Abstract
Herbicides are used worldwide to protect agricultural crops, glyphosate being among the most frequently applied. In 2019 the European Commission approved the use of this herbicide for another 5 years and is now preparing its re-authorization for marketing. It is known that glyphosate (an active ingredient—AI) is usually less toxic than its commercial formulations, which may be related to adjuvants presents in such formulas. In this context, this work aimed to evaluate and compare the effects of glyphosate, as the commercial formulation Roundup® Ready (RR), and the AI in the Hydra viridissima life cycle, namely: mortality, morphology, feeding, reproduction, and regeneration. To attain this goal, H. viridissima was exposed to an environmentally relevant concentration of glyphosate (5.2 mg AI/L, both for RR and the AI) and to its culture medium (control). The mortality was lower than 0.03% for both RR and AI. Regarding morphological alterations, these were more severe on organisms exposed to RR, while a high recovery capacity in hydras exposed to AI was observed. No hydra was able to completely regenerate its body parts when exposed to RR, while 95% of the organisms exposed to AI were able to regenerate completely. The feeding rates of hydras exposed to RR decreased by from ~20% to ~50% compared to AI. As for reproduction, hydras exposed to RR released ~70% less buds than those exposed to AI. These timely results suggest that adjuvants present in the commercial formulation of glyphosate may cause higher toxicity to biota than the AI at environmentally relevant concentrations. Though the commercial formulation exerted higher toxicity in hydra, the effects AI induced in the morphology of the hydra cannot be disregarded, suggesting that a deeper understanding on the long-term toxicity of this AI is still needed to further support the decision on its marketing authorization and environmentally safe use.
1. Introduction
Herbicide application is commonly used to protect agricultural crops against weed, however, this is also considered a cause of soil and environmental pollution. Although many studies have been published, the individual and combined effects of herbicides on multitrophic interactions within agrosystems can still be considered limited given the wide range of chemicals being commercialized [1]. The use of herbicides is one of the main causes of rural aquatic agroecosystem contamination of adjacent aquatic systems, since its commercial formulations have high solubility in water that facilitates leaching from fields to the nearest surface waters [2]. Glyphosate is the most widely used herbicide in the world. It is a systemic and non-selective herbicide used to control weeds in crops. Glyphosate inhibits 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) in the shikimate acid pathway, thereby interfering with the production of proteins and other molecules that require tryptophan, phenylalanine, or tyrosine as precursors [3]. Some of the blocked molecules act as plant growth promoters (e.g., indoleacetic acid, IAA) or defense metabolites (e.g., tannins, anthocyanins, flavonoids, and lignin). The shikimate pathway can be found in plants and microbes, some of which play key roles in the soil nutrient cycle [1,3,4].
To assess the contamination of aquatic ecosystems by glyphosate, some studies have been conducted to determine the concentrations of this herbicide in surface waters. Values ranging between 0.00002 and 5.153 mg/L of glyphosate have been reported in previous studies [5,6,7,8]. In addition, the toxicity of glyphosate has been studied in some non-target freshwater organisms (both non-photosynthetic and photosynthetic) such as cnidarians, insects, fish, amphibians, algae, and vascular plants. These reports indicate a toxicity pattern with higher toxicity in low-complexity organisms and photosynthetic organisms, and low toxicity in fish and amphibians, e.g., [9,10,11,12,13,14,15], resumed on Supplementary Table S1.
Taking into account the concentrations causing 50% effect (IC50, EC50, and LC50) in some freshwater species, commercial formulations have, in general, been shown to be more toxic than the active ingredient. Some exceptions were observed for Hydra atenuatta where the toxicity was lower in the commercial formulation than in the active ingredient [9,10,12,13] (Supplementary Table S1). For amphibians, two different commercial formulations were tested, with the same amount of active ingredient (36%) but different adjuvants in their formulation. For one of the formulations (Roundup® Herbicide), the toxicity was between 63 and 110 times higher than that of the active ingredient for the different species tested, while in the other formulation (Roundup® Biactive), the values were almost equal to those of the active ingredient (maximum 1.3 times more toxic; Supplementary Table S1) [10]. In studies with fish, insects, and algae comparing the effects of glyphosate, a commercial formulation, and its adjuvants, toxicity increased as follows: adjuvants > commercial formulation > active ingredient (confront with Supplementary Table S1). Moreover, some level of risk may be uncovered as most studies have chosen to perform dose–response approaches, and therefore the derived effective concentrations may have surpassed in at least one order of magnitude of the suggested concentrations of application or those measured in environmental matrices (confront with Supplementary Table S1). Also, it is to highlight that the standard procedures carried out do not usually consider relevant scenarios of exposure such as that represented by pulses [16,17].
