Beyond Tailpipe Emissions: Life Cycle Assessment Unravels Battery’s Carbon Footprint in Electric Vehicles
Abstract
1. Introduction
2. Materials and Methods
2.1. Conventional Technologies
- Lead–Acid Batteries
- Nickel–Metal Hydride
- Lithium-Ion
- NCM—Lithium Nickel Cobalt Manganese Oxide (LiNiMnCoO2)
- NCA—Lithium Nickel Cobalt Aluminum Oxide (LiNiCoAlO2)
- LFP—Lithium Iron Phosphate (LiFePO4)
- Zn-Ion batteries
2.2. Emerging Technologies
- Solid state batteries (SSB’s)
- Sodium ion batteries (SIB’s)
2.3. Life Cycle Assessment Methodology
- Comparative analysis: Highlighting the environmental strengths and weaknesses of different battery chemistries (e.g., NCM, LFP, LMO).
- Informed decision-making: Guiding manufacturers and policymakers in developing sustainable practices for battery production and use.
- Continuous improvement: Identifying hotspots (processes with significant environmental impacts) across the life cycle, promoting innovation and optimization.
3. Discussion
3.1. Regional Differences in Life Cycle Greenhouse Gas Emissions of NCM Batteries
3.2. Research Gaps and Recommendations
3.2.1. Life Cycle Analysis of Conventional Batteries for Automobiles
Lithium Nickel Cobalt Manganese Oxide (NMC) Batteries
Lithium Iron Phosphate (LFP) and Lithium-Ion Manganese Oxide (LMO) Batteries
3.2.2. Life Cycle Assessment of Emerging Batteries for Automobiles
Solid State Batteries (SSBs)
Sodium Ion Batteries (SIBs)
3.3. Recommendations
- Transport stakeholders should invest in research and development to reduce the reliance on critical materials like cobalt and nickel in battery production and promote hybridization since our study reported lower greenhouse gas emissions on new emerging technologies compared to conventional batteries with cobalt and nickel. Minimal usage of batteries helps to reduce the footprint such as with its implementation in hybrid vehicles.
- Our review suggests that the manufacturers should prioritize the development of sustainable sourcing practices and ethical mining for critical materials to reduce the carbon intensity of battery production and overall impacts of electrical vehicles.
- Our study found that the SSB’s and SIB’s have the lowest emissions due to the materials, which will suggest that (a) the manufacturers and researchers should work towards broadening their spectrum towards designing battery technologies more sustainably with enhanced results. (b) There should be continued research into solid-state battery technology which should focus on improving the environmental footprint and scalability of solid electrolytes. (c) The transport industry key stakeholders should support the development and adoption of sodium-ion batteries as a more sustainable alternative to lithium-ion batteries.
- Battery recycling and second-life applications should be encouraged to minimize waste and resource depletion in the electric vehicle industry.
4. Conclusions
- -
- NCM/NCA batteries, while offering high energy density, pose significant challenges due to the limited availability of cobalt and nickel.
- -
- LFP batteries, with their lower environmental impact and abundance of iron and phosphate, present a more sustainable option for electric vehicles, especially in applications where high energy density is not critical.
- -
- Research related to combinations/blending of different battery technologies has significant potential to produce synergic effects on their electrodes, enriching their energy densities, improving their rate capabilities, and increasing their life cycle.
- -
- SSB technology holds promise in terms of safety and energy density but requires further research and development to address the environmental impact and scalability challenges associated with solid electrolytes.
- -
- SIBs, utilizing abundant sodium, offer a viable alternative to lithium-ion batteries, especially in regions where sodium resources are more accessible.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
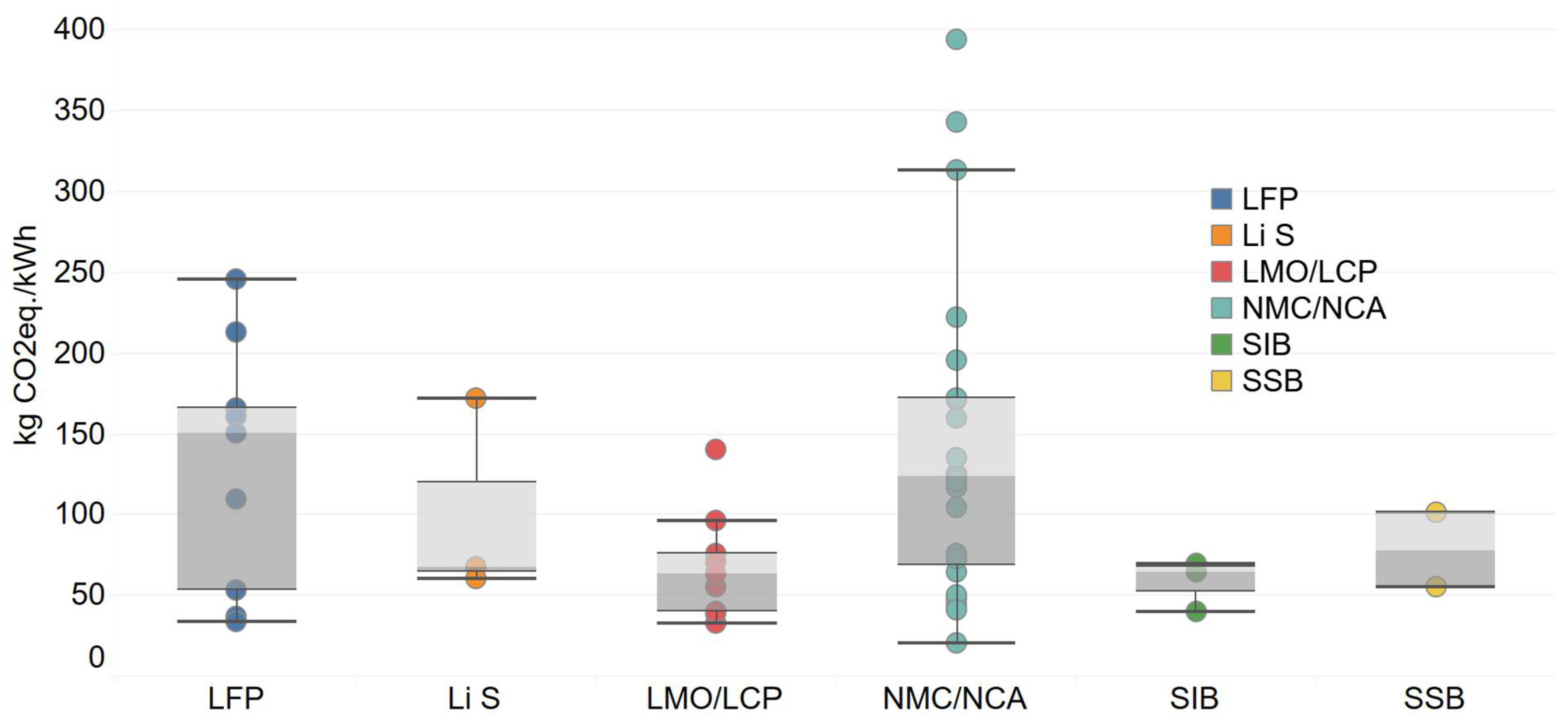
| Battery Technology | Literature | kg CO2-eq/kwh | References | Functional Unit | Impact Assessment Method | System Boundary |
|---|---|---|---|---|---|---|
| LFP | Amarakoon et al., 2013 | 151 | [96] | Distance travelled by vehicles lifetime | - | Cradle to grave |
| LMO/LCP | Amarakoon et al., 2013 | 63.4 | [96] | Distance travelled by vehicles lifetime | - | Cradle to grave |
| NMC/NCA | Amarakoon et al., 2013 | 121 | [96] | Distance travelled by vehicles lifetime | - | Cradle to grave |
| LFP | Ambrose and Kendall, 2016 | 33.9 | [48] | 1 metric ton of batteries | GWP, TETP and HTP | End-of-Life (Recycling phase) |
| LMO/LCP | Ambrose and Kendall, 2016 | 39.83 | [48] | 1 metric ton of batteries | GWP, TETP and HTP | End-of-Life (Recycling phase) |
| NMC/NCA | Ambrose and Kendall, 2016 | 41.39 | [48] | 1 metric ton of batteries | GWP, TETP and HTP | End-of-Life (Recycling phase) |
| Li S | Barke et al., 2022 | 60.4 | [82] | 1 battery pack | ReCiPe Midpoint (H) V1.13 method | cradle-to-gate |
| NMC/NCA | Bauer et al., 2010 | 135 | [97] | - | GWP, HTP, AP, EP and ETP/CML and EI99 | Cradle-to-Gate |
