The Natural Anthraquinone Parietin Inactivates Candida tropicalis Biofilm by Photodynamic Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solvents
2.2. Yeast Strain and Growth Conditions
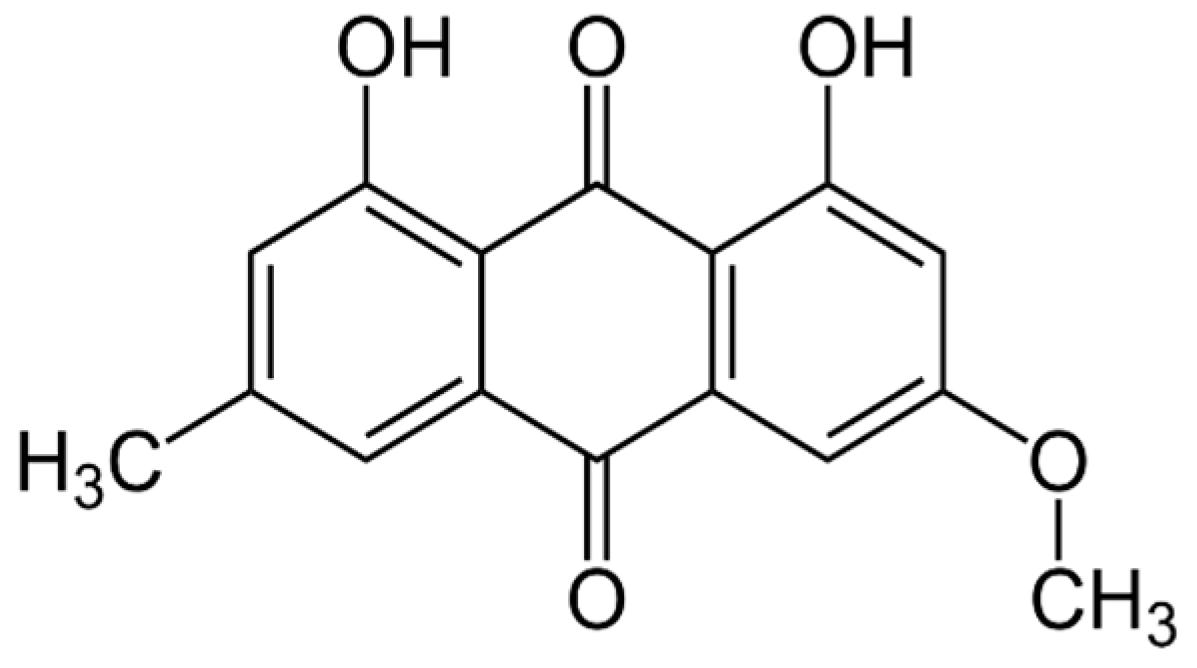
2.3. Natural Photosensitizer Tested
2.4. Irradiation System
2.5. Photoactive Minimum Inhibitory Concentration
2.6. Photoinactivation Biofilm Procedures
2.7. Mechanism Action Studies
2.8. Statistical Analysis
2.9. Declaration of Generative AI–AI-Assisted Technologies
3. Results
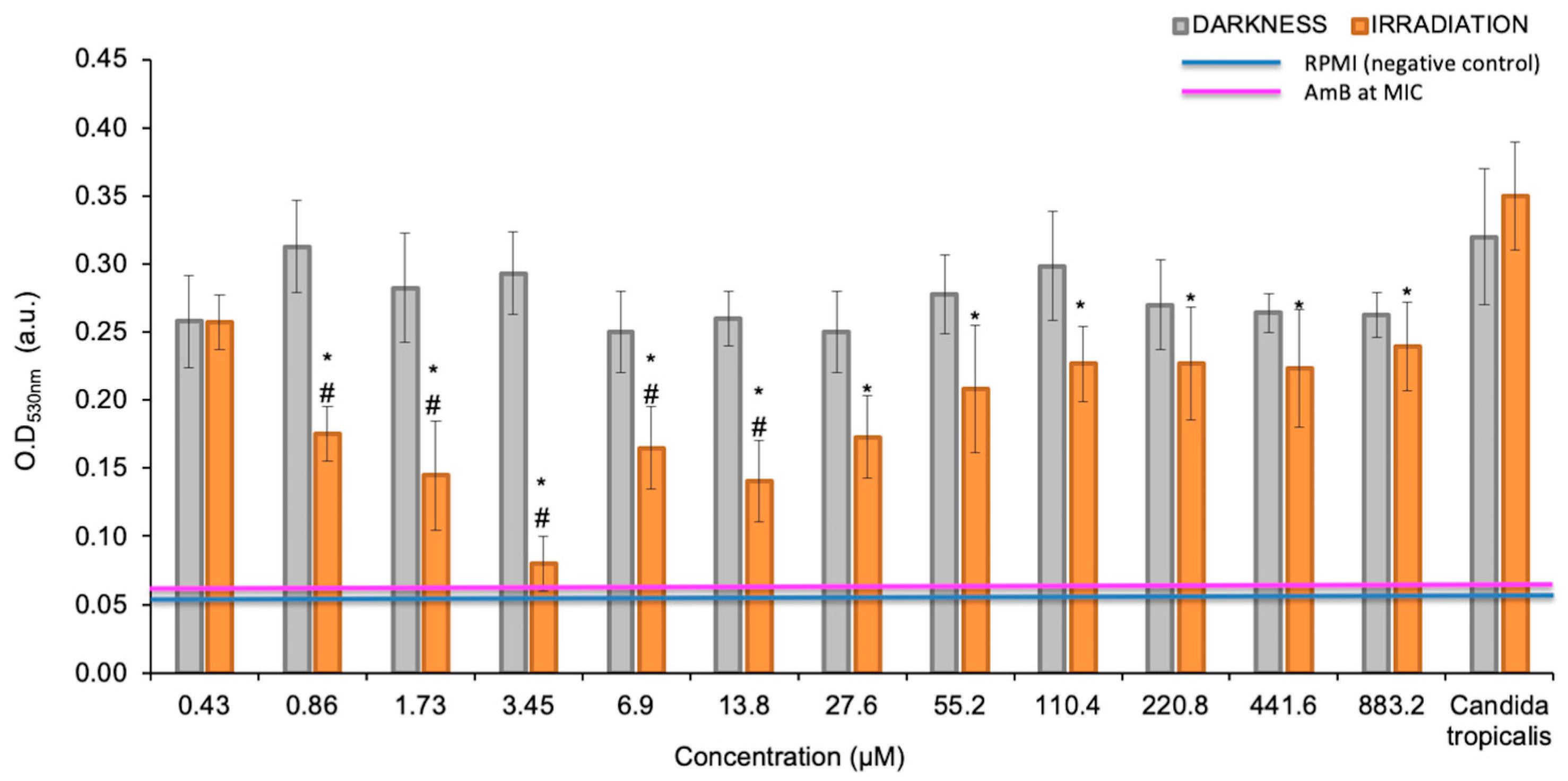
3.1. Photoactive Minimum Inhibitory Concentration (pMIC)
3.2. PTN Antimicrobial Photodynamic Therapy (APDT)
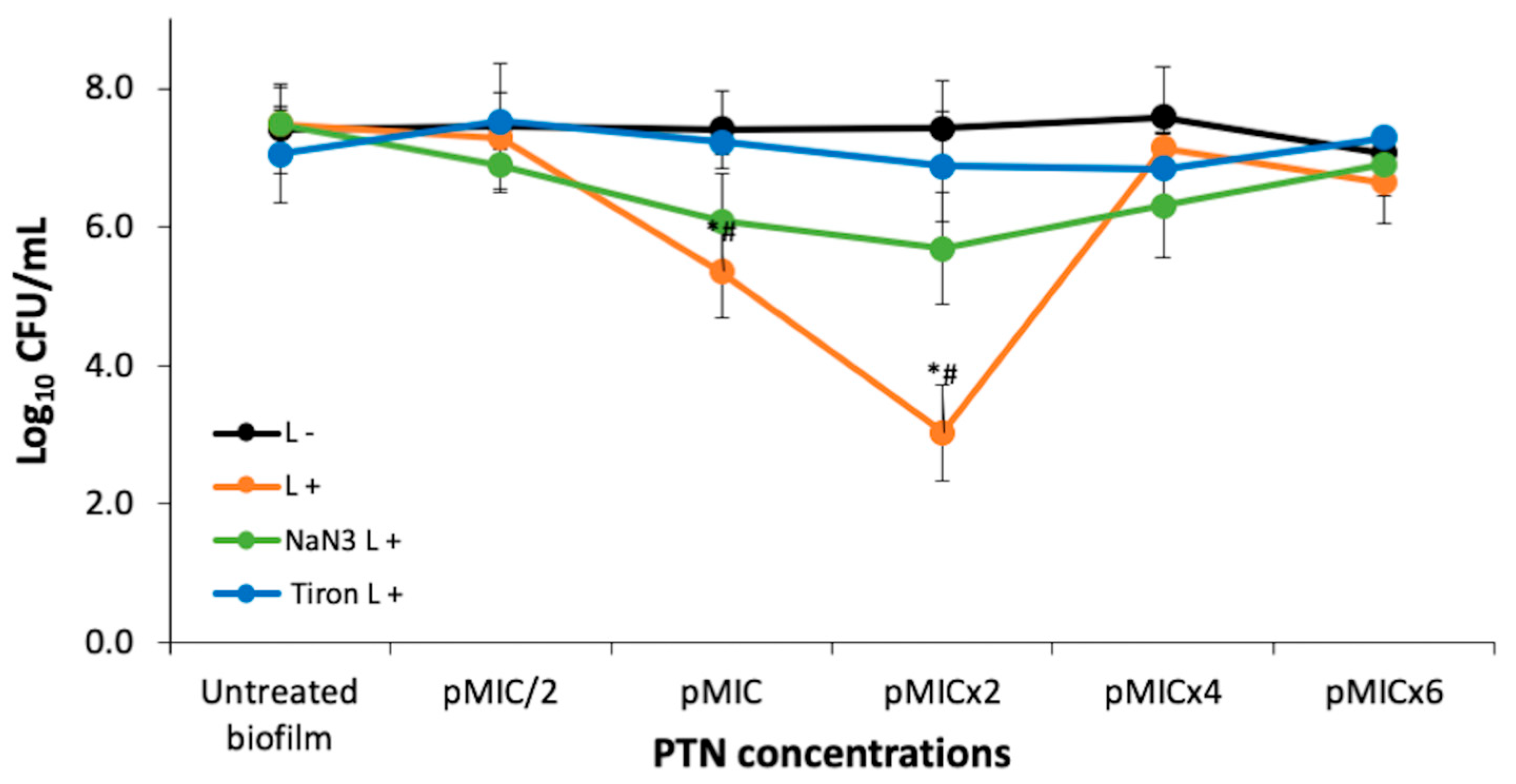
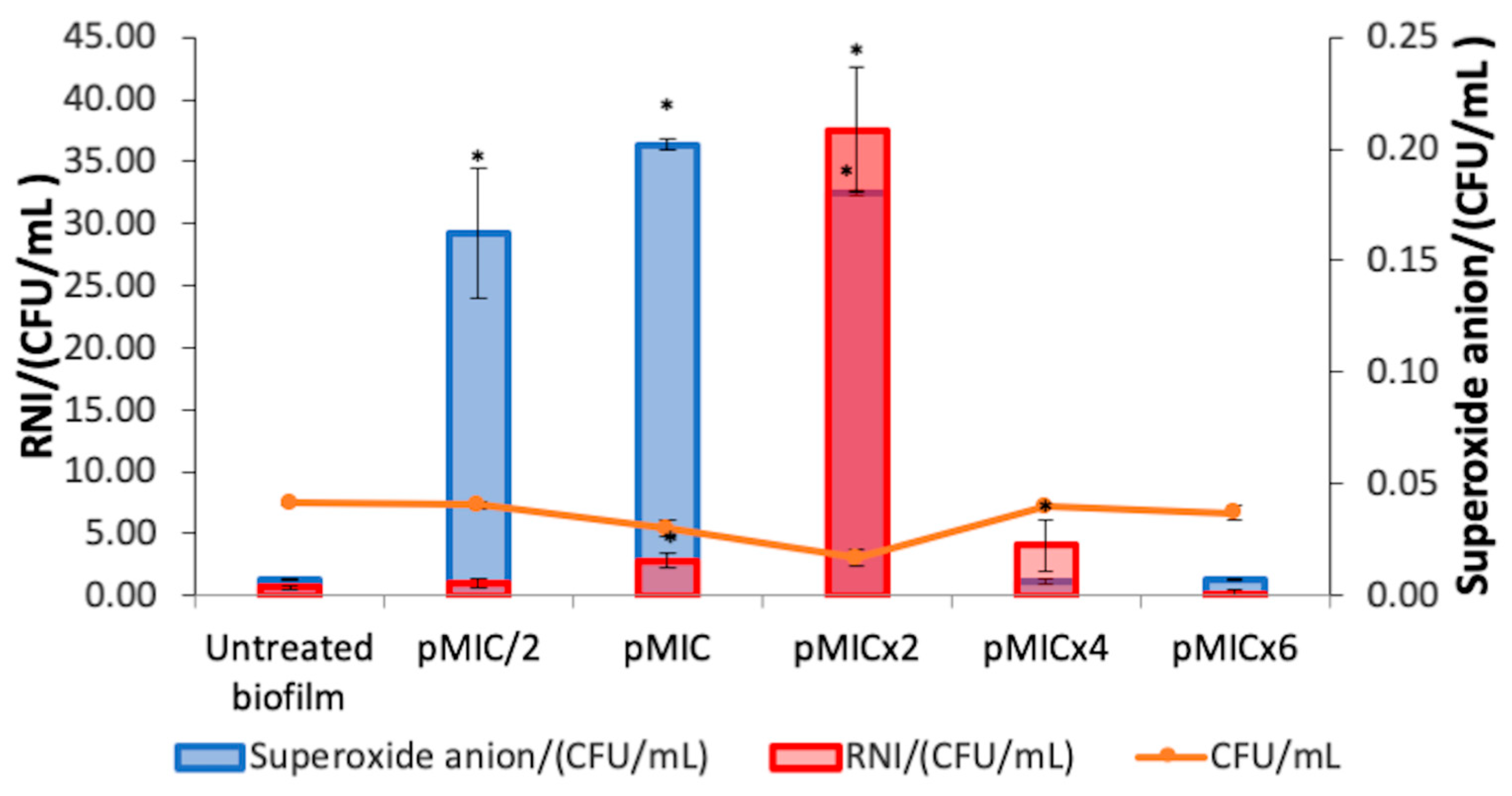
3.2.1. Photodynamic Mechanism on Analysis After PTN-APDT
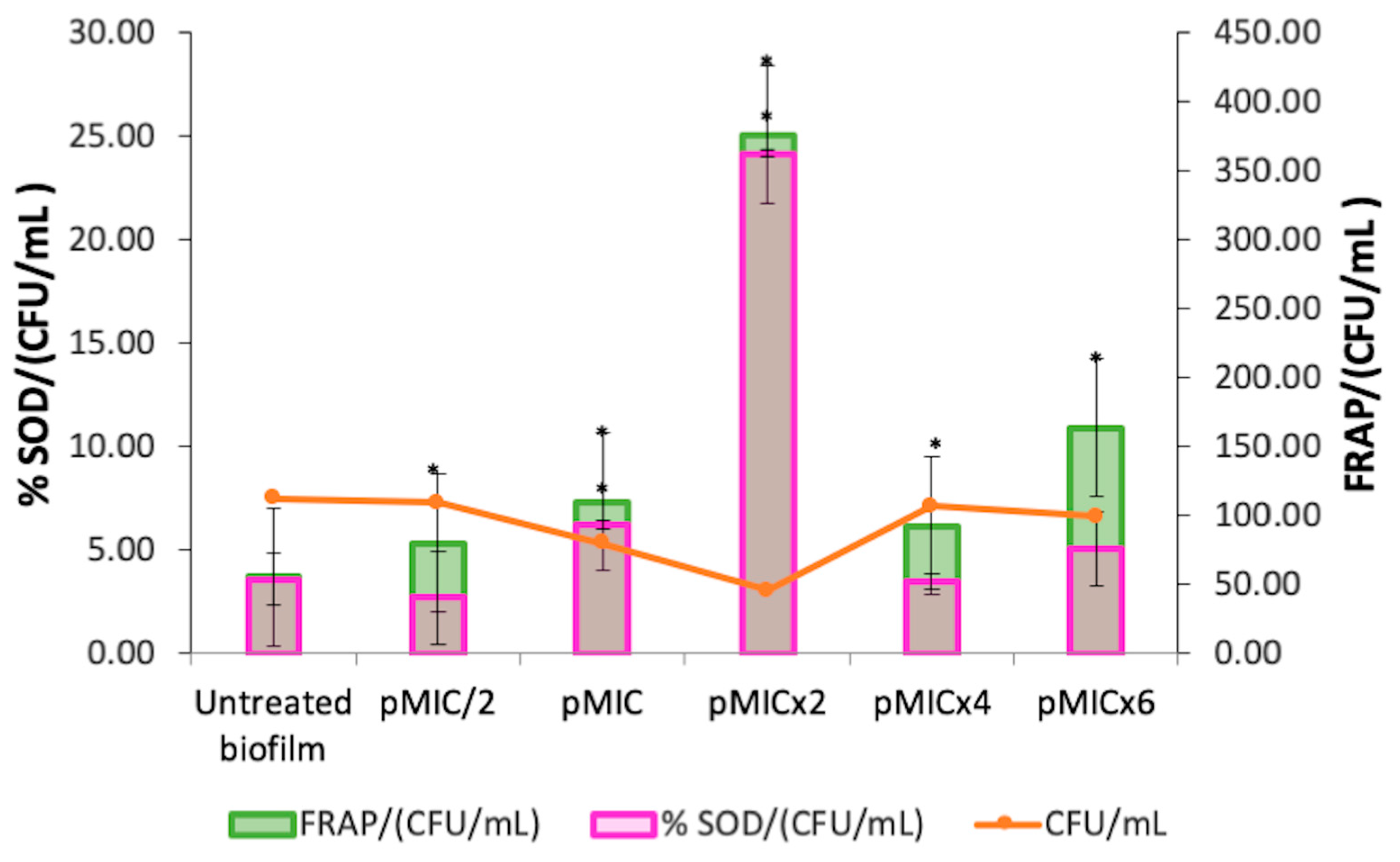
3.2.2. Biofilm Stress Response After PTN-APDT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ΦΔ | Quantum yield of singlet oxygen |
| +C | Positive control |
| 1O2 | Singlet oxygen |
| 3O2 | Molecular oxygen |
| AmB | Amphotericin B |
| aPDI | Antimicrobial photodynamic inactivation |
| aPDT | Antimicrobial photodynamic therapy |
| AQ/s | Anthraquinone/s |
| ATM | Antimicrobial |