Based on the above, it cannot be concluded that the toxicity of Roundup® varies with the perceived active ingredients or adjuvants [9,10,12,13] (Supplementary Table S1). Uncertainties associated with glyphosate effects (whether the active ingredient is more toxic than the commercial formulation) led to its approval for another five years in Europe in 2017. However, not all member states agreed with such a measure, claiming that glyphosate had a high endocrine disruption and carcinogenic potential by the International Agency for Research on Cancer [18]; therefore, the precautionary principle should have been invoked as a mitigation or risk reduction measure. In March 2019, the European Food Safety Authority invited member states to act as co-rapporteurs for the risk assessment of glyphosate effects with a view to its re-approval (or not) in 2023. In July 2023, the EFSA published its first conclusions on the peer review made for glyphosate, indicating that no critical areas of concern were identified regarding its impacts on the health of Humans, animals, and the environment. Though, it is simultaneously stated that the risks associated with glyphosate use for biodiversity are complex and influenced by multiple factors. Accordingly, the aim of this study is to examine the impact of glyphosate on Hydra viridissima by evaluating its toxicity under various ecologically relevant exposure scenarios (continuous and pulsed) and considering the recommended application dose. Furthermore, this study aims to determine if the observed toxic effects are primarily attributed to the active ingredient (AI) itself or the adjuvants present in the commercial formulation (Roundup® Ready-RR). The choice for the invertebrate Hydra viridissima was based in the increasing attention that this cnidarian has attracted in aquatic ecotoxicology as a sensitive species of the epibenthonic community and as a bioindicator species [19,20,21]. Together with its small size, simple anatomy, and easy maintenance of the culture, the multiple physiological targets that can be evaluated during tests with this organism are very attractive characteristics, which include asexual reproduction, feeding behavior (predators), and morphological and regenerative characteristics [13,19,22,23,24,25], which may provide a broader understanding on the effects of both Round® Ready and the active ingredient.
2. Materials and Methods
2.1. Test Species Maintenance: Hydra viridissima
Cultures of H. viridissima were kept in glass vessels with hydra culture medium at a temperature of approximately 20 ± 2 °C and a photoperiod of 16 h light and 8 h dark. The hydras were fed once a week with Artemia salina nauplii (instar II) and Daphnia longispina neonates (less than 24 h-old). Hydra medium was changed twice a week to avoid evaporation and fungal growth [26].
2.2. Chemicals Tested
Glyphosate active ingredient (AI) and its commercial formulation were studied in the present work. Glyphosate Pestanal® (purity: 97.5%) was obtained through the company Sigma-Aldrich while the commercial formulation Roundup® Ready (RR) was purchased at a supermarket as ready to use and its composition presents 7.2 g/L or 0.72% (p/p) of glyphosate (in the form of isopropylammonium salt) [27]. The other components of this commercial formulation were not provided by the company.
Roundup® Ready (RR), a trademark of the company Monsanto, is a commercial formulation of glyphosate widely used is a systemic herbicide which penetrates through the leaves of the plants and circulates in its sap reaching the entire plant. This is a product authorized for non-professional use, namely on gardens and family vegetable gardens. The concentrations used in the ecotoxicological tests were chosen to expose the organisms to an ecologically relevant value. Firstly, preliminary assays were run with the recommended application rates concentration described in the package (15 to 60 mL/m2) and with dilutions of these recommended doses. Also, assays were carried out by exposing hydras to the highest concentration of glyphosate that was measured in environmental samples of superficial water: 5.153 mg/L [5].
2.3. Experimental Design
2.3.1. Morphological and Feeding Assays
The experimental design involved two different exposure regimes: continuous exposure (recommended by traditional ecological risk assessment) and pulsed-exposure (aiming at a more realistic scenario since application of glyphosate and its leaching to water systems is expected to result in a single, short-pulse with a peak concentration). As there was no literature available on the species studied here, several preliminary, range-finding tests were performed to achieve the final concentrations either for continuous exposure or pulsed exposure. Definitive assays were carried out by exposing hydras to 5.2 mg/L of glyphosate as active ingredient (both for Glyphosate Pestanal, and for RR). The 5.2 mg/L of AI concentration also corresponded to the highest concentration found in superficial waters [5]. Six exposure treatments were performed to each chemical—AI of Glyphosate Pestanal or RR (please see Figure 1): (a) control treatment, where organisms were exposed continuously for 96 h to hydra medium (HM); (b) early single pulse, where organisms were exposed to AI or RR in the first 24 h of the assay and then transferred to clean HM; (c) two sequential pulses, where organisms were exposed to two sequential pulses of AI or RR (from 0 to 24 h and from 48 to 72 h) and between pulses and after the two pulses were transferred to clean HM; (d) late single pulse, where organisms were exposed to a pulse of AI or RR for 24 h (from exposure time 48 to 72 h), prior and after the pulse organisms were exposed to HM; (e) prolonged exposure, where organisms were exposed for 72 h to AI or RR and then transferred to HM; and (f) continuous exposure, where organisms were exposed to AI or RR for 96 h.
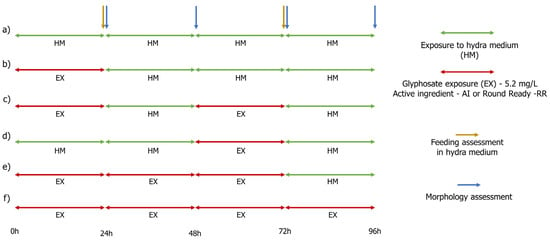
Figure 1.
Schematic representation of the exposure treatments (a–f) carried out during the pulsed and continuous exposure assay.
Assays were performed at 20 ± 2 °C and a photoperiod of 16:8 h L:D by exposing young individuals with the same age (1 day) to 5.2 mg/L of AI. Assays were run in 12-well plates, by introducing 3 mL of test solution and 1 hydra per well. For each treatment 8 replicates were performed. Medium was changed every 24 h and morphology of the organisms as well as feeding rates were evaluated, according to the method described below. Morphology: morphology was evaluated by observing the visible malformations under a magnifying glass every 24 h by using the Wilby [25] (Figure 1). Feeding: feeding rates assessment was made at times of exposure 24 h and 72 h of the test. To determine feeding rates, each hydra was provided with ten nauplii of A. salina (II instar) that were placed away above its tentacles. After a period of 30 min in the dark, the nauplii remaining in the test vessel were counted to calculate feeding rates (Figure 1).
