Real-World Evidence of 3D Printing of Personalised Paediatric Medicines and Evaluating Its Potential in Children with Cancer: A Scoping Review
Abstract
1. Introduction
2. Review Question
3. Methods
3.1. Inclusion Criteria
- Population
- Concept
- Context
3.2. Types of Sources
3.3. Search Strategy
3.4. Search Process
3.5. Data Extraction
3.6. Quality Assessment
3.7. Data Synthesis
4. Results
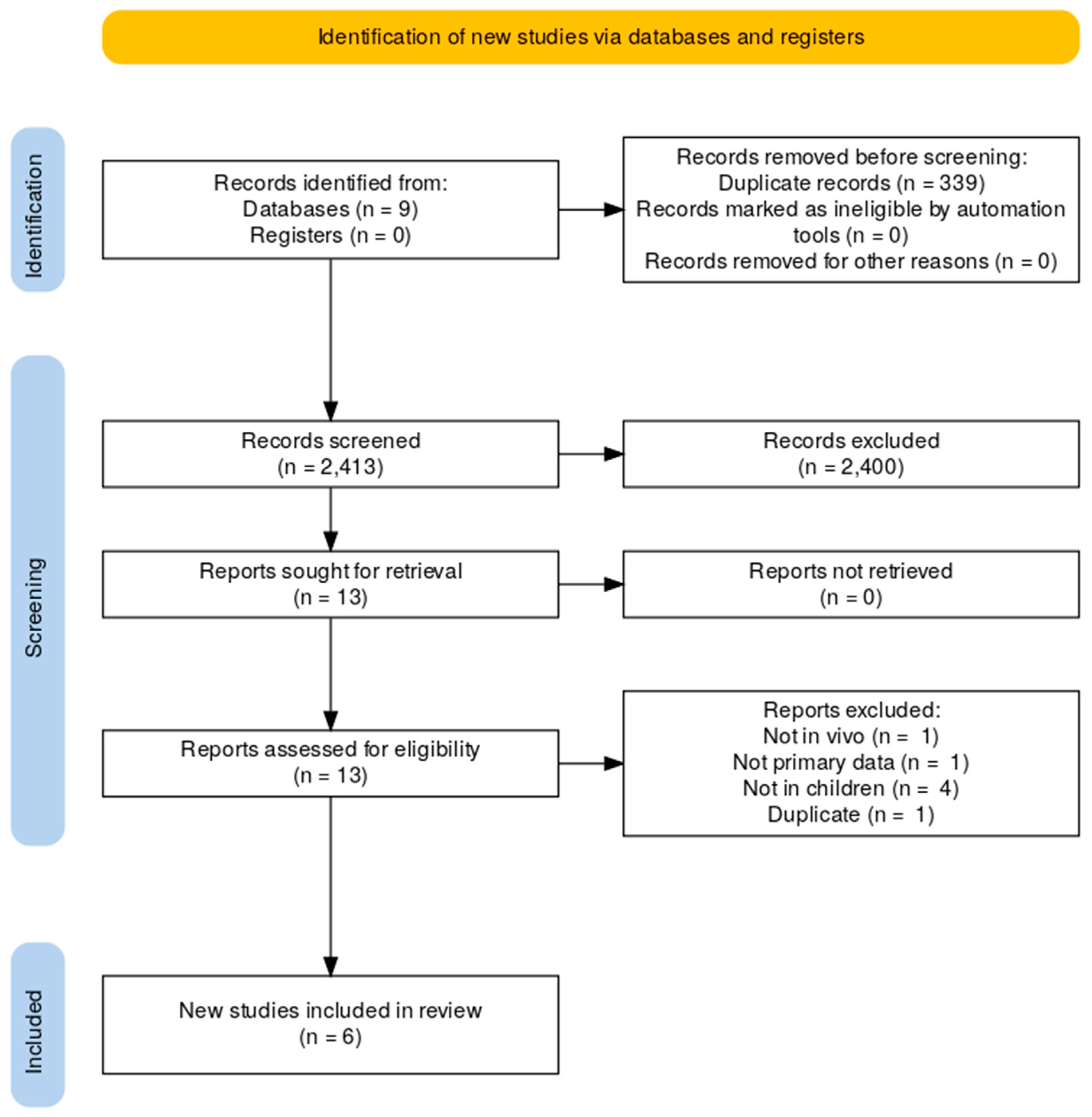
4.1. Study Inclusion
4.2. Characteristics of Included Studies
4.3. Quality of Included Papers
4.4. Synthesis of Results
Thematic Analysis
- Clinical application:
- 2.
- Acceptability of 3D-printed dosage forms in paediatric population:
- 3.
- Benefits of 3D-printed drug products:All included studies highlighted the perceived benefits of 3D-printed drug products over conventional formulations. These included precise and personalised doses [47,48,50,51], polypills [48], improved drug acceptance [46,47,48,50,51], reduced labour intensity [51], cost savings [48,51], improved safety [49,50,51], and rapid manufacture [47,50].
- Precise and personalised doses: Healthcare professionals highlighted the advantage of 3D printing technology in producing precise and patient-specific doses, enhancing medication adherence and treatment outcomes [48]. Healthcare professionals also considered that it was useful to have an option of on-demand new and modified doses based on the patient’s new requirements [47]. Preliminary studies focusing on the feasibility of producing 3D-printed tablets have found that 3D printing has the potential to overcome challenges in pharmaceutical manufacturing, such as the ability to produce complex geometries and personalised dosage forms [50]. The benefits of 3D printing over conventional manufacturing [35] were found to be accurate dosing and compliance with the specifications of the regional Pharmacopoeia [51]. The 3D printing technology allowed for precise control over the dose of the subdivided tablets [51]. Moreover, the model parameters of the 3D printer were able to be adjusted to obtain tablets with any desired dose, ensuring accurate dosing for individual patients [51].