| SIB | Peters et al., 2017 | 40 | [93] | 1 kg and 1kWh | GWP, AP, EP, ODP, HTP, ADP and CED/Ecoinvent 3.4 ReCiPe 2008, Eco-Indicator 99/minerals and CML-IA 2002 | - |
| NMC/NCA | Benveniste et al., 2022 | 394 | [31] | 1 kWh | CML and ReCiPe 2008 | cradle to the grave |
| NMC/NCA | Cusenza et al., 2019 | 313 | [98] | One LMO-NMC battery pack 140,000 km | CED, ADP, ODP, PMFP, IR, GWP, HTP, POFP, AP and EP/IPCC 2007 | End-of-Life (Recycling phase) |
| NMC/NCA | Dai et al., 2019 | 72.9 | [99] | 1 kWh | GWP, AP and PMFP/GREET 2018 | Cradle-to-gate |
| NMC/NCA | Deng et al., 2018 | 343 | [100] | km | 13 impact categories measured using ReCiPe method | Cradle-to-grave |
| LMO/LCP | Dunn et al., 2012 | 39 | [101] | kg battery | GWP using GREET | cradle-to-gate and recycling stages |
| NMC/NCA | Dunn et al., 2012 | 50 | [101] | kg battery | GWP using GREET | cradle-to-gate and recycling stages |
| NMC/NCA | Ellingsen et al., 2014 | 172 | [102] | 1 battery | GWP, FDP, ODP, POFP. PMFP, TAP, FEP, MEP, FETP, METP, TETP, HTP and MDP/ReCiPe Midpoint | Cradle-to-gate |
| LMO/LCP | Faria et al., 2014 | 70.9 | [103] | 200,000 vehicle km (service life of the vehicle) | ADP, AP, EP and GWP/CML-IA 2001 | well-to-wheel |
| LFP | GREET, 2018 | 36.5 | [66] | 1 kWh | GWP, AP and PMFP/GREET 2018 | Cradle-to-Gate |
| LMO/LCP | GREET, 2018 | 32.9 | [66] | 1 kWh | GWP, AP and PMFP/GREET 2018 | Cradle-to-Gate |
| LFP | Hao et al., 2017 | 109.3 | [50] | 1 kWh | GREET 2015 | Cradle-to-Gate |
| LMO/LCP | Hao et al., 2017 | 96.6 | [50] | 1 kWh | GREET 2015 | Cradle-to-Gate |
| NMC/NCA | Hao et al., 2017 | 104 | [50] | 1 kWh | GREET 2015 | Cradle-to-Gate |
| NMC/NCA | Hendrickson et al., 2015 | 44 | [104] | 1 battery | GREET 2015 | End-of-Life (Recycling phase) |
| NMC/NCA | Jenu et al., 2020 | 172 | [105] | 1 kWh | GWP, Ecoinvent 3.5 | Cradle-to-Gate |
| NMC/NCA | Kallitsis et al., 2020 | 171 | [106] | Production of one traction battery | GWP, FDP, ODP, POFP, PMFP, TAP, FEP, HTP, MEP, FETP, METP, TETP and MDP | Cradle-to-Gate- |
| NMC/NCA | Kelly et al., 2020 | 65 | [57] | 1 kWh | GREET | well-to-wheels |
| LMO/LCP | Kim et al., 2016 | 140 | [72] | 1 kWh | GWP, AP, EP, PMFP and POFP | Cradle-to-Gate |
| SSB | Lastoskie et al., 2015 | 55 | [71] | 120,000 km | CED, GWP, HTP, WDP, MDP, PMFP, POFP and FEP | Cradle-to-Gate |
| NMC/NCA | Philippot et al., 2019 | 123 | [107] | 1 kWh | IPCC 2013 method V1.03. | Cradle-to-Gate |
| LFP | Majeau-Bettez et al., 2011 | 246 | [108] | 50 MJ (100 km) | GWP, CED, FDP, FETP, FEP, HTP, METP, MEP, MDP, ODP, PMFP, TAP and TETP/ReCiPe Midpoint | well-to-wheel |
| NMC/NCA | Majeau-Bettez et al., 2011 | 196 | [108] | 50 MJ (100 km) | GWP, CED, FDP, FETP, FEP, HTP, METP, MEP, MDP, ODP, PMFP, TAP and TETP/ReCiPe Midpoint | well-to-wheel |
| LMO/LCP | Raugei et al., 2019 | 76.1 | [109] | 17 kWh battery pack | CED, GWP | ‘cradle-to-gate’ + End-of-Life boundary |
| NMC/NCA | Mohr et al., 2020 | 75.5 | [110] | 1 kWh | GWP and ADP/ILCD midpoint, OpenLCA 1.7.4 and Ecoinvent 3.4 | - |
| LFP | Notter et al., 2010 | 53 | [111] | 1 kg | CED, AP, ODP, EP, PMFP, GWP and ADP/EI 99 Endpoint and CML-IA 2002 | well-to-wheels |
| SIB | Peters et al., 2016 | 70 | [112] | 1 kg and 1 kWh | GWP, AP, EP, ODP, HTP, ADP and CED/Ecoinvent 3.4 ReCiPe 2008, Eco- Indicator 99/minerals and CML-IA 2002 | - |
| LFP | Peters et al., 2016 | 161 | [112] | 1 kg and 1 kWh | GWP, AP, EP, ODP, HTP, ADP and CED/Ecoinvent 3.4 ReCiPe 2008, Eco-Indicator 99/minerals and CML-IA 2002 | - |
| LMO/LCP | Peters et al., 2016 | 55 | [112] | 1 kg and 1 kWh | GWP, AP, EP, ODP, HTP, ADP and CED/Ecoinvent 3.4 ReCiPe 2008, Eco-Indicator 99/minerals and CML-IA 2002 | - |
| NMC/NCA | Peters et al., 2016 | 160 | [112] | 1 kg and 1 kWh | GWP, AP, EP, ODP, HTP, ADP and CED/Ecoinvent 3.4 ReCiPe 2008, Eco-Indicator 99/minerals and CML-IA 2002 | - |
| SSB | Popien et al., 2023 | 101 | [78] | 1 traction battery | climate change, human toxicity, mineral resource depletion, photochemical oxidant formation | cradle-to-gate |
| NMC/NCA | Qiao et al., 2017 | 117 | [113] | - | CED, GWP | Cradle-to-Gate |
| NMC/NCA | Sun et al., 2020 | 124.5 | [114] | 1 kWh | PED, GWP, AP, POCP, PMFP, MDP, FDP, EP and HTP/CML-IA baseline V3.02 | cradle-to-grave |
| LFP | Thomas et al., 2020 | 213 | [115] | 8.1 kWh | CED, GWP and MRS/ReCiPe Endpoint (H) V1.13 and World ReCiPe H/A | cradle-to-gate |
| Li S | Wang et al., 2020 | 67.94 | [116] | 200,000 km | GWP, ODP, PMFP, TAP, TETP, METP, FEP, FETP, HTP, MEP, METP, POFP and MDP/ReCiPe midpoint (H) and Gabi 9.2 software | Cradle-to-grave |
| SIB | Wang et al., 2020 | 64.35 | [116] | 200,000 km | GWP, ODP, PMFP, TAP, TETP, METP, FEP, FETP, HTP, MEP, METP, POFP and MDP/ReCiPe midpoint (H) and Gabi 9.2 software | Cradle-to-grave |
| Li S | Wickerts et al., 2023 | 172.5 | [81] | 1 MWh | ReCiPe 2016 | cradle-to-gate and cradle-to-grave |
| NMC/NCA | Sun et al., 2020 | 124.5 | [114] | 1 kWh | PED, GWP, AP, POCP, PMFP, MDP, FDP, EP and HTP/CML-IA baseline V3.02 | cradle-to-grave |
| LFP | Zackrisson et al., 2010 | 166 | [117] | 10 kWh | GWP, AP, EP, ODP and POFP/CML | well-to-wheel |
References
- IEA. Tracking Clean Energy Progress 2023, IEA, Paris. Available online: https://www.iea.org/reports/tracking-clean-energy-progress-2023 (accessed on 16 March 2024).