2.3.2. Regenerative Capacity Assay
Glyphosate effects (AI or RR) were also evaluated in a regenerative capacity 96 h assay, following the protocol of Traversetti et al. [28], with some minor adaptations instead of using a gastric section, as suggested by Wilby [25]. Here, each hydra was cut in half and just the head regeneration was evaluated. Before starting the assay, all specimens were cut with a scalpel under a magnifying glass in two parts: hypostoma (mouthpart, head, and tentacles) and columna (remaining body portion). After this procedure, 40 columnae per treatment were immediately submerged for 96 h in eight 55 mm Petri dishes (5 specimens per well) containing 10 mL of the HM, or solution of AI or RR (concentration of 5.2 mg/L of AI). The regeneration was monitored every 24 h during 96 h, since hydra may regenerate a whole specimen within 4 days, and scoring was attributed according to Wilby [25].
2.3.3. Reproduction Assays
To assess the effect of glyphosate in the reproduction of hydra, organisms were exposed continuously for 7 days to the two following treatments: (a) hydra medium culture and (b) 5.2 mg/L of AI or RR. The reproduction endpoint followed the protocol of Holdway [29]. For each treatment 8 replicates were carried out. Each replica consisted of a 55 mm Petri dish filled with 10 mL of HM or test solution (AI or RR) with 5 hydras each, each hydra with a bud. Hydra were fed daily with A. salina nauplii for a 30 min period, after which HM or test solutions (AI or RR) were changed. Every day, the number of produced buds was counted and discarded. The mean relative population growth rate (K) was calculated using the following Equation (1)
where nx is the number of hydroids at the beginning of the assay, ny is the total number of hydras produced during the assay, and T is the length of the assay (7 days). The test was considered valid if the mean relative population growth rate (K) of the controls (replicates pooled after 7 days) was greater than 0.25.
K = (lnny − lnnx) T,
2.4. Data Analysis
All the data analysis was performed in the software Rstudio 3.0.1 and SigmaStat 4.0. Significant differences were attested using univariate statistics, where RR or AI concentrations were used as factors.
2.4.1. Morphology
All the statistical tests performed to analyze the morphology were non-parametric, since the assumptions to run parametric analysis of variance were not met: Shapiro–Wilk normality test or the Brown-Forsythe were performed for the homogeneity of variances. The Friedman test followed by the Holm–Sidak multiple comparison test was performed to determine if there were differences between the type of chemical (AI or RR) and exposure scenarios (Figure 1a–f) in the hydras response at the end of the test (96 h).
To determine if the duration of the exposure influenced the morphology of the hydras, the Kruskal–Wallis test was performed for each of the chemicals, AI and RR, followed by a Dunn’s multiple comparisons test for the treatments (b) (short-term exposure—24 h), (e) (medium-term exposure—72 h), and (f) (long-term exposure—96 h) using the morphology scores at the end of the respective exposition period. For each chemical, RR and AI, the Mann–Whitney test (t-test) was performed to perceive the difference between an early pulse and a late-pulse comparing treatment (b) at 24 h with treatment (d) at 72 h.
The hydras recovery capacity was evaluated using the mean of the scores (MS). For a short-term recovery (StR) (24 h in HM) the following treatments were evaluated using the following equations:
After an early pulse (0 h to 24 h), treatment (b) plus (c):
StR1 = (MS after 48 h)/(MS after 24 h)
Treatment (b) was evaluated together with treatment (c) because they were in the same conditions until 48 h, the relevant period for this analysis, and thus increased the robustness of the results.
After a late pulse (48 h to 72 h), treatment (d):
StR2 = (MS after 96 h)/(MS after 72 h)
After a prolonged pulse (0 h to 72 h), treatment (e):
StR3 = (MS after 96 h)/(MS after 72 h)
After a sequence of two pulses (0 h to 24 h and 48 h to 72 h), treatment (c):
StR4 = (MS after 96 h)/(MS after 72 h)
For a long-term recovery (LtR) (72 h in HM) only treatment (b) was evaluated, so for an early pulse (0 h to 24 h), the following Equation (6) was used:
LtR1 = (MS after 96 h)/(MS after 24 h)
Only recoveries with a score of 6 or higher were counted since scores below 5 are considered irreversible [30]. If the result of the equations was greater than 1, we consider that there was a recovery, if it was equal to 1 no recovery occurred and if it was lower than 1 we consider that the effects on hydras morphology intensified (values rounded to 1 decimal place).
2.4.2. Feeding
All the statistical tests performed to analyze the feeding rate were non-parametric, as data showed a non-normal distribution (Shapiro–Wilk test). For the feeding test, at 24 h the feeding rates of treatments (a) plus (d) were compared, the two groups exposed to HM for 24 h, with the treatments (d) plus (c) plus (e) and (f), the four groups exposed to AI or RR, using Friedman test followed by the Holm–Sidak multiple comparison test, to see if there are differences between the types of exposure (factor 1—AI or RR) and the type of treatment (factor 2—exposed or not exposed to glyphosate). At 72 h the Friedman test was also performed followed by the Holm–Sidak multiple comparison test, to verify if there was difference between the chemicals (AI or RR) and the different exposure scenarios (Figure 1a–f).
2.4.3. Reproduction and Regeneration
To assess the effects on reproduction rate, a one-way ANOVA was performed followed by Tukey’s multiple comparison test to see if there were differences in reproduction rates depending on the chemical being tested. Prior to the ANOVA, assumptions regarding data normal distribution and homoscedasticity of variance were confirmed by the Shapiro–Wilk test and by the Brown-Forsythe test, respectively. For the regeneration capacity data, since the assumptions to run the parametric ANOVA were not met, the non-parametric test, Kruskal–Wallis, was used followed by Dunn’s multiple comparison test to see if there were differences in regenerative capacity depending on the chemical.