- Polypills: Healthcare professionals were of the view that incorporating multiple drug substances into one product simplifies medication regimens, particularly for children with polypharmacy, thereby improving adherence [48].
- Improved drug acceptance: Children [47,50] and healthcare professionals [48,50] were of the view that customizing the shape, size, and colour of solid dosage forms can enhance drug acceptability, especially among paediatric patients. Evidence for the preference for shape and colour is limited. Swallowability, palatability, such as mouthfeel, and taste positively influenced acceptability in a sample of children [46]. Suggestions from healthcare professionals included using funny or appealing shapes to make medication more appealing to children [48]. Accurate doses and good appearance were considered by nurses and pharmacists to contribute to improving drug acceptability [51].
- Reduced labour intensity: 3D printing is considered by healthcare professionals to have benefits over the traditional methods of manipulating dosage forms by hand, such as splitting, crushing, powdering, or making an extemporaneous liquid preparation—all require manual labour and can be time consuming for healthcare professionals and caregivers [51]. Healthcare professionals were of the view that 3D printing could potentially improve efficiency by reducing the workload through the automation process [51].
- Cost savings: In the view of healthcare professionals, automation will eliminate the need for additional equipment such as tablet cutters and crushers, extemporaneous liquid formulations, and reduce the costs of human resources and materials [51]. Healthcare professionals, especially pharmacists, were of the view that 3D printing technology could potentially reduce the costs associated with making tablets by reducing waste costs [48]. Nevertheless, significant upfront costs might be necessary for advanced 3D printers, as well as ongoing maintenance expenses and expenditures for materials, equipment, and personnel, could result in manufacturing being costly [48]. As the evidence is still limited, further studies are required to demonstrate the cost effectiveness of implementing 3D printing technology in hospital settings.
- Improved safety: Results from the preliminary studies indicate that 3D printing technology will ensure the accuracy and consistency of dosages with drug uniformity [51]. The laboratory tests performed showed exceptionally low variations in mass, drug content, and uniformity compared to manual splitting, reducing the risk of dosage errors from subdividing doses [49,51]. Improved safety results were also obtained from the quality control and monitoring of 3D-printed tablets [51] and the elimination of cross-contamination [49,50]. Personalised dosing will contribute to improved safety by reducing the side effects from inappropriate doses.
- Rapid manufacture: 3D printing allows for on-demand production of drug dosage forms at the point of dispensing with a small-batch and rapid manufacturing process. This can improve access to medicines and reduce lead times, especially for children with specific needs [47]. It is fast and more efficient, reducing the time and resources needed for making tablets compared to conventional manufacturing [50]. Twenty-eight 3D-printed tablets, sufficient for one month of treatment, were printed in approximately 8 min [50].
- 4.
- Concerns regarding the implementation of 3D printing of drug products [48]:Concerns were raised by healthcare professionals surrounding the implementation of 3D-printed pharmaceutical dosage forms [48]. The main concerns were:
- Medication safety: Healthcare professionals (physicians, nurses, pharmacists) expressed concerns about the even distribution of drug substances within printed dosage forms (verification of drug contents), accuracy of doses, quality control, stability, and shelf-life and storage conditions of formulations [48].
- Drug administration: Healthcare professionals also expressed their concerns on drug administration. Challenges include administering dosage forms to all paediatric patients, especially infants and those with enteral feeding tubes, as well as concerns about functionality and the dissolution characteristics of patient-specific dosage forms [48].
- Production and delivery on demand: The logistics and response time for the production and delivery of on-demand prepared dosage forms (the ability to react quickly to dose changes) were noted concerns by healthcare professionals, particularly in hospital settings [48].
- Cost: Concerns were raised on the cost effectiveness of personalised drug products by healthcare professionals [48]
- 5.
- Feasibility of 3D-printed dosage forms:
- 6.
- Suggestions for printed medicines
- 7.