- IEA. Global CO2 Emissions from Transport by Sub-Sector in the Net Zero Scenario, 2000–2030, IEA, Paris. Available online: https://www.iea.org/data-and-statistics/charts/global-co2-emissions-from-transport-by-sub-sector-in-the-net-zero-scenario-2000-2030-2 (accessed on 16 March 2024).
- McCollum, D.L.; Wilson, C.; Bevione, M.; Carrara, S.; Edelenbosch, O.Y.; Emmerling, J.; Guivarch, C.; Karkatsoulis, P.; Keppo, I.; Krey, V.; et al. Interaction of consumer preferences and climate policies in the global transition to low-carbon vehicles. Nat. Energy 2018, 3, 664–673. [Google Scholar] [CrossRef]
- Ellingsen, L.A.-W.; Singh, B.; Strømman, A.H. The size and range effect: Lifecycle greenhouse gas emissions of electric vehicles. Environ. Res. Lett. 2016, 11, 054010. [Google Scholar] [CrossRef]
- Hawkins, T.R.; Singh, B.; Majeau-Bettez, G.; Strømman, A.H. Comparative Environmental Life Cycle Assessment of Conventional and Electric Vehicles. J. Ind. Ecol. 2013, 17, 53–64. [Google Scholar] [CrossRef]
- IEA. Comparative Life-Cycle Greenhouse Gas Emissions of a Mid-Size BEV and ICE Vehicle, IEA, Paris. 2021. Available online: https://www.iea.org/data-and-statistics/charts/comparative-life-cycle-greenhouse-gas-emissions-of-a-mid-size-bev-and-ice-vehicle (accessed on 16 March 2024).
- Lattanzio, R.K.; Clark, C.E. No. R46420; Environmental Effects of Battery Electric and Internal Combustion Engine Vehicles. 2020. Available online: https://crsreports.congress.gov/product/pdf/R/R46420 (accessed on 16 March 2024).
- Bieker, G. A global comparison of the life-cycle greenhouse gas emissions of combustion engine and electric passenger cars. Communications 2021, 49, 1877590. [Google Scholar]
- Raugei, M.; Aleix, P.O.N.S.; Vasileiadis, N.; Hugo, O.N.G.; Casullo, L. Research for TRAN Committee—Environmental Challenges through the Life Cycle of Battery Electric Vehicles; European Parliament, Policy Department for Structural and Cohesion Policies: Brussels, Belgium, 2023. [Google Scholar]
- European Parliament; Directorate-General for Internal Policies of the Union; Hill, N.; Raugei, M.; Pons, A. Environmental Challenges through the Life Cycle of Battery Electric Vehicles: Research for TRAN Committee, European Parliament. 2023; Available online: https://data.europa.eu/doi/10.2861/43673 (accessed on 16 March 2024).
- IEA. By 2030 EVs Represent More than 60% of Vehicles Sold Globally, and Require an Adequate Surge in Chargers Installed in Buildings, IEA, Paris. 2022. Available online: https://www.iea.org/reports/by-2030-evs-represent-more-than-60-of-vehicles-sold-globally-and-require-an-adequate-surge-in-chargers-installed-in-buildings (accessed on 16 March 2024).
- Atlas EV Hub. Quarterly U.S. Light-Duty Electric Vehicle Sales by Automaker. Available online: https://www.atlasevhub.com/materials/automakers-dashboard/ (accessed on 16 March 2024).
- Global EV Sales for 2023. EV Volumes. Available online: https://ev-volumes.com/news/ev/global-ev-sales-for-2023/ (accessed on 27 February 2024).
- Colin McKerracher. Electric Vehicle Outlook 2023. BloombergNEF. Available online: https://about.bnef.com/electric-vehicle-outlook/ (accessed on 16 March 2024).
- IEA. Global EV Outlook 2023, IEA, Paris. 2023. Available online: https://www.iea.org/reports/global-ev-outlook-2023 (accessed on 16 March 2024).
- Rajper, S.Z.; Albrecht, J. Prospects of Electric Vehicles in the Developing Countries: A Literature Review. Sustainability 2020, 12, 1906. [Google Scholar] [CrossRef]
- Liu, X.; Elgowainy, A.; Vijayagopal, R.; Wang, M. Well-to-Wheels Analysis of Zero-Emission Plug-In Battery Electric Vehicle Technology for Medium- and Heavy-Duty Trucks. Environ. Sci. Technol. 2020, 55, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Wang, M.; Lu, Z.; Kelly, J. Taking into account greenhouse gas emissions of electric vehicles for transportation de-carbonization. Energy Policy 2021, 155, 112353. [Google Scholar] [CrossRef]
- Mastoi, M.S.; Zhuang, S.; Munir, H.M.; Haris, M.; Hassan, M.; Usman, M.; Bukhari, S.S.H.; Ro, J.-S. An in-depth analysis of electric vehicle charging station infrastructure, policy implications, and future trends. Energy Rep. 2022, 8, 11504–11529. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, H.; Lin, F. Sustainable Electric Vehicle Batteries for a Sustainable World: Perspectives on Battery Cathodes, Environment, Supply Chain, Manufacturing, Life Cycle, and Policy. Adv. Energy Mater. 2022, 12, 2200383. [Google Scholar] [CrossRef]
- Xu, C.; Steubing, B.; Hu, M.; Harpprecht, C.; van der Meide, M.; Tukker, A. Future greenhouse gas emissions of automotive lithium-ion battery cell production. Resour. Conserv. Recycl. 2022, 187, 106606. [Google Scholar] [CrossRef]
- Andreasi Bassi, S.; Peters, J.F.; Candelaresi, D.; Valente, A.; Ferrara, N.; Mathieux, F.; Ardente, F. Rules for the calculation of the Carbon Footprint of Electric Vehicle Batteries (CFB-EV), Publications Office of the European Union, Luxembourg. 2023. Available online: https://eplca.jrc.ec.europa.eu/permalink/battery/GRB-CBF_CarbonFootprintRules-EV_June_2023.pdf (accessed on 16 March 2024).
- Timms, P.; King, M. EU Battery Regulations 2023: UK Readiness for Battery Passports and Smart Labelling-Report on a Round Table Discussion and Follow-Up Interviews; Loughborough University: Loughborough, UK, 2023. [Google Scholar]
- Berger, K.; Schöggl, J.-P.; Baumgartner, R.J. Digital battery passports to enable circular and sustainable value chains: Conceptualization and use cases. J. Clean. Prod. 2022, 353, 131492. [Google Scholar] [CrossRef]
- OU. State of the Art of Life Cycle Inventory Data for Electric Vehicle Batteries. In KTH, School of Architecture and the Built Environment (ABE), Sustainable Development, Environmental Science and Engineering. 15 March 2018. Available online: https://www.diva-portal.org/smash/get/diva2:1163424/FULLTEXT01.pdf (accessed on 10 January 2024).