3. Results
3.1. Morphology Assay
The hydras exposed to RR exhibited scores between 4 and 10, whereas those exposed to AI Glyphosate Pestanal® exhibited scores between 7 and 10, nevertheless, at this latter group a death was registered (Figure 2a,b). When comparing the toxicity caused by AI and RR to hydra at the end of the assay, no significant differences were registered for organisms exposed to an early pulse treatment (p-value = 0.198); for all the other treatments significant differences were observed between the two chemicals (p-value < 0.05), with RR presenting the highest toxicity (Figure 2b). Also, at the end of the test, no significant differences were observed between treatments performed with AI and the control (p-value > 0.05; Figure 2a). However, for hydra exposed to RR treatments, no significant differences relatively to the control were reported for the treatment b (early pulse) (p-value = 0.294), all other treatments were significantly different from the control (p-values < 0.05) (Figure 2b).
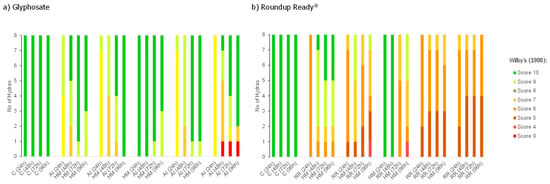
Figure 2.
Number of Hydra viridissima specimens exhibiting morphological alterations after being exposed to pulses of (a) glyphosate as active ingredient (AI) or (b) Roundup Ready®. Scoring is attributed according to Wilby [25]. C—control; HM—hydra medium; AI—active ingredient; RR—Roundup Ready®.
The duration of exposure (treatments b, d, and e) did not significantly alter the intensity of responses of hydra exposed to RR (p-value = 0.141; Figure 2b). However, for hydra exposed to AI, significant differences in the intensity of effects were observed between a short-term exposure (24 h) and a medium-term exposure (72 h) (p-value = 0.013). (Figure 2a) Short-term exposure (24 h) compared with long-term exposure (96 h), and medium-term exposure (72 h) compared to long-term exposure (96 h) did not show significant differences (p-values > 0.05). To assess if the age of hydras influenced their sensitivity to the pesticide, toxicity observed after the early pulse (treatment b) was compared with that observed after the late pulse (treatment d). In the case of RR, the age of hydra did not influence their sensitivity (p-value = 0.234). However, younger hydras were significantly more sensitive to AI than older ones (p-value < 0.05) (Figure 2a).
As for the recovery capacity, after being exposed to AI, recovery only occurred after a sequential pulse followed by a short-term recovery (StR4 = 1.1) and then an early pulse followed by a long-term recovery (LtR1 = 1.1) (Table 1). In the RR exposure, there was no recovery after a prolonged pulse followed by a short-term recovery (StR3 = 1.0), in all other cases there was recovery (Table 1). There was no record of hydras that showed increased intensity of effects after a period of recovery (Table 1).

Table 1.
Score results for regeneration capacity (according to Wilby [25]) of Hydra viridissima when exposed to pulses of glyphosate as active ingredient of Glyphosate Pestanal—AI or when exposed to pulses of glyphosate as commercial formulation Roundup Ready—RR.
In comparing hydras of the control group with the group exposed to the RR, large differences were observed. In Figure 3a is shown a hydra of the control group with score 10, and in Figure 3b, a hydra exposed to RR exhibiting a score 7, which presents that the body contracted and the tentacles began to present atrophy. In Figure 3c, a hydra also exposed to RR shows a score of 5 with a very contracted body. Finally, in Figure 3d is shown a hydra with score 0, disintegrated.
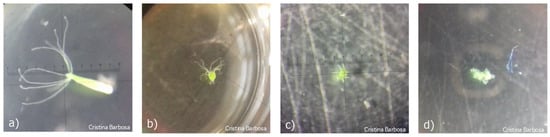
Figure 3.
Images of Hydra viridissima (a) after being exposed to the control medium, exhibiting a score 10; (b) after being exposed to Round Ready (RR) exhibiting a score 7; (c) after being exposed to RR exhibiting a score 5; (d) after being exposed to RR exhibiting a score 0, disintegrated.
3.2. Feeding Assay
Feeding was assessed at the time point of 24 h and 72 h of the 96 h pulse test. In general, hydras exposed to AI—Glyphosate Pestanal, ate 85% more than hydras exposed to the commercial formulation (RR) which almost exhibited feeding rates close to zero (Figure 4a,b).
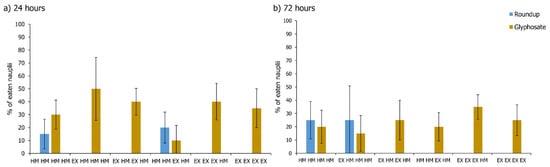
For all results, by comparing the feeding rates at 24 h with those at 72 h, we found that, overall, they were 20% higher at 24 h. At 24 h, there are significant differences between the RR group and the AI group (p-value < 0.05). Comparing both AI exposure and RR exposure with control, there were significant differences (p-values < 0.05), so hydras exposed to RR eat much less than the control and AI hydras, and that the hydras exposed to AI eat much more than those of the control group and the RR group. At 72 h all treatments were significantly different when comparing AI and RR exposure (p-values < 0.05) except for (a) control treatment. Within AI exposure, no significant difference was found between treatments (p-values > 0.05). In the RR exposure, differences were found comparing early exposure treatment (EX-HM-HM-HM, scenario (b) in Figure 1) with late exposure treatment (HM-HM-EX-HM, scenario (d) in Figure 1), sequential pulse treatment (EX-HM-EX-HM, scenario) in Figure 1), and prolonged exposure treatments (EX-EX-EX-HM and EX-EX-EX-EX, scenarios (e) and (f in Figure 1). The early pulse hydras ate much more than the rest (p values < 0.05; Figure 4b). No further significant differences were recorded.