- Potential applications and implications:
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A. Search Strategy
- Embase
| Search Number | Search Terms Used | Number of Results |
| #1 | exp ‘Printing, Three-Dimensional’/ or ‘Stereolithography’:tw.mp. or ‘Stereolithogr*’:tw.mp. or ((“3 D:tw” or ‘3D’:tw or ‘3 Dimensional’:tw or ‘3Dimensional’:tw or ‘Three Dimensional’:tw or ‘ThreeDimensional’:tw) and (‘Printing’:tw or ‘Print’:tw or ‘Print*’:tw)).mp. or ‘3D Manufacturing’:tw.mp. or ‘Three Dimensional Manufacturing’:tw.mp. or ‘3D Manufactur*’:tw.mp. or ‘Three Dimensional Manufactur*’:tw.mp. or ‘3D Produced’:tw.mp. or ‘3D Produc*’:tw.mp. or ‘Three Dimensional Produc*’:tw.mp. or ‘Printlets’:tw.mp. or ‘additive manufacturing’:tw.mp. or ‘additive manufactur*’:tw.mp. or ‘additive production’:tw.mp. or ‘additive produc*’:tw.mp. or ‘additive printing’:tw.mp. or ‘additive print*’:tw.mp. or ‘rapid prototyping’:tw.mp. or ‘rapid prototyp*’:tw.mp. or ‘bioprinting’:tw.mp. or ‘bioprint*’:tw.mp. or ‘Binder deposit*’:tw.mp. or ‘Binder deposition’:tw.mp. or ‘Binder jet*’:tw.mp. or ‘Binder jetting’:tw.mp. or ‘Direct ink writ*’:tw.mp. or ‘Direct ink writing’:tw.mp. or ‘Direct powder extru*’:tw.mp. or ‘Direct powder extrusion’:tw.mp. or ‘Drop on demand’:tw.mp. or ‘Drop on demand*’:tw.mp. or ‘Drop on drop’:tw.mp. or ‘Drop on drop*’:tw.mp. or ‘Drop on solid’:tw.mp. or ‘Drop on solid*’:tw.mp. or ‘Fused deposition model*’:tw.mp. or ‘Fused deposition modelling ‘:tw.mp. or ‘Fused filament fabric*’:tw.mp. or ‘Fused filament fabrication ‘:tw.mp. or ‘Ink jet*’:tw.mp. or ‘Ink jetting’:tw.mp. or ‘Inkjet print*’:tw.mp. or ‘Inkjet printing’:tw.mp. or ‘Micro extru*’:tw.mp. or ‘Micro extrusion’:tw.mp. or ‘On demand manufact*’:tw.mp. or ‘On demand manufacturing’:tw.mp. or ‘Pressure assisted microsyring*’:tw.mp. or ‘Pressure assisted microsyringe ‘:tw.mp. or ‘Rapid manufactur*’:tw.mp. or ‘Rapid manufacturing’:tw.mp. or ‘Selective laser sinter*’:tw.mp. or ‘Selective laser sintering ‘:tw.mp. or ‘Semi solid extru*’:tw.mp. or ‘Semi solid extrusion ‘:tw.mp. or ‘Vat photopolymer*’:tw.mp. or ‘Vat photopolymerization’:tw.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] | 29,668 |
| #2 | (‘Tablets’:exp or ‘Dosage Forms’:exp or ‘Pharmaceutical Preparations’:exp or ‘Drug Therapy’:exp or ‘Pharmacology’:exp or ‘Pharmacokinetics’:exp or ‘Technology, Pharmaceutical’:exp or ‘Precision Medicine’:exp or ‘Personalized Medicine’:exp or ‘Individualized Medicine’:exp or ‘Extemporaneous preparations’:exp or ‘Extemporaneous preparation’:exp or (‘Tablets’ or ‘Dosage Forms’ or ‘Pharmaceutical Preparations’ or ‘Drug Therapy’ or ‘Pharmacology’ or ‘Pharmacokinetics’ or ‘Technology, Pharmaceutical’ or ‘chemotherapy’ or ‘dosage forms’ or ‘drug’ or ‘drug therapy’ or ‘drugs’ or ‘medication’ or ‘medications’ or ‘pharmaceutical’ or ‘pharmacokinetics’ or ‘pharmacology’ or ‘tablets’ or ‘chemotherap*’ or ‘dosage form’ or ‘medicat*’ or ‘pharmac*’ or ‘pharmacokinetic*’ or ‘pharmacol*’ or ‘formulation’ or ‘formulations’ or ‘pill’ or ‘pills’ or ‘Printlets’ or ‘Precision Medicine’ or ‘Personalized Medicine’ or ‘Personalised Medicine’ or ‘Individualized Medicine’ or ‘Individualised Medicine’ or ‘P Health’)).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] | 1,444,235 |
| #3 | exp ‘Child’/ or ‘child’:tw.mp. or ‘children’:tw.mp. or exp ‘Infant’/ or ‘infant’:tw.mp. or ‘infants’:tw.mp. or ‘newborn’:tw.mp. or ‘newborns’:tw.mp. or ‘new-born’:tw.mp. or ‘newborns’:tw.mp. or ‘neonate’:tw.mp. or ‘neonates’:tw.mp. or ‘neonatal’:tw.mp. or ‘neo-nate’:tw.mp. or ‘neo-nates’:tw.mp. or ‘neo-natal’:tw.mp. or ‘neonatology’:tw.mp. or ‘NICU’:ti.mp. or ‘premature’:tw.mp. or ‘prematures’:tw.mp. or ‘pre-mature’:tw.mp. or ‘pre-matures’:tw.mp. or ‘preterm’:tw.mp. or ‘pre-term’:tw.mp. or ‘postnatal’:tw.mp. or ‘post-natal’:tw.mp. or ‘baby’:tw.mp. or ‘babies’:tw.mp. or ‘suckling’:tw.mp. or ‘sucklings’:tw.mp. or ‘toddler’:tw.mp. or ‘toddlers’:tw.mp. or ‘childhood’:tw.mp. or ‘schoolchild’:tw.mp. or ‘schoolchildren’:tw.mp. or ‘childcare’:tw.mp. or ‘child-care’:tw.mp. or ‘young’:ti.mp. or ‘youngster’:tw.mp. or ‘youngsters’:tw.mp. or ‘preschool’:tw.mp. or ‘pre-school’:tw.mp. or ‘kid’:tw.mp. or ‘kids’:tw.mp. or ‘boy’:tw.mp. or ‘boys’:tw.mp. or ‘girl’:tw.mp. or ‘girls’:tw.mp. or exp ‘Adolescent’/ or ‘adolescent’:tw.mp. or ‘adolescents’:tw.mp. or ‘adolescence’:tw.mp. or ‘pre-adolescent’:tw.mp. or ‘pre-adolescents’:tw.mp. or ‘pre-adolescence’:tw.mp. or ‘schoolage’:tw.mp. or ‘schoolboy’:tw.mp. or ‘schoolboys’:tw.mp. or ‘schoolgirl’:tw.mp. or ‘schoolgirls’:tw.mp. or ‘pre-puber’:tw.mp. or ‘pre-puberty’:tw.mp. or ‘prepuber’:tw.mp. or ‘prepubers’:tw.mp. or ‘prepuberty’:tw.mp. or ‘puber’:tw.mp. or ‘puberty’:tw.mp. or ‘puberal’:tw.mp. or ‘teenager’:tw.mp. or ‘teenagers’:tw.mp. or ‘teens’:tw.mp. or ‘youth’:tw.mp. or ‘youths’:tw.mp. or ‘underaged’:tw.mp. or ‘under-aged’:tw.mp. or exp ‘Pediatrics’/ or ‘Pediatric’:tw.mp. or ‘Pediatrics’:tw.mp. or ‘Paediatric’:tw.mp. or ‘Paediatrics’:tw.mp. or ‘PICU’:ti.mp. or (‘child’:ab,ti not child’:au).mp. or children*:ab,ti.mp. or schoolchild*:ab,ti.mp. or ‘infant’:ab,ti.mp. or ‘infants’:ab,ti.mp. or adolesc*:ab,ti.mp. or pediat*:ab,ti.mp. or paediat*:ab,ti.mp. or neonat*:ab,ti.mp. or toddler*:ab,ti.mp. or ‘teen’:ab,ti.mp. or ‘teens’:ab,ti.mp. or teenager*:ab,ti.mp. or preteen*:ab,ti.mp. or newborn*:ab,ti.mp. or postneonat*:ab,ti.mp. or postnatal*:ab,ti.mp. or ‘puberty’:ab,ti.mp. or preschool*:ab,ti.mp. or suckling*:ab,ti.mp. or ‘juvenile’:ab,ti.mp. or ‘new born’:ab,ti.mp. or ‘new borns’:ab,ti.mp. or new-born*:ab,ti.mp. or neo-nat*:ab,ti.mp. or neonat*:ab,ti.mp. or perinat*:ab,ti.mp. or underag*:ab,ti.mp. or ‘under age’:ab,ti.mp. or ‘under aged’:ab,ti.mp. or youth*:ab,ti.mp. or kinder*:ab,ti.mp. or pubescen*:ab,ti.mp. or prepubescen*:ab,ti.mp. or ‘prepuberty’:ab,ti.mp. or ‘school age’:ab,ti.mp. or ‘schoolage’:ab,ti.mp. or ‘school ages’:ab,ti.mp. or schoolage*:ab,ti.mp. or ‘one year old’:ab,ti.mp. or ‘two year old’:ab,ti.mp. or ‘three year old’:ab,ti.mp. or ‘four year old’:ab,ti.mp. or ‘five year old’:ab,ti.mp. or ‘six year old’:ab,ti.mp. or ‘seven year old’:ab,ti.mp. or ‘eight year old’:ab,ti.mp. or ‘nine year old’:ab,ti.mp. or ‘ten year old’:ab,ti.mp. or ‘eleven year old’:ab,ti.mp. or ‘twelve year old’:ab,ti.mp. or ‘thirteen year old’:ab,ti.mp. or ‘fourteen year old’:ab,ti.mp. or ‘fifteen year old’:ab,ti.mp. or ‘sixteen year old’:ab,ti.mp. or ‘seventeen year old’:ab,ti.mp. or ‘eighteen year old’:ab,ti.mp. or ‘1 year old’:ab,ti.mp. or ‘2 year old’:ab,ti.mp. or ‘3 year old’:ab,ti.mp. or ‘4 year old’:ab,ti.mp. or ‘5 year old’:ab,ti.mp. or ‘6 year old’:ab,ti.mp. or ‘7 year old’:ab,ti.mp. or ‘8 year old’:ab,ti.mp. or ‘9 year old’:ab,ti.mp. or ‘10 year old’:ab,ti.mp. or ‘11 year old’:ab,ti.mp. or ‘12 year old’:ab,ti.mp. or ‘13 year old’:ab,ti.mp. or ‘14 year old’:ab,ti.mp. or ‘15 year old.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] | 4,048,728 |
| #4 | exp ‘Neoplasms’/ or ‘Cancer’:tw.mp. or ‘haematolog*’:tw.mp. or ‘hematolog*’:tw.mp. or ‘oncolog*’:tw.mp. or ‘Tumour’:tw.mp. or ‘Tumor’:tw.mp. or ‘Tumo*’:tw.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] | 5,607,776 |
Appendix B. Studies Excluded Following Full Text Review
- Boudriau S, Hanzel C, et al. Randomized Comparative Bioavailability of a Novel Three-Dimensional Printed Fast-Melt Formulation of Levetiracetam Following the Administration of a Single 1000-mg Dose to Healthy Human Volunteers Under Fasting and Fed Conditions. Drugs R D. 2016 Jun;16(2):229-38. doi: 10.1007/s40268-016-0132-1. PMID: 27028750; PMCID: PMC4875927.Reason for exclusion: Not in children
- Karavasili C, Zgouro P, et al. Cereal-Based 3D Printed Dosage Forms for Drug Administration During Breakfast in Pediatric Patients within a Hospital Setting. J Pharm Sci. 2022 Sep;111(9):2562-2570. doi: 10.1016/j.xphs.2022.04.013. Epub 2022 Apr 22. PMID: 35469835.Reason for exclusion: Not in vivo
- van Kampen EEM, Willemsteijn L, et al. 3D printing of drugs: expanding the options for child-tailored pharmacotherapy. Arch Dis Child. 2021 Jul 14:archdischild-2021-321629. doi: 10.1136/archdischild-2021-321629. Epub ahead of print. PMID: 34261670.Reason for exclusion: Not primary data
- Tabriz AG, Hui HW, et al. 3D Printed Flavor-Rich Chewable Pediatric Tablets Fabricated Using Microextrusion for Point of Care Applications. Mol Pharm. 2023 Jun 5;20(6):2919-2926. doi: 10.1021/acs.molpharmaceut.2c01061. Epub 2023 Apr 6. PMID: 37022302.Reason for exclusion: Palatability assessment, but only in healthy adult volunteers not in children.
