Yang, S.-Y.; Fang, J.-Y. Evaluation of Drug and Sunscreen Permeation via Skin Irradiated with UVA and UVB: Comparisons of Normal Skin and Chronologically Aged Skin. J. Dermatol. Sci. 2012, 68, 135–148. [Google Scholar] [CrossRef]
- Zhu, X.; Zeng, X.; Zhang, X.; Cao, W.; Wang, Y.; Chen, H.; Wang, T.; Tsai, H.-I.; Zhang, R.; Chang, D.; et al. The Effects of Quercetin-Loaded PLGA-TPGS Nanoparticles on Ultraviolet B-Induced Skin Damages in Vivo. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.C.; Rodrigues, D.; Sequeira, J.A.D.; Pereira, I.; Simões, A.; Costa, D.; Peixoto, D.; Costa, G.; Veiga, F. Nanotechnological Breakthroughs in the Development of Topical Phytocompounds-Based Formulations. Int. J. Pharm. 2019, 572, 118787. [Google Scholar] [CrossRef] [PubMed]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: A Review Emphasizing on Particle Structure and Drug Release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Akbari, J.; Saeedi, M.; Ahmadi, F.; Hashemi, S.M.H.; Babaei, A.; Yaddollahi, S.; Rostamkalaei, S.S.; Asare-Addo, K.; Nokhodchi, A. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: A Review of the Methods of Manufacture and Routes of Administration. Pharm. Dev. Technol. 2022, 27, 525–544. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, H.; Yu, L.; Zhao, X.; Yu, X.; Fu, Y. Preparation of Resveratrol-Nanostructured Lipid Carrier and Evaluation of in vitro Antioxidant Activity. Bull. Bot. Res. 2020, 40, 623. [Google Scholar]
- Radmard, A.; Saeedi, M.; Morteza-Semnani, K.; Hashemi, S.M.H.; Nokhodchi, A. An Eco-Friendly and Green Formulation in Lipid Nanotechnology for Delivery of a Hydrophilic Agent to the Skin in the Treatment and Management of Hyperpigmentation Complaints: Arbutin Niosome (Arbusome). Colloids Surf. B Biointerfaces 2021, 201, 111616. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, H.; Kohli, K.; Kumar, V. Nano-Transfersomes as a Novel Carrier for Transdermal Delivery. Int. J. Pharm. 2013, 454, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Simrah; Hafeez, A.; Usmani, S.A.; Izhar, M.P. Transfersome, an Ultra-Deformable Lipid-Based Drug Nanocarrier: An Updated Review with Therapeutic Applications. Naunyn Schmiedeberg’s Arch. Pharmacol. 2024, 397, 639–673. [Google Scholar]
- Avadhani, K.S.; Manikkath, J.; Tiwari, M.; Chandrasekhar, M.; Godavarthi, A.; Vidya, S.M.; Hariharapura, R.C.; Kalthur, G.; Udupa, N.; Mutalik, S. Skin Delivery of Epigallocatechin-3-Gallate (EGCG) and Hyaluronic Acid Loaded Nano-Transfersomes for Antioxidant and Anti-Aging Effects in UV Radiation Induced Skin Damage. Drug Deliv. 2017, 24, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Tartaro, G.; Mateos, H.; Schirone, D.; Angelico, R.; Palazzo, G. Microemulsion Microstructure(s): A Tutorial Review. Nanomaterials 2020, 10, 1657. [Google Scholar] [CrossRef]
- Nikolaev, B.; Yakovleva, L.; Fedorov, V.; Li, H.; Gao, H.; Shevtsov, M. Nano- and Microemulsions in Biomedicine: From Theory to Practice. Pharmaceutics 2023, 15, 1989. [Google Scholar] [CrossRef]
- Souto, E.B.; Cano, A.; Martins-Gomes, C.; Coutinho, T.E.; Zielińska, A.; Silva, A.M. Microemulsions and Nanoemulsions in Skin Drug Delivery. Bioengineering 2022, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Liu, T.; Ma, H.; Tian, Y.; Li, L.; Li, Z.; Gao, M.; Zhang, J.; Tang, Z. Preparation of Essential Oil-Based Microemulsions for Improving the Solubility, pH Stability, Photostability, and Skin Permeation of Quercetin. Aaps Pharmscitech 2017, 18, 3097–3104. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, J.; Ding, Y. Gold Nanoparticle-Conjugated Nanomedicine: Design, Construction, and Structure–Efficacy Relationship Studies. J. Mater. Chem. B 2020, 8, 4813–4830. [Google Scholar] [CrossRef] [PubMed]
