Abstract
Despite the numerous benefits of intensive air transport development, many activities associated with the operation of airports contribute to environmental pollution. The purpose of this research was the development, optimization, and validation of a headspace–solid-phase microextraction–comprehensive two-dimensional gas chromatography–time of flight–mass spectrometry (HS-SPME-GC × GC-TOF-MS)-based procedure for determining anti-corrosive compounds in airport stormwater. Optimized HS-SPME conditions include: 45 min extraction time, 100 °C temperature, 1.0 g salt addition, and 10 min desorption time at 270 °C. The developed procedure is sensitive, selective, accurate (recoveries ≥ 80.0%), and precise (the coefficient of variation (CV) ≤ 14.9%), making it a highly suitable tool for extensive airport stormwater quality monitoring. The validated analytical protocol was successfully used to detect pollutants, including 1H-BT, 4-MeBT, 5-MeBT, and 5,6-diMe-1H-BT, in stormwater from various European airports with different flight capacities. Throughout the sampling period at the investigated airports, 1H-benzotriazole was found in the highest concentrations, ranging from below the MQL to 467 mg/L. An ecotoxicological risk assessment revealed that 69% of the sites exhibited high risk levels (Risk Quotient ≥ 1). The developed procedure and carried out environmental risk assessments of benzotriazoles in airport stormwater enable an evidence-based approach to sustainable airport stormwater management.
1. Introduction
One of the significant environmental problems is the increase in anthropogenic pressure associated with the operation of airports on a global scale [1,2,3,4]. Despite the numerous benefits of intensive air transport development, many activities associated with the operation of airports contribute to environmental pollution [5,6,7,8]. In this regard, airport stormwater, also known as airport runoff water, poses a growing environmental problem [9,10,11,12,13]. Airport stormwater is formed when atmospheric deposits or precipitation flush the airport’s surfaces during everyday activities [14,15,16]. Streams that receive airport runoff can contain a wide spectrum of hazardous contaminants, such as polycyclic aromatic hydrocarbons (PAHs), benzotriazoles (BTs or BTR), polychlorinated biphenyls (PCBs), glycols, per- and polyfluoroalkyl substances (PFAS), pesticides, phenols, detergents, formaldehyde, and metals [17,18,19]. Moreover, stormwater from airports during heavy precipitation events can lead to microbiological contamination of the source water for public drinking resources [19,20]. Runoff water following intense rainfall, especially from regions surrounding airports, has been associated with an increased risk of acute gastrointestinal illness (AGI) in highly developed countries, such as the USA, Canada, England, France, and Australia [20,21]. Contaminated surface stormwater from airports can enter municipal sewage systems, and from there, it is directed to wastewater treatment plants (WWTPs). In cases where WWTPs are either non-existent or malfunctioning, this contaminated stormwater may then seep into surface water, soil, and groundwater, potentially affecting public drinking water supplies [21,22].
Chemical compounds from the benzotriazoles group are recognized as particularly threatening to all environmental compartments [23,24,25]. BTs are the fourth most abundant individual aquatic contaminants [26]. BTs are ubiquitous aquatic contaminants applied in a wide range of industrial and domestic applications. The major applications of BTs include use as de-icing/anti-icing fluids (ADAFs), corrosion inhibitors, the photography industry, ultraviolet light stabilizers, antifogging agents, coolants, surface coatings, hydraulic fluids, and cutting fluids [27,28,29]. Additionally, BTs are widely used in pigments, greases, lubricants, dishwasher detergents, dry cleaning equipment, and also in the synthesis of pharmaceuticals [30]. The benzotriazoles group of compounds has been classified as high-production volume (HPV) substances [27]. The annual usage of benzotriazoles in the United States (USA) exceeds 9000 tons per year, and global usage is much greater [27,30]. The global market for benzotriazoles was estimated at USD 348.1 million in the year 2020, and it is projected to reach a revised size of USD 610.1 million by 2027. They are detected globally in all environmental elements, especially in water supplies [27,28,31]. In the case of airports, these isomers of benzotriazoles are corrosion inhibitors added to aircraft de-icing and anti-icing fluids [9,24,32,33]. These compounds are primarily discharged during operations to de-ice or prevent re-icing of aircraft, taxiways, runways, and other paved surfaces on airport platforms during periods of freezing precipitation. According to Directive 67/548/EEC, the BTs group of compounds is classified as dangerous to the environment and can cause long-term adverse effects in the aquatic environment [34]. Considering the available literature data, it has been noted that 1H-benzotriazole (1H-BT), 4-methyl-1H-benzotriazole (4-MeBT), 5-methyl-1H-benzotriazole (5-MeBT), 5,6-dimethyl-1H-benzotriazole (5,6-diMe-1H-BT or xylyltriazole-XTR), and a mixture of isomers of 4-MeBT and 5-MeBT have been shown to be phytotoxic and mutagenic [28,29]. They are also classified as suspected human carcinogens with estrogenic potential [30,35,36,37]. Additionally, recent research indicates that exposure to benzotriazoles during pregnancy could potentially increase the risk of developing gestational diabetes mellitus (GDM) [38].
In this context, developing analytical protocols to identify benzotriazoles in airport runoff water samples is essential. These protocols are key for monitoring the environmental impact of these compounds and understanding how airports affect the surrounding environment [36,37,38]. Nevertheless, the determination of benzotriazoles in airport runoff water is a great analytical challenge. Critical issues in analyzing airport stormwater involve complex sample matrices, the presence of analytes at low or trace levels, and the potential overlap of various compounds with similar physicochemical properties within a single sample. Further complicating matters are the difficulties in standardizing measurements, affected by factors such as the diverse sizes of airports and fluctuating weather conditions [2,10,11]. Given this, the essential steps in the proper analytical methodology include sample preparation and the subsequent determination stage. Based on the literature reports, it can be summarized that the field of the carried out research is very limited. To date, the analysis of target benzotriazoles in airport runoff water has primarily utilized methodologies based on liquid–liquid extraction (LLE) or solid-phase extraction (SPE) coupled with either gas chromatography–mass spectrometry (GC-MS), flame ionization detection (FID), or liquid chromatography–tandem mass spectrometry (LC–MS/MS). This approach is detailed in the works of the Cancilla and Corsi research teams [9,24,39,40], as well as in our previous study [34]. However, using conventional techniques to extract and determine benzotriazoles from complex matrices, such as airport runoff water samples, presents significant challenges.
It is recommended that new procedures be developed that not only offer advantages over existing methods but also ensure their effective implementation. The increased interest of our research team in the development and optimization of modern, reliable, and powerful analytical protocols resulted in intensive research in this field. SPME is an alternative to traditional extraction methods, which have been applied to isolate benzotriazoles from runoff water samples. Solvent-free SPME is a simple and convenient extraction technique that merges extraction, concentration, and sample introduction in one step. Thus, solid-phase microextraction can often be faster, more sensitive, easier to automate, economical, and meet the requirements of green chemistry. Then, the selection of a proper separation technique is another crucial step of a relevant analytical methodology applied for the detection, identification, and quantitative determination of target compounds in the samples at the trace and even ultratrace concentration levels. Stormwater and other environmental samples often have high viscosity and a complex, variable matrix composition; analyzing them with just one chromatographic technique, such as one-dimensional gas chromatography, may be insufficient. The target compounds may be indistinguishable from the baselines, or the focusing chromatographic peak can be missing through larger co-eluting peaks. This fundamental problem can resolved by applying comprehensive two-dimensional gas chromatography (GC × GC). The suggested technique is recognized as a powerful high-resolution technique for the separation of a broad spectrum of organic compounds in highly complex sample matrices [41]. The advantages of GC × GC multidimensional systems include highly improved sensitivity, large separation power, as well as high selectivity [42,43,44]. Furthermore, GC × GC chromatograms are characterized by very narrow peaks that require the application of a fast scanning detector. For this purpose, the time-of-flight mass spectrometry (TOF-MS) detector can be efficiently applied for the identification of a wide range of organic compounds [45,46,47,48,49].
Furthermore, incorporating an additional approach to assess the environmental risk of benzotriazoles can help identify and understand the sources, pathways, and consequences of these environmental stressors. This approach can also facilitate the development of suitable strategies to prevent or mitigate their impacts. Risk assessment offers technical support for decision-making under uncertainty. Accordingly, the findings from lab-scale toxicological studies are employed in this process [36,50]. This assessment aims to determine the probable impact of certain chemicals contaminating a specific environment on the life processes of organisms living in that environment [36,50]. The chronic effect of BTs on living organisms and ecosystems is a relatively recent research topic. Furthermore, there is a lack of comprehensive knowledge about the toxicological data of BTs, which is further complicated by their widespread use and the low toxicity indices of many of these compounds. This could potentially have unspecified chronic effects at the sublethal level and potentially lead to environmental contamination [50,51]. Environmental risk assessment can also help ensure compliance with existing environmental policies and obligations, promote sustainability and environmental stewardship, and ensure the health and safety of all stakeholders.
In this work, we hypothesize that the application of solid-phase microextraction–comprehensive two-dimensional gas chromatography–time of flight–mass spectrometry-based procedure significantly enhances the determination of benzotriazoles in airport runoff water, leading to more accurate environmental risk assessments and informed management decisions for sustainable airport operations. The main aim of the present research was to develop, optimize, validate, and apply HS-SPME-GC × GC-TOF-MS-based procedure for the determination of benzotriazoles in the samples of runoff water. Stormwater samples were collected from four international airports, each located in a distinct geographical region and characterized by varying sizes and capacities, as indicated by the number of flights and passengers per year. Attempts were made to estimate environmental risk assessments. To our knowledge, this is the first time that such an investigation was carried out.
2. Materials and Methods
2.1. Airport Runoff Water Samples
Pilot samples of stormwater were collected from four international airports, encompassing three locations in Poland and one in the United Kingdom. During the 2012–2013 period, stormwater samples were collected from international airports characterized by varying passenger movement capacities—namely, a large-capacity airport in the UK (Large Airport UK), and high (High Airport PL), medium (Medium Airport PL), and low (Small Airport PL) capacity airports in Poland. There have been no significant changes in the monitored airport conditions that would affect the relevance of the data. Specifically, there have been no major expansions or renovations at these airports, and the intensity of air traffic has remained comparable to the period during which the samples were collected. Additionally, there have been no alterations in the operational procedures, including changes in the quantity or types of de-icing agents used. This consistency over the years ensures that the samples remain representative of current conditions. The generic names used for the airports are the result of cooperation agreements signed with these airports.