- Tabriz AG, Fullbrook DHG, et al. Personalised Tasted Masked Chewable 3D Printed Fruit-Chews for Paediatric Patients. Pharmaceutics. 2021 Aug 20;13(8):1301. doi: 10.3390/pharmaceutics13081301. PMID: 34452262; PMCID: PMC8400795.Reason for exclusion: Palatability assessment, but only in healthy adult volunteers not in children.
- Blazhenkova O, Dogerlioglu-Demir K. The shape of the pill: Perceived effects, evoked bodily sensations and emotions. PLoS One. 2020 Sep 8;15(9):e0238378. doi: 10.1371/journal.pone.0238378. PMID: 32898184; PMCID: PMC7478620.Reason for exclusion: Palatability assessment, but in healthy adult volunteers not in children.
References
- Wojtyłko, M.; Lamprou, D.A.; Froelich, A.; Kuczko, W.; Wichniarek, R.; Osmałek, T. 3D-Printed Solid Oral Dosage Forms for Mental and Neurological Disorders: Recent Advances and Future Perspectives. Expert Opin. Drug Deliv. 2023, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Ding, S.; Zhou, X.; Zheng, N.; Zheng, M.; Wang, J.; Yang, Q.; Yang, G. 3D-Printed Dosage Forms for Oral Administration: A Review. Drug Deliv. Transl. Res. 2024, 14, 312–328. [Google Scholar] [CrossRef] [PubMed]
- Preis, M.; Öblom, H. 3D-Printed Drugs for Children—Are We Ready Yet? AAPS PharmSciTech 2017, 18, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Scarpa, M.; Kamlow, M.; Gaisford, S.; Basit, A.W.; Orlu, M. Patient Acceptability of 3D Printed Medicines. Int. J. Pharm. 2017, 530, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Fastø, M.M.; Genina, N.; Kaae, S.; Sporrong, S.K. Perceptions, Preferences and Acceptability of Patient Designed 3D Printed Medicine by Polypharmacy Patients: A Pilot Study. Int. J. Clin. Pharm. 1234, 41, 1290–1298. [Google Scholar] [CrossRef]
- Carou-Senra, P.; Rodríguez-Pombo, L.; Monteagudo-Vilavedra, E.; Awad, A.; Alvarez-Lorenzo, C.; Basit, A.W.; Goyanes, A.; Couce, M.L. 3D Printing of Dietary Products for the Management of Inborn Errors of Intermediary Metabolism in Pediatric Populations. Nutrients 2024, 16, 61. [Google Scholar] [CrossRef]
- Patel, H.; Raje, V.; Maczko, P.; Patel, K. Application of 3D Printing Technology for the Development of Dose Adjustable Geriatric and Pediatric Formulation of Celecoxib. Int. J. Pharm. 2024, 655, 123941. [Google Scholar] [CrossRef]
- Windolf, H.; Chamberlain, R.; Quodbach, J. Dose-Independent Drug Release from 3D Printed Oral Medicines for Patient-Specific Dosing to Improve Therapy Safety. Int. J. Pharm. 2022, 616, 121555. [Google Scholar] [CrossRef]
- El Aita, I.; Rahman, J.; Breitkreutz, J.; Quodbach, J. 3D-Printing with Precise Layer-Wise Dose Adjustments for Paediatric Use via Pressure-Assisted Microsyringe Printing. Eur. J. Pharm. Biopharm. 2020, 157, 59–65. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D Printing of Five-in-One Dose Combination Polypill with Defined Immediate and Sustained Release Profiles. J. Control. Release 2015, 217, 308–314. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D Printing of Tablets Containing Multiple Drugs with Defined Release Profiles. Int. J. Pharm. 2015, 494, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Goh, W.J.; Tan, S.X.; Pastorin, G.; Ho, P.C.L.; Hu, J.; Lim, S.H. 3D Printing of Four-in-One Oral Polypill with Multiple Release Profiles for Personalized Delivery of Caffeine and Vitamin B Analogues. Int. J. Pharm. 2021, 598, 120360. [Google Scholar] [CrossRef] [PubMed]
- Gioumouxouzis, C.I.; Baklavaridis, A.; Katsamenis, O.L.; Markopoulou, C.K.; Bouropoulos, N.; Tzetzis, D.; Fatouros, D.G. A 3D Printed Bilayer Oral Solid Dosage Form Combining Metformin for Prolonged and Glimepiride for Immediate Drug Delivery. Eur. J. Pharm. Sci. 2018, 120, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Sadia, M.; Isreb, A.; Abbadi, I.; Isreb, M.; Aziz, D.; Selo, A.; Timmins, P.; Alhnan, M.A. From ‘Fixed Dose Combinations’ to ‘a Dynamic Dose Combiner’: 3D Printed Bi-Layer Antihypertensive Tablets. Eur. J. Pharm. Sci. 2018, 123, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Robles-Martinez, P.; Xu, X.; Trenfield, S.J.; Awad, A.; Goyanes, A.; Telford, R.; Basit, A.W.; Gaisford, S. 3D Printing of a Multi-Layered Polypill Containing Six Drugs Using a Novel Stereolithographic Method. Pharmaceutics 2019, 11, 274. [Google Scholar] [CrossRef]
