Effect of Farming System Type on Broilers’ Antioxidant Status, Performance, and Carcass Traits: An Industrial-Scale Production Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Broiler Chickens, Housing, and Diet
2.2. Growth Performance
2.3. Materials
2.4. Sampling and Preparation of Serum and Homogenized Muscle Tissue Extracts
2.5. Antioxidant Status
2.5.1. Total Antioxidant Capacity (TAC)
2.5.2. TBARS Assay
2.5.3. α-Tocopherol
2.6. Meat Quality
2.6.1. Meat Chemical Analysis
2.6.2. Organoleptic Characteristics
2.7. Statistical Analysis
3. Results
3.1. Performance
3.2. Total Antioxidant Capacity
3.3. Thiobarbituric Acid Reactive Substances (TBARS)
3.4. α-Tocopherol
3.5. Meat Quality
3.5.1. Chemical Analysis of the Meat
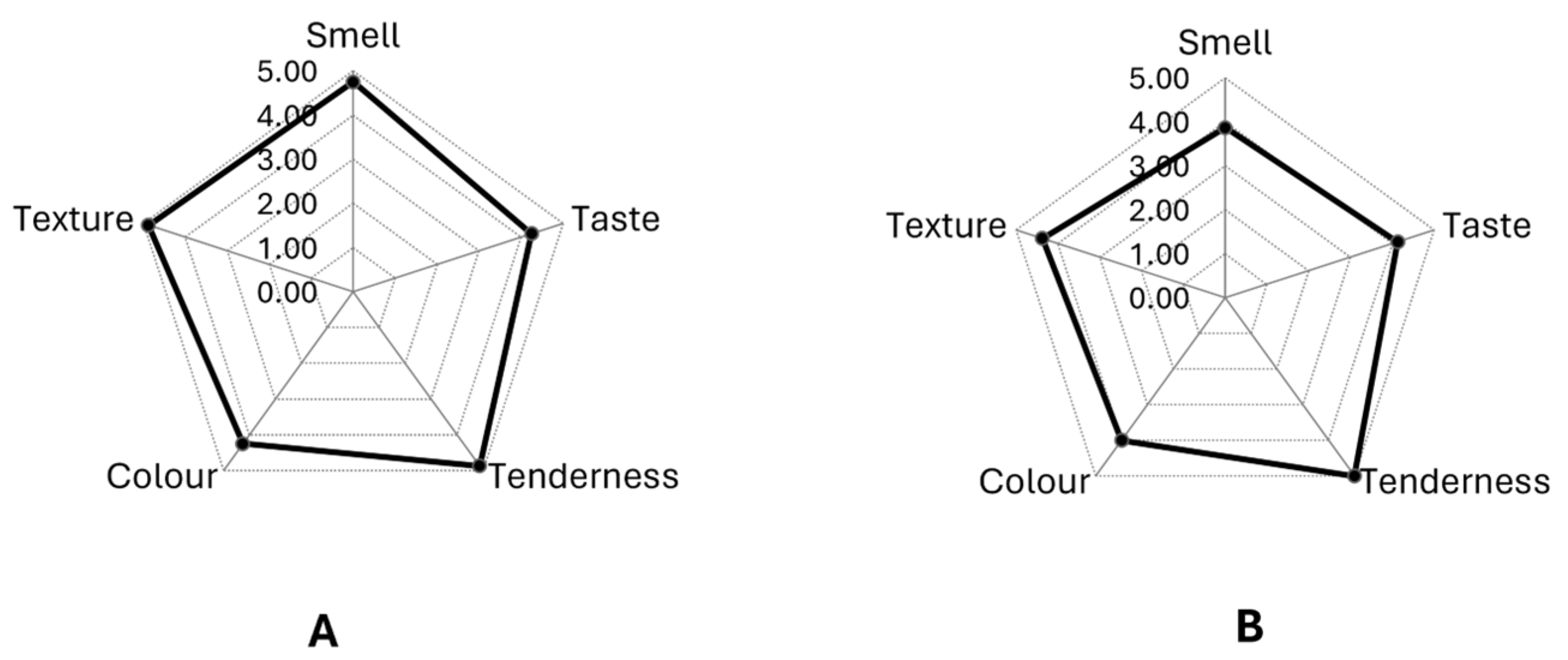
3.5.2. Organoleptic Characteristics
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mir, N.A.; Rafiq, A.; Kumar, F.; Singh, V.; Shukla, V. Determinants of Broiler Chicken Meat Quality and Factors Affecting Them: A Review. J. Food Sci. Technol. 2017, 54, 2997–3009. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Mattioli, S.; Cartoni Mancinelli, A.; Cotozzolo, E.; Castellini, C. Extensive Rearing Systems in Poultry Production: The Right Chicken for the Right Farming System. A Review of Twenty Years of Scientific Research in Perugia University, Italy. Animals 2021, 11, 1281. [Google Scholar] [CrossRef]
- da Silva, D.C.F.; de Arruda, A.M.V.; Gonçalves, A.A. Quality Characteristics of Broiler Chicken Meat from Free-Range and Industrial Poultry System for the Consumers. J. Food Sci. Technol. 2017, 54, 1818–1826. [Google Scholar] [CrossRef]
- OECD; FAO. OECD-FAO Agricultural Outlook 2016–2025; OECD-FAO Agricultural Outlook; OECD: Paris, France; FAO: Rome, Italy, 2016; ISBN 9789264253223. [Google Scholar]
- Dawkins, M.S. Animal Welfare and Efficient Farming: Is Conflict Inevitable? Anim. Prod. Sci. 2017, 57, 201–208. [Google Scholar] [CrossRef]
- Feddes, J.J.R.; Emmanuel, E.J.; Zuidhof, M.J. Broiler Performance, Body Weight Variance, Feed and Water Intake, and Carcass Quality at Different Stocking Densities. Poult. Sci. 2002, 81, 774–779. [Google Scholar] [CrossRef]
- Karavolias, J.; Salois, M.J.; Baker, K.T.; Watkins, K. Raised without Antibiotics: Impact on Animal Welfare and Implications for Food Policy. Transl. Anim. Sci. 2018, 2, 337–348. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Schmidt, C.G.; Herskin, M.S.; et al. Welfare of Broilers on Farm. EFSA J. 2023, 21, e07788. [Google Scholar] [CrossRef]
- European Commission. The Welfare of Chickens Kept for Meat Production (Broilers). Report of the Scientific Committee on Animal Health and Animal Welfare; European Commission: Brussels, Belgium, 2000. [Google Scholar]
- Fanatico, A.C.; Pillai, P.B.; Cavitt, L.C.; Emmert, J.L.; Meullenet, J.F.; Owens, C.M. Evaluation of Slower-Growing Broiler Genotypes Grown with and Without Outdoor Access: Sensory Attributes. Poult. Sci. 2006, 85, 337–343. [Google Scholar] [CrossRef]