- Rónavári, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green Silver and Gold Nanoparticles: Biological Synthesis Approaches and Potentials for Biomedical Applications. Molecules 2021, 26, 844. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Hwang, S.J.; Kang, Y.S.; Jung, J.; Park, S.; Hong, J.E.; Park, Y.; Lee, H.-J. Synthesis of Arbutin-Gold Nanoparticle Complexes and Their Enhanced Performance for Whitening. Arch. Pharm. Res. 2019, 42, 977–989. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Yu, H.; Li, Z.; Lin, H.; Wu, F.; Tan, L.; Wang, C.; Li, P.; Liu, J. Comprehensive Phytochemicals Analysis and Anti-myocardial Ischemia Activity of Total Saponins of American Ginseng Berry. J. Food Biochem. 2022, 46, e14042. [Google Scholar] [CrossRef]
- Jiménez Pérez, Z.E.; Mathiyalagan, R.; Markus, J.; Kim, Y.-J.; Kang, H.M.; Abbai, R.; Seo, K.H.; Wang, D.; Soshnikova, V.; Yang, D.C. Ginseng-Berry-Mediated Gold and Silver Nanoparticle Synthesis and Evaluation of Their in Vitro Antioxidant, Antimicrobial, and Cytotoxicity Effects on Human Dermal Fibroblast and Murine Melanoma Skin Cell Lines. Int. J. Nanomed. 2017, 12, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Avcil, M.; Akman, G.; Klokkers, J.; Jeong, D.; Celik, A. Clinical Efficacy of Dissolvable Microneedles Armed with Anti-Melanogenic Compounds to Counter Hyperpigmentation. J. Cosmet. Dermatol. 2021, 20, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A Smart Approach and Increasing Potential for Transdermal Drug Delivery System. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef]
- Zhao, Y.; Ning, H.; Zhang, Y.; Li, X.; Li, M.; Li, Y.; Hou, W.; Wang, Y. Research progress on traditional Chinese medicine percutaneous microneedle preparation. Chin. Herb. Med. 2022, 53, 2550–2559. [Google Scholar]
- Liang, Y.; Xi, X.; Liu, Q.; Huang, P.; Li, J.; Lin, Q. Research progress on the physiological activity and application of ferulic acid and its derivatives. J. Food Sci. Biotechnol. 2018, 37, 449–454. [Google Scholar]
- Yang, B.; Du, S.; Bai, J.; Shang, K.; Lu, Y.; Li, P. Studies on transdermal delivery of ferulic acid through rat skin treated by microneedle arrays. Chin. J. Tradit. Chin. Med. 2014, 39, 4773–4777. [Google Scholar]
- Mozafari, M.R.; Javanmard, R.; Raji, M. Tocosome: Novel Drug Delivery System Containing Phospholipids and Tocopheryl Phosphates. Int. J. Pharm. 2017, 528, 381–382. [Google Scholar] [CrossRef] [PubMed]
- Mehrarya, M.; Gharehchelou, B.; Kabarkouhi, Z.; Ataei, S.; Esfahani, F.N.; Wintrasiri, M.N.; Mozafari, M.R. Functionalized Nanostructured Bioactive Carriers: Nanoliposomes, Quantum Dots, Tocosome, and Theranostic Approach. Curr. Drug Deliv. 2022, 19, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Jhan, S.; Pethe, A.M. Double-loaded liposomes encapsulating lycopene β-cyclodextrin complexes: Preparation, optimization, and evaluation. J. Liposome Res. 2020, 30, 80–92. [Google Scholar] [CrossRef]
- Baccarin, T.; Lemos-Senna, E. Potential Application of Nanoemulsions for Skin Delivery of Pomegranate Peel Polyphenols. Aaps Pharmscitech 2017, 18, 3307–3314. [Google Scholar] [CrossRef] [PubMed]