- Ghanizadeh Tabriz, A.; Nandi, U.; Hurt, A.P.; Hui, H.W.; Karki, S.; Gong, Y.; Kumar, S.; Douroumis, D. 3D Printed Bilayer Tablet with Dual Controlled Drug Release for Tuberculosis Treatment. Int. J. Pharm. 2021, 593, 120147. [Google Scholar] [CrossRef]
- Awad, A.; Yao, A.; Trenfield, S.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printed Tablets (Printlets) with Braille and Moon Patterns for Visually Impaired Patients. Pharmaceutics 2020, 12, 172. [Google Scholar] [CrossRef]
- Rodríguez-Pombo, L.; Awad, A.; Basit, A.W.; Alvarez-Lorenzo, C.; Goyanes, A. Innovations in Chewable Formulations: The Novelty and Applications of 3D Printing in Drug Product Design. Pharmaceutics 2022, 14, 1732. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Hui, H.W.; Boersen, N.; Roberts, S.; Jones, J.; Douroumis, D. 3D Printed Flavor-Rich Chewable Pediatric Tablets Fabricated Using Microextrusion for Point of Care Applications. Mol. Pharm. 2023, 20, 2919–2926. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Nandi, U.; Scoutaris, N.; Sanfo, K.; Alexander, B.; Gong, Y.; Hui, H.W.; Kumar, S.; Douroumis, D. Personalised Paediatric Chewable Ibuprofen Tablets Fabricated Using 3D Micro-Extrusion Printing Technology. Int. J. Pharm. 2022, 626, 122135. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Fullbrook, D.H.G.; Vilain, L.; Derrar, Y.; Nandi, U.; Grau, C.; Morales, A.; Hooper, G.; Hiezl, Z.; Douroumis, D. Personalised Tasted Masked Chewable 3d Printed Fruit-Chews for Paediatric Patients. Pharmaceutics 2021, 13, 1301. [Google Scholar] [CrossRef] [PubMed]
- Karavasili, C.; Gkaragkounis, A.; Moschakis, T.; Ritzoulis, C.; Fatouros, D.G. Pediatric-Friendly Chocolate-Based Dosage Forms for the Oral Administration of Both Hydrophilic and Lipophilic Drugs Fabricated with Extrusion-Based 3D Printing. Eur. J. Pharm. Sci. 2020, 147, 105291. [Google Scholar] [CrossRef] [PubMed]
- Rouaz-El Hajoui, K.; Herrada-Manchón, H.; Rodríguez-González, D.; Fernández, M.A.; Aguilar, E.; Suñé-Pou, M.; Nardi-Ricart, A.; Pérez-Lozano, P.; García-Montoya, E. Pellets and Gummies: Seeking a 3D Printed Gastro-Resistant Omeprazole Dosage for Paediatric Administration. Int. J. Pharm. 2023, 643, 123289. [Google Scholar] [CrossRef] [PubMed]
- Scoutaris, N.; Ross, S.A.; Douroumis, D. 3D Printed “Starmix” Drug Loaded Dosage Forms for Paediatric Applications. Pharm. Res. 2018, 35, 34. [Google Scholar] [CrossRef] [PubMed]
- Parulski, C.; Bya, L.A.; Goebel, J.; Servais, A.C.; Lechanteur, A.; Evrard, B. Development of 3D Printed Mini-Waffle Shapes Containing Hydrocortisone for Children’s Personalized Medicine. Int. J. Pharm. 2023, 642, 123131. [Google Scholar] [CrossRef]
- Wang, S.; Chen, X.; Han, X.; Hong, X.; Li, X.; Zhang, H.; Li, M.; Wang, Z.; Zheng, A. A Review of 3D Printing Technology in Pharmaceutics: Technology and Applications, Now and Future. Pharmaceutics 2023, 15, 416. [Google Scholar] [CrossRef]
- Thabet, Y.; Sibanc, R.; Breitkreutz, J. Printing Pharmaceuticals by Inkjet Technology: Proof of Concept for Stand-Alone and Continuous in-Line Printing on Orodispersible Films. J. Manuf. Process 2018, 35, 205–215. [Google Scholar] [CrossRef]
- Rahman, Z.; Khuroo, T.; Mohamed, E.M.; Dharani, S.; Kayalar, C.; Kuttolamadom, M.A.; Sangaré, L.O.; Khan, M.A. Pyrimethamine 3D Printlets for Pediatric Toxoplasmosis: Design, Pharmacokinetics, and Anti-Toxoplasma Activity. Expert. Opin. Drug Deliv. 2023, 20, 301–311. [Google Scholar] [CrossRef]
- Iurian, S.M.; Bogdan, C.; Casian, T.; Hu, J.; Fitaihi, R.; Abukhamees, S.; Abdelhakim, H.E. Formulation and Characterisation of Carbamazepine Orodispersible 3D-Printed Mini-Tablets for Paediatric Use. Pharmaceutics 2023, 15, 250. [Google Scholar] [CrossRef]
- Han, X.; Kang, D.; Liu, B.; Zhang, H.; Wang, Z.; Gao, X.; Zheng, A. Feasibility of Developing Hospital Preparation by Semisolid Extrusion 3D Printing: Personalized Amlodipine Besylate Chewable Tablets. Pharm. Dev. Technol. 2022, 27, 164–174. [Google Scholar] [CrossRef]
- Isreb, A.; Baj, K.; Wojsz, M.; Isreb, M.; Peak, M.; Alhnan, M.A. 3D Printed Oral Theophylline Doses with Innovative “radiator-like” Design: Impact of Polyethylene Oxide (PEO) Molecular Weight. Int. J. Pharm. 2019, 564, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Öblom, H.; Sjöholm, E.; Rautamo, M.; Sandler, N. Towards Printed Pediatric Medicines in Hospital Pharmacies: Comparison of 2D and 3D-Printed Orodispersible Warfarin Films with Conventional Oral Powders in Unit Dose Sachets. Pharmaceutics 2019, 11, 334. [Google Scholar] [CrossRef] [PubMed]