- Torabi, F.; Ahmadi, P. Battery Technologies; Elsevier eBooks: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Hammou, A.; Meng, J.; Diallo, D.; Petrone, R.; Gualous, H. State-of-health prediction of Li-ion NMC Batteries Using Kalman Filter and Gaussian Process Regression. In Proceedings of the 2023 Prognostics and Health Management Conference (PHM), Paris, France, 31 May–2 June 2023; pp. 226–231. [Google Scholar]
- Hasan, A.; Hossain, R.; Sahajwalla, V. Critical metals (Lithium and Zinc) recovery from battery waste, ores, brine, and steel dust: A review. Process. Saf. Environ. Prot. 2023, 178, 976–994. [Google Scholar] [CrossRef]
- Erakca, M.; Bautista, S.P.; Moghaddas, S.; Baumann, M.; Bauer, W.; Leuthner, L.; Weil, M. Closing gaps in LCA of lithium-ion batteries: LCA of lab-scale cell production with new primary data. J. Clean. Prod. 2023, 384, 135510. [Google Scholar] [CrossRef]
- Zhang, T.; Bai, Y.; Shen, X.; Zhai, Y.; Ji, C.; Ma, X.; Hong, J. Cradle-to-gate life cycle assessment of cobalt sulfate production derived from a nickel–copper–cobalt mine in China. Int. J. Life Cycle Assess. 2021, 26, 1198–1210. [Google Scholar] [CrossRef]
- Benveniste, G.; Sánchez, A.; Rallo, H.; Corchero, C.; Amante, B. Comparative life cycle assessment of Li-Sulphur and Li-ion batteries for electric vehicles. Resour. Conserv. Recycl. Adv. 2022, 15, 200086. [Google Scholar] [CrossRef]
- Mandade, P.; Weil, M.; Baumann, M.; Wei, Z. Environmental life cycle assessment of emerging solid-state batteries: A review. Chem. Eng. J. Adv. 2023, 13, 100439. [Google Scholar] [CrossRef]
- Lai, X.; Chen, Q.; Tang, X.; Zhou, Y.; Gao, F.; Guo, Y.; Bhagat, R.; Zheng, Y. Critical review of life cycle assessment of lithium-ion batteries for electric vehicles: A lifespan perspective. eTransportation 2022, 12, 100169. [Google Scholar] [CrossRef]
- Lajunen, A.; Sainio, P.; Laurila, L.; Pippuri-Mäkeläinen, J.; Tammi, K. Overview of Powertrain Electrification and Future Scenarios for Non-Road Mobile Machinery. Energies 2018, 11, 1184. [Google Scholar] [CrossRef]
- The Future of High Energy Density Batteries|Hard Reset. (n.d.). [Video]. YOUTUBE. Available online: https://www.youtube.com/watch?v=KMP0×0DpxSY (accessed on 6 January 2024).
- MSE PRO Lithium Nickel Cobalt Aluminum Oxide NCA Cathode Powder 500 g. (n.d.). MSE Supplies LLC. Available online: https://www.msesupplies.com/products/mse-pro-lithium-nickel-cobalt-aluminum-oxide-nca-cathode-powder-500g-lini-sub-0-8-sub-co-sub-0-15-sub-al-sub-0-05-sub-o-sub-2-sub?variant=31013998428218 (accessed on 7 January 2024).
- Lai, X.; Gu, H.; Chen, Q.; Tang, X.; Zhou, Y.; Gao, F.; Han, X.; Guo, Y.; Bhagat, R.; Zheng, Y. Investigating greenhouse gas emissions and environmental impacts from the production of lithium-ion batteries in China. J. Clean. Prod. 2022, 372, 133756. [Google Scholar] [CrossRef]
- Zackrisson, M.; Fransson, K.; Hildenbrand, J.; Lampic, G.; O’Dwyer, C. Life cycle assessment of lithium-air battery cells. J. Clean. Prod. 2016, 135, 299–311. [Google Scholar] [CrossRef]
- Ding, Y.; Cano, Z.P.; Yu, A.; Lu, J.; Chen, Z. Automotive Li-Ion Batteries: Current Status and Future Perspectives. Electrochem. Energy Rev. 2019, 2, 1–28. [Google Scholar] [CrossRef]
- Mierlo, J.; Messagie, M.; Rangaraju, S. Comparative Environmental Assessment of Alternative Fueled Vehicles Using a Life Cycle Assessment. Transp. Res. Procedia 2017, 25, 3435–3445. [Google Scholar] [CrossRef]
- Kelly, J. Iron-Air Batteries Promise Higher Energy Density than Lithium-Ion Batteries. SciTechDaily. Available online: https://scitechdaily.com/iron-air-batteries-promise-higher-energy-density-than-lithium-ion-batteries/ (accessed on 24 March 2023).
- Imanishi, N.; Yamamoto, O. Perspectives and challenges of rechargeable lithium–air batteries. Mater. Today Adv. 2019, 4, 100031. [Google Scholar] [CrossRef]
- He, P.; Zhang, T.; Jiang, J.; Zhou, H. Lithium–Air Batteries with Hybrid Electrolytes. J. Phys. Chem. Lett. 2016, 7, 1267–1280. [Google Scholar] [CrossRef] [PubMed]
- HELIS Project—We Put Quality in Your Project. Available online: https://www.helis-project.eu/ (accessed on 8 June 2021).
- LIND. Primary Lithium Batteries. Electronic Products. 1 August 2010. Available online: https://www.electronicproducts.com/primary-lithium-batteries/ (accessed on 7 January 2024).
- Arshad, F.; Lin, J.; Manurkar, N.; Fan, E.; Ahmad, A.; Tariq, M.-U.; Wu, F.; Chen, R.; Li, L. Life Cycle Assessment of Lithium-ion Batteries: A Critical Review. Resour. Conserv. Recycl. 2022, 180, 106164. [Google Scholar] [CrossRef]
- Bielewski, M. Clean Energy Technology Observatory: Batteries for Energy Storage in the European Union—2022 Status Report on Technology Development, Trends, Value Chains and Markets; JRC Publications Repository, Publications Office of the European Union: Luxembourg, 2022. [Google Scholar] [CrossRef]
- Ambrose, H.; Kendall, A. Effects of battery chemistry and performance on the life cycle greenhouse gas intensity of electric mobility. Transp. Res. Part D Transp. Environ. 2016, 47, 182–194. [Google Scholar] [CrossRef]
- Zackrisson, M. Life Cycle Assessment of Electric Vehicle Batteries and New Technologies MATS ZACKRISSON Kth Royal Institute of Technology. Ph.D. Thesis, Royal Institute of Technology, Stockholm, Sweden, 2021. [Google Scholar] [CrossRef]
- Hao, H.; Mu, Z.; Jiang, S.; Liu, Z.; Zhao, F. GHG Emissions from the Production of Lithium-Ion Batteries for Electric Vehicles in China. Sustainability 2017, 9, 504. [Google Scholar] [CrossRef]
- Ali, R.; Chowdhury, M.A.; Rahman, M.; Ali, O.; Mahmud, S.; Rana, M.; Roy, B.K. Performance enhancement of lithium-ion battery using modified LiMn2O4 cathode followed by ultrasonic-assisted electrochemically synthesized graphene. Results Eng. 2023, 20, 101578. [Google Scholar] [CrossRef]
- Arvidsson, R.; Tillman, A.; Sandén, B.A.; Janssen, M.; Nordelöf, A.; Kushnir, D.; Molander, S. Environmental Assessment of Emerging Technologies: Recommendations for Prospective LCA. J. Ind. Ecol. 2017, 22, 1286–1294. [Google Scholar] [CrossRef]
- Messagie, M.; Da Quinta ECosta Neves De Oliveira, L.M.; Rangaraju, S.; Forner, J.; Rivas, M.H. Environmental Performance of Lithium Batteries; Elsevier eBooks: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Bonsu, N.O. Towards a circular and low-carbon economy: Insights from the transitioning to electric vehicles and net zero economy. J. Clean. Prod. 2020, 256, 120659. [Google Scholar] [CrossRef]
- ISO 14040; International Organization for Standardization (ISO). The New International Standards for Life Cycle Assessment. ISO: Geneva, Switzerland, 2006.
- ISO 14044; International Organization for Standardization (ISO). The New International Standards for Life Cycle Assessment. ISO: Geneva, Switzerland, 2006.