- Stadig, L.M.; Bas Rodenburg, T.; Reubens, B.; Aerts, J.; Duquenne, B.; Tuyttens, F.A.M. Effects of Free-Range Access on Production Parameters and Meat Quality, Composition and Taste in Slow-Growing Broiler Chickens. Poult. Sci. 2016, 95, 2971–2978. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.; Richmond, A.; Lavery, U.; O’Connell, N.E. A Comparison of Fast Growing Broiler Chickens with a Slower-Growing Breed Type Reared on Higher Welfare Commercial Farms. PLoS ONE 2021, 16, e0259333. [Google Scholar] [CrossRef] [PubMed]
- Pappas, A.C.; Tsiplakou, E.; Papadomichelakis, G.; Mitsiopoulou, C.; Sotirakoglou, K.; Mpekelis, V.; Haroutounian, S.A.; Fegeros, K.; Zervas, G. Effects of Olive Pulp Addition to Broiler Diets on Performance, Selected Biochemical Parameters and Antioxidant Enzymes. J. Hell. Vet. Med. Soc. 2019, 70, 1687. [Google Scholar] [CrossRef]
- de Oliveira Sans, E.C.; Dahlke, F.; Freitas Federici, J.; Tuyttens, F.A.M.; Forte Maiolino Molento, C. Welfare of broiler chickens in Brazilian free-range versus intensive indoor production systems. J. Appl. Anim. Welf. Sci. 2023, 26, 505–517. [Google Scholar] [CrossRef]
- Vissers, L.S.M.; Saatkamp, H.W.; Oude Lansink, A.G.J.M. Analysis of synergies and trade-offs between animal welfare, ammonia emission, particulate matter emission and antibiotic use in Dutch broiler production systems. Agric. Syst. 2021, 189, 103070. [Google Scholar] [CrossRef]
- EC (European Commission). Council Directive 2007/43/EC of 28 June 2007 Laying down Minimum Rules for the Protection of Chickens Kept for Meat Production (Text with EEA Relevance); European Commission: Brussels, Belgium, 2007. [Google Scholar]
- Janaszewska, A.; Bartosz, G. Assay of Total Antioxidant Capacity: Comparison of Four Methods as Applied to Human Blood Plasma. Scand. J. Clin. Lab. Investig. 2002, 62, 231–236. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. [42] Determination of Aldehydic Lipid Peroxidation Products: Malonaldehyde and 4-Hydroxynonenal. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1990; Volume 186, pp. 407–421. ISBN 0076-6879. [Google Scholar]
- Fotou, E.; Moulasioti, V.; Angelis, I.; Tsiouris, V.; Patsias, A.; Tellis, C.; Moussis, V.; Boti, M.E.; Tsoukatos, D. Influence of Dietary Olive Paste Flour on the Performance and Oxidative Stress in Chickens Raised in Field Conditions. J. Hell. Vet. Med. Soc. 2021, 72, 3239–3248. [Google Scholar] [CrossRef]
- Hansen, L.G.; Warwick, W.J. A Fluorometric Micro Method for Fat Tocopherol. Clin. Biochem. 1969, 229, 225–229. [Google Scholar]
- AOAC. Official, Methods of Analysis of AOAC International, 16th ed.; AOAC International: Arlington, VA, USA, 1995; 547p, ISBN 0935584420. [Google Scholar]