- Aung, N.N.; Pengnam, S.; Ngawhirunpat, T.; Rojanarata, T.; Patrojanasophon, P.; Opanasopit, P.; Pamornpathomkul, B. Fabrication of Polyvinyl Pyrrolidone-K90/Eudragit RL100-Based Dissolving Microneedle Patches Loaded with Alpha-Arbutin and Resveratrol for Skin Depigmentation. Biomater. Sci. 2023, 11, 4583–4601. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liao, L.; Liao, S.; Hu, Q.; Han, B.; Qiu, Y. The development of glabridin-loaded sustained microneedles and the evaluation of drug release in vitro and in vivo. Chin. J. New Drugs 2022, 31, 455–463. [Google Scholar]
- Aung, N.; Ngawhirunpat, T.; Rojanarata, T.; Patrojanasophon, P.; Opanasopit, P.; Pamornpathomkul, B. HPMC/PVP dissolving microneedles: A promising delivery platform to promote trans-epidermal delivery of alpha-arbutin for skin lightening. Aaps Pharmscitech 2020, 21, 25. [Google Scholar] [CrossRef]
- Aktary, Z.; Raymond, J.H.; Pouteaux, M.; Delmas, V.; Petit, V.; Larue, L. Derivation and Use of Cell Lines from Mouse Models of Melanoma. J. Investig. Dermatol. 2023, 143, 538–544.e2. [Google Scholar]
- Qin, Y.; Cheng, S.; Huang, J.; Zhang, J. Whitening efficacy assessment for cosmetic materials in animal model. Chin. J. Comp. Med. 2013, 23, 21–24+35. [Google Scholar]
- Liu, X.; Su, Z.; Fan, Q.; Gao, J.; Zhu, P.; Liu, S.; Zhao, R.; Wang, T. Visualization Analysis of Research Status and Trends of Melasma Treated by Chinese Medicine in Recent 20 Years Based on Citespace. J. Tradit. Chin. Med. 2020, 35, 2480–2486. [Google Scholar]
- Feng, N.; Zhu, Q. Transdermal administration of traditional Chinese medicine and functional cosmetics [M]. China Med. Sci. Technol. Press 2019, 45–71. [Google Scholar]
- Ren, Q.; Wu, H.; Jin, J. Botanical cosmetic ingredient (I) Research and development of tyrosinase inhibitors from plant extracts in skin whitening. Dly. Chem. Ind. 2021, 51, 178–185. [Google Scholar]
- Liu, S.-C.; Sheu, M.-L.; Tsai, Y.-C.; Lin, Y.-C.; Chang, C.-W.; Lai, D.-W. Attenuation of in Vitro and in Vivo Melanin Synthesis Using a Chinese Herbal Medicine through the Inhibition of Tyrosinase Activity. Phytomedicine 2022, 95, 153876. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.-S.; Yang, J.Y.; Lee, J.; Lee, Y.-R.; Kim, S.S.; Kim, G.R.; Chae, J.S.; Ahn, J.H.; Shin, D.-S.; Choi, T.-Y.; et al. A Novel Anti-Melanogenic Agent, KDZ-001, Inhibits Tyrosinase Enzymatic Activity. J. Dermatol. Sci. 2018, 89, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin Whitening Agents: Medicinal Chemistry Perspective of Tyrosinase Inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Roulier, B.; Pérès, B.; Haudecoeur, R. Advances in the Design of Genuine Human Tyrosinase Inhibitors for Targeting Melanogenesis and Related Pigmentations. J. Med. Chem. 2020, 63, 13428–13443. [Google Scholar] [CrossRef] [PubMed]
- Ghani, U. Azole Inhibitors of Mushroom and Human Tyrosinases: Current Advances and Prospects of Drug Development for Melanogenic Dermatological Disorders. Eur. J. Med. Chemistry 2022, 239, 114525. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Sun, X.; Wu, H.; Jin, J. Botanical cosmetic ingredients (V) Research and development of plant antioxidants. China Surfactant Deterg. Cosmet. 2019, 51, 817. [Google Scholar]
