Pharmacometrics to Evaluate Dosing of the Patient-Friendly Ivermectin CHILD-IVITAB in Children ≥ 15 kg and <15 kg
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Pharmacometric Population PK Modeling
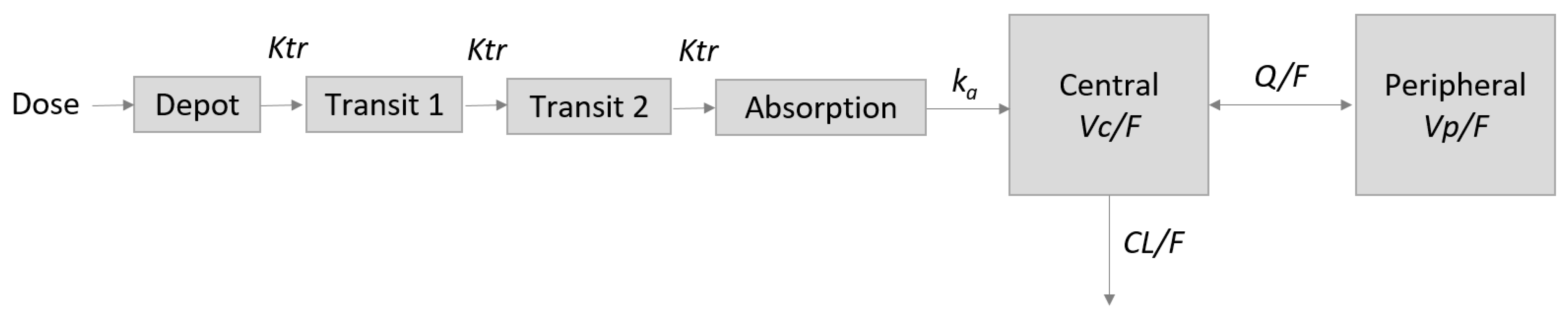
2.3. Base Pharmacokinetic (PK) Model
2.4. Alternative Investigated Model Structures
2.5. Correlation between Parameters
2.6. Investigation of a Formulation Effect and Potential Other Covariates
2.7. Evaluation of Population PK Model