Sampling was conducted during two distinct seasons, autumn and winter, to account for seasonal variations. This monitoring was conducted over two seasons because antifreeze fluids containing compounds from the benzotriazole group are primarily discharged during operations to de-ice aircraft and paved surfaces at airports in periods of low temperatures, particularly during the winter and autumn. Due to safety regulations at the airports and atmospheric constraints related to the appropriate amount of precipitation, only a very limited number of samples could be collected.
The runoff water samples were usually collected within 30 min of the start of the precipitation event (first flush). During the study period, the amount of rainfall ranged from 2 to 10 mm, and the duration of the events was from 3 to 5 h. During periods of low temperature, stormwater samples were collected from ground depressions adjacent to drainage inlets and airport drainage systems—locations where rainwater had naturally accumulated. Sampling sites were strategically chosen in the vicinity of airport zones predominantly associated with technical services, specifically areas involving the application of de-icing and antifreeze fluids on aircraft and aircraft refueling stations. Consequently, these sampling sites were identified as primary points where pollutants are transported via stormwater into drainage ditches and, thereafter, dispersed into other environmental components.
Due to safety regulations, installing and locating any sampling devices on the airport platform is not possible. These requirements stem from the necessity to uphold regular operational schedules at the airport and to adhere to enhanced, more rigorous security protocols implemented at international airports. Consequently, runoff water samples were manually collected in 1000 mL dark glass bottles using a 100 mL syringe equipped with Teflon tubing. The structure and characteristics of the selected benzotriazoles for this study are presented in Table S1. The reagents, standards, and equipment used in the proposed procedure for the determination of analytes from the benzotriazole group in airport runoff water samples are listed in Table 1. Before use, the syringes and tubing were first rinsed with Milli-Q water and then with the sampled airport runoff water. After each sample collection campaign, the airport runoff water samples were transported to the laboratory and stored at 4 °C in the dark until extraction [34].

Table 1.
The reagents, standards, and equipment used in the proposed analytical methods.
2.2. Development of Analytical Procedures
Growing concerns about obtaining accurate data on the state of individual environmental elements and the processes occurring within them often necessitate the application of complex, time-consuming, and labor-intensive analytical protocols. The analysis of pollutants generated during airport operations is encountering new challenges, particularly the necessity to detect analytes at trace and even ultratrace concentrations in samples with highly complex and often variable matrix compositions. Consequently, there is a need to develop new analytical protocols for identifying the most toxic pollutants in airport runoff water. These protocols must consider the diverse range of compounds present and the potential coexistence of components with similar physical and chemical properties within a single sample. Figure 1 presents a general outline of the steps involved in developing a new procedure for the determination of benzotriazoles in airport runoff water samples.
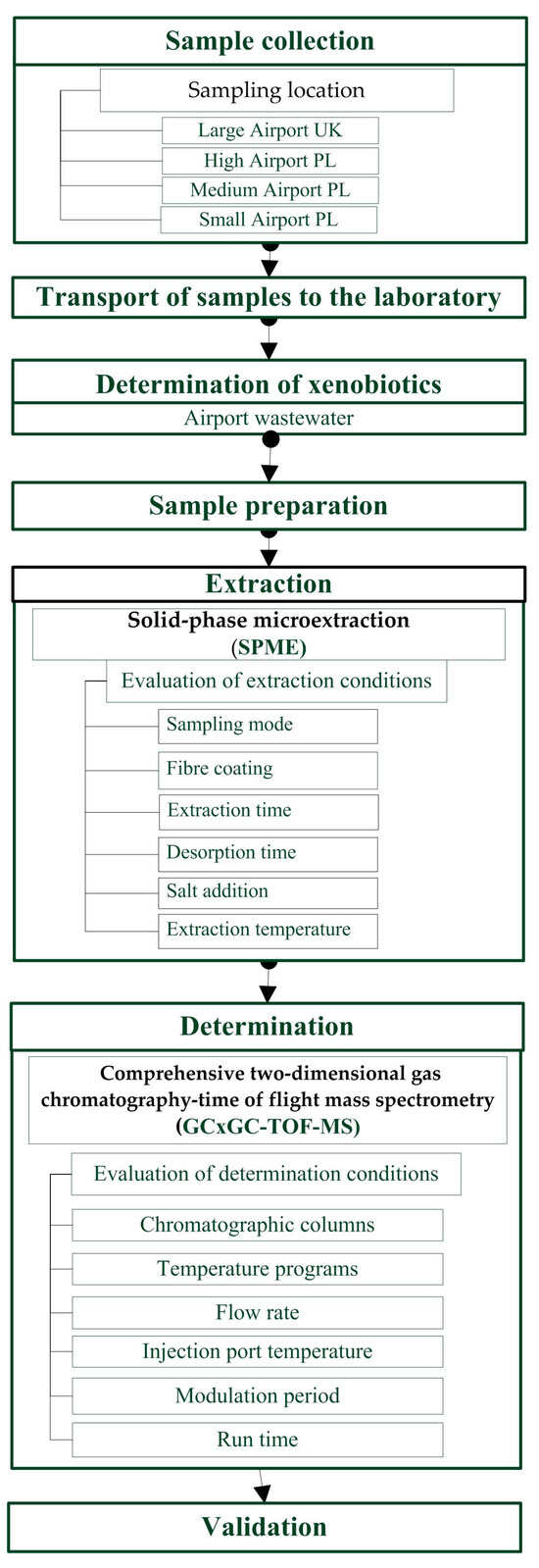
Figure 1.
General outline of the steps involved in developing a new procedure for the determination of benzotriazoles in airport runoff water samples.
2.2.1. Sample Preparation
Sample preparation is a crucial step in the analytical methodology used for detecting, identifying, and quantifying benzotriazole compounds in runoff water samples, which often have a highly complex matrix composition. Due to interactions between components in the samples, it is imperative that the collected runoff water samples should be transported to the laboratory and analyzed as soon as possible.
The initial step in preparing the stormwater samples involved removing solid impurities, such as leaves, sand, and dust, through filtration using a Millex®-HV filter with a pore size of 0.45 mm.
The use of various extraction techniques in the preparation of airport stormwater samples for benzotriazole analysis could have an impact on the final results. In the conducted research, solid-phase microextraction was used to isolate/enrich analytes. SPME fiber coated with a 65 µm-thick film of polydimethylsiloxane/divinylbenzene (PDMS-DVB) was applied [51,52]. The SPME extractions were carried out in 20 mL amber glass vials closed with stainless-steel screw caps fitted with a PTFE/silicone-coated septum. The headspace-to-sample ratio was 2.
To establish the best extraction conditions, a sample of airport runoff water was enriched with the appropriate amounts of a 1-MeBT/4-MeBT/5-MeBT mixture and 5,6-dimethylo-1H-benzotriazole, each at a concentration of 1000 µg/mL. Additionally, decafluorobiphenyl and an isotopically labeled standard (1H-benzotriazol-d4) were used as internal standards in this model sample. For the analysis of real samples and to calculate the recovery, only the isotopically labeled standard was used to spike the stormwater samples.
The SPME fiber was conditioned inside the injection port at 250 °C for 30 min, according to the manufacturer’s specifications. Before extraction, the model sample solution was kept in the vial at the target temperature for 5 min and stirred at a speed of 700 rpm. The incubation step was performed to enhance the transfer of volatile compounds from the sample solution to the headspace. All the model solutions were agitated at a constant maximum speed of 700 rpm throughout the extraction period to enhance analyte extraction and reduce extraction time. The target compounds were adsorbed onto the SPME fiber’s stationary phase from the gaseous phase. Subsequently, the adsorbed analytes were thermally desorbed by inserting the fiber into the GC injector port. To avoid potential sample carryover, the SPME fiber was subjected to post-baking at 270 °C for 5 min in the GC injector, using the splitless mode. Additionally, to confirm the cleanliness of the GC × GC system, SPME fiber and chromatographic blanks were periodically run during the analysis of extracts. Each extraction was carried out in triplicate. All extraction steps were automated using a Gerstel MultiPurpose Sampler (MPS), operated with the Gerstel MAESTRO software.
2.2.2. GC × GC-TOF-MS Analysis
Two-dimensional gas chromatography with time-of-flight mass spectrometry (GC × GC-TOF-MS) was applied to determine benzotriazoles in the extracts. Based on data from the literature and conclusions from the authors’ previous experience, an appropriate set of chromatographic columns was selected, along with the basic operating parameters for the GC × GC system. The technical specifications and operating conditions are detailed in Table 2. Additionally, ions used for quantifying the target analytes are provided in Table S1.

Table 2.
The technical specifications and operating conditions in GC × GC-TOF-MS analysis of benzotriazole extracts.
The obtained chromatographic data were processed and visualized as chromatograms using LECO ChromaTOF™ software version 4.44 (LECO Corporation, St. Joseph, MI, USA). After data acquisition, the obtained results were submitted to a data processing module, where the individual peaks were detected automatically based on a 10:1 signal-to-noise ratio (S/N). The area of each individual peak was automatically acquired, and the identification of the analyte was conducted by evaluating and comparing its mass spectra with those in the National Institute of Standards and Technology (NIST) library. This process also involved comparisons with standards, elution order, and retention times as described in the existing literature data. The amount of target compound was calculated by using the most abundant ions in the mass spectrum of the compound.
2.2.3. Quality Assurance/Quality Control (QA/QC)
A validation process was conducted to determine the selectivity, linearity, range, method detection limits (MDLs), method quantification limits (MQLs), accuracy, inter-day (between-run) precision, and intra-day (within-run) precision of the methodology.
A quantitative analysis based on the peak areas was performed by applying the internal standard method. The concentrations of target compounds were calculated by applying the standard calibration curves, which had been created by using the detector response. The target analyte concentrations were defined as the ratio of the analyte ion (the most abundant, specific, product ion) to the internal standard base peak ion.
Methodology selectivity was assessed by examining the chromatograms for the presence of any interfering peaks at the retention time of the analyte. The procedure exhibited satisfactory selectivity.