- Kelly, J.C.; Dai, Q.; Wang, M. Globally regional life cycle analysis of automotive lithium-ion nickel manganese cobalt batteries. Mitig. Adapt. Strat. Glob. Chang. 2020, 25, 371–396. [Google Scholar] [CrossRef]
- Winjobi, O.; Kelly, J.C.; Dai, Q. Life-cycle analysis, by global region, of automotive lithium-ion nickel manganese cobalt batteries of varying nickel content. Sustain. Mater. Technol. 2022, 32, e00415. [Google Scholar] [CrossRef]
- Petrauskienė, K.; Skvarnavičiūtė, M.; Dvarionienė, J. Comparative environmental life cycle assessment of electric and conventional vehicles in Lithuania. J. Clean. Prod. 2020, 246, 119042. [Google Scholar] [CrossRef]
- Porzio, J.; Scown, C.D. Life-Cycle Assessment Considerations for Batteries and Battery Materials. Adv. Energy Mater. 2021, 11, 2100771. [Google Scholar] [CrossRef]
- Iturrondobeitia, M.; Akizu-Gardoki, O.; Minguez, R.; Lizundia, E. Environmental Impact Analysis of Aprotic Li–O2 Batteries Based on Life Cycle Assessment. ACS Sustain. Chem. Eng. 2021, 9, 7139–7153. [Google Scholar] [CrossRef]
- Accardo, A.; Dotelli, G.; Musa, M.L.; Spessa, E. Life Cycle Assessment of an NMC Battery for Application to Electric Light-Duty Commercial Vehicles and Comparison with a Sodium-Nickel-Chloride Battery. Appl. Sci. 2021, 11, 1160. [Google Scholar] [CrossRef]
- Elgowainy, A.; Burnham, A.; Wang, M.; Molburg, J.; Rousseau, A. Well-to-Wheels Energy Use and Greenhouse Gas Emissions Analysis of Plug-in Hybrid Electric Vehicles. In Argonne National Laboratory. Energy Systems Division. 2009. Available online: https://publications.anl.gov/anlpubs/2009/03/63740.pdf (accessed on 11 January 2024).
- Degen, F.; Schuette, M. Life cycle assessment of the energy consumption and GHG emissions of state-of-the-art automotive battery cell production. J. Clean. Prod. 2022, 330, 129798. [Google Scholar] [CrossRef]
- Júnior, C.A.R.; Sanseverino, E.R.; Gallo, P.; Koch, D.; Schweiger, H.-G.; Zanin, H. Blockchain review for battery supply chain monitoring and battery trading. Renew. Sustain. Energy Rev. 2022, 157, 112078. [Google Scholar] [CrossRef]
- Dai, Q.; Kelly, J.C.; Dunn, J.; Benavides, P.T. Update of Bill-of-Materials and Cathode Materials Production for Lithium-Ion Batteries in the GREET Model. U.S. Department of Energy. 2018. Available online: https://greet.anl.gov/files/update_bom_cm (accessed on 5 January 2020).
- Gaines, L.; Sullivan, J.L.; Burnham, A. Paper No. 11-3891 Life-Cycle Analysis for Lithium-Ion Battery Production and Recycling. ResearchGate. Available online: https://www.researchgate.net/publication/265158823_Paper_No_11-3891_Life-Cycle_Analysis_for_Lithium-Ion_Battery_Production_and_Recycling (accessed on 1 January 2011).
- Romare, M.; Dahllöf, L.; IVL Swedish Environmental Research Institute. The Life Cycle Energy Consumption and Greenhouse Gas Emissions from Lithium-Ion Batteries: A Study with Focus on Current Technology and Batteries for Light-Duty Vehicles. 2017. Available online: https://www.energimyndigheten.se/globalassets/forskning--innovation/transporter/c243-the-life-cycle-energy-consumption-and-co2-emissions-from-lithium-ion-batteries-.pdf (accessed on 6 January 2020).
- Wu, H.; Hu, Y.; Yu, Y.; Huang, K.; Wang, L. The environmental footprint of electric vehicle battery packs during the production and use phases with different functional units. Int. J. Life Cycle Assess. 2020, 26, 97–113. [Google Scholar] [CrossRef]
- Kim, H.C.; Wallington, T.J.; Arsenault, R.; Bae, C.; Ahn, S.; Lee, J. Cradle-to-Gate Emissions from a Commercial Electric Vehicle Li-Ion Battery: A Comparative Analysis. Environ. Sci. Technol. 2016, 50, 7715–7722. [Google Scholar] [CrossRef]
- Lastoskie, C.M.; Dai, Q. Comparative life cycle assessment of laminated and vacuum vapor-deposited thin film solid-state batteries. J. Clean. Prod. 2015, 91, 158–169. [Google Scholar] [CrossRef]
- Zhao, S.; You, F. Comparative Life-Cycle Assessment of Li-Ion Batteries through Process-Based and Integrated Hybrid Approaches. ACS Sustain. Chem. Eng. 2019, 7, 5082–5094. [Google Scholar] [CrossRef]
- Wang, S.; Yu, J. A comparative life cycle assessment on lithium-ion battery: Case study on electric vehicle battery in China considering battery evolution. Waste Manag. Res. J. Sustain. Circ. Econ. 2020, 39, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Bae, C.; Denlinger, A.; Miller, T. Electric Vehicles Batteries: Requirements and Challenges. Joule 2020, 4, 511–515. [Google Scholar] [CrossRef]
- Duffner, F.; Kronemeyer, N.; Tübke, J.; Leker, J.; Winter, M.; Schmuch, R. Post-lithium-ion battery cell production and its compatibility with lithium-ion cell production infrastructure. Nat. Energy 2021, 6, 123–134. [Google Scholar] [CrossRef]
- Placke, T.; Kloepsch, R.; Dühnen, S.; Winter, M. Lithium ion, lithium metal, and alternative rechargeable battery technologies: The odyssey for high energy density. J. Solid State Electrochem. 2017, 21, 1939–1964. [Google Scholar] [CrossRef]