- Fu, W.; Wu, Z.; Zheng, R.; Yin, N.; Han, F.; Zhao, Z.; Dai, M.; Han, D.; Wang, W.; Niu, L. Inhibition Mechanism of Melanin Formation Based on Antioxidant Scavenging of Reactive Oxygen Species. Analyst 2022, 147, 2703–2711. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; He, R. Review of anti-oxidative evaluation methods for catechins and therapeutic mechanism of catechins. Tradit. Chin. Drug Res. Clin. Pharmacol. 2016, 27, 295–302. [Google Scholar]
- Amorati, R.; Valgimigli, L. Advantages and Limitations of Common Testing Methods for Antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tang, W.; Chu, B.; Chen, C.; Jin, L.A.; Zhang, Y. Whitening Efficacy of Bamboo Leaf Flavonoids Nanoparticles Based on B16 Melanoma Cell Evaluation System. Fine Chem. Ind. 2016, 33, 1375–1380. [Google Scholar]
- Liu, H.; Liao, W.; Fan, L.; Zheng, Z.; Liu, D.; Zhang, Q.-W.; Yang, A.; Liu, F. Ethanol Extract of Ophiorrhiza Pumila Suppresses Liver Cancer Cell Proliferation and Migration. Chin. Med. 2020, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Goff, P.S.; Castle, J.T.; Kohli, J.S.; Sviderskaya, E.V.; Bennett, D.C. Isolation, Culture, and Transfection of Melanocytes. Curr. Protoc. 2023, 3, e774. [Google Scholar] [CrossRef] [PubMed]
- Lajis, A.F.B. A Zebrafish Embryo as an Animal Model for the Treatment of Hyperpigmentation in Cosmetic Dermatology Medicine. Medicina 2018, 54, 35. [Google Scholar] [CrossRef] [PubMed]
- Neuffer, S.J.; Cooper, C.D. Zebrafish Syndromic Albinism Models as Tools for Understanding and Treating Pigment Cell Disease in Humans. Cancers 2022, 14, 1752. [Google Scholar] [CrossRef] [PubMed]
- Usui, Y.; Kondo, S.; Watanabe, M. Melanophore Multinucleation Pathways in Zebrafish. Dev. Growth Differ. 2018, 60, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fan, Q.; Gao, J.; Su, Z.; Zhang, J.; Zhao, B.; Zhu, P.; Wang, T. Preparation and reflection on animal models of melasma. Chin. J. Comp. Med. 2021, 31, 54–60. [Google Scholar]
- Day, C.-P.; Marchalik, R.; Merlino, G.; Michael, H. Mouse Models of UV-Induced Melanoma: Genetics, Pathology, and Clinical Relevance. Lab. Investig. 2017, 97, 698–705. [Google Scholar] [CrossRef]
- Nakano, S.; Abe, Y.; Nakajima, K.; Sano, S.; Yamamoto, O.; Wakamatsu, K.; Ito, S.; Hayashi, M.; Suzuki, T. Establishment of a Mouse Model for Post-inflammatory Hyperpigmentation. Pigment Cell Melanoma Res. 2021, 34, 101–110. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, X.; Ma, Y.; Yan, C.; Wei, X.; Zhang, L.; Jiang, H.; Ma, Y.; Zhang, S.; Xing, M.; Gao, Y. Mechanism, Formulation, and Efficacy Evaluation of Natural Products for Skin Pigmentation Treatment. Pharmaceutics 2024, 16, 1022. https://doi.org/10.3390/pharmaceutics16081022
Peng X, Ma Y, Yan C, Wei X, Zhang L, Jiang H, Ma Y, Zhang S, Xing M, Gao Y. Mechanism, Formulation, and Efficacy Evaluation of Natural Products for Skin Pigmentation Treatment. Pharmaceutics. 2024; 16(8):1022. https://doi.org/10.3390/pharmaceutics16081022
Chicago/Turabian StylePeng, Xueli, Yuning Ma, Chenxin Yan, Xiaocen Wei, Linlin Zhang, Hehe Jiang, Yuxia Ma, Suohui Zhang, Mengzhen Xing, and Yunhua Gao. 2024. "Mechanism, Formulation, and Efficacy Evaluation of Natural Products for Skin Pigmentation Treatment" Pharmaceutics 16, no. 8: 1022. https://doi.org/10.3390/pharmaceutics16081022
APA StylePeng, X., Ma, Y., Yan, C., Wei, X., Zhang, L., Jiang, H., Ma, Y., Zhang, S., Xing, M., & Gao, Y. (2024). Mechanism, Formulation, and Efficacy Evaluation of Natural Products for Skin Pigmentation Treatment. Pharmaceutics, 16(8), 1022. https://doi.org/10.3390/pharmaceutics16081022