3.3. Reproduction Assay
The mean relative population growth rate (K) of the controls was 0.491, which means that the test was valid. For the AI and RR exposure the K values were 0.496 and 0.323, respectively. On average, H. viridissima from the control produced 151 buds, while those exposed to AI and RR produced 161 and 48 buds, respectively (Figure 5a). Hydras exposed to RR produced significantly less buds than the ones exposed to the control and the AI group (Figure 5; p < 0.05). Any significant differences were registered between the number of buds produced hydras exposed to AI or to the control (p-value = 0.646) (Figure 5a).
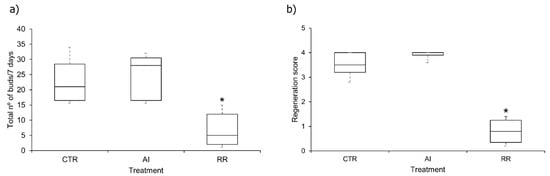
Figure 5.
Boxplots representing (a) the mean number of buds produced per Hydra viridissima in a 7-day reproduction assay and (b) the regeneration scores of H. viridissima in a 96 h regeneration assay, after exposure to hydra medium (CTR), glyphosate as active ingredient (AI) and Roundup Ready® (RR). * Means significant differences from control.
3.4. Regeneration Assay
In this test, there was a high mortality rate in the RR group, which translates into a very low mean score (=0.8); this occurs because when disintegration occurs, the score value of 0 is registered. In contrast, most of the hydras exposed to AI were able to regenerate completely, obtaining a mean score of 4 (the highest value), which was even higher than the control group (mean = 3.5) results visible in the boxplot of the three groups (Figure 5b). Significant differences were found when comparing hydras exposed to RR with those exposed to the control and AI (p-value = 0.01 and 0.0001, respectively). No significant differences were observed between control and AI (p-value = 0.73).
4. Discussion
The principal objective of this study was to compare and evaluate the effects of Glyphosate Pestanal® active ingredient (AI), and the commercial formulation, Roundup® Ready (RR), in the freshwater cnidarian H. viridissima using concentrations with relevant ecological interest.
The results obtained suggest that glyphosate concentrations found in the environment are toxic to H. viridissima if the RR commercial formulation is used, since at all endpoints there were significant differences when comparing the results with the control and AI. In its condition, the tested concentration of the active ingredient Glyphosate Pestanal® did not exhibit a significant toxicity to this species. The results are then in agreement with the most part of the literature presented previously, wherein the commercial formulation presented to be more toxic than the active ingredient [9,10,12,13], reviewed by [31]. Notwithstanding, similar values were expected to be attained with H. viridissima as those delivered by Demetrio et al. [13] with H. attenuata, given the similarities between the two species. In Demetrio et al. [13], the commercial formulation proved to be less toxic than the active ingredient as confirmed by the computation of the median lethal concentrations (LC50) of 18.2 mg/L for AI and 21.8 mg/L for Roundup® Max [13]. On one hand, this highlights that in the present study the objective was not to deliver benchmarks as presented by Demetrio et al. [13], and thus, studying different glyphosate levels may end up on different outcomes; but, on the other hand these differences may be due to the adjuvants of the different formulations tested: Roundup® Max was used for H. attenuata, while in this study Roundup® Ready was used. Unfortunately, this statement cannot be confirmed since Roundup® Ready adjuvants studied here are not provided by the company. Nevertheless, there is growing concern regarding the evaluation of potential toxic effects caused by adjuvants, as they could potentially account for the majority of observed toxic effects [31,32].
For the RR-exposed group, the effects observed were dependent on the duration of exposure, with a greater number of days of continuous exposure resulting in lower scores of the hydras (drastic morphological changes). However, when short-term or pulsed exposure scenarios were evaluated, recovery was seen in all situations. In the AI group, there were differences between a short-term exposure and a medium-term exposure, although in both cases morphological scores increased during exposure. Since the malformations caused by the AI were not very severe (the scores were high), the averages at the end of the recovery periods did not vary much from the averages at the beginning of recovery. These results showing differences between RR and AI point once again to the issue of the so-called “inert ingredient” in Roundup® formulations due to the clear evidence on RR- and AI-induced different toxicity profiles in continuous exposure. The lack of recovery after prolonged exposure to RR compared to AI most likely indicates that detoxification mechanisms in the hydras were overwhelmed, adding to the fact that significant morphological changes (greatly shrunken and atrophied tentacles, supported by the low morphological scores and the results of the feeding trials) substantially limited their ability to feed, and thus prevented the restoration of energy budgets to cope with the detoxification mechanisms. It should be considered that many adjuvants present in these formulations are quite toxic for aquatic biota, a fact for which attention was already called for [33]. For instance, surfactants are generally included in pesticide formulations to improve their effectiveness, and application properties may not only increase the bioavailability and uptake of the active ingredient (glyphosate) into aquatic organisms, but they themselves may cause toxicity to non-target organisms, partially explaining the results obtained for RR [34].