- Randau, S.; Weber, D.A.; Koetz, O.; Koerver, R.; Braun, P.; Weber, A.; Ivers-Tiffee, E.; Adermann, T.; Kulisch, J.; Zeier, W.G.; et al. Benchmarking the performance of all-solid-state lithium batteries. Nat. Energy 2020, 5, 259–270. [Google Scholar] [CrossRef]
- Popien, J.-L.; Thies, C.; Barke, A.; Spengler, T.S. Comparative sustainability assessment of lithium-ion, lithium-sulfur, and all-solid-state traction batteries. Int. J. Life Cycle Assess. 2023, 28, 462–477. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M.; Goodenough, J.B.; Zaghib, K. Tribute to Michel Armand: From Rocking Chair—Li-ion to Solid-State Lithium Batteries. J. Electrochem. Soc. 2020, 167, 070507. [Google Scholar] [CrossRef]
- Knehr, K.W.; Kubal, J.J.; Nelson, P.A.; Ahmed, S. Battery Performance and Cost Modeling for Electric-Drive Vehicles: A Manual for BatPaC v5. 0. No. ANL/CSE-22/1; Argonne National Lab. (ANL): Argonne, IL, USA, 2022. [Google Scholar]
- Wickerts, S.; Arvidsson, R.; Nordelöf, A.; Svanström, M.; Johansson, P. Prospective Life Cycle Assessment of Lithium-Sulfur Batteries for Stationary Energy Storage. ACS Sustain. Chem. Eng. 2023, 11, 9553–9563. [Google Scholar] [CrossRef]
- Barke, A.; Cistjakov, W.; Steckermeier, D.; Thies, C.; Popien, J.; Michalowski, P.; Melo, S.P.; Cerdas, F.; Herrmann, C.; Krewer, U.; et al. Green batteries for clean skies: Sustainability assessment of lithium-sulfur all-solid-state batteries for electric aircraft. J. Ind. Ecol. 2022, 27, 795–810. [Google Scholar] [CrossRef]
- Schnell, J.; Günther, T.; Knoche, T.; Vieider, C.; Köhler, L.; Just, A.; Keller, M.; Passerini, S.; Reinhart, G. All-solid-state lithium-ion and lithium metal batteries—Paving the way to large-scale production. J. Power Sources 2018, 382, 160–175. [Google Scholar] [CrossRef]
- Schnell, J.; Tietz, F.; Singer, C.; Hofer, A.; Billot, N.; Reinhart, G. Prospects of production technologies and manufacturing costs of oxide-based all-solid-state lithium batteries. Energy Environ. Sci. 2019, 12, 1818–1833. [Google Scholar] [CrossRef]
- Troy, S.; Schreiber, A.; Reppert, T.; Gehrke, H.-G.; Finsterbusch, M.; Uhlenbruck, S.; Stenzel, P. Life Cycle Assessment and resource analysis of all-solid-state batteries. Appl. Energy 2016, 169, 757–767. [Google Scholar] [CrossRef]
- Vandepaer, L.; Cloutier, J.; Amor, B. Environmental impacts of Lithium Metal Polymer and Lithium-ion stationary batteries. Renew. Sustain. Energy Rev. 2017, 78, 46–60. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Luo, L.; Fan, Y.; Du, Z. A review on thermal management of lithium-ion batteries for electric vehicles. Energy 2022, 238, 121652. [Google Scholar] [CrossRef]
- Smith, L.; Ibn-Mohammed, T.; Astudillo, D.; Brown, S.; Reaney, I.M.; Koh, S.C.L. The Role of Cycle Life on the Environmental Impact of Li6.4La3Zr1.4Ta0.6O12 based Solid-State Batteries. Adv. Sustain. Syst. 2020, 5, 2000241. [Google Scholar] [CrossRef]
- Peters, J.F.; Cruz, A.P.; Weil, M. Exploring the Economic Potential of Sodium-Ion Batteries. Batteries 2019, 5, 10. [Google Scholar] [CrossRef]
- Rey, I.; Iturrondobeitia, M.; Akizu-Gardoki, O.; Minguez, R.; Lizundia, E. Environmental Impact Assessment of Na3V2(PO4)3 Cathode Production for Sodium-Ion Batteries. Adv. Energy Sustain. Res. 2022, 3, 2200049. [Google Scholar] [CrossRef]
- Mozaffarpour, F.; Hassanzadeh, N.; Vahidi, E. Comparative life cycle assessment of synthesis routes for cathode materials in sodium-ion batteries. Clean Technol. Environ. Policy 2022, 24, 3319–3330. [Google Scholar] [CrossRef]
- Liu, W.; Liu, H.; Liu, W.; Cui, Z. Life cycle assessment of power batteries used in electric bicycles in China. Renew. Sustain. Energy Rev. 2021, 139, 110596. [Google Scholar] [CrossRef]
- Peters, J.F.; Baumann, M.; Zimmermann, B.; Braun, J.; Weil, M. The environmental impact of Li-Ion batteries and the role of key parameters—A review. Renew. Sustain. Energy Rev. 2017, 67, 491–506. [Google Scholar] [CrossRef]
- Trotta, F.; Wang, G.J.; Guo, Z.; Xu, Z.; Ribadeneyra, M.C.; Au, H.; Edge, J.S.; Titirici, M.M.; Lander, L. A Comparative Techno-Economic and Lifecycle Analysis of Biomass-Derived Anode Materials for Lithium- and Sodium-Ion Batteries. Adv. Sustain. Syst. 2022, 6, 2200047. [Google Scholar] [CrossRef]
- Peters, J.; Buchholz, D.; Passerini, S.; Weil, M. Life cycle assessment of sodium-ion batteries. Energy Environ. Sci. 2016, 9, 1744–1751. [Google Scholar] [CrossRef]
- Shanika, A.; Smith, J.; Segal, B. No. EPA 744-R-12-001; Application of Life-Cycle Assessment to Nanoscale Technology: Lithium-ion Batteries for Electric Vehicles. US EPA, USA. 2013. Available online: https://archive.epa.gov/epa/sites/production/files/2014-01/documents/lithium_batteries_lca.pdf (accessed on 16 March 2024).
- Bauer, C. Ökobilanz von Lithium-Ionen Batterien; Paul Scherrer Institut, Labor für Energiesystem-Analysen (LEA): Villingen, Switzerland, 2010. [Google Scholar]