The linearity and concentration range of the developed procedure were estimated by serial dilution of a stock solution of the target analytes. The calibration curves for the analytes were examined under optimal HS–SPME and GC × GC-MS conditions. A linear calibration curve was determined for each analyte by plotting the peak area against the concentration of standard solutions with the calculated coefficients of determination (R2). The linear range of the calibration curve was assessed in the ranges of 5.0–50 µg/L, 50–500 µg/L, and 500–50,000 µg/L, respectively. The first point of the calibration curve was always the instrumental blank sample. The calibration curves were freshly prepared before each analysis by diluting the analyte stock solution. Three independent measurements were performed for each calibration point.
The limits of detection (LODs) were estimated based on three independent measurements. The LODs were established using the equation LOD = 3.3SD/b, where b is the slope of the calibration curve, while SD is the standard deviation. The limits of quantification (LOQs) were estimated as three times the LODs. The obtained results were applied to establish the method detection limits (MDLs) as well as method quantification limits (MQLs) of the procedure, considering all analytical steps. The MDL was defined as the lowest concentration of the target analyte that could be detected with a specified probability by applying a proposed analytical methodology. The MQL was the lowest concentration of the analyte that could be quantified by applying the developed procedure with certain precision, accuracy, and uncertainty levels.
Accuracy studies were performed by testing airport runoff water samples containing low, medium, and high concentration levels of the investigated compounds. Blank airport runoff water samples were spiked with 10 µg/L, 100 µg/L, and 1000 µg/L of target analytes. Moreover, the airport stormwater samples were spiked with 100 µg/L of isotopically labeled analyte (1H-benzotriazol-d4) before each filtration and extraction process. The concentration levels of BTs in spiked stormwater samples were calculated based on the expected concentrations of target analytes in real samples. It was difficult to receive genuine blank samples of airport runoff water. Blank samples were collected from the airport’s peripheral areas, which have the lowest maintenance activity, where lower concentrations of target analytes were expected compared to areas with more frequent technical service operations. These blank samples were tested before each analysis. The recovery of the proposed analytical methodology was verified by analyzing runoff water samples based on triplicate measurements at each spiked concentration. As mentioned earlier, the recovery values were estimated using the standard addition method. The known amounts of target analytes and isotopically labeled standard were added to one sample, whilst parallel samples were only spiked with isotopically labeled standard. After finishing the analysis of the investigated sample without the added analytes (x) and the sample containing the added analytes (x + si), the recovery value was calculated by applying the equation of % recovery = [(x + si) − (x)/s] × 100, where si is the amount of the assessed analyte.
The precision of the developed methodology was assessed by spiking stormwater samples with the analytes and labeled standard, as described earlier in the section on accuracy. The precision of the procedure was expressed as the coefficient of variation (CV) calculated for five replicate measurements according to the equation CV = SD/X × 100%, where SD is the standard deviation of the concentration of the analyte, while X is the average concentration of the analyte in a tested sample. The evaluation of precision included both intra-day and inter-day assays. The intra-day precision included three independent measurements of each quality control (QC) sample at three concentration levels in a single batch for one day. In turn, the inter-day assay was assessed based on the analysis of three independent measurements of each QC sample at three concentration levels on separate days during a three-day period.
2.2.4. Statistical Analysis
The significance of differences between various parameter levels or sample categories was tested using the Kruskal–Wallis ANOVA due to the assumptions of the regular ANOVA not being fulfilled in the collected dataset (the assumption of equal variance between groups or normality within groups or both, not being satisfied). We checked the equal variance assumption with Levene’s test and the normality assumption in categorized histograms. Following the general Kruskal–Wallis test, pairwise comparisons were also conducted. All calculations were perfomed with Statistica v. 13 (StatSoft, TIBCO Software Inc., Palo Alto, CA, USA).
2.2.5. Evaluation of the Ecotoxicological Risk
The assessment of the ecotoxicological risk to the environment posed by benzotriazoles in stormwater from airports has been conducted using the risk quotient (RQ) method. The RQ calculation method was defined by the European technical guidance document on risk assessment for various trophic levels within the water ecosystem, including producers such as algae or plants, consumers such as invertebrates (Crustacea; Branchiopoda), vertebrates (Actinopterygii), and decomposers (Bacteria). The RQx values were determined by calculating the ratio between the highest environmental concentration (MEC) measured for each investigated compound and the short-term predicted no-effect concentration (PNEC) [36,50,51].
where MECx—the highest measured concentration of the pollutant in airport runoff water sample; PNECx—predicted no-effect-concentration [mg/L] of the pollutant x towards the given trophic level in the environmental conditions; AF—the assessment factor selected to include the differences between laboratory results and natural conditions, considering both interspecies and intraspecies differences; ECx,y[%]—the measure of toxicity (also available as IC for inhibition or LC for lethality), the calculated concentration [mg/L] of chemical compound x in which a given percent of a laboratory population of model organism y (representing a given trophic level) shows the observed effect or the percentile of this effect that is achieved. This study used available literature data to establish the reference values.
There are three criteria for low, medium, and high risk, which are based on the hazard quotient (HQ). The HQ is the ratio between the MEC and the PNEC: HQ < 0.1 (low risk), 0.1 ≤ HQ < 1 (medium), and HQ ≥ 1 (high) [50,53].
This research considers RQx < 0.1 as an acceptable level of risk, 0.1 ≤ RQx < 1 as medium risk, and RQx ≥ 1 as an unacceptable level of ecological risk for the aquatic ecosystem. This is based on the established guidelines of Perrodin et al. [54] and the European Chemical Bureau (ECB) [55] and in accordance with the ranking categories outlined in Table S1 based on the available literature.
The predicted no-effect concentration (PNEC) was determined by using the median lethal/inhibition/effect concentration (EC/IC/LC50) data from acute exposure times (ranging from 15 min for bacteria to 96 h for certain fish) or from chronic toxicity data based on EC10. This value was then divided by an appropriate safety factor (AF). In this study, the AF adhered to the guidelines recommended by the European Parliament and the Council [56], typically employing a factor of 1000 for acute studies and 100 for chronic studies. These AF values can be modified to suit different regions, subject to the specific requirements of local legislation [36,50].
When multiple toxicity factor values for a given compound were identified across a representative trophic level, the smallest concentration value, indicating the highest toxicity, was chosen. The chosen toxicity factor values incorporated into the calculations were established based on data extracted from available literature [31,32,39,57,58], as detailed in Table S1.
3. Results and Discussion
3.1. Optimization of the Extraction Procedure
The SPME extraction efficiency is associated with a considerable number of variables, including the sampling mode, the coating of the SPME fiber, the extraction temperature, the extraction time, the desorption time and temperature, as well as the ionic strength. In this study, several different SPME operating conditions were tested to obtain the optimal performance of the benzotriazoles analysis procedure.
3.1.1. Selection of the Sampling Mode and Fiber Coating
Based on the properties of the analytes, literature data, and conclusions resulting from the authors’ prior experience, the headspace (HS) mode of SPME was applied for the determination of analytes from the benzotriazoles group [51,52]. The HS-SPME has been demonstrated to be a useful tool for the efficient enrichment and isolation of benzotriazoles from water samples, particularly when the sample preheating step and sampling of analyte at higher temperatures are used [51,52]. This type of SPME technique enables the analysis of high-viscosity samples with a complex and sometimes variable matrix composition, such as the airport runoff water. The fiber coating is protected from damage caused by non-volatile contaminants present in the sample matrix. This significantly reduces the operating costs associated with using this type of extraction technique.
The crucial criteria, such as the polarity of the analytes, octanol–water partition coefficients, boiling points, and water solubility of analytes, were considered in selecting a suitable SPME fiber coating [25,59]. Considering the available extraction results of the SPME fiber coatings, it can be concluded that the SPME fiber coated with PDMS/DVB was clearly more effective than the other commercially available fibers. Based on the available literature data and conclusions resulting from our previous experience, SPME fiber coated with a 65 µm-thick film of polydimethylsiloxane/divinylbenzene (PDMS-DVB) was used to determine the analytes belonging to the benzotriazole group [51,52].