| CFU | Colony-forming units |
| CLSI | Clinical and Laboratory Standards Institute |
| EtOH | Ethanol |
| FeSO4 | Ferrous sulfate |
| FRAP | Ferrous Reduction Antioxidant Potency assay |
| H2O2 | Hydrogen peroxide |
| HO• | Hydroxyl radical |
| MIC | Minimal inhibitory concentration |
| MOPS | Morpholine propane sulfonic acid |
| NaN3 | Sodium azide |
| NaNO2 | Sodium nitrate |
| NaOH | Sodium hydroxide |
| NBT | Nitro-blue tetrazolium |
| NCPF | National Collection of Pathogenic Fungi |
| NO | Nitric oxide |
| O2•− | Superoxide radical anion |
| OD | Optical density |
| PBS | Phosphate buffered saline |
| pMIC | Photoactive minimal inhibitory concentration |
| PS | Photosensitizer |
| PTN | Parietin |
| RNI | Reactive nitrogen intermediates |
| ROS | Reactive oxygen species |
| RPMI | Roswell Park Memorial Institute 1640 |
| SDA | Sabouraud dextrose agar |
| SDB | Sabouraud dextrose broth |
| SOD | Superoxide dismutase |
| MFC | Minimum fungicidal concentration |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| -OH | Hydroxyl groups |
| DNA | Deoxyribonucleic acid |
| −OONO− | Peroxynitrite |
References