- Cusenza, M.A.; Bobba, S.; Ardente, F.; Cellura, M.; Di Persio, F. Energy and environmental assessment of a traction lithium-ion battery pack for plug-in hybrid electric vehicles. J. Clean. Prod. 2019, 215, 634–649. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Kelly, J.C.; Gaines, L.; Wang, M. Life Cycle Analysis of Lithium-Ion Batteries for Automotive Applications. Batteries 2019, 5, 48. [Google Scholar] [CrossRef]
- Deng, Y.; Ma, L.; Li, T.; Li, J.; Yuan, C. Life Cycle Assessment of Silicon-Nanotube-Based Lithium Ion Battery for Electric Vehicles. ACS Sustain. Chem. Eng. 2018, 7, 599–610. [Google Scholar] [CrossRef]
- Dunn, J.B.; Gaines, L.; Sullivan, J.; Wang, M.Q. Impact of Recycling on Cradle-to-Gate Energy Consumption and Greenhouse Gas Emissions of Automotive Lithium-Ion Batteries. Environ. Sci. Technol. 2012, 46, 12704–12710. [Google Scholar] [CrossRef]
- Ellingsen, L.A.; Majeau-Bettez, G.; Singh, B.; Srivastava, A.K.; Valøen, L.O.; Strømman, A.H. Life Cycle Assessment of a Lithium-Ion Battery Vehicle Pack. J. Ind. Ecol. 2013, 18, 113–124. [Google Scholar] [CrossRef]
- Faria, R.; Marques, P.; Garcia, R.; Moura, P.; Freire, F.; Delgado, J.; de Almeida, A.T. Primary and secondary use of electric mobility batteries from a life cycle perspective. J. Power Sources 2014, 262, 169–177. [Google Scholar] [CrossRef]
- Hendrickson, T.P.; Kavvada, O.; Shah, N.; Sathre, R.; Scown, C.D. Life-cycle implications and supply chain logistics of electric vehicle battery recycling in California. Environ. Res. Lett. 2015, 10, 014011. [Google Scholar] [CrossRef]
- Jenu, S.; Deviatkin, I.; Hentunen, A.; Myllysilta, M.; Viik, S.; Pihlatie, M. Reducing the climate change impacts of lithium-ion batteries by their cautious management through integration of stress factors and life cycle assessment. J. Energy Storage 2020, 27, 101023. [Google Scholar] [CrossRef]
- Kallitsis, E.; Korre, A.; Kelsall, G.; Kupfersberger, M.; Nie, Z. Environmental life cycle assessment of the production in China of lithium-ion batteries with nickel-cobalt-manganese cathodes utilising novel electrode chemistries. J. Clean. Prod. 2020, 254, 120067. [Google Scholar] [CrossRef]
- Philippot, M.; Smekens, J.; VAN Mierlo, J.; Messagie, M. Life cycle assessment of silicon alloy-based lithium-ion battery for electric vehicles. WIT Trans. Built Environ. 2019, 182, 129–139. [Google Scholar]
- Majeau-Bettez, G.; Hawkins, T.R.; Strømman, A.H. Life Cycle Environmental Assessment of Lithium-Ion and Nickel Metal Hydride Batteries for Plug-In Hybrid and Battery Electric Vehicles. Environ. Sci. Technol. 2011, 45, 4548–4554. [Google Scholar] [CrossRef] [PubMed]
- Raugei, M.; Winfield, P. Prospective LCA of the production and EoL recycling of a novel type of Li-ion battery for electric vehicles. J. Clean. Prod. 2019, 213, 926–932. [Google Scholar] [CrossRef]
- Mohr, M.; Peters, J.F.; Baumann, M.; Weil, M. Toward a cell-chemistry specific life cycle assessment of lithium-ion battery recycling processes. J. Ind. Ecol. 2020, 24, 1310–1322. [Google Scholar] [CrossRef]
- Notter, D.A.; Gauch, M.; Widmer, R.; Wäger, P.; Stamp, A.; Zah, R.; Althaus, H.-J. Contribution of Li-Ion Batteries to the Environmental Impact of Electric Vehicles. Environ. Sci. Technol. 2010, 44, 6550–6556. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.F.; Weil, M. A Critical Assessment of the Resource Depletion Potential of Current and Future Lithium-Ion Batteries. Resources 2016, 5, 46. [Google Scholar] [CrossRef]
- Qiao, Q.; Zhao, F.; Liu, Z.; Jiang, S.; Hao, H. Cradle-to-gate greenhouse gas emissions of battery electric and internal combustion engine vehicles in China. Appl. Energy 2017, 204, 1399–1411. [Google Scholar] [CrossRef]
- Sun, X.; Luo, X.; Zhang, Z.; Meng, F.; Yang, J. Life cycle assessment of lithium nickel cobalt manganese oxide (NCM) batteries for electric passenger vehicles. J. Clean. Prod. 2020, 273, 123006. [Google Scholar] [CrossRef]
- Le Varlet, T.; Schmidt, O.; Gambhir, A.; Few, S.; Staffell, I. Comparative life cycle assessment of lithium-ion battery chemistries for residential storage. J. Energy Storage 2020, 28, 101230. [Google Scholar] [CrossRef]
- Wang, F.; Deng, Y.; Yuan, C. Life cycle assessment of lithium oxygen battery for electric vehicles. J. Clean. Prod. 2020, 264, 121339. [Google Scholar] [CrossRef]
- Zackrisson, M.; Avellán, L.; Orlenius, J. Life cycle assessment of lithium-ion batteries for plug-in hybrid electric vehicles—Critical issues. J. Clean. Prod. 2010, 18, 1519–1529. [Google Scholar] [CrossRef]


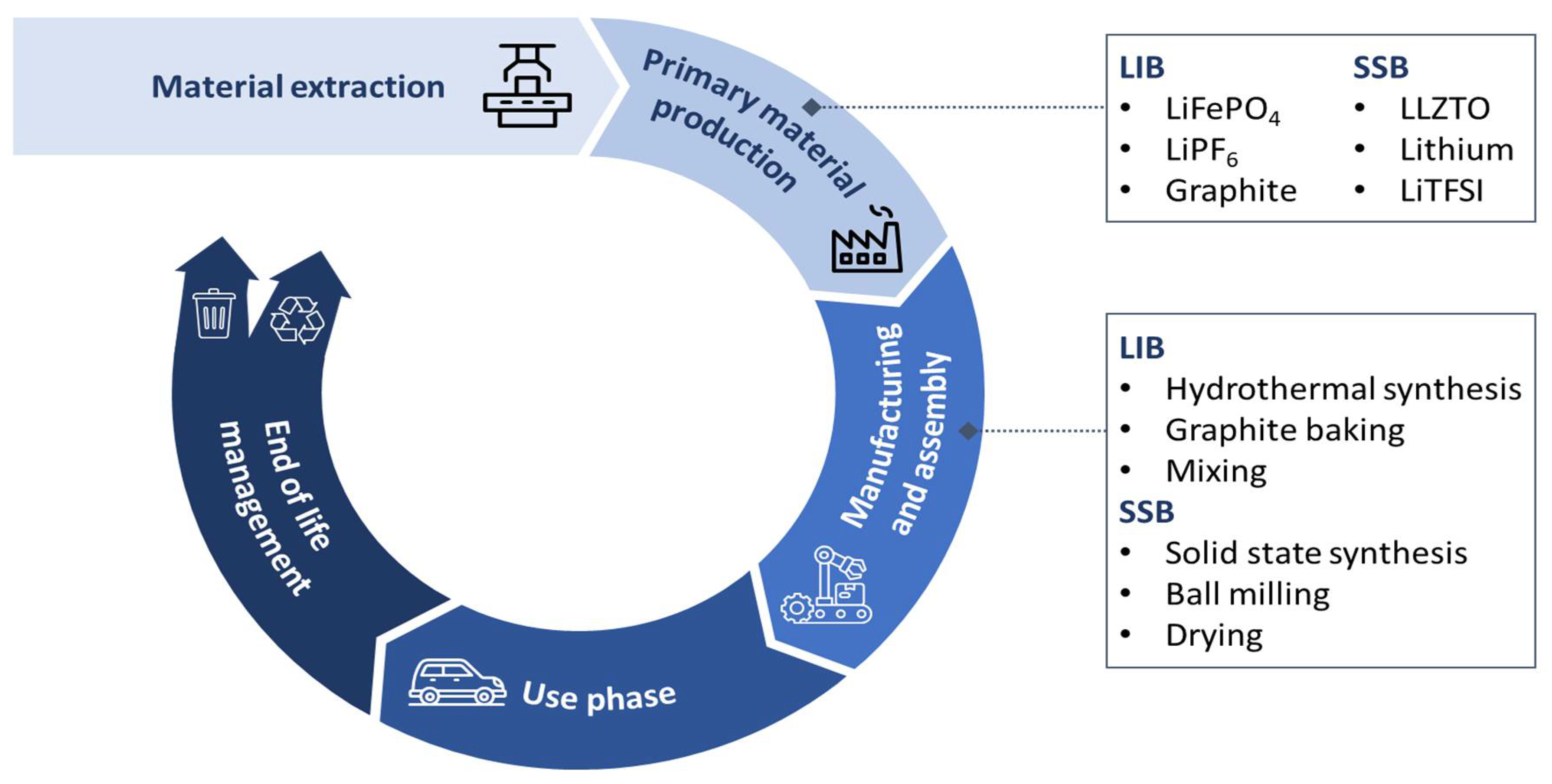
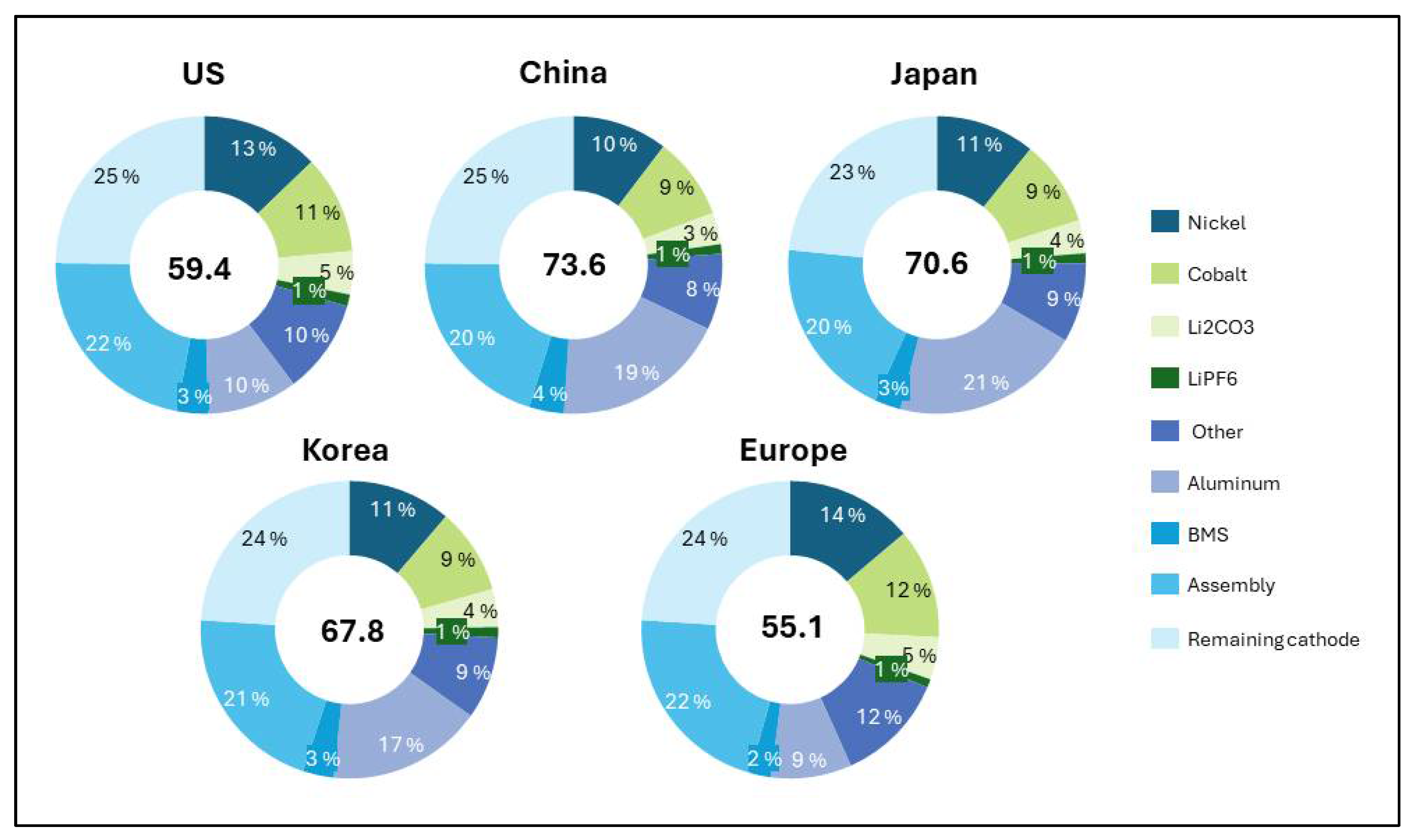

| Technology Type | Battery Technologies | Energy Density Ranges | Reference |
|---|---|---|---|
| Conventional Technology | NCA (Nickel Cobalt Aluminum Oxide) | 200–260 Wh/kg 700 Wh/L | [36] |
| LFP (Lithium Iron Phosphate) | 90–165 Wh/kg | [37] | |