3.1.2. Effects of Extraction Time
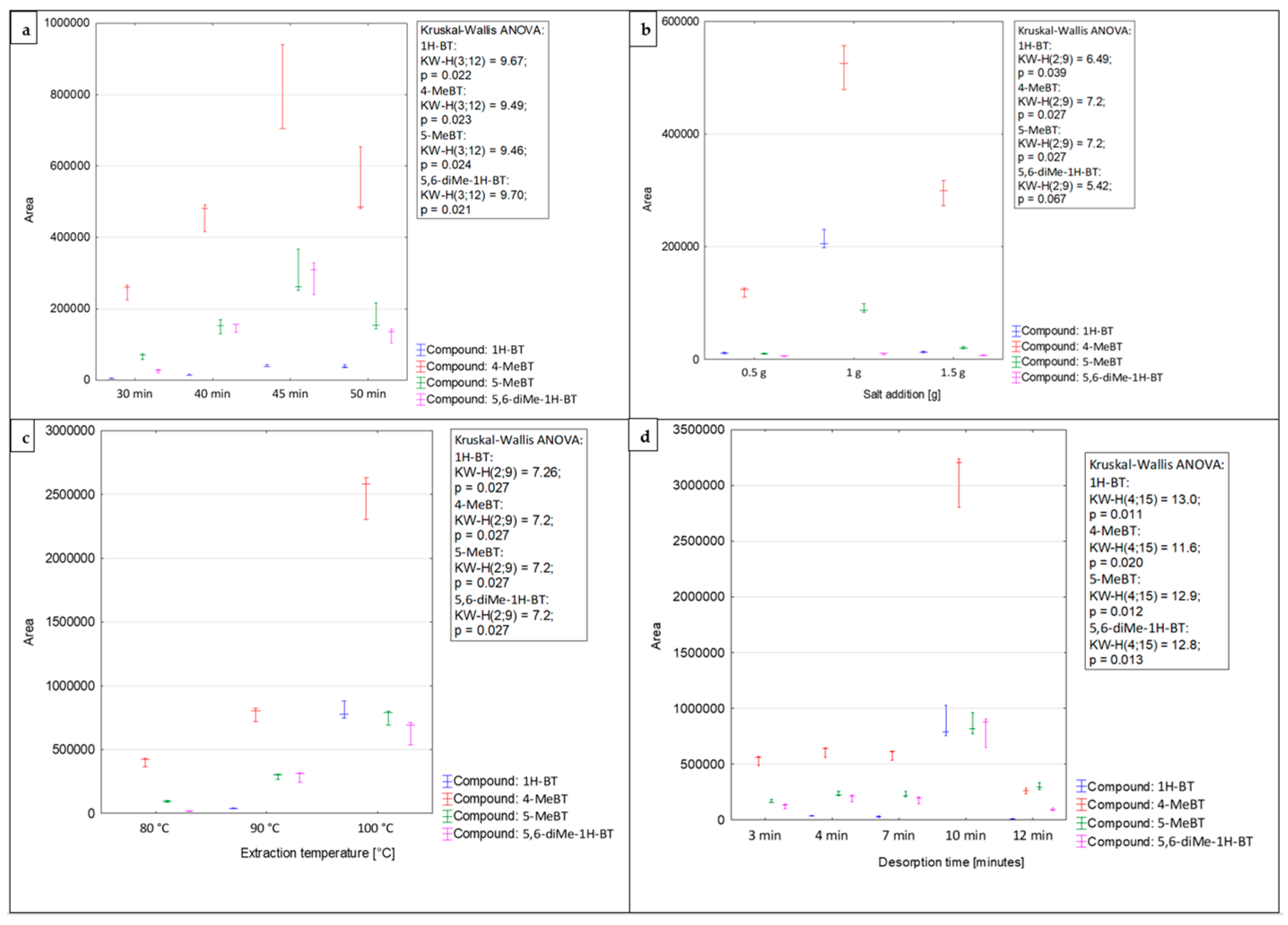
HS–SPME is an equilibrium extraction technique. This means that the mass of the analyte present in the extracting sorbent depends on its initial concentration in the sample [60]. For a system in the equilibrium state, the number of analytes is constant and equal to the amount introduced into the system together with the original sample at any time during the extraction [61]. In this case, the extraction time is a fundamental parameter governing the process efficiency. The time needed to reach equilibrium indicates the maximum amount of the target compounds that can be extracted by the SPME fiber. This has an impact on the sensitivity of the extraction technique [60]. Thus, it is essential to determine the extraction time necessary to achieve the equilibrium state. Nevertheless, the practical limitations related to the analysis should also be considered [62]. The extraction time depends on various parameters, such as the sample temperature, partition coefficient of the analyte, and means of stirring. Times required to achieve an equilibrium state are usually long and may be shortened by intensive stirring [62]. Considering this aspect, the investigated samples were stirred at a constant maximum rate before every extraction process. As previously mentioned, extraction times can be shortened in the HS mode by applying a sample preheating step. In this case, the time required to reach equilibrium is shorter through the more rapid diffusion of target compounds in a gaseous medium [62]. Hence, the investigated samples were incubated in a vial at the target temperature before each extraction process. In this study, the sorption time profiles were tested by measuring the chromatographic peak area as a function of exposure time [62]. The SPME fiber was exposed to the HS of model samples during four different extraction periods (30–50 min). The extraction times longer than 50 min were not used to avoid the extended time of routine analysis. Figure 2a shows the equilibrium time profiles of analytes from the benzotriazoles group and the significance of differences between the groups (Kruskal–Wallis test, p < 0.05 for all compounds). The average recovery values achieved by applying a 30 min extraction time were 55% lower than those obtained after a 40 min extraction. The highest response values for all investigated compounds were attained for the 45 min extraction time, and the difference between the 45 and 30 min extraction times was the single significant pairwise comparison within the whole experiment. Therefore, the extraction time of 45 min was applied to determine the analytes from the benzotriazoles group in airport runoff water samples.
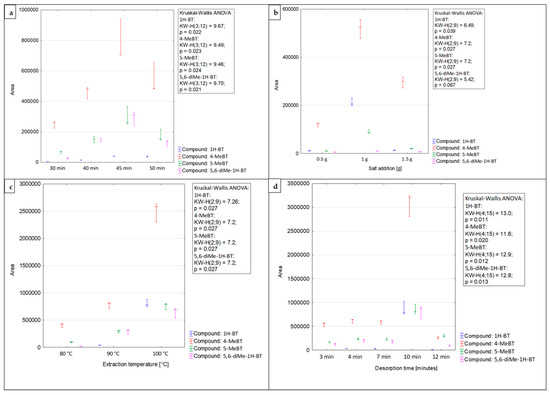
Figure 2.
Effects of the extraction time (a), ionic strength (b), extraction temperature (c), and de-sorption time (d) on the efficiency of the HS-SPME procedure for the determination of benzotriazoles in airport runoff water.
3.1.3. Effects of Ionic Strength
The addition of small amounts of salts, such as sodium chloride, potassium chloride, or sodium sulfate, impacts the extraction efficiency by increasing the ionic strength of the solution, leading to a ‘salting-out’ effect [60]. Organic compounds become less soluble; thus, their partition coefficients increase. As a result, the amount of analyte adsorbed on the SPME fiber increases. However, it should be underlined that the extraction efficiency depends on the analyte and salt concentration in the sample [63]. Furthermore, it should be noted that the salt addition can significantly increase the risk of contamination of the investigated sample [62,64]. In this work, sodium chloride was applied to adjust the ionic strength of the solution. To determine the optimal ionic strength, different amounts of NaCl (0.5, 1.0, and 1.5 g) were added to the model solution. Higher doses of the salt were not examined due to the possible saturation of the sample solution. The effect of salt addition on the HS–SPME extraction is presented in Figure 2b. The obtained results indicate that the addition of NaCl had a positive effect on the extraction efficiency, resulting in increased sensitivity of the analytical method (the presented differences are significant for all compounds except 5,6-diMe-1H-BT, with p < 0.05 and driven by the pairwise significant difference between 0.5 g and 1 g salt addition). All the investigated benzotriazoles achieved the highest extraction yield when 1.0 g of NaCl was added to the model sample, and while the difference for 5,6-diMe-1H-BT was not significant, it was the best salt addition setting for the entire range of compounds. Thus, the addition of 1.0 g of sodium chloride has been selected as the optimal extraction parameter for the determination of benzotriazoles in the samples of airport stormwater.
3.1.4. Effects of Extraction Temperature
During the headspace mode of SPME, the distribution and adsorption equilibria of analytes must be established between the gaseous and aqueous phases, and between the gaseous and solid phases [61]. As previously mentioned, the equilibrium depends on a number of parameters, such as the types of target analytes, the type of fiber coating, the extraction time, and the temperature [65,66]. The extraction temperature is the next key parameter, which has an impact on the extraction efficiency. An increase in extraction temperature accelerates the analyte transport from the solution or solid to the headspace [67]. Considering that an increase in temperature affects the partition coefficient, selecting an optimal extraction temperature is crucial. This selection should take into account several factors: the matrix composition of the medium under investigation, the volatility of the analytes (since higher temperatures increase vapor pressure, facilitating the extraction of medium- or low-volatility compounds), and the nature of the SPME fiber coating [62]. In this study, the influence of the extraction temperature was tested at three different levels: 80 °C, 90 °C, and 100 °C (Figure 2c), yielding a significant difference within the assay at p < 0.05 (Kruskal-Wallis ANOVA). The results of the carried out research indicate that the amount of analyte adsorbed significantly increases with increasing extraction temperature. The extraction temperature of 100 °C provided the highest efficiency for the target analytes, and its pairwise comparison at extraction temperature of 80 °C drove the significant difference within the entire comparison (for all compounds). Thus, extraction at 100 °C was applied to determine the benzotriazole levels.
3.1.5. Effects of Desorption Time
Immediately after HS-SPME, the target analytes were introduced into the GC inlet and then heated to an appropriate temperature. This heating increases the volatility of the target analytes, enabling their release through the thermal desorption process [42]. It is crucial to select suitable desorption conditions that ensure the complete removal of target analytes from the SPME fiber. These conditions should prevent carryover while simultaneously avoiding degradation of the investigated compounds or damage to the fiber coating [68]. Appropriate desorption time and temperature are crucial parameters that ensure complete desorption of analytes from the SPME fiber while also preventing the occurrence of memory effects or carryover [69]. In this study, desorption times were evaluated by inserting the fiber into the GC injection port for a period of 3–12 min. Additionally, to avoid sample carryover, the post-baking of the fiber for the additional 5 min in the injection port was used. Desorption of target analytes was performed at a temperature of 270 °C. Considering the thermal instability of the septum and the possibility of the occurrence of a gas leak or a noticeable blank signal, desorption temperatures above 270 °C are not recommended. Moreover, the blank sample runs for the fiber were carried out between the analysis of two consecutive samples to check the background level of the GC × GC system. Under these analytical conditions, no sample carryover was noticed. The optimal response values were obtained for the desorption time of 10 min (Figure 2d), with a significant difference within an assay for each compound at p < 0.05 (Kruskal–Wallis ANOVA). The pairwise comparison, which was significant within such an experiment, was either 10 min to 3 min desorption time (for 1H-BT and 5-MeBT) or between 10 min and 12 min desorption time (for 4-MeBT and 5,6-diMe-1H-BT).