- Surai, P.F.; Kochish, I.I. Nutritional Modulation of the Antioxidant Capacities in Poultry: The Case of Selenium. Poult. Sci. 2019, 98, 4231–4239. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, H.J.; Jeon, J.J.; Nam, K.C.; Shim, K.S.; Jung, J.H.; Kim, K.S.; Choi, Y.; Kim, S.H.; Jang, A. Comparison of the Quality Characteristics of Chicken Breast Meat from Conventional and Animal Welfare Farms under Refrigerated Storage. Poult. Sci. 2020, 99, 1788–1796. [Google Scholar] [CrossRef]
- Fanatico, A.C.; Pillai, P.B.; Emmert, J.L.; Owens, C.M. Meat Quality of Slow- and Fast-Growing Chicken Genotypes Fed Low-Nutrient or Standard Diets and Raised Indoors or with Outdoor Access. Poult. Sci. 2007, 86, 2245–2255. [Google Scholar] [CrossRef] [PubMed]
- Mikulski, D.; Celej, J.; Jankowski, J.; Majewska, T.; Mikulska, M. Growth Performance, Carcass Traits and Meat Quality of Slower-Growing and Fast-Growing Chickens Raised with and without Outdoor Access. Asian-Australas. J. Anim. Sci. 2011, 24, 1407–1416. [Google Scholar] [CrossRef]
- Martinez, D.A.; Ponce-de-Leon, C.L.; Vilchez, C. Meta-Analysis of Commercial-Scale Trials as a Means to Improve Decision-Making Processes in the Poultry Industry: A Phytogenic Feed Additive Case Study. Int. J. Poult. Sci. 2020, 19, 513–523. [Google Scholar] [CrossRef]
- Singh, M.; Lim, A.J.; Muir, W.I.; Groves, P.J. Comparison of Performance and Carcass Composition of a Novel Slow-Growing Crossbred Broiler with Fast-Growing Broiler for Chicken Meat in Australia. Poult. Sci. 2021, 100, 100966. [Google Scholar] [CrossRef]
- Bogosavljević-Bošković, S.; Rakonjac, S.; Dosković, V.; Petrović, M.D. Broiler Rearing Systems: A Review of Major Fattening Results and Meat Quality Traits. Worlds Poult. Sci. J. 2012, 68, 217–228. [Google Scholar] [CrossRef]
- Duy Hoan, N.; Khoa, M.A. Meat Quality Comparison Between Fast Growing Broiler Ross 308 and Slow Growing Sasso Laying Males Reared in Free Range System. J. Sci. Dev. 2016, 14, 101–108. [Google Scholar]
- Hellmeister Filho, P.; Machado Menten, J.F.; Neves da Silva, M.A.; Domingos Coelho, A.A.; Savino, V.J.M. Effect of Genotype and Rearing System on Performance of Alternative Lines of Broiler Chickens. Rev. Bras. Zootec. 2003, 32, 1883–1889. [Google Scholar] [CrossRef]
- Durali, T.; Singh, M.; Groves, P.; Cowieson, A.J. Comparison of Free-Range and Conventional Broiler Performace and Digestibility. In Proceedings of the 24th Annual Australian Poultry Science Symposium, Sydney, NSW, Australia, 17–20 February 2013; pp. 150–153. [Google Scholar]
- Yamak, U.S.; Sarica, M.; Boz, M.A. Comparing Slow-Growing Chickens Produced by Two- and Three-Way crossings with Commercial Genotypes. 1. Growth and Carcass Traits. Eur. Poult. Sci. 2014, 78, 1–10. [Google Scholar] [CrossRef]
- Ponte, P.I.P.; Rosado, C.M.C.; Crespo, J.P.; Crespo, D.G.; Mourão, J.L.; Chaveiro-Soares, M.A.; Brás, J.L.A.; Mendes, I.; Gama, L.T.; Prates, J.A.M.; et al. Pasture Intake Improves the Performance and Meat Sensory Attributes of Free-Range Broilers. Poult. Sci. 2008, 87, 71–79. [Google Scholar] [CrossRef]