| NCM (Nickel-Cobalt-Manganese) | 150–300 Wh/kg | [37] | |
| 260 Wh/kg | [32] | ||
| 770 Wh/L | [32] | ||
| Li-ion | 250 Wh/kg | [38] | |
| 200–250 Wh/kg | [39] | ||
| 450 Wh/kg (Expected by 2030) | [40] | ||
| Emerging Technology | Li-Air | 11,400 Wh/kg (Theoretical) | [41] |
| Li2O2 | 3505 Wh/kg (Theoretical) | [42] | |
| 700 Wh/kg (Achieved) | [43] | ||
| SIB’s | 100–150 Wh/kg | [37] | |
| Li-S | 2600 Wh/kg (Theoretical) | [31] | |
| 500–550 Wh/kg | [44] | ||
| Li-MnO2 | 150–250 Wh/kg | [45] | |
| 500–650 Wh/L | [45] | ||
| Li-(CF)n | 200–300 Wh/kg | [45] | |
| 500–600 Wh/L | [45] | ||
| Li-SO2 | 240–315 Wh/kg | [45] | |
| 350–450 Wh/L | [45] | ||
| Li-SOCl2 | 500–700 Wh/kg | [45] | |
| 600–900 Wh/L | [45] |
| Year | Source of Study | GHG Emissions | Battery Format Type | Cathode Type | Research Insights |
|---|---|---|---|---|---|
| 2016 | Troy et al. [85] | 0.2–5.4 kg CO2-eq per pouch | Pouch with 43.75 mAH | Inorganic | LLZO electrolyte production accounts for the majority of energy consumption. |
| 2017 | Vandepaer et al. [86] | 70-98.12 kg CO2-eq./kWh of storage | LMP-75 kWh LMP-6MWh for stationary storage | Polymer (LMP) | Pilot scale project with low ionic conductivity and low Temperatures |
| 2022 | Zhang et al. [87] | 0.103 gm CO2 eq./km | Coin cell using primary data literature | ASSB with inorganic electrolyte LATP | Lowering thickness improves energy efficiency and reduces impacts |
| 2020 | Smith et al. [88] | 79.11 kg of CO2-eq./kg cell material | Li7La3Zr2O12 (LLZO) garnet-structured electrolyte | Inorganic | Electrolyte production has major impacts due to the high temperature sintering process during manufacturing. |
| 2014 | Lastoskie et al. [71] | 25–85 kg CO2-eq/kWh | Pack | Inorganic | Silver, Nickel, or Cobalt have high emissions. LVO and LNMO have the least |
| 2023 | Popien et al. [78] | 79–123 kg CO2-eq/kWh | SSB-NCA, SSB-LFP, SSB-NMC, and SSB-Li-S | ASSB with inorganic electrolyte | More environmental benefits to LSB, even more with the use of renewables in production process |
| 2023 | Wickerts et al. [81] | 40–305 kg CO2-eq/kWh | LIS | SSB with LiTFSI as electrolyte | LiTFSI as electrolyte production was key contributor |
| 2023 | Barke et al. [82] | 56.6–64.3 kg CO2-eq/kWh | LIS | With different electrolyte configurations | LiS-ASSB[Ge] performs worst, and LiS-ASSB[Cl] performs best in all categories |
| Source of Study | Recorded GHG Emissions in the Proposed Study | Type of Electrode | Research Interpretation |
|---|---|---|---|
| Peters et al. [89] | 50–90 kg CO2-eq./kWh | NaNMC, NaMMO, NaNMMT, NaPBA, and NaMVP | The manganese and nickel–manganese-based SIB chemistries show promising environmental benefits compared to other chemistries |
| Rey et al. [90] | 423.9–1380.0 kg CO2-eq./ kg cathode, 18–38% reductions can be achieved by shifting to renewable electricity sources | Na3V2(PO4)3 Cathode active material (CAM) | The Na3V2(PO4)3 cathode code is as follows: 1: “hierarchical carbon-NVP” 2: “rGO-LbL NVP” 3: “μPorous NVP” 4: “N-doped carbon NVP” 5: “N,B-doped carbon/NVP” 6: “La3+-doped NVP” 7: “3D NVP nanofiber” 8: “Nanoplatelet NVP” 9:“Agarose carbon NVP” and 10: “Glucomannan NVP” |
| Mozaffarpor et al. [91] | 15.3, 14.2, and 20.0 kg CO2-eq. per kg NMCP | NMCP CAM | NMCP materials produced via ball milling, hydrothermal, and stirring-assisted hydrothermal methods |
| Liu et al. [92] | 4.07 and 4.61 kg CO2 eq/kg anode | Different hard carbon anodes | Hydrothermal carbonization (HTC), followed by pyrolysis and direct pyrolysis (5.82 industry scale graphite) |
| Peters et al. [93] | 10–70 CO2-eq/kWh | 42 different cathode materials | Emissions related to cell material and cell manufacturing are ignored |
| Trotta et al. [94] | 615, 500 and 5.5 kg CO2/kg anode | Glucose, Kuranode, and graphite Anode material | Due to well-established industry process, graphite anode has lower emissions compared to other anode material |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ankathi, S.K.; Bouchard, J.; He, X. Beyond Tailpipe Emissions: Life Cycle Assessment Unravels Battery’s Carbon Footprint in Electric Vehicles. World Electr. Veh. J. 2024, 15, 245. https://doi.org/10.3390/wevj15060245
Ankathi SK, Bouchard J, He X. Beyond Tailpipe Emissions: Life Cycle Assessment Unravels Battery’s Carbon Footprint in Electric Vehicles. World Electric Vehicle Journal. 2024; 15(6):245. https://doi.org/10.3390/wevj15060245
Chicago/Turabian StyleAnkathi, Sharath K., Jessey Bouchard, and Xin He. 2024. "Beyond Tailpipe Emissions: Life Cycle Assessment Unravels Battery’s Carbon Footprint in Electric Vehicles" World Electric Vehicle Journal 15, no. 6: 245. https://doi.org/10.3390/wevj15060245
APA StyleAnkathi, S. K., Bouchard, J., & He, X. (2024). Beyond Tailpipe Emissions: Life Cycle Assessment Unravels Battery’s Carbon Footprint in Electric Vehicles. World Electric Vehicle Journal, 15(6), 245. https://doi.org/10.3390/wevj15060245