2.8. Model-Based Simulations to Evaluate Dosing of CHILD-IVITAB in Persons ≥ 15 kg and Children < 15 kg
3. Results
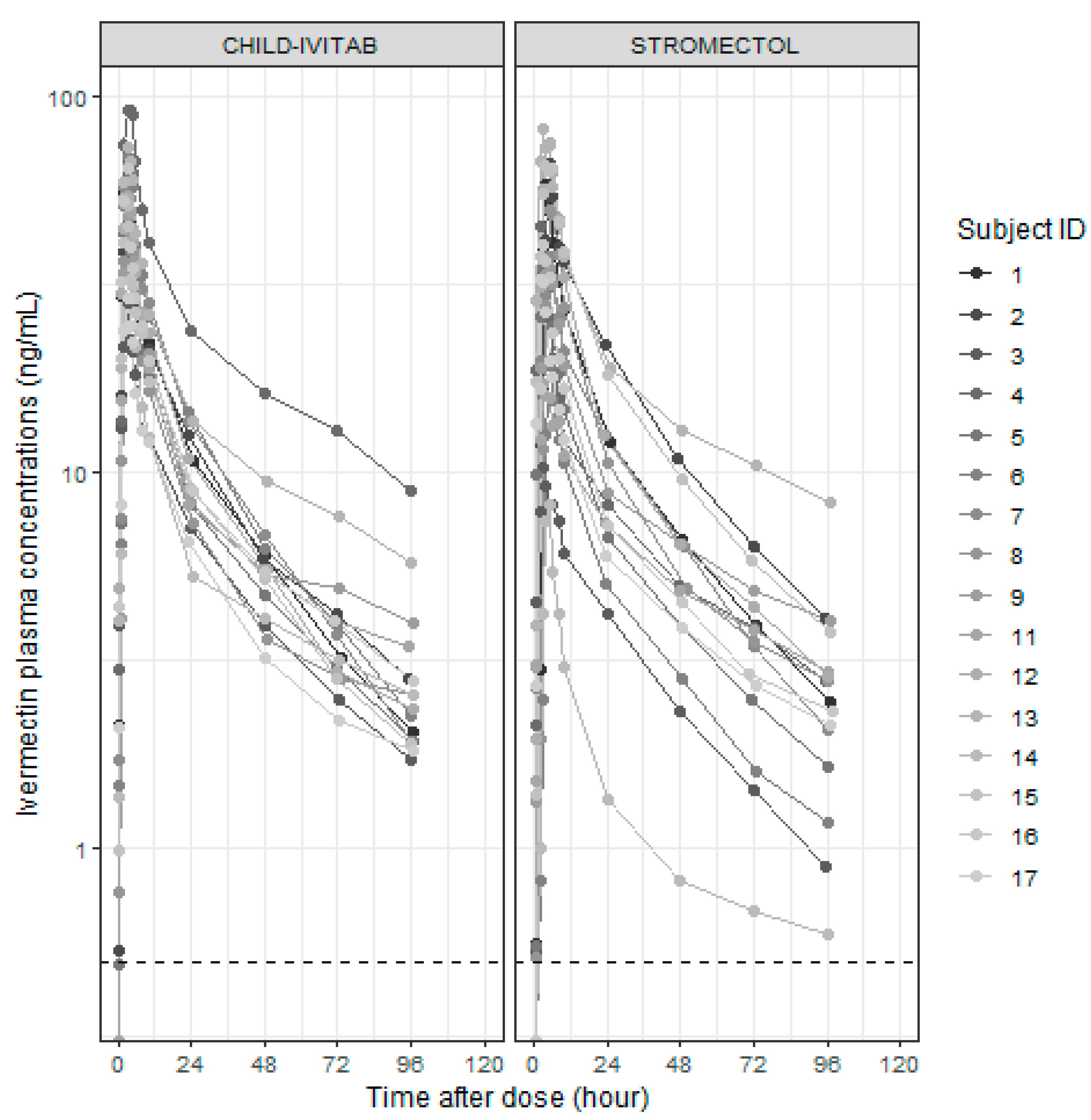
3.2. Pharmacometric Population PK Modeling
3.3. Alternative Investigated Model Structures
3.4. Investigation of a Formulation Effect and Potential Other Covariates
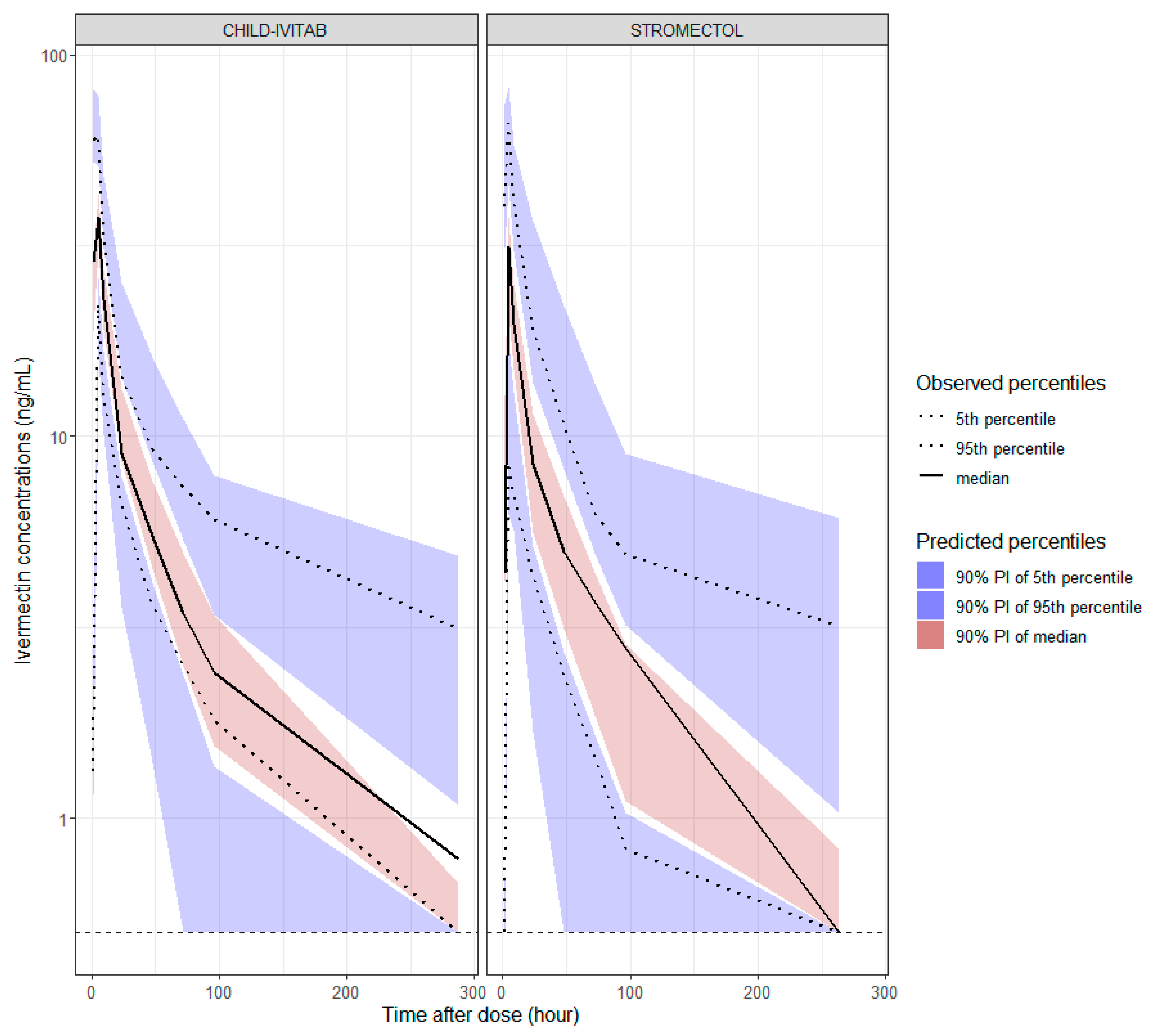
3.5. Evaluation of Population PK Model
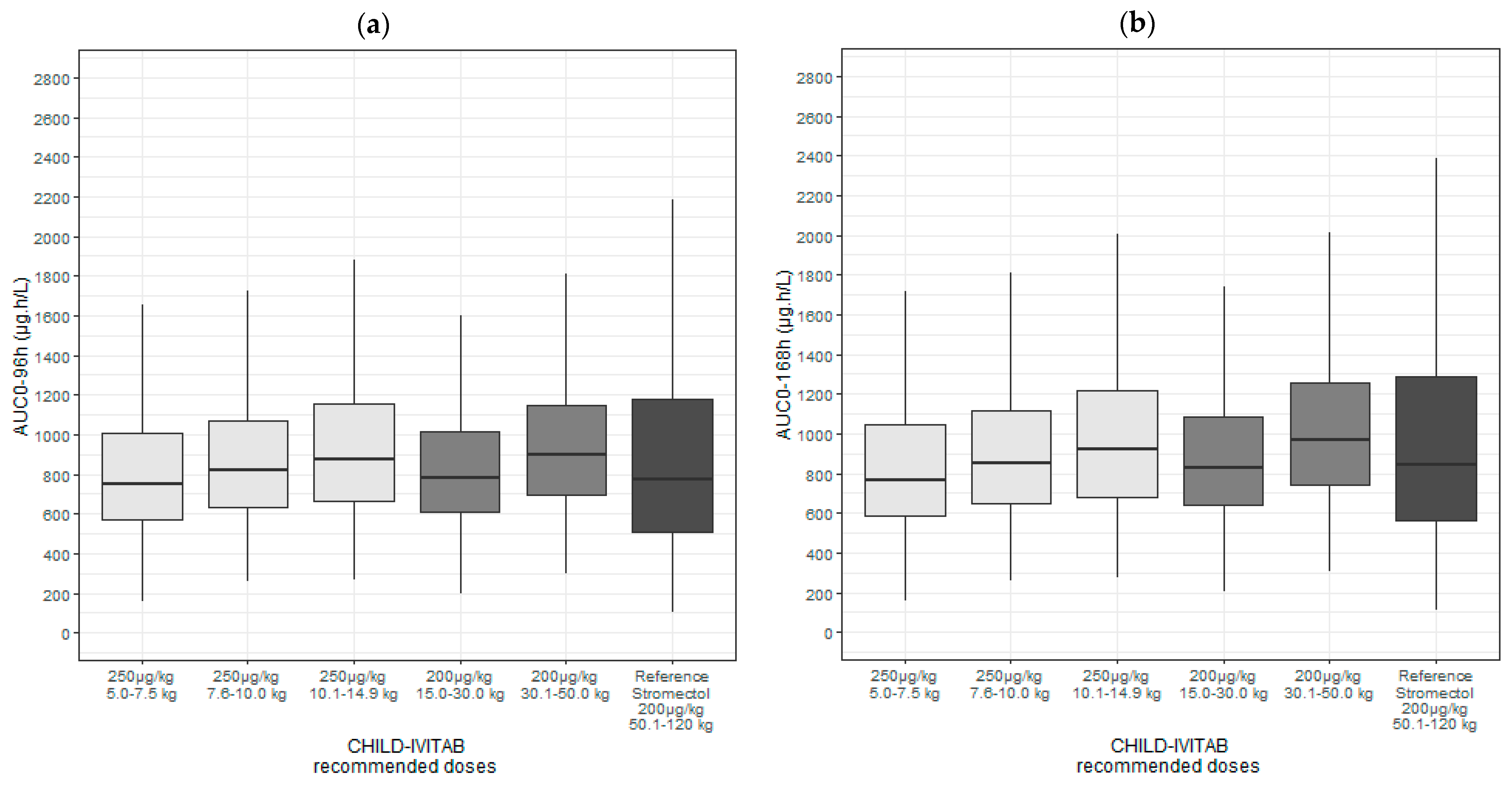
3.6. Model-Based Simulations to Evaluate Dosing of CHILD-IVITAB in Persons ≥ 15 kg and Children < 15 kg
4. Discussion
4.1. Modeling and Simulation to Facilitate Dose Selection for a Pediatric Study in Children with Scabies
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molyneux, D.; Taylor, H.R. The Discovery of Ivermectin. Trends Parasitol. 2015, 31, 1. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Model List of Essential Medicines for Children; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Sodahlon, Y. 2022 Annual Highlights. Available online: https://mectizan.org/wp-content/uploads/2023/05/2022-Annual-Highlights-of-Mectizan-Donation-Program.pdf (accessed on 1 July 2024).
- Buettcher, M.; Stebler, A.K.; Theiler, M.; Kobylinski, K.; Pfister, M. National Survey in Switzerland Calls for Improved Diagnosis and Treatment in Children with Scabies. Swiss. Med. Wkly. 2023, 153, 40129. [Google Scholar] [CrossRef] [PubMed]
- Compendium. Subvectin. Available online: https://compendium.ch (accessed on 23 July 2024).