- Katekhaye, A. Review on Slow and Fast Growing Chicken Varieties Physicochemical Qualities. Agric. Rev. 2019, 40, 150–156. [Google Scholar] [CrossRef]
- Qiao, M.; Fletcher, D.L.; Smith, D.P.; Northcutt, J.K. The Effect of Broiler Breast Meat Color on PH, Moisture, Water-Holding Capacity, and Emulsification Capacity. Poult. Sci. 2001, 80, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, S.; Cartoni Mancinelli, A.; Menchetti, L.; Dal Bosco, A.; Madeo, L.; Guarino Amato, M.; Moscati, L.; Cotozzolo, E.; Ciarelli, C.; Angelucci, E.; et al. How the Kinetic Behavior of Organic Chickens Affects Productive Performance and Blood and Meat Oxidative Status: A Study of Six Poultry Genotypes. Poult. Sci. 2021, 100, 101297. [Google Scholar] [CrossRef] [PubMed]
- Castellini, C. Organic Poultry Production System and Meat Characteristics. In Proceedings of the XVII European Symposium on the Quality of Poultry Meat, Doorwerth, The Netherlands, 23–26 May 2005; pp. 47–52. [Google Scholar]
- Castellini, C.; Mugnai, C.; Dal Bosco, A. Effect of Organic Sysytem on Broiler Carcass and Meat Quality. Meat Sci. 2002, 60, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.; Sakomura, N.K.; Freitas, E.R.; Fortes, C.M.S.; Carrilho, E. Comparison of Free Range Broiler Chicken Strains Raised in Confined or Semi-Confined Systems. Braz. J. Poult. Sci. 2005, 7, 85–92. [Google Scholar] [CrossRef]
- Fanatico, A.C.; Pillai, P.B.; Cavitt, L.C.; Owens, C.M.; Emmert, J.L. Evaluation of Slower-Growing Broiler Genotypes Grown with and without Outdoor Access: Growth Performance and Carcass Yield. Poult. Sci. 2005, 84, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Debut, M.; Bihan-Duval, E.; Berri, C. Impacts des Conditions de Pré-Abattage sur la Qualité Technologique de La Viande de Volaille. Sci. Tech. Avic. 2004, 48, 4–13. [Google Scholar]
- Tang, H.; Gong, Y.Z.; Wu, C.X.; Jiang, J.; Wang, Y.; Li, K. Variation of Meat Quality Traits among Five Genotypes of Chicken. Poult. Sci. 2009, 88, 2212–2218. [Google Scholar] [CrossRef]
| System | Extensive 1 | Intensive 2 | |
|---|---|---|---|
| Genotype | Sasso | Ross 308 | |
| Farming type | Free-range | Conventional | |
| Growth type | Slow growing | Fast growing | |
| Diet | Standard 3 | Standard 4 | |
| Stocking density | Indoor | outdoor | indoor |
| 13 broiler chickens/m2 | 1 broiler chicken/m2 | 15 broiler chickens/m2 | |
| N 5 | 6.000 | 20.000 | |
| Slaughter age | 67 | 47 | |
| Ingredients (kg/ton) | Starter (Days 1–17) | Grower (Days 17–35) | Finisher (Days 36–Slaughter) | |||
|---|---|---|---|---|---|---|
| A 1 | B 2 | A 1 | B 2 | A 1 | B 2 | |
| Corn | 329 | 200 | 423 | 150 | 650 | 0 |
| Wheat | 300 | 392 | 250 | 478 | 68 | 714 |
| Soya-meal | 314 | 335 | 273 | 305 | 236 | 230 |
| Phosphoric acid | 6.0 | 8.5 | 6.8 | 5.8 | 7.0 | 3.8 |
| Limestone | 14 | 14 | 13 | 12 | 11 | 10 |