3.2. GC × GC-TOF-MS Analysis
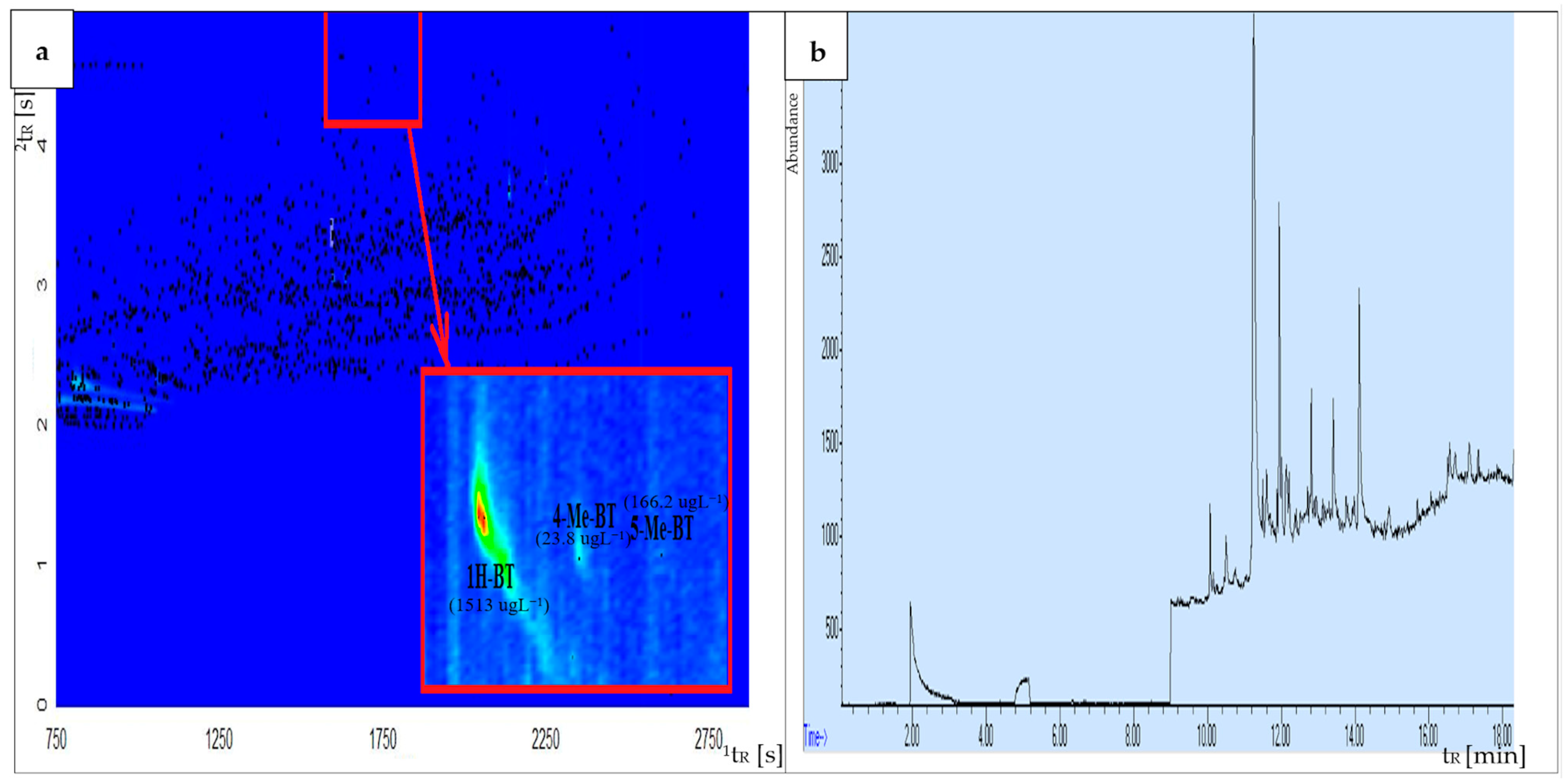
Gas chromatography is a commonly applied technique to separate and identify organic compounds. Despite the high resolution of capillary columns, GC is often insufficient for the separation of a wide spectrum of interfering substances that are commonly present in environmental samples. The peak capacity of GC × GC is significantly higher in comparison with the one-dimensional GC. The application of comprehensive two-dimensional gas chromatography enables significantly improved analyte separation. Moreover, it is possible to separate target compounds from interfering components in wastewater, such as petroleum-derived substances, polycyclic aromatic compounds, and polychlorinated biphenyls. It should be emphasized that the principal advantage of the trapping and refocusing processes occurring during modulation is a 3–10-fold increase in the signal-to-noise ratio (S/N) in comparison with the one-dimensional GC, which results in an enhanced sensitivity of GC × GC. Furthermore, the use of GC × GC enhances the reliability of identifying and quantifying target compounds. This method provides two distinct retention times for each compound and yields well-organized compound group bands, aiding in the analysis of chromatographic fingerprints [42,43,44,70,71]. Based on our previous research, we found that separating BTs from interfering compounds in airport runoff water samples using one-dimensional gas chromatography presents significant challenges, particularly in distinguishing between 4-MeBT and 5-MeBT [34]. Figure 3 presents a comparative analysis of chromatograms obtained from the same airport runoff water sample, collected from the aircraft de-icing area during the winter sampling campaign at the Large Airport UK, using (a) GC × GC-TOF-MS and (b) GC-MS systems. Based on the obtained results, it can be stated that the separation of compounds from the BT group using the GC × GC-TOF-MS system was significantly improved in comparison to results obtained using one-dimensional GC. These exemplary results confirm that comprehensive two-dimensional gas chromatography is an effective technique for separating the investigated analytes from interfering compounds present in the complex matrix of airport runoff water samples. Thus, the GC × GC technique with a high peak capacity and separation efficiency is recommended as a tool for analyzing samples characterized by complex matrix composition, such as airport stormwater.
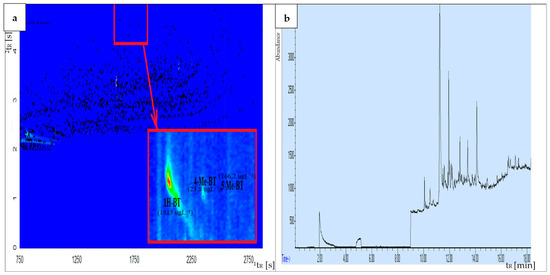
Figure 3.
Comparison of chromatograms obtained from the same airport runoff water sample, collected at Large Airport, UK (aircraft de-icing area, winter sampling campaign), using (a) GC × GC-TOF-MS and (b) GC-MS systems.
A multitude of parameters were evaluated and optimized to enhance chromatographic performance. These parameters encompassed the temperature gradients applied to both the primary and secondary columns in the GC × GC system, injection port temperature, the modulation period, the run time, and the dynamic profiles of carrier gas flow rates. This optimization was critical for ensuring not only the comprehensive resolution of target analytes but also their effective separation from co-eluting, non-target constituents in complex matrices such as airport runoff water samples. The optimal operating conditions used for GC × GC-TOF-MS analysis of benzotriazoles in airport stormwater are summarized in Table 2. A 2D chromatogram of the model sample, extracted via HS-SPME under the optimized conditions, is shown in Figure S1.
3.3. Quality Assurance/Quality Control (QA/QC)
After the optimization process, the methodology for the determination of benzotriazoles in airport stormwater samples was validated to provide an adequate level of quality assurance and quality control of measurements (QA/QC). The obtained validation parameters of the developed analytical procedure are summarized in Table 3.

Table 3.
Metrological parameters of the newly developed analytical procedure for determining benzotriazoles using HS-SPME and GC × GC-MS system.
The values of the coefficient of determination (R2) of the calibration curves were higher than 0.990, which indicated good linearity. Only in the case of the calibration curve for 1H-BT in the range of 5.0–50 µg/L did the coefficient of determination equal 0.9899. Moreover, linearity was examined by applying the Visual Linearity Test and Mandel’s Fitting Test with a probability p = 0.99. Mandel’s Fitting Tests were passed in all cases.
The obtained MDL and MQL values ranged from 0.0042 to 0.0062 μg/L and from 0.012 to 0.019 μg/L, respectively. The results obtained indicate that the MDL values for the HS-SPME-GC × GC-TOF-MS-based procedure were significantly lower than those reported by Breedved for the SPE/GC-MS (0.1–0.35 μg/L) [24,72] and our research team (0.01 μg/L for 4-MeBT, 5-MeBT) [34].
The recovery values were sufficient for all tested compounds at every fortification level (≥80.0%) (Table 3). The studies mentioned above also demonstrate that the efficiency of the developed analytical procedure is comparable to that of the SPE/GC-MS method, with recoveries ranging from 62 to 102% [1]. The obtained recoveries met the requirements of analytical methods, where the recovery values should range from 70 to 120%, depending on matrix complexity [34]. The obtained recovery values indicate the suitability of the SPME technique for the efficient extraction of BTs from airport stormwater samples.
The values of intra-day precision for the airport stormwater samples ranged from 5.9 to 9.8%. In turn, the values of inter-day precision ranged between 6.2 and 14.9%. The precision data for each analyte are shown in Table 3. Similar numerical data on the reproducibility by applying the SPME have been achieved by other research teams [51,52]. Validation parameters determined in the presented study are similar or better compared to the validation results achieved by other research teams using SPE-GC–MS-based methodology [9,72].