- Jittamala, P.; Monteiro, W.; Smit, M.R.; Pedrique, B.; Specht, S.; Chaccour, C.J.; Dard, C.; Del Giudice, P.; Khieu, V.; Maruani, A.; et al. A Systematic Review and an Individual Patient Data Meta-Analysis of Ivermectin Use in Children Weighing Less than Fifteen Kilograms: Is It Time to Reconsider the Current Contraindication? PLoS Negl. Trop. Dis. 2021, 15, e0009144. [Google Scholar] [CrossRef]
- Brussee, J.M.; Schulz, J.D.; Coulibaly, J.T.; Keiser, J.; Pfister, M. Ivermectin Dosing Strategy to Achieve Equivalent Exposure Coverage in Children and Adults. Clin. Pharmacol. Ther. 2019, 106, 661–667. [Google Scholar] [CrossRef]
- Schulz, J.D.; Coulibaly, J.T.; Schindler, C.; Wimmersberger, D.; Keiser, J. Pharmacokinetics of Ascending Doses of Ivermectin in Trichuris Trichiura-Infected Children Aged 2–12 Years. J. Antimicrob. Chemother. 2019, 74, 1642–1647. [Google Scholar] [CrossRef] [PubMed]
- Duthaler, U.; Suenderhauf, C.; Karlsson, M.O.; Hussner, J.; Meyer zu Schwabedissen, H.; Krähenbühl, S.; Hammann, F. Population Pharmacokinetics of Oral Ivermectin in Venous Plasma and Dried Blood Spots in Healthy Volunteers. Br. J. Clin. Pharmacol. 2019, 85, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Duthaler, U.; Leisegang, R.; Karlsson, M.O.; Krähenbühl, S.; Hammann, F. The Effect of Food on the Pharmacokinetics of Oral Ivermectin. J. Antimicrob. Chemother. 2020, 75, 438–440. [Google Scholar] [CrossRef]
- Levy, M.; Martin, L.; Bursztejn, A.; Chiaverini, C.; Miquel, J.; Mahé, E.; Maruani, A.; Boralevi, F.; Groupe de Recherche de la Société Française de Dermatologie Pédiatrique. Ivermectin Safety in Infants and Children under 15 Kg Treated for Scabies: A Multicentric Observational Study. Br. J. Dermatol. 2020, 182, 1003–1006. [Google Scholar] [CrossRef]
- Kost, J.; Huwyler, J.; Puchkov, M. Calcium Phosphate Microcapsules as Multifunctional Drug Delivery Devices. Adv. Funct. Mater. 2023, 33, 2303333. [Google Scholar] [CrossRef]
- Wagner-Hattler, L.; Kiene, K.; Bielicki, J.; Pfister, M.; Puchkov, M.; Huwyler, J. High Acceptability of an Orally Dispersible Tablet Formulation by Children. Children 2021, 8, 194. [Google Scholar] [CrossRef]
- Wagner-Hattler, L.; Wyss, K.; Schoelkopf, J.; Huwyler, J.; Puchkov, M. In Vitro Characterization and Mouthfeel Study of Functionalized Calcium Carbonate in Orally Disintegrating Tablets. Int. J. Pharm. 2017, 534, 50–59. [Google Scholar] [CrossRef]
- Wagner-Hattler, L.; Schoelkopf, J.; Huwyler, J.; Puchkov, M. Stability Investigation of FCC-Based Tablets for Oral Suspension with Caffeine and Oxantel Pamoate as Model Drugs. Drug Dev. Ind. Pharm. 