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial Resistance: Impacts, Challenges, and Future Prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Riera, F.O.; Caeiro, J.P.; Angiolini, S.C.; Vigezzi, C.; Rodriguez, E.; Icely, P.A.; Sotomayor, C.E. Invasive Candidiasis: Update and Current Challenges in the Management of This Mycosis in South America. Antibiotics 2022, 11, 877. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef]
- Aroso, R.T.; Schaberle, F.A.; Arnaut, L.G.; Pereira, M.M. Photodynamic Disinfection and Its Role in Controlling Infectious Diseases. Photochem. Photobiol. Sci. 2021, 20, 1497–1545. [Google Scholar] [CrossRef]
- Klausen, M.; Ucuncu, M.; Bradley, M. Design of Photosensitizing Agents for Targeted Antimicrobial Photodynamic Therapy. Molecules 2020, 25, 5239. [Google Scholar] [CrossRef]
- Aware, C.B.; Patil, D.N.; Suryawanshi, S.S.; Mali, P.R.; Rane, M.R.; Gurav, R.G.; Jadhav, J.P. Natural Bioactive Products as Promising Therapeutics: A Review of Natural Product-Based Drug Development. S. Afr. J. Bot. 2022, 151, 512–528. [Google Scholar] [CrossRef]
- Ranković, B.; Kosanić, M. Lichens as a Potential Source of Bioactive Secondary Metabolites. In Lichen Secondary Metabolites: Bioactive Properties and Pharmaceutical Potential; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–29. [Google Scholar]