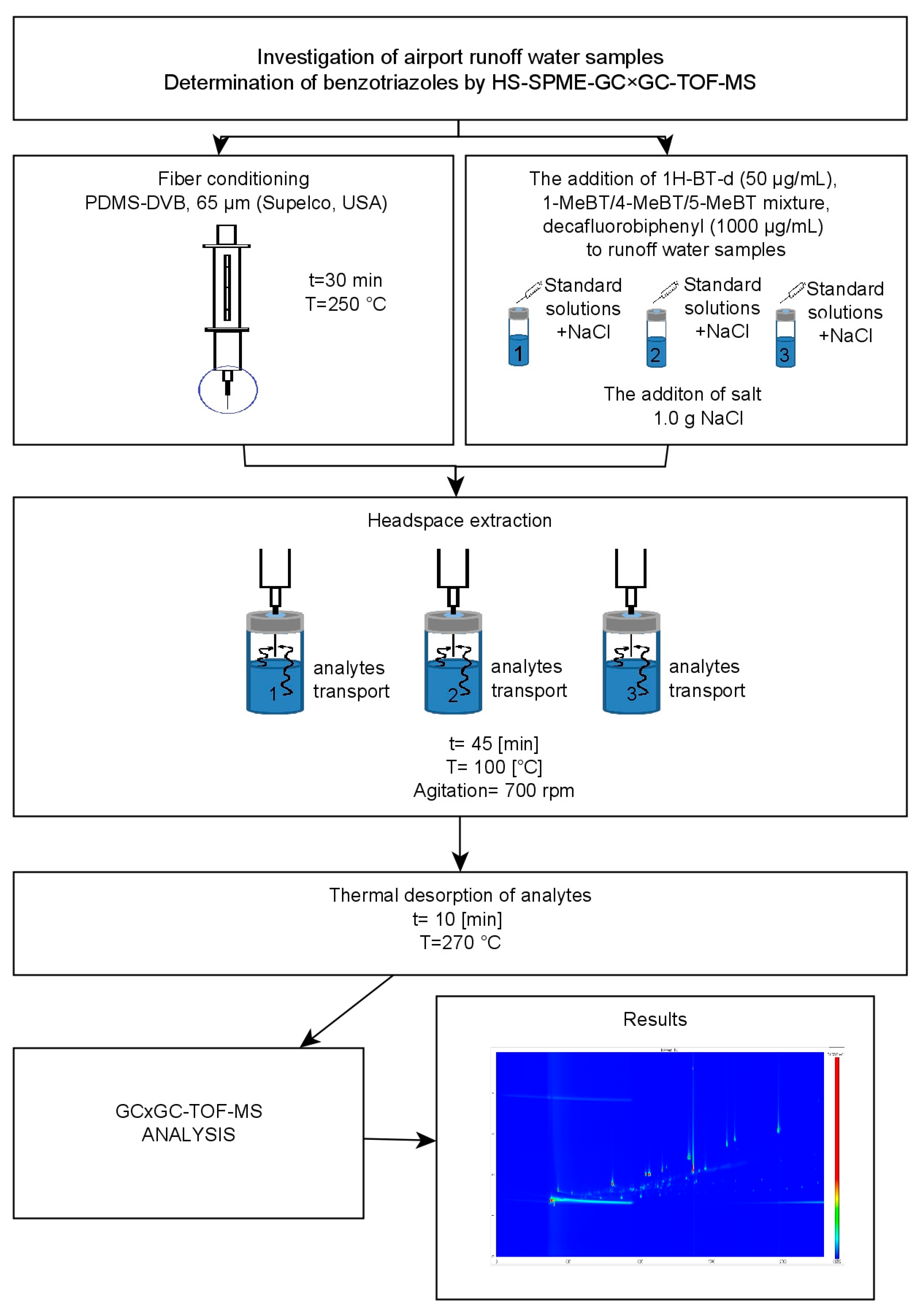
A schematic representation of the optimized and validated analytical procedure applied for the determination of benzotriazoles in airport stormwater samples is shown in Figure 4.
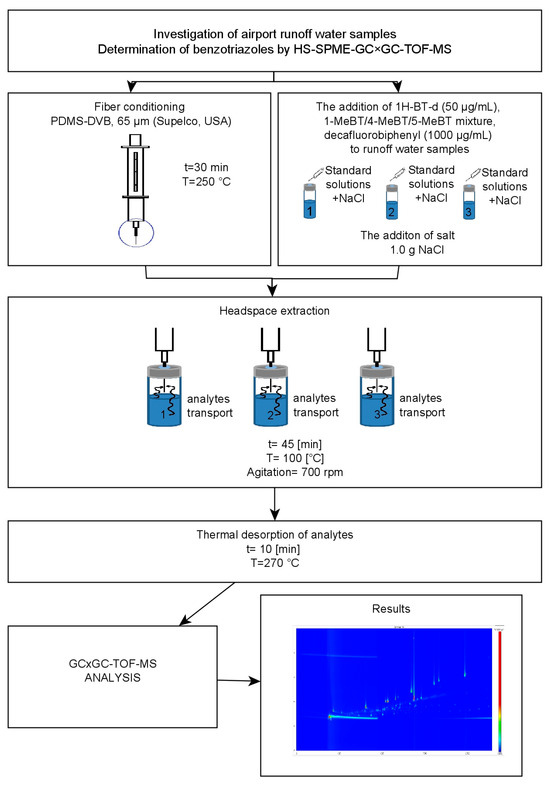
Figure 4.
Schematic representation of the analytical procedure for the determination of benzotriazoles in airport runoff water samples using HS-SPME-GC × GC-TOF-MS system.
3.4. Environmental Application
The developed HS-SPME-GC × GC-TOF-MS-based methodology was employed for determining anti-corrosive compounds in airport stormwater samples collected from four European airports, each characterized by varying levels of activity (number of flights/passengers per year). The runoff water samples were collected during the period from autumn 2012 to winter 2013.
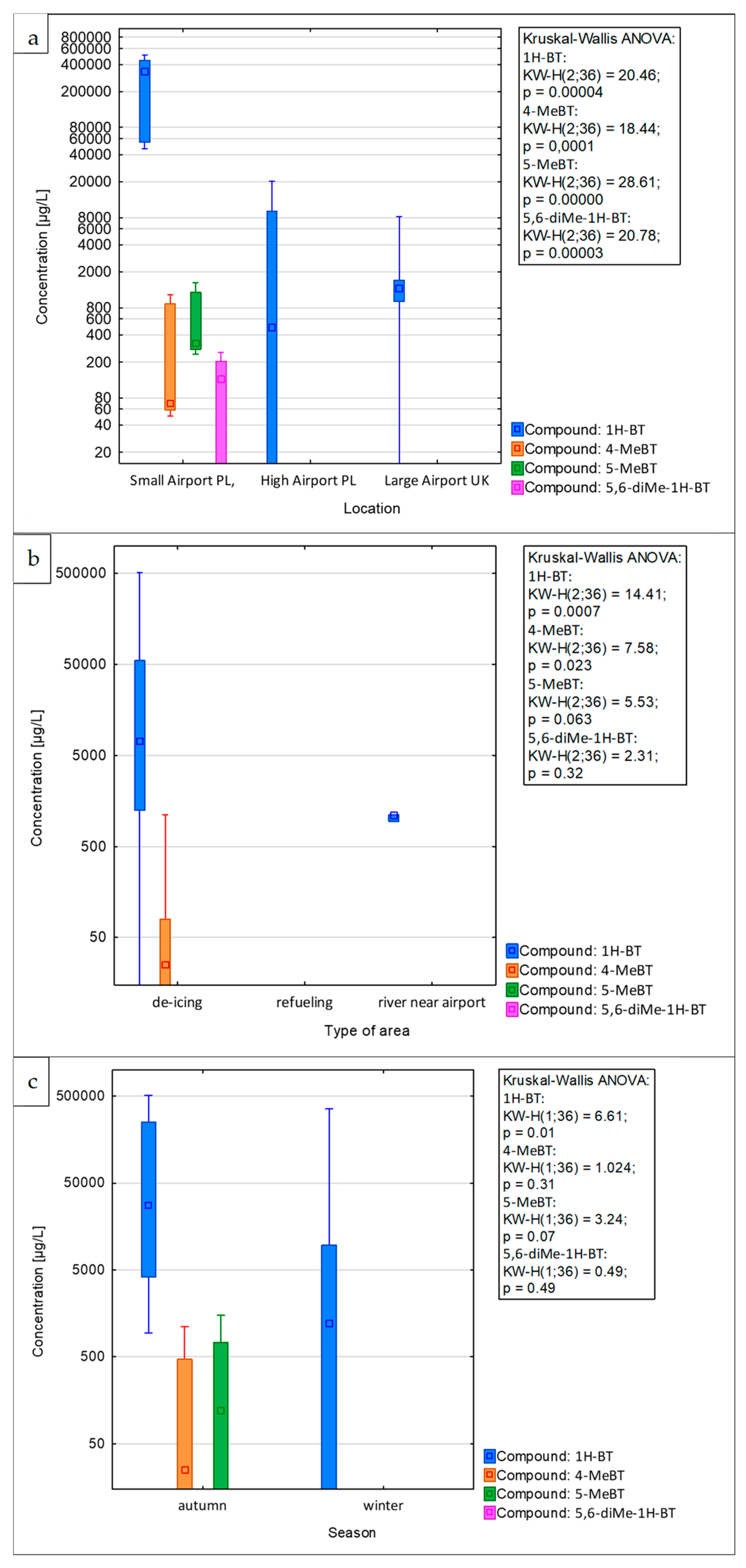
The concentration levels of various benzotriazole analytes identified in stormwater samples collected from European airports are depicted in Figure 5. These levels were recorded over the course of thirteen different sampling campaigns. During the entire sampling period at the investigated airports, 1H-benzotriazole was the most abundant analyte, with concentrations ranging from below the MQL to 467 mg/L.
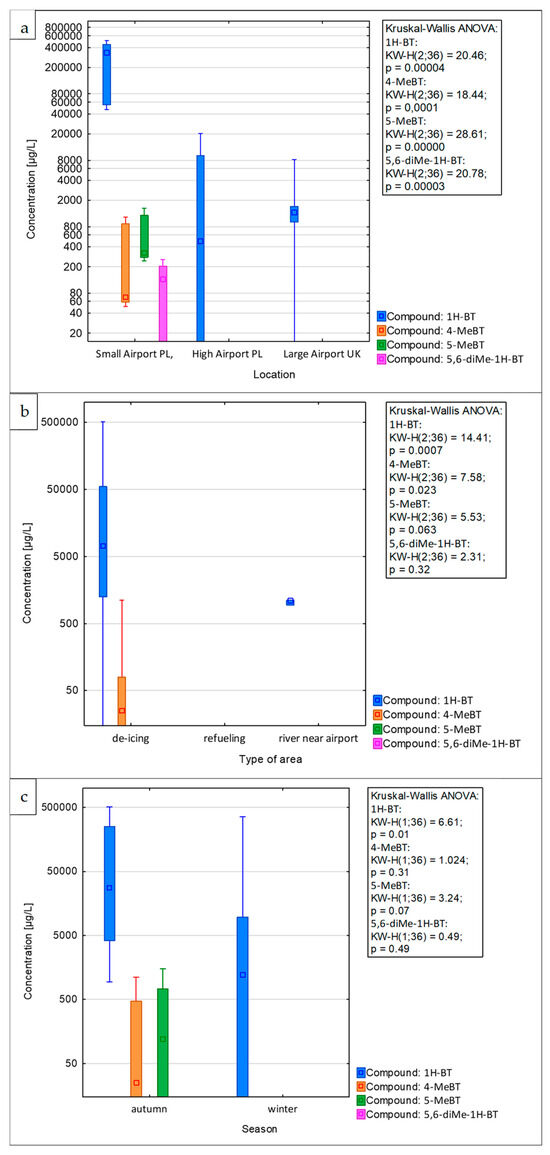
Figure 5.