2019, 45, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Dao, K.; Buettcher, M.; Golhen, K.; Kost, J.; Schittny, A.; Duthaler, U.; Atkinson, A.; Haefliger, D.; Guidi, M.; Bardinet, C.; et al. Novel Patient-Friendly Orodispersible Formulation of Ivermectin Is Associated with Enhanced Palatability, Controlled Absorption, and Less Variability: High Potential for Pediatric Use. J. Clin. Pharmacol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Duthaler, U.; Suenderhauf, C.; Gaugler, S.; Vetter, B.; Krähenbühl, S.; Hammann, F. Development and Validation of an LC-MS/MS Method for the Analysis of Ivermectin in Plasma, Whole Blood, and Dried Blood Spots Using a Fully Automatic Extraction System. J. Pharm. Biomed. Anal. 2019, 172, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Bergstrand, M.; Karlsson, M.O. Handling Data below the Limit of Quantification in Mixed Effect Models. AAPS J. 2009, 11, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Krzyzanski, W.; Pérez-Ruixo, J.J.; Schropp, J. Modeling of Delays in PKPD: Classical Approaches and a Tutorial for Delay Differential Equations. J. Pharmacokinet. Pharmacodyn. 2014, 41, 291–318. [Google Scholar] [CrossRef]
- Nicolas, P.; Maia, M.F.; Bassat, Q.; Kobylinski, K.C.; Monteiro, W.; Rabinovich, N.R.; Menéndez, C.; Bardají, A.; Chaccour, C. Safety of Oral Ivermectin during Pregnancy: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2020, 8, e92–e100. [Google Scholar] [CrossRef]
- Kimland, E.; Odlind, V. Off-Label Drug Use in Pediatric Patients. Clin. Pharmacol. Ther. 2012, 91, 796–801. [Google Scholar] [CrossRef]
- Vallet, T.; Elhamdaoui, O.; Berraho, A.; Cherkaoui, L.O.; Kriouile, Y.; Mahraoui, C.; Mouane, N.; Pense-Lheritier, A.-M.; Ruiz, F.; Bensouda, Y. Medicines Acceptability in Hospitalized Children: An Ongoing Need for Age-Appropriate Formulations. Pharmaceutics 2020, 12, 766. [Google Scholar] [CrossRef]
- Wohlrab, J.; Stadie, L.; Neubert, R.H.H.; Bosse, K. Development of an Ivermectin-Containing Syrup as an Extemporaneous Preparation for Treatment of Scabies in Children. Hautarzt 2021, 72, 720–728. [Google Scholar] [CrossRef]
- Rowland Yeo, K.; Wesche, D. PBPK Modeling of Ivermectin—Considerations for the Purpose of Developing Alternative Routes to Optimize Its Safety Profile. CPT Pharmacomet. Syst. Pharmacol. 2023, 12, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Gwee, A.; Duffull, S.; Zhu, X.; Tong, S.Y.C.; Cranswick, N.; McWhinney, B.; Ungerer, J.; Francis, J.; Steer, A.C. Population Pharmacokinetics of Ivermectin for the Treatment of Scabies in Indigenous Australian Children. PLoS Negl. Trop. Dis. 2020, 14, e0008886. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.N.; Rostami-Hodjegan, A.; Tucker, G.T. Prediction of the Clearance of Eleven Drugs and Associated Variability in Neonates, Infants and Children. Clin. Pharmacokinet. 2006, 45, 931–956. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, K.; Aleksunes, L.; Brandys, B.; Giacoia, G.; Knipp, G.; Lukacova, V.; Meibohm, B.; Nigam, S.; Rieder, M.; de Wildt, S. Human Ontogeny of Drug Transporters: Review and Recommendations of the Pediatric Transporter Working Group. Clin. Pharmacol. Ther. 2015, 98, 266–287. [Google Scholar] [CrossRef]
| Demographic Characteristics of Study Participants (N = 16) | |
|---|---|
| Age (years) | 24.0 [20.8, 28.0] |
| Weight (kg) | 63.7 [58.0, 71.5] |
| Height (cm) | 171 [168, 178] |
| BMI (kg/m2) | 22.3 [19.7, 23.1] |
| Gender | |
| Female | 7 (43.8%) |