- Thakur, M.; Chander, H. Potential of lichens: A Review of Bioactive Compounds with Biological Activities. Biol. Forum Int. J. 2021, 13, 39–47. [Google Scholar]
- Poulsen-Silva, E.; Otero, M.C.; Diaz-Cornejo, S.; Atala, C.; Fuentes, J.A.; Gordillo-Fuenzalida, F. Secondary Metabolites of Lichens: The Untapped Biomedical and Pharmaceutical Potential of Antimicrobial Molecules. Fungal Biol. Rev. 2025, 51, 100410. [Google Scholar] [CrossRef]
- Kocovic, A.; Jeremic, J.; Bradic, J.; Sovrlic, M.; Tomovic, J.; Vasiljevic, P.; Andjic, M.; Draginic, N.; Grujovic, M.; Mladenovic, K.; et al. Phytochemical Analysis, Antioxidant, Antimicrobial, and Cytotoxic Activity of Different Extracts of Xanthoparmelia Stenophylla Lichen from Stara Planina, Serbia. Plants 2022, 11, 1624. [Google Scholar] [CrossRef]
- Kello, M.; Goga, M.; Kotorova, K.; Sebova, D.; Frenak, R.; Tkacikova, L.; Mojzis, J. Screening Evaluation of Antiproliferative, Antimicrobial and Antioxidant Activity of Lichen Extracts and Secondary Metabolites In Vitro. Plants 2023, 12, 611. [Google Scholar] [CrossRef]
- Aoussar, N.; Laasri, F.E.; Bourhia, M.; Manoljovic, N.; Mhand, R.A.; Rhallabi, N.; Ullah, R.; Shahat, A.A.; Noman, O.M.; Nasr, F.A.; et al. Phytochemical Analysis, Cytotoxic, Antioxidant, and Antibacterial Activities of Lichens. Evid. Based Complement. Alternat. Med. 2020, 2020, 8104538. [Google Scholar] [CrossRef]