Concentrations of benzotriazoles in airport stormwater samples collected from small airport and high airport in Poland, and large airport in the UK: (a) overall concentrations, (b) differences between airport areas tested, and (c) differences between sampling seasons. Note the logarithmic scale for concentrations on the y-axis.
The concentration levels of 4-methyl-1H-benzotriazole and 5-methyl-1H-benzotriazole were found to range from below the MQL to 979.7 µg/L and from below the MQL to 1312.6 µg/L, respectively. In turn, 5,6-dimethyl-1H-benzotriazole was detected only in the runoff water collected at the small airport in Poland during the autumn and winter sampling campaigns, with concentrations of 133.6 ± 10.7 µg/L and 222.2 ± 17.8 µg/L, respectively. Analysis of the results from all sampling campaigns revealed that the highest concentration of the target anti-corrosive analytes was found in the runoff water collected at the small airport in Poland, as shown in Figure 5a. Moreover, there were significant differences (p < 0.001, Kruskal–Wallis ANOVA) between the sampled airports, in pairwise comparisons revealed to be resulting from significant differences for all compound concentrations between the small airport in Poland and the other airports. The concentrations of 1H-BT in stormwater samples from the small airport in Poland, collected during all sampling campaigns, were substantially higher, ranging from 51.8 to 467 mg/L. This is in contrast to the lower levels found in samples from other investigated airports, as detailed in Figure 5. The increased concentration of benzotriazoles detected in the samples monitored at the small airport in Poland might be attributable to the local weather conditions. During the research period, the ambient temperature at small airport PL varied between −4.5 °C and −2.5 °C. In contrast, the air temperature at the other investigated airports ranged from −0.5 °C to 7.0 °C. This could be linked to the higher frequency of operations, such as aircraft and airport platform de-icing, necessitated by the lower ambient temperatures. In all sampling campaigns, the concentration levels of the compounds under investigation in runoff water samples from medium airports in Poland were below the MDLs. For large airports in the UK, the concentration levels of benzotriazoles measured in samples from all sampling campaigns were unexpectedly low, ranging from below the MQL to 7567 µg/L. The results obtained suggest that the degree of environmental contamination at airport infrastructures cannot be consistently correlated with the size or capacity of the airport.
Overall, the concentrations of anti-corrosive compounds found in runoff water samples from the de-icing area were significantly higher than those in samples from the other areas (Figure 5b, Kruskal–Wallis ANOVA p < 0.001). In pairwise comparisons, only two compounds (1H-BT and 4-MeBT) showed significant differences (p < 0.05), and only in the case of 1H-BT was there a significant difference (p < 0.05) between de-icing and refueling area. For instance, during the first winter sampling campaign at the high airport in Poland, the concentration of 1H-BT in stormwater samples from the de-icing area was 18,579 ± 929 µg/L. In contrast, the concentration of 1H-BT in samples from the refueling area collected on the same day was below the MQL, as illustrated in Figure 5b.
Based on the data shown in Figure 5c, it is observed that between the different seasons, there were significant differences (p < 0.01); however, only 1H-BT showed a significant pairwise difference (p < 0.05) between autumn and winter, with autumn concentrations being higher. The studies conducted indicate that toxic analytes are entering the surface waters in the vicinity of the airport; for example, during the autumn campaign at the large airport in the UK, the concentration of 1H-BT in samples from the river near the airport was 1053 ± 53 µg/L. Given these findings, it becomes crucial to develop more effective management strategies for handling airport de-icing runoff water, as well as to enhance the efficiency of waste management.
3.5. The Ecotoxicological Risk of BTs
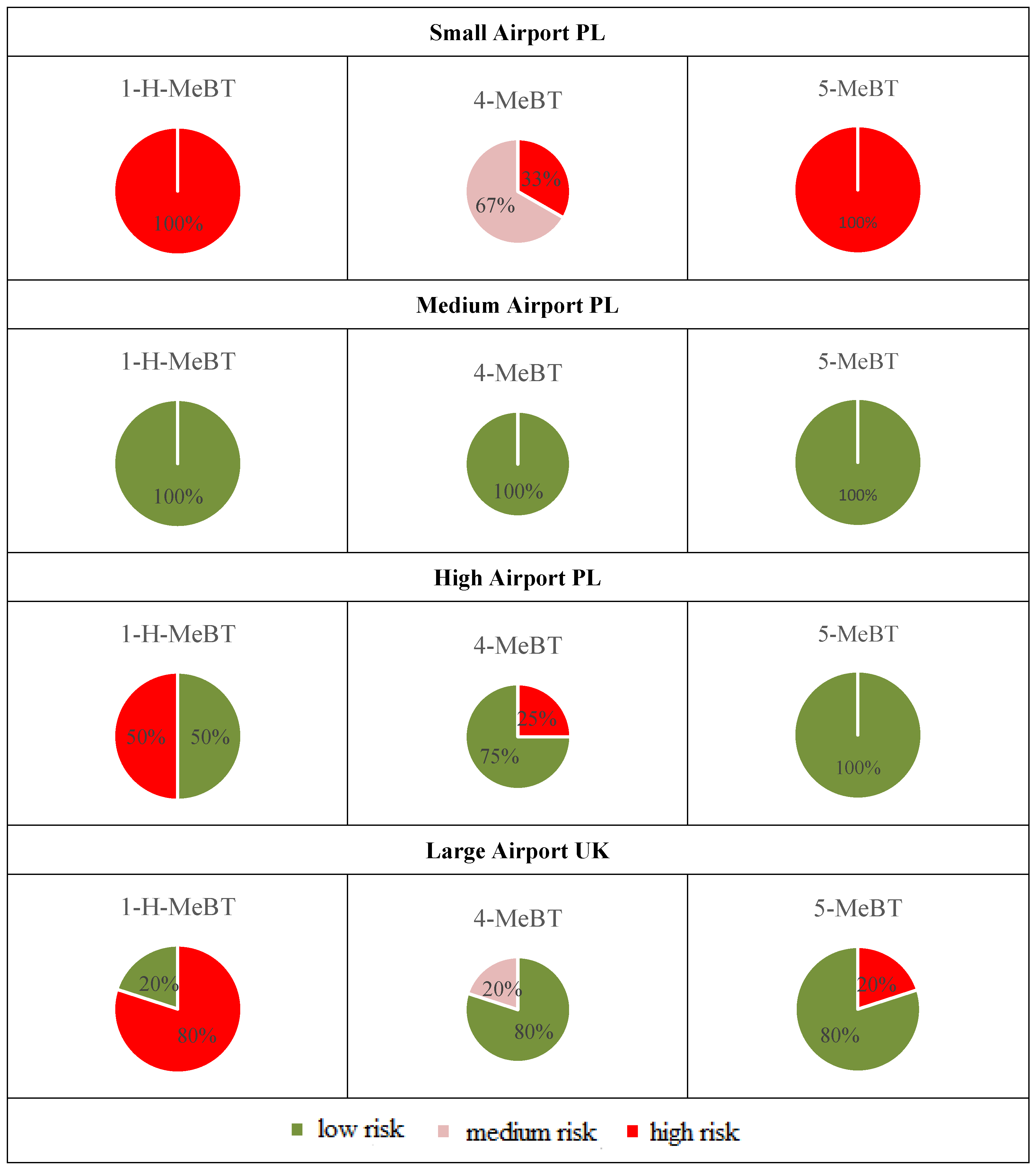
The potential risks associated with BTs at monitored airports are influenced by various factors, including the geographical location of the airport, weather conditions, airport capacity, sampling location, and season. As previously mentioned, the assessment of risk level was conducted using the RQ formula (see Section 2.2.4), based on the highest measured concentrations of BTs in airport runoff water samples and ecotoxicological data presented in Table S1. The potential risk associated with BTs is low since all analyzed samples from the specified location showed an RQ below 0.1. Summarizing all the examined runoff water samples across all studied airports, 69% of the tested sites exhibited high-risk levels (RQ ≥ 1). It should be emphasized that, even in samples collected from a river outside the large UK airport area, the RQ values, determined in relation to organisms from various trophic levels (Lemna minor, Desmodesmus subspicatus, Daphnia galeata, Pimephales promelas, Aliivibrio fischeri), consistently exceeded 1 in every instance. Additionally, the location of sample collection during the same survey period proved to be critically influential. For example, samples from the de-icing zone at high airport PL registered RQ values above 1, indicating a high-risk potential. In contrast, samples from the aircraft refueling area at the same airport, gathered during the identical sampling campaign, demonstrated RQ values below 0.1.
Risk frequencies of BTs in runoff water samples collected from international airports are presented in Figure 6. An analysis of the proportional distribution of risk quotient levels across all examined airports yields a comprehensive overview. Particular attention should be given to the small airport PL, where 100% of the tested sites showed high-risk levels for 1-H-BT and 5-MeBT. In the context of the risk assessment for 1-H-BT, attention must be drawn to the observations at large airports in the UK. In these areas, a substantial 80% of the evaluated sites were classified as possessing a high-risk level in relation to 1-H-BT exposure. In turn, the estimated RQ values for 1-H-BT, 4-MeBT, and 5-MeBT in all runoff water samples collected from the medium airport in Poland were below 0.1. This indicates a minimal detrimental impact on both surface and benthic organisms. As previously noted in Table S1, toxicity data for 5,6-diMe-1H-BT is currently unavailable. Generally, the calculation of RQ values suggested that BTs posed a significant threat to the ecosystem around airports. Due to their high lipophilicity, these organic pollutants are likely to enter the food chain and pose a substantial risk to aquatic organisms at higher trophic levels.
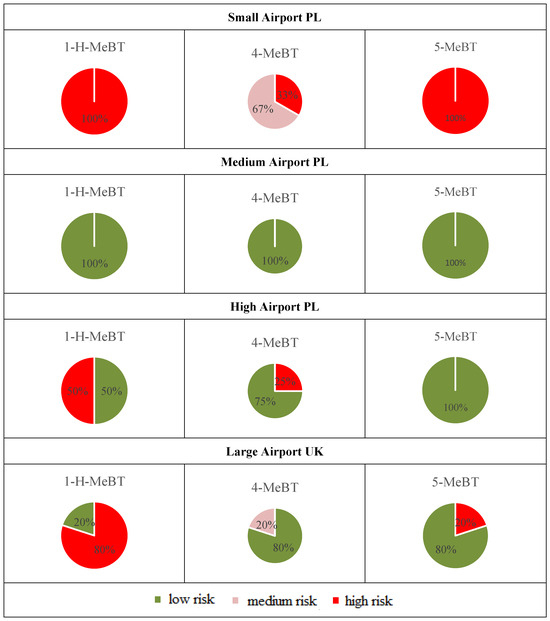
Figure 6.