| Male | 9 (56.3%) |
| Ethnicity | |
| African | 2 (12.5%) |
| Caucasian | 10 (62.5%) |
| Hispanic/Latin American | 1 (6.3%) |
| Multiracial | 3 (18.8%) |
| Parameter (Unit) | Value (RSE %) (Shrinkage) | |
|---|---|---|
| CHILD-IVITAB absorption rate constant and transit rate constant (h−1) | ka = ktr | 2.41 (6%) |
| STROMECTOL® absorption rate constant and transit rate constant (h−1) | 1.56 (12%) | |
| CHILD-IVITAB mean transit time (h) | MTT = 3/ktr | 1.24 |
| STROMECTOL® mean transit time (h) | 1.92 | |
| Clearance (L/h) | CL | 5.8 (17%) × (WT/18)0.75 |
| Volume of distribution (L) | Vc | 60.29 (24%) × (WT/18) |
| Vp | 103.56 (16%) × (WT/18) | |
| Intercompartmental clearance (L/h) | Q | 9.73 (18%) × (WT/18)0.75 |
| CHILD-IVITAB relative bioavailability | Frel | 1.30 (16%) |
| IIV ka CHILD-IVITAB (CV%) | 0.19 (22%) (14%) | |
| IIV ka STROMECTOL (CV%) | 0.43 (19%) (0.70%) | |
| IIV CL (CV%) | 0.67 (27%) (1.6%) | |
| IIV Vc (CV%) | 0.84 (28%) (1.1%) | |
| IIV Vp (CV%) | 0.57 (41%) (5.9%) | |
| IIV Frel (CV%) | 0.61 (24%) (2.3%) |
| Weight-Based Ivermectin Dosing Regimen | ||||
|---|---|---|---|---|
| Ivermectin Formulation | Body Weight (kg) | Recommended Dosing Regimen (µg/kg) | Simulated Median AUC0–96h [IQR] (µg·h/L) | Simulated Median AUC0–168h [IQR] (µg·h/L) |
| STROMECTOL® (reference in adults) | 50.1–120 | 200 | 800 [516, 1230] | 884 [572, 1380] |
| CHILD-IVITAB | 5.0–7.5 | 250 | 752 [574, 1010] | 770 [589, 1040] |
| 7.6–10.0 | 250 | 822 [635, 1070] | 853 [651, 1120] | |
| 10.1–14.9 | 250 | 879 [661, 1150] | 919 [682, 1220] | |
| 15.0–30.0 | 200 | 780 [612, 1010] | 832 [639, 1080] | |
| 30.1–50.0 | 200 | 899 [698, 1140] | 971 [740, 1260] | |
| Body Weight (kg) | CHILD-IVITAB 1 mg Tablets | CHILD-IVITAB 3 mg Tablets | Effective Dose Range (µg/kg/dose) |
|---|---|---|---|
| 5.0–7.5 | 1 to 2 | - | 133–400 |
| 7.6–10.0 | 2 | - | 200–263 |
| 10.1–14.9 | - | 1 | 201–297 |
| 15.0–30.0 | - | 2 | 200–400 |
| 30.1–50.0 | - | 3 | 180–300 |
| 50.1–120 | - | 4 | 100–240 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golhen, K.; Buettcher, M.; Huwyler, J.; van den Anker, J.; Gotta, V.; Dao, K.; Rothuizen, L.E.; Kobylinski, K.; Pfister, M. Pharmacometrics to Evaluate Dosing of the Patient-Friendly Ivermectin CHILD-IVITAB in Children ≥ 15 kg and <15 kg. Pharmaceutics 2024, 16, 1186. https://doi.org/10.3390/pharmaceutics16091186
Golhen K, Buettcher M, Huwyler J, van den Anker J, Gotta V, Dao K, Rothuizen LE, Kobylinski K, Pfister M. Pharmacometrics to Evaluate Dosing of the Patient-Friendly Ivermectin CHILD-IVITAB in Children ≥ 15 kg and <15 kg. Pharmaceutics. 2024; 16(9):1186. https://doi.org/10.3390/pharmaceutics16091186
Chicago/Turabian StyleGolhen, Klervi, Michael Buettcher, Jörg Huwyler, John van den Anker, Verena Gotta, Kim Dao, Laura E. Rothuizen, Kevin Kobylinski, and Marc Pfister. 2024. "Pharmacometrics to Evaluate Dosing of the Patient-Friendly Ivermectin CHILD-IVITAB in Children ≥ 15 kg and <15 kg" Pharmaceutics 16, no. 9: 1186. https://doi.org/10.3390/pharmaceutics16091186