- Christina Pires Gonçalves, L. Photophysical Properties and Therapeutic Use of Natural Photosensitizers. J. Photochem. Photobiol. 2021, 7, 100052. [Google Scholar] [CrossRef]
- Polat, E.; Kang, K. Natural Photosensitizers in Antimicrobial Photodynamic Therapy. Biomedicines 2021, 9, 584. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Marín, B.; Artetxe, U.; Becerril, J.M.; Martínez-Abaigar, J.; Núñez-Olivera, E.; García-Plazaola, J.I. Can Parietin Transfer Energy Radiatively to Photosynthetic Pigments? Molecules 2018, 23, 1741. [Google Scholar] [CrossRef]
- Comini, L.R.; Morán Vieyra, F.E.; Mignone, R.A.; Páez, P.L.; Laura Mugas, M.; Konigheim, B.S.; Cabrera, J.L.; Núñez Montoya, S.C.; Borsarelli, C.D. Parietin: An Efficient Photo-Screening Pigment in Vivo with Good Photosensitizing and Photodynamic Antibacterial Effects in Vitro. Photochem. Photobiol. Sci. 2017, 16, 201–210. [Google Scholar] [CrossRef]
- Hurtado Bredda, F.J.; Nin Vaeza, N.; Rubbo Amonini, H. Estrés oxidativo y nitrosativo en la sepsis. Med. Intensive 2005, 29, 159–165. [Google Scholar] [CrossRef]