The increase in scores in AI exposure may be partially explained by the hydras’ mechanisms of detoxification of organic xenobiotics [19,35,36]. Hydras are simple aquatic organisms with a limited detoxification mechanism compared to more complex organisms, and their detoxification processes are less well-studied, especially in relation to specific compounds like glyphosate. However, hydras may use general detoxification mechanisms commonly found in other organisms, namely the enzyme glutathione S transferase (GST) that acts in phase II of the biotransformation [35,36], and the oxidative metabolism that has shown to be significantly increased in both heme oxidase (HO) and lipid peroxidation [19]. These mechanisms were discovered when hydras were exposed to the antiepileptic drug and common environmental pollutant carbamazepine [19] and are believed here to have played a key role in AI detoxification. Other authors have reported that upon exposure to xenobiotics, an increase occurs in cnidarians cell protection biomarkers (such as glycoproteins), which induces the generation of multiple resistance phenotypes (or MRX; [37]). It is possible that hydras may excrete glyphosate or its metabolites through these mechanisms. In addition, hydras exposed to AI were the group that ate the most prey, compared to the control and RR groups, which may also have been an advantage since the acquisition of food can compensate for energy losses with detoxification mechanisms and therefore help to tolerate the AI and balance the lack of resources that could be obtained from the symbiosis with the algae-like carbohydrates [38]. In the case of RR, the morphological structures observed were so drastic that they very possibly prevented the hydra from either capturing the food item or ingesting it.
The evaluation of the reproductive capacity of hydras is an often-disregarded endpoint of hydras lifecycles, despite the bridge it offers between the effects at the suborganismal level and the population level. The reproductive capacity was proven to be very sensitive, with hydras exposed to RR suffering from suppressions on budding formation around 70%, a result which was not observed for AI-exposed hydras. In this case, relating the obtained results in the morphological assay and the feeding assays, in which highly damaged hydras were not able to feed properly, may help explain this result. The feeding rates may well have influenced the asexual reproduction rates, since the energy obtained through food items is partially diverted to produce excess cells that are channeled into the production of buds [39]. In the reproduction assay with RR, although the feeding rates were not evaluated, it does call for attention that hydras were fed daily ad libitum, but most probably due to prey capture hampering (sustained by the scoring results on pulse and continuous exposure assays) they were most likely unable to successfully capture the prey, and subsequently reproduced less. In summary, the malformations influence the feeding which in turn influences the asexual reproduction.
The effect of glyphosate on hydra can be brought to the ecosystem level. Studies on the identification on the major predators and food preference of H. viridissima have indicated that few predators are found to attack cnidarians due to their nematocysts, which make them unpalatable [22,40], whilst also observing that H. viridissima positively selected the nauplii and copepodites of calanoid copepods and small cladocerans and rejected large prey, such as the adults of calanoid copepods and ostracods [22]. Since both the defense against predators and feeding process are dependent on the nematocysts present in the tentacles and it has been verified that the malformations caused by RR not only make it impossible to catch prey but also make it impossible to defend against the predators. This evidence highlights that RR exposure may not only cause irreversible damage to hydras, impairing feeding (by which they help regulate the abundance of other groups of organisms) and reproduction, but may also make them more vulnerable to predators, which may further result in unforecasted changes on ecosystems normal functioning.
5. Conclusions
The environmentally simulated concentration used in this study was lower than the recommended dose authorized by the European Commission for the commercial product. The actual glyphosate concentrations found in the environment was then found to be toxic to H. viridissima if the Roundup® Ready commercial formulation is used, since in all parameters there were significant differences when comparing the results with the formulation and the AI. These results suggest that the commercial formulation adjuvants may induce higher toxicity to biota than the AI itself, so the use of such compounds and the sale to the public for non-professional use should be reviewed and rethought to be clearly in line with Directive 2009/128/EC of 2009, which supports the proposal of new solutions for a more sustainable agricultural practice.
Pulsed exposure is the most expected scenario under environmental situations compared to continuous exposure, but it should be noted that basing regulatory decisions on continuous exposure scenarios should result in more conservative and therefore more environmentally protective agendas. Freshwater cnidarians proved to be very sensitive organisms to contamination posed by this herbicide and were able to provide many sensitive and rapid endpoints covering effects from the individual to the population. Note that the highly cost-effective freshwater cnidarian assays can be used as organisms for the rapid screening of new compounds, and therefore assays with freshwater cnidarians may be integrated into alternative management strategies for the protection of soils and surrounding environments, such as freshwater reservoirs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su151612428/s1, Table S1: Median lethal concentrations causing 50% of effect on freshwater species for glyphosate, commercial formulations and adjuvants in freshwater organisms. IC50, LC50, EC50 (mg/L). Abbreviations stand for: AI—active ingredient; GR—growth rate; IMM—immobilization. References [9,10,11,12,13,14,15,26,41,42] are cited in supplementary materials.