| Palm oil | 0 | 4 | 10 | 14 | 17 | 18 |
| Soya oil | 17 | 25 | 9 | 19 | 0 | 10 |
| Premix 3 | 19.9 | 20.0 | 15.7 | 16.3 | 11.8 | 14.4 |
| System | Extensive 1 | Intensive 2 | p Value |
|---|---|---|---|
| Mortality% | 4.60 ± 0.62 a | 3.96 ± 0.28 a | 0.365 |
| BW (kg) | 2.48 ± 0.04 a | 2.60 ± 0.06 a | 0.142 |
| ADFI | 89.70 ± 5.34 a | 106.82 ± 8.79 a | 0.096 |
| BWG (g) | 36.55 ± 1.75 A | 61.51 ± 3.57 B | <0.001 |
| FCR | 2.43 ± 0.01 A | 1.74 ± 0.02 B | <0.001 |
| EPEF | 145 ± 2.31 A | 314 ± 3.66 B | <0.001 |
| System | Extensive 1 | Intensive 2 | p Value |
|---|---|---|---|
| Serum RSA % | 33.27 ± 1.03 A | 29.19 ± 0.87 B | <0.001 |
| Thigh RSA% | 50.42 ± 0.77 a | 44.29 ± 1.14 b | 0.004 |
| Serum MDA (nmol/mL) | 4.70 ± 0.16 a | 3.98 ± 0.14 b | 0.005 |
| Thigh Muscle MDA (nmol/mL) | 2.63 ± 0.08 a | 3.03 ± 0.11 b | 0.017 |
| α-tocopherol (μg/mL) | 21.38 ± 1.18 a | 21.47 ± 0.93 a | 0.261 |
| System | Extensive 1 | Intensive 2 | p Value |
|---|---|---|---|
| Protein% | 21.20 ± 0.03 a | 20.90 ± 0.11 b | 0.016 |
| Fat% | 12.20 ± 0.25 a | 13.20 ± 0.21 b | 0.019 |
| Moisture% | 65.20 ± 0.11 A | 66.20 ± 0.12 B | <0.001 |
| Ash% | 1.10 ± 0.01 a | 1.20 ± 0.02 b | 0.002 |
| pH | 5.20 ± 0.01 A | 5.60 ± 0.07 B | <0.001 |
| WHC% | 3.10 ± 0.02 A | 4.10 ± 0.01 B | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotou, E.; Moulasioti, V.; Papadopoulos, G.A.; Kyriakou, D.; Boti, M.-E.; Moussis, V.; Papadami, M.; Tellis, C.; Patsias, A.; Sarrigeorgiou, I.; et al. Effect of Farming System Type on Broilers’ Antioxidant Status, Performance, and Carcass Traits: An Industrial-Scale Production Study. Sustainability 2024, 16, 4782. https://doi.org/10.3390/su16114782
Fotou E, Moulasioti V, Papadopoulos GA, Kyriakou D, Boti M-E, Moussis V, Papadami M, Tellis C, Patsias A, Sarrigeorgiou I, et al. Effect of Farming System Type on Broilers’ Antioxidant Status, Performance, and Carcass Traits: An Industrial-Scale Production Study. Sustainability. 2024; 16(11):4782. https://doi.org/10.3390/su16114782
Chicago/Turabian StyleFotou, Evgenia, Vasiliki Moulasioti, Georgios A. Papadopoulos, Dimitra Kyriakou, Maria-Eleni Boti, Vassilios Moussis, Maria Papadami, Constantinos Tellis, Apostolos Patsias, Ioannis Sarrigeorgiou, and et al. 2024. "Effect of Farming System Type on Broilers’ Antioxidant Status, Performance, and Carcass Traits: An Industrial-Scale Production Study" Sustainability 16, no. 11: 4782. https://doi.org/10.3390/su16114782
APA StyleFotou, E., Moulasioti, V., Papadopoulos, G. A., Kyriakou, D., Boti, M.-E., Moussis, V., Papadami, M., Tellis, C., Patsias, A., Sarrigeorgiou, I., Theodoridis, A., Lymberi, P., Tsiouris, V., Tsikaris, V., & Tsoukatos, D. (2024). Effect of Farming System Type on Broilers’ Antioxidant Status, Performance, and Carcass Traits: An Industrial-Scale Production Study. Sustainability, 16(11), 4782. https://doi.org/10.3390/su16114782