- Roche, A.; Sanchez-Ballester, N.M.; Aubert, A.; Rossi, J.C.; Begu, S.; Soulairol, I. Preliminary Study on the Development of Caffeine Oral Solid Form 3D Printed by Semi-Solid Extrusion for Application in Neonates. AAPS PharmSciTech 2023, 24, 122. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ting, Y.H.; Youssef, S.H.; Song, Y.; Garg, S. Three-Dimensional Printing for Cancer Applications: Research Landscape and Technologies. Pharmaceuticals 2021, 14, 787. [Google Scholar] [CrossRef]
- Shi, K.; Tan, D.K.; Nokhodchi, A.; Maniruzzaman, M. Drop-On-Powder 3D Printing of Tablets with an Anti-Cancer Drug, 5-Fluorouracil. Pharmaceutics 2019, 11, 150. [Google Scholar] [CrossRef]
- Abeysekera, N.; Whitmore, K.A.; Abeysekera, A.; Pang, G.; Laupland, K.B. Applications of 3D Printing in Critical Care Medicine: A Scoping Review. Anaesth. Intensive Care 2021, 49, 164–172. [Google Scholar] [CrossRef]
- Cooke, C.M.; Flaxman, T.E.; Sikora, L.; Miguel, O.; Singh, S.S. Individualized Medicine Using 3D Printing Technology in Gynecology: A Scoping Review. 3D Print Med. 2023, 9, 6. [Google Scholar] [CrossRef]
- Ribeiro, D.; Cimino, S.R.; Mayo, A.L.; Ratto, M.; Hitzig, S.L. 3D Printing and Amputation: A Scoping Review. Disabil. Rehabil. Assist. Technol. 2021, 16, 221–240. [Google Scholar] [CrossRef]
- Pradíes, G.; Morón-Conejo, B.; Martínez-Rus, F.; Salido, M.P.; Berrendero, S. Current Applications of 3D Printing in Dental Implantology: A Scoping Review Mapping the Evidence. Clin. Oral Implant. Res. 2023, 35, 1011–1032. [Google Scholar] [CrossRef]
- Ianno, V.; Vurpillot, S.; Prillieux, S.; Espeau, P. Pediatric Formulations Developed by Extrusion-Based 3D Printing: From Past Discoveries to Future Prospects. Pharmaceutics 2024, 16, 441. [Google Scholar] [CrossRef]
- Lafeber, I.; Ruijgrok, E.J.; Guchelaar, H.J.; Schimmel, K.J.M. 3D Printing of Pediatric Medication: The End of Bad Tasting Oral Liquids?—A Scoping Review. Pharmaceutics 2022, 14, 416. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.J.; Godfrey, C.; McInerney, P.; Munn, Z.; Tricco, A.C.; Khalil, H. Chapter 11: Scoping Reviews. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Adelaide, Australia, 2020; Available online: https://synthesismanual.jbi.global (accessed on 4 September 2024). [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Sirriyeh, R.; Lawton, R.; Gardner, P.; Armitage, G. Reviewing Studies with Diverse Designs: The Development and Evaluation of a New Tool. J. Eval. Clin. Pract. 2012, 18, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Harden, A. Methods for the Thematic Synthesis of Qualitative Research in Systematic Reviews. BMC Med. Res. Methodol. 2008, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Bracken, L.; Habashy, R.; McDonough, E.; Wilson, F.; Shakeshaft, J.; Ohia, U.; Garcia-Sorribes, T.; Isreb, A.; Alhnan, M.A.; Peak, M. Creating Acceptable Tablets 3D (CAT 3D): A Feasibility Study to Evaluate the Acceptability of 3D Printed Tablets in Children and Young People. Pharmaceutics 2022, 14, 516. [Google Scholar] [CrossRef]
- Januskaite, P.; Xu, X.; Ranmal, S.R.; Gaisford, S.; Basit, A.W.; Tuleu, C.; Goyanes, A. I Spy with My Little Eye: A Paediatric Visual Preferences Survey of 3d Printed Tablets. Pharmaceutics 2020, 12, 1100. [Google Scholar] [CrossRef]
- Rautamo, M.; Kvarnström, K.; Sivén, M.; Airaksinen, M.; Lahdenne, P.; Sandler, N. Benefits and Prerequisites Associated with the Adoption of Oral 3D-Printed Medicines for Pediatric Patients: A Focus Group Study among Healthcare Professionals. Pharmaceutics 2020, 12, 229. [Google Scholar] [CrossRef]
- Liu, L.; Fu, K.; Hong, S.; Wang, Z.; Mo, M.; Li, S.; Yu, Y.; Chen, J.; Chen, J.; Zeng, W.; et al. Improving the Quality and Clinical Efficacy of Subdivided Levothyroxine Sodium Tablets by 3D Printing Technology. J. Drug Deliv. Sci. Technol. 2023, 89, 105008. [Google Scholar] [CrossRef]
- Goyanes, A.; Madla, C.M.; Umerji, A.; Duran Piñeiro, G.; Giraldez Montero, J.M.; Lamas Diaz, M.J.; Gonzalez Barcia, M.; Taherali, F.; Sánchez-Pintos, P.; Couce, M.L.; et al. Automated Therapy Preparation of Isoleucine Formulations Using 3D Printing for the Treatment of MSUD: First Single-Centre, Prospective, Crossover Study in Patients. Int. J. Pharm. 2019, 567, 118497. [Google Scholar] [CrossRef]