- Baltazar, L.M.; Ray, A.; Santos, D.A.; Cisalpino, P.S.; Friedman, A.J.; Nosanchuk, J.D. Antimicrobial Photodynamic Therapy: An Effective Alternative Approach to Control Fungal Infections. Front. Microbiol. 2015, 6, 202. [Google Scholar] [CrossRef]
- Ong, K.S.; Mawang, C.I.; Daniel-Jambun, D.; Lim, Y.Y.; Lee, S.M. Current Anti-Biofilm Strategies and Potential of Antioxidants in Biofilm Control. Expert Rev. Anti Infect. Ther. 2018, 16, 855–864. [Google Scholar] [CrossRef]
- M27-A2; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard—Second Edition. NCCLS: Wayne, PA, USA, 2002.
- Mugas, M.L.; Calvo, G.; Marioni, J.; Céspedes, M.; Martinez, F.; Sáenz, D.; Di Venosa, G.; Cabrera, J.L.; Montoya, S.N.; Casas, A. Photodynamic Therapy of Tumour Cells Mediated by the Natural Anthraquinone Parietin and Blue Light. J. Photochem. Photobiol. B 2021, 214, 112089. [Google Scholar] [CrossRef]
- Marioni, J.; Arce, J.E.; Cabrera, J.L.; Paraje, M.G.; Núñez Montoya, S.C. Reduction of Candida tropicalis Biofilm by Photoactivation of a Heterophyllaea pustulata Extract. Pharm. Biol. 2016, 54, 2791–2801. [Google Scholar] [CrossRef]
- M27-A3; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard—Third Edition. CLSI: Wayne, PA, USA, 2008.