Author Contributions
Conceptualization, C.V. and I.L.; methodology, C.V. and C.B.; validation, C.V. and C.B.; formal analysis, C.B.; resources, I.L.; writing—original draft preparation, C.V. and C.B.; writing—review and editing, C.V., C.B., and I.L.; supervision, I.L.; funding acquisition, I.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CESAM financial support through national funding by Foundation for Science and Technology (UIDB/50017/2020+UIDP/50017/2020+LA/P/0094/2020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nivelle, E.; Verzeaux, J.; Chabot, A.; Roger, D.; Chesnais, Q.; Ameline, A.; Lacoux, J.; Nava-Saucedo, J.E.; Tétu, T.; Catterou, M. Effects of glyphosate application and nitrogen fertilization on the soil and the consequences on aboveground and belowground interactions. Geoderma 2018, 311, 45–57. [Google Scholar] [CrossRef]
- Smedbol, É.; Gomes, M.P.; Paquet, S.; Labrecque, M.; Lepage, L.; Lucotte, M.; Juneau, P. Effects of low concentrations of glyphosate-based herbicide factor 540® on an agricultural stream freshwater phytoplankton community. Chemosphere 2018, 192, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Helander, M.; Saloniemi, I.; Saikkonen, K. Glyphosate in northern ecosystems. Trends Plant Sci. 2012, 17, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Tuffi Santos, L.D.; Graça, R.N.; Alfenas, A.C.; Ferreira, F.A.; Melo, C.A.; Machado, M.S. Glyphosate reduces urediniospore development and Puccinia psidii disease severity on Eucalyptus grandis. Pest Manag. Sci. 2011, 67, 876–880. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Glyphosate and AMPA in Drinking-Water Background Document for Development of WHO Guidelines for Drinking-Water Quality. World Health Organization, (WHO/SDE/WSH/03.04/97). 2005. Available online: http://www.who.int/water_sanitation_health/dwq/chemicals/glyphosateampa290605.pdf (accessed on 29 May 2023).
- Scribner, E.A.; Battaglin, W.A.; Gilliom, R.J.; Meyer, M.T. Concentrations of Glyphosate, Its Degradation Product, Aminomethylphosphonic Acid, and Glufosinate in Ground- and Surface-Water, Rainfall, and Soil Samples Collected in the United States, 2001–2006; U.S. Geological Survey Investigations Report 2007-5122; United States Geological Survey: Reston, VA, USA, 2007; p. 111. [Google Scholar]
- Tsui, M.T.K.; Chu, L.M. Environmental fate and non-target impact of glyphosate-based herbicide (Roundup®) in a subtropical wetland. Chemosphere 2008, 71, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Rendon-von Osten, J.; Dzul-Caamal, R. Glyphosate Residues in Groundwater, Drinking Water and Urine of Subsistence Farmers from Intensive Agriculture Localities: A Survey in Hopelchén, Campeche, Mexico. Int. J. Environ. Res. Public Health 2017, 14, 595. [Google Scholar] [CrossRef]
- Folmar, L.C.; Sanders, H.O.; Julin, A.M. Toxicity of the herbicide glyphosate and several of its formulations to fish and aquatic invertebrates. Arch. Environ. Contam. Toxicol. 1979, 8, 269–278. [Google Scholar] [CrossRef]
- Mann, R.M.; Bidwell, J.R. The Toxicity of Glyphosate and Several Glyphosate Formulations to Four Species of Southwestern Australian Frogs. 1998. Available online: http://www.bison-m.org/documents/41_Mann1999.pdf (accessed on 29 May 2023).
- Tsui, M.T.K.; Chu, L.M. Aquatic toxicity of glyphosate-based formulations: Comparison between different organisms and the effects of environmental factors. Chemosphere 2003, 52, 1189–1197. [Google Scholar] [CrossRef]
- Sobrero, M.C.; Rimoldi, F.; Ronco, A.E. Effects of the Glyphosate Active Ingredient and a Formulation on Lemna gibba L. at Different Exposure Levels and Assessment End-Points. Bull. Environ. Contam. Toxicol. 2007, 79, 537–543. [Google Scholar] [CrossRef]
- Demetrio, P.M.; Bulus Rossini, G.D.; Bonetto, C.A.; Ronco, A.E. Effects of pesticide formulations and active ingredients on the coelenterate Hydra attenuata (Pallas, 1766). Bull. Environ. Contam. Toxicol. 2012, 88, 15–19. [Google Scholar] [CrossRef]
- Cuhra, M.; Traavik, T.; Bøhn, T. Clone- and age-dependent toxicity of a glyphosate commercial formulation and its active ingredient in Daphnia magna. Ecotoxicology 2013, 22, 251–262. [Google Scholar] [CrossRef]
- von Fumetti, S.; Blaurock, K. Effects of the herbicide Roundup® on the metabolic activity of Gammarus fossarum Koch, 1836 (Crustacea; Amphipoda). Ecotoxicology 2018, 27, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Sesin, V.; Davy, C.M.; Stevens, K.J.; Hamp, R.; Freeland, J.R. Glyphosate toxicity to native nontarget macrophytes following three different routes of incidental exposure. Integr. Environ. Assess. Manag. 2021, 17, 597–613. [Google Scholar] [CrossRef]
- Venâncio, C.; Ribeiro, R.; Soares, A.M.V.M.; Lopes, I. Survival recovery rates by six clonal lineages of Daphnia longispina after intermittent exposures to copper. Chemosphere 2021, 264, 128403. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. The IARC Monograph on the Herbicide Glyphosate; International Agency for Research on Cancer: Lyon, France, 2015; p. 92. [Google Scholar]
- Quinn, B.; Gagné, F.; Blaise, C. Hydra, a model system for environmental studies. Int. J. Dev. Biol. 2012, 56, 613–625. [Google Scholar] [CrossRef]
- Nanieva, A.V.; Pelishenko, A.V.; Kovalenko, V.F.; Goncharuk, V.V. Hydra attenuata, a model system for determining the acute lethal and chronic toxicity of drinking and natural waters and aqueous solutions of chemicals. J. Water Chem. Technol. 2019, 41, 329–333. [Google Scholar] [CrossRef]
- Gabriel, A.; Venâncio, C.; Sousa, J.P.; Leston, S.; Ramos, F.; Soares, A.M.; Lopes, I. Ecotoxicity of eluates obtained from Basamid® contaminated soils is pH dependent: A study with Hydra viridissima, Xenopus laevis and Danio rerio. Sci. Total Environ. 2023, 868, 161640. [Google Scholar] [CrossRef]
- Pollino, C.A.; Holdway, D.A. Potential of two hydra species as standard toxicity test animals. Ecotoxicol. Environ. Saf. 1999, 43, 309–316. [Google Scholar] [CrossRef]
- Massaro, F.C.; Negreiros, N.F.; Rocha, O.; Massaro, F.C.; Negreiros, N.F.; Rocha, O. A search for predators and food selectivity of two native species of Hydra (Cnidaria: Hydrozoa) from Brazil. Biota Neotrop. 2013, 13, 35–40. [Google Scholar] [CrossRef]
- Hoffmeister-Ullerich, S.A.H. Hydra—Ancient model with modern outfit. Cell Mol. Life Sci. 2007, 64, 3012–3016. [Google Scholar] [CrossRef] [PubMed]
- Wilby, O.K. The Hydra Regeneration Assay. In Proceedings of the Workshop Organised by Association Francaise de Teratologie, Royaumont, France, 3 June 1988; pp. 108–124. [Google Scholar]
- Relyea, R.A.; Jones, D.K. The toxicity of Roundup Original Max to 13 species of larval amphibians. Environ. Toxicol. Chem. 2009, 28, 2004–2008. [Google Scholar] [CrossRef] [PubMed]
- Liscampo. 2018. Available online: http://www.liscampo.com/uploads/documentos/produtos/eba_ROUNDUP_PRONTO.pdf (accessed on 23 June 2022).