- Zheng, Z.; Lv, J.; Yang, W.; Pi, X.; Lin, W.; Lin, Z.; Zhang, W.; Pang, J.; Zeng, Y.; Lv, Z.; et al. Preparation and Application of Subdivided Tablets Using 3D Printing for Precise Hospital Dispensing. Eur. J. Pharm. Sci. 2020, 149, 105293. [Google Scholar] [CrossRef]
- Rodríguez-Pombo, L.; de Castro-López, M.J.; Sánchez-Pintos, P.; Giraldez-Montero, J.M.; Januskaite, P.; Duran-Piñeiro, G.; Dolores Bóveda, M.; Alvarez-Lorenzo, C.; Basit, A.W.; Goyanes, A.; et al. Paediatric Clinical Study of 3D Printed Personalised Medicines for Rare Metabolic Disorders. Int. J. Pharm. 2024, 657, 124140. [Google Scholar] [CrossRef] [PubMed]
| Framework Element | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Children ≤ 18 years old | Adults (>18 years old) All non-human subjects |
| Concept | Manufacturing of pharmaceutical dosage forms using 3D printer | Medical devices, regenerative medicine, 3D printing in imaging, scaffolding, surgical implants, organs/tissue modelling. |
| Context | Neoplasm—all types | All other disease types |
| Study | Year of Publication | Active Drug | Population and Sample | Outcome | Study Design |
|---|---|---|---|---|---|
| Bracken et al. [46] | 2022 | placebo | 30 participants (aged 4–12 years), disease not specified and included healthy volunteers. | Acceptability (swallowability, acceptability, mouthfeel, volume of water consumed, and taste) | Observational and Questionnaire |
| Januskaite et al. [47] | 2020 | placebo | 368 participants (4–11 years old) school children | Acceptability (visual preference) | Questionnaire |
| Rautamo et al. [48] | 2020 | n/a | 19 participants, health care professionals | Benefits, concerns, and risks of 3D; 3D drug of choice and disease area | Focus Group Discussions |
| Liu et al. [49] | 2023 | levothyroxine sodium | 91 preterm infants with transient hypothyroxinaemia | Personalised dosing | Observational Study |
| Goyanes et al. [50] | 2019 | isoleucine | 4 patients (3–16 years); maple syrup urine disease (MSUD) | Acceptability, flavours/colours | Prospective Crossover Experimental Study |
| Zheng et al. [51] | 2020 | spironolactone | 11 hospital inpatients (<1 months–9 months) | Acceptability | Observational Study |
| QATSDD Criteria * | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | Total Score | % Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bracken et al. [46] | 3 | 3 | 3 | 1 | 2 | 3 | 3 | 2 | 0 | 3 | 3 | 3 | 3 | n/a | 3 | 2 | 37/48 | 77 |
| Januskaite et al. [47] | 0 | 3 | 3 | 3 | 3 | 3 | 3 | 0 | n/a | n/a | 3 | 3 | 3 | 0 | 0 | 0 | 27/48 | 56 |
| Rautamo et al. [48] | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | n/a | n/a | 3 | 3 | 3 | 3 | 0 | 3 | 39/48 | 81 |
| Liu et al. [49] | 0 | 3 | 3 | 0 | 2 | 3 | 1 | 3 | 3 | 3 | n/a | 3 | 1 | n/a | 0 | 3 | 28/48 | 58 |
| Goyanes et al. [50] | 1 | 3 | 3 | 0 | 1 | 3 | 1 | 0 | 1 | 3 | n/a | 3 | 1 | n/a | 0 | 2 | 23/48 | 48 |
| Zheng et al. [51] | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | n/a | 0 | 1 | n/a | 0 | 0 | 5/48 | 10 |
| Mean | 1.2 | 2.5 | 2.7 | 1.2 | 1.8 | 2.8 | 2.0 | 1.3 | 1.0 | 2.3 | 3.0 | 2.5 | 2.0 | 1.5 | 0.5 | 1.7 | 55.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, M.; Tomlin, S.; Tuleu, C.; Garfield, S. Real-World Evidence of 3D Printing of Personalised Paediatric Medicines and Evaluating Its Potential in Children with Cancer: A Scoping Review. Pharmaceutics 2024, 16, 1212. https://doi.org/10.3390/pharmaceutics16091212
Ahmed M, Tomlin S, Tuleu C, Garfield S. Real-World Evidence of 3D Printing of Personalised Paediatric Medicines and Evaluating Its Potential in Children with Cancer: A Scoping Review. Pharmaceutics. 2024; 16(9):1212. https://doi.org/10.3390/pharmaceutics16091212
Chicago/Turabian StyleAhmed, Munsur, Stephen Tomlin, Catherine Tuleu, and Sara Garfield. 2024. "Real-World Evidence of 3D Printing of Personalised Paediatric Medicines and Evaluating Its Potential in Children with Cancer: A Scoping Review" Pharmaceutics 16, no. 9: 1212. https://doi.org/10.3390/pharmaceutics16091212
APA StyleAhmed, M., Tomlin, S., Tuleu, C., & Garfield, S. (2024). Real-World Evidence of 3D Printing of Personalised Paediatric Medicines and Evaluating Its Potential in Children with Cancer: A Scoping Review. Pharmaceutics, 16(9), 1212. https://doi.org/10.3390/pharmaceutics16091212