- Marioni, J.; Da Silva, M.A.; Cabrera, J.L.; Montoya, S.C.N.; Paraje, M.G. The Anthraquinones Rubiadin and Its 1-Methyl Ether Isolated from Heterophyllaea Pustulata Reduces Candida Tropicalis Biofilms Formation. Phytomedicine 2016, 23, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Marioni, J.; Bresolí-Obach, R.; Agut, M.; Comini, L.R.; Cabrera, J.L.; Paraje, M.G.; Nonell, S.; Montoya, S.C.N. On the Mechanism of Candida Tropicalis Biofilm Reduction by the Combined Action of Naturally-Occurring Anthraquinones and Blue Light. PLoS ONE 2017, 12, e0181517. [Google Scholar] [CrossRef]
- Mattila, H.; Khorobrykh, S.; Havurinne, V.; Tyystjärvi, E. Reactive Oxygen Species: Reactions and Detection from Photosynthetic Tissues. J. Photochem. Photobiol. B 2015, 152, 176–214. [Google Scholar] [CrossRef]
- Tsikas, D. Analysis of Nitrite and Nitrate in Biological Fluids by Assays Based on the Griess Reaction: Appraisal of the Griess Reaction in the l-Arginine/Nitric Oxide Area of Research. J. Chromatogr. B 2007, 851, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Youf, R.; Müller, M.; Balasini, A.; Thétiot, F.; Müller, M.; Hascoët, A.; Jonas, U.; Schönherr, H.; Lemercier, G.; Montier, T.; et al. Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics 2021, 13, 1995. [Google Scholar] [CrossRef]
- Fiala, J.; Roach, T.; Holzinger, A.; Husiev, Y.; Delueg, L.; Hammerle, F.; Armengol, E.S.; Schöbel, H.; Bonnet, S.; Laffleur, F.; et al. The Light-Activated Effect of Natural Anthraquinone Parietin against Candida Auris and Other Fungal Priority Pathogens. Planta Med. 2024, 90, 588–594. [Google Scholar] [CrossRef]
- Ma, W.; Liu, C.; Li, J.; Hao, M.; Ji, Y.; Zeng, X. The Effects of Aloe Emodin-Mediated Antimicrobial Photodynamic Therapy on Drug-Sensitive and Resistant Candida Albicans. Photochem. Photobiol. Sci. 2020, 19, 485–494. [Google Scholar] [CrossRef]
- Quishida, C.C.C.; De Oliveira Mima, E.G.; Jorge, J.H.; Vergani, C.E.; Bagnato, V.S.; Pavarina, A.C. Photodynamic Inactivation of a Multispecies Biofilm Using Curcumin and LED Light. Lasers Med. Sci. 2016, 31, 997–1009. [Google Scholar] [CrossRef]
- Alshehri, A.H. Mechanical and Antimicrobial Effects of Riboflavin-Mediated Photosensitization of in Vitro C. Albicans Formed on Polymethyl Methacrylate Resin. Photodiagnosis Photodyn. Ther. 2021, 36, 102488. [Google Scholar] [CrossRef]
- Janeczko, M.; Masłyk, M.; Kubiński, K.; Golczyk, H. Emodin, a Natural Inhibitor of Protein Kinase CK2, Suppresses Growth, Hyphal Development, and Biofilm Formation of Candida albicans. Yeast 2017, 34, 253–265. [Google Scholar] [CrossRef]
- Manoharan, R.K.; Lee, J.-H.; Kim, Y.-G.; Lee, J. Alizarin and Chrysazin Inhibit Biofilm and Hyphal Formation by Candida Albicans. Front. Cell. Infect. Microbiol. 2017, 7, 447. [Google Scholar] [CrossRef]
- Tsang, P.W.-K.; Bandara, H.M.H.N.; Fong, W.-P. Purpurin Suppresses Candida Albicans Biofilm Formation and Hyphal Development. PLoS ONE 2012, 7, e50866. [Google Scholar] [CrossRef]
- Galinari, C.B.; Biachi, T.D.P.; Gonçalves, R.S.; Cesar, G.B.; Bergmann, E.V.; Malacarne, L.C.; Kioshima Cotica, É.S.; Bonfim-Mendonça, P.D.S.; Svidzinski, T.I.E. Photoactivity of Hypericin: From Natural Product to Antifungal Application. Crit. Rev. Microbiol. 2023, 49, 38–56. [Google Scholar] [CrossRef]
- Łopaciński, M.; Fiegler-Rudol, J.; Niemczyk, W.; Skaba, D.; Wiench, R. Riboflavin- and Hypericin-Mediated Antimicrobial Photodynamic Therapy as Alternative Treatments for Oral Candidiasis: A Systematic Review. Pharmaceutics 2024, 17, 33. [Google Scholar] [CrossRef]
- Amorim, C.F.; Iglesias, B.A.; Pinheiro, T.R.; Lacerda, L.E.; Sokolonski, A.R.; Pedreira, B.O.; Moreira, K.S.; Burgo, T.A.L.; Meyer, R.; Azevedo, V.; et al. Photodynamic Inactivation of Different Candida Species and Inhibition of Biofilm Formation Induced by Water-Soluble Porphyrins. Photodiagnosis Photodyn. Ther. 2023, 42, 103343. [Google Scholar] [CrossRef]