- Traversetti, L.; Del Grosso, F.; Malafoglia, V.; Colasanti, M.; Ceschin, S.; Larsen, S.; Scalici, M. The Hydra regeneration assay reveals ecological risks in running waters: A new proposal to detect environmental teratogenic threats. Ecotoxicology 2017, 26, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Holdway, D.A. Hydra population reproduction toxicity test method. In Small-Scale Freshwater Toxicity Investigations; Volume 1—Toxicity Test Methods; Springer: Dordrecht, The Netherlands, 2005; Volume 1, pp. 395–411. [Google Scholar]
- Biaise, C.; Kusui, T. Acute toxicity assessment of industrial effluents with a microplate-based Hydra attenuata assay. Environ. Toxicol. Water Qual. 1997, 12, 53–60. [Google Scholar]
- Hao, Y.; Zhang, Y.; Cheng, J.; Xu, W.; Xu, Z.; Gao, J.; Tao, L. Adjuvant contributes Roundup’s unexpected effects on A549 cells. Envir. Resear. 2020, 184, 109306. [Google Scholar] [CrossRef]
- Martins-Gomes, C.; Silva, T.L.; Andreani, T.; Silva, A.M. Glyphosate vs. glyphosate-based herbicides exposure: A review on their toxicity. J. Xenobiot. 2022, 12, 21–40. [Google Scholar] [CrossRef]
- Bach, N.C.; Marino, D.J.G.; Natale, G.S.; Somoza, G.M. Effects of glyphosate and its commercial formulation, Roundup®Ultramax, on liver histology of tadpoles of the neotropical frog, Leptodactylus latrans (amphibia: Anura). Chemosphere 2018, 202, 289–297. [Google Scholar] [CrossRef]
- Simões, A.M.; Venâncio, C.; Alves, L.; Antunes, F.E.; Lopes, I. Hydrophobic modifications of hydroxyethyl cellulose polymers: Their influence on the acute toxicity to aquatic biota. J. Hazard. Mater. 2021, 409, 124966. [Google Scholar] [CrossRef]
- Stenersen, J.; Kobro, S.; Bjerke, M.; Arend, U. Glutathione transferases in aquatic and terrestrial animals from nine phyla. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1987, 86, 73–82. [Google Scholar] [CrossRef]
- Hoarau, P.; Garello, G.; Gnassia-Barelli, M.; Roméo, M.; Girard, J.P. Effect of three xenobiotic compounds on Glutathione S-Transferase in the clam Ruditapes decussatus. Aquat. Toxicol. 2004, 68, 87–94. [Google Scholar] [CrossRef]
- Anjos, V.A.; da Silva-Júnior, F.M.; Souza, M.M. Cell damage induced by copper: An explant model to study anemone cells. Toxicol. Vitr. 2014, 28, 365–372. [Google Scholar] [CrossRef]
- Germond, A.; Kunihiro, T.; Inouhe, M.; Nakajima, T. Physiological changes of a green alga (Micractinium sp.) involved in an early-stage of association with Tetrahymena thermophila during 5-year microcosm culture. Biosystems 2013, 114, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.A. Pattern formation in the immortal Hydra. Trends Genet. 1996, 12, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Slobodkin, L.B.; Bossert, P.; Matessi, C.; Gatto, M. A review of some physiological and evolutionary aspects of body size and bud size of Hydra. Hydrobiologia 1991, 216–217, 377–382. [Google Scholar] [CrossRef]
- Deepananda, K.H.M.A.; Gajamange, D.; De Silva, W.A.J.P.; Wegiriya, H.C.E. Acute toxicity of a glyphosate herbicide, Roundup®, to two freshwater crustaceans. J. Natl. Sci. Found. Sri Lanka 2011, 39, 169–173. [Google Scholar] [CrossRef]
- Yadav, S.S.; Giri, S.; Singha, U.; Boro, F.; Giri, A. Toxic and genotoxic effects of Roundup on tadpoles of the Indian skittering frog (Euflictis cyanophlyctis) in the presence and absence of predator stress. Aquat. Toxicol. 2013, 132, 1–8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).