- Souza, S.O.; Raposo, B.L.; Sarmento-Neto, J.F.; Rebouças, J.S.; Macêdo, D.P.C.; Figueiredo, R.C.B.Q.; Santos, B.S.; Freitas, A.Z.; Cabral Filho, P.E.; Ribeiro, M.S.; et al. Photoinactivation of Yeast and Biofilm Communities of Candida Albicans Mediated by ZnTnHex-2-PyP4+ Porphyrin. J. Fungi 2022, 8, 556. [Google Scholar] [CrossRef]
- Bors, W. Pulse-Radiolytic Investigations of Catechols and Catecholamines II. Reactions of Tiron with Oxygen Radical Species. Biochim. Biophys. Acta BBA Gen. Subj. 1979, 582, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.W.; Skovsen, E.; Lambert, J.D.C.; Poulsen, L.; Ogilby, P.R. Optical Detection of Singlet Oxygen from Single Cells. Phys. Chem. Chem. Phys. 2006, 8, 4280. [Google Scholar] [CrossRef] [PubMed]
- Vatansever, F.; De Melo, W.C.M.A.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial Strategies Centered around Reactive Oxygen Species—Bactericidal Antibiotics, Photodynamic Therapy, and Beyond. FEMS Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef] [PubMed]
- Elian, C.; Méallet, R.; Versace, D. Photoactive Dye–Loaded Polymer Materials: A New Cutting Edge for Antibacterial Photodynamic Therapy. Adv. Funct. Mater. 2024, 34, 2407228. [Google Scholar] [CrossRef]
- Wink, D.A.; Mitchell, J.B. Chemical Biology of Nitric Oxide: Insights into Regulatory, Cytotoxic, and Cytoprotective Mechanisms of Nitric Oxide. Free Radic. Biol. Med. 1998, 25, 434–456. [Google Scholar] [CrossRef] [PubMed]
- Fraix, A.; Sortino, S. Combination of PDT Photosensitizers with NO Photodononors. Photochem. Photobiol. Sci. 2018, 17, 1709–1727. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, H.; Tao, X.; Xie, Y.; Yang, L.; Mao, Z.; Xia, W. Light-Triggered Nitric Oxide Release by a Photosensitizer to Combat Bacterial Biofilm Infections. Chem. Eur. J. 2021, 27, 5453–5460. [Google Scholar] [CrossRef]
- Tavares, A.; Carvalho, C.M.B.; Faustino, M.A.; Neves, M.G.P.M.S.; Tomé, J.P.C.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Gomes, N.C.M.; Alves, E.; et al. Antimicrobial Photodynamic Therapy: Study of Bacterial Recovery Viability and Potential Development of Resistance after Treatment. Mar. Drugs 2010, 8, 91–105. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marioni, J.; Romero, B.C.; Mugas, M.L.; Martinez, F.; Gómez, T.I.; Morales, J.M.N.; Konigheim, B.S.; Borsarelli, C.D.; Nuñez-Montoya, S.C. The Natural Anthraquinone Parietin Inactivates Candida tropicalis Biofilm by Photodynamic Mechanisms. Pharmaceutics 2025, 17, 548. https://doi.org/10.3390/pharmaceutics17050548
Marioni J, Romero BC, Mugas ML, Martinez F, Gómez TI, Morales JMN, Konigheim BS, Borsarelli CD, Nuñez-Montoya SC. The Natural Anthraquinone Parietin Inactivates Candida tropicalis Biofilm by Photodynamic Mechanisms. Pharmaceutics. 2025; 17(5):548. https://doi.org/10.3390/pharmaceutics17050548
Chicago/Turabian StyleMarioni, Juliana, Bianca C. Romero, Ma. Laura Mugas, Florencia Martinez, Tomas I. Gómez, Jesús M. N. Morales, Brenda S. Konigheim, Claudio D. Borsarelli, and Susana C. Nuñez-Montoya. 2025. "The Natural Anthraquinone Parietin Inactivates Candida tropicalis Biofilm by Photodynamic Mechanisms" Pharmaceutics 17, no. 5: 548. https://doi.org/10.3390/pharmaceutics17050548
APA StyleMarioni, J., Romero, B. C., Mugas, M. L., Martinez, F., Gómez, T. I., Morales, J. M. N., Konigheim, B. S., Borsarelli, C. D., & Nuñez-Montoya, S. C. (2025). The Natural Anthraquinone Parietin Inactivates Candida tropicalis Biofilm by Photodynamic Mechanisms. Pharmaceutics, 17(5), 548. https://doi.org/10.3390/pharmaceutics17050548