Pie charts depicting the frequency of BT risks in stormwater samples collected from international airports.
There is insufficient data regarding the prevalence and impact of airport pollutants on the environment [73,74,75,76]. In European or Polish regulations, there are no specific provisions regarding the necessity of the determination or removal of benzotriazoles from runoff water generated upon the use of de-icing agents for airports [7,75,76]. The key issue is the inclusion of compounds from the benzotriazole group in the lists of substances that are particularly dangerous to the aquatic environment, which are supplied as runoff water from airports, in the regulations of the European Parliament and Council (EC), the Food and Agriculture Organization (FAO), and the International Civil Aviation Organization. The findings of our research underscore the necessity of implementing routine monitoring for benzotriazole concentrations in airport runoff water samples. Continuous monitoring and observation of both seasonal and long-term trends are crucial.
By implementing methodologies for the determination of the diverse pollutants released into runoff water as a result of airport operations, it will be feasible to assess the detrimental environmental impact of airport maintenance activities. The newly developed pro-quality analytical procedure (HS-SPME-GC × GC-TOF-MS) combined with additional environmental risk assessments of benzotriazoles in airport runoff water enables the collection of more comprehensive data on environmental quality. It is possible to create and complete databases of international environmental information systems and company environmental information systems as instruments for analyzing the environmental impact of companies. A newly developed analytical procedure enables the swift assessment of the ecological hazards associated with airport runoff water. The methodology can be used as a tool for monitoring and controlling the quality of airport runoff water. This proposal proposes a novel approach to be considered in the management process of airport infrastructure in terms of its environmental impact. This includes suggestions for reducing the load of pollutants in runoff water, recommended waste recycling techniques, substitutes for currently used de-icing substances, and proposals for wastewater treatment technology. This will lead to the development of environmentally conscious and sustainable protocols [77,78,79].
4. Conclusions
As a result of the conducted research, the HS-SPME-GC × GC-TOF-MS-based procedure for the determination of the anti-corrosive compounds in the samples of airport stormwater was developed, optimized, and validated. The established methodology is sensitive, selective, accurate, precise, and easily applicable to the analysis of airport runoff water samples. The application of the developed HS-SPME-GC × GC-TOF-MS-based methodology substantially improves the identification and quantification of benzotriazoles in airport stormwater. The developed and validated analytical protocol was used as a tool to estimate the occurrence of the anti-corrosive compounds in runoff water from airports with varying capacities. The research results confirmed the presence of 1H-BT, 4-MeBT, 5-MeBT, and 5,6-diMe-1H-BT in airport runoff water collected from European airports. This is the first time that a HS-SPME-GC × GC-TOF-MS-based procedure has been applied for the determination of benzotriazoles in any matrix. Furthermore, this study is characterized by new scientific elements in terms of the scale of the performed research regarding international airports located in different geographical regions. The newly developed procedure, aligned with green analytical chemistry principles, serves as an effective tool for comprehensive monitoring and quality control in the water and wastewater sector. These objectives align with the priority objectives of the United Nations 2030 Agenda for Sustainable Development.
The results of the performed study allow for a swift ecological risk assessment related to airport stormwater. The estimation of ecotoxicological risk assessment for BTs in water samples is exceptionally rare, making it a novel research topic. The research enhances environmental quality assessments and databases, facilitating swift and accurate ecological risk evaluations for sustainable airport management, thereby reducing environmental impacts and improving quality of life.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su16125152/s1, Table S1: Properties and eco-toxicity profiles of selected compounds for research; Figure S1: The 2D total ion chromatogram of the model sample of airport runoff water, spiked with a standard mixture of benzotriazoles, under the optimized HS-SPME conditions.
Author Contributions
Conceptualization, methodology, software, validation, formal analysis, investigation, data curation, writing—original draft preparation, review & editing, visualization, project administration, A.M.S.-S.; statistical calculations, writing—statistical analysis, review & editing, K.K.; conceptualization, supervision, visualization, funding acquisition, Ż.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Gdynia Maritime University under grant no. WZNJ/2024/PZ/04, titled ‘Systemic Management of Quality, Environment, and Safety in the Product Lifecycle’.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors gratefully acknowledge P. Przybyłowski for his invaluable help and support during the preparation of this project. The authors express their gratitude to Krzysztof Suchomski for his help in preparing the graphic side of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kiss, A.; Fries, E. Occurence of benzotriazoles in the rivers Main, Hengstbach, and Hegbach (Germany). Environ. Sci. Pollut. Res. 2009, 16, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Mohiley, A.; Franzaring, J.; Calvo, O.C.; Fangmeier, A. Potential toxic effects of aircraft de-icers and wastewater samples containing these compounds. Environ. Sci. Pollut. Res. 2015, 22, 13094–13101. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Chiarotti, M.; Bergmaschi, A.; Marsili, R. Determination of airborne polycyclic aromatic hydrocarbons at an airport by gas chromatography-mass spectrometry and evaluation of occupational exposure. J. Chromatogr. A 2007, 1150, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Zhelnovach, G.; Belokon, K.; Barabash, O.; Dychko, A. Airport Runoff Management: Engineering Solutions. Ecol. Eng. Environ. Technol. 2022, 23, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yu, L.; Zhong, X.; Dong, T. Study on Runoff Control Effect of Different Drainage Schemes in Sponge Airport. Water Resour. Manag. 2022, 36, 1043–1055. [Google Scholar] [CrossRef]
- Peng, J.; Wang, Q.Q.; Yang, X.S.; Yu, L.; Zhong, X. Application and evaluation of LID facilities in sponge airport, China. Water Sci. Technol. 2022, 85, 756–768. [Google Scholar]
- Rodziewicz, J.; Mielcarek, A.; Janczukowicz, W.; Bryszewski, K.; Ostrowska, K. Treatment of wastewater containing runway de-icing agents in biofilters as a part of airport environment management system. Sustainability 2020, 12, 3608. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, X.; Liang, S.; Zhu, B.; Yao, Y.; Meng, S.; Lu, C. Quantifying the response of potential flooding risk to urban growth in. Sci. Total Environ. 2020, 705, 135868. [Google Scholar] [CrossRef]
- Corsi, S.R.; Zitomer, D.J.; Field, J.; Cancilla, D.A. Nonylphenol Ethoxylates and Other Additives in Aircraft Deicers, Antiicers, and Waters Receiving Airport Runoff. Environ. Sci. Technol. 2003, 37, 4031–4037. [Google Scholar] [CrossRef] [PubMed]
- Corsi, S.R.; Geis, S.W.; Bowman, G.; Failey, G.; Di Rutter, T. Aquatic Toxicity of Airfield-Pavement Deicer Materials and Implications for Airport Runoff. Environ. Sci. Technol. 2009, 43, 40–46. [Google Scholar] [CrossRef]
- Calvo, O.C.; Quaglia, G.; Mohiley, A.; Cesarini, M.; Fangmeier, A. Assessing potential aquatic toxicity of airport runoff using physicochemical parameters and Lemna gibba and Aliivibrio fischeri bioassays. Environ. Sci. Pollut. Res. 2020, 27, 40604–40617. [Google Scholar] [CrossRef]
- Magomedova, D.S.; Alimirzayeva, Z.M.; Magomedova, A.G.; Isaev, A.B.; Kharlamova, T.A. Electrochemical Treatment of Airport Runoff Water Containing Ethylene Glycol. Chem. Probl. 2022, 20, 109–115. [Google Scholar] [CrossRef]
- Pressl, A.; Pucher, G.; Scharf, B.; Langergraber, G. Treatment of de-icing contaminated surface water runoff along an airport runway using in-situ soil enriched with structural filter materials. Sci. Total Environ. 2019, 660, 321–328. [Google Scholar] [CrossRef]
- Corsi, S.R.; Harwell, G.R.; Geis, S.W.; Bergman, D. Impacts of aircraft deicer and anti-icer runoff on receiving waters from dallas/Fort Worth Intrnational Airport, Texas, USA. Environ. Toxicol. Chem. 2006, 25, 2890–2900. [Google Scholar] [CrossRef]
- Milley, S.A.; Koch, I.; Fortin, P.; Archer, J.; Reynolds, D.; Weber, K.P. Estimating the number of airports potentially contaminated with perfluoroalkyl and polyfluoroalkyl substances from aqueous film forming foam: A Canadian example. J. Environ. Manag. 2018, 222, 122–131. [Google Scholar] [CrossRef]
- Barash, S.; Covington, J.; Tamulonis, C. Preliminary Data Summary Airport Deicing Operations (Revised); United States Environmental Protection Agency: Washington, DC, USA, 2000; Volume 4303.
- Hartwell, S.I.; Jordahl, D.M.; Evans, J.E.; May, E.B. Toxicity of aircraft de-icer and anti-icer solutions to aquatic organisms. Environ. Toxicol. Chem. 1995, 14, 1375–1386. [Google Scholar]
- Iervolino, I.; Accardo, D.; Tirri, A.E.; Pio, G.; Salzano, E. Quantitative risk analysis for the Amerigo Vespucci (Florence, Italy) airport including domino effects. Saf. Sci. 2019, 113, 472–489. [Google Scholar] [CrossRef]
- Nguyen, M.A.; Norström, K.; Wiberg, K.; Gustavsson, J.; Josefsson, S.; Ahrens, L. Seasonal trends of per- and polyfluoroalkyl substances in river water affected by fire training sites and wastewater treatment plants. Chemosphere 2022, 308, 136467. [Google Scholar] [CrossRef]
- De Roos, A.J.; Kondo, M.C.; Robinson, L.F.; Rai, A.; Ryan, M.; Haas, C.N.; Lojo, J.; Fagliano, J.A. Heavy precipitation, drinking water source, and acute gastrointestinal illness in Philadelphia, 2015–2017. PLoS ONE 2020, 15, e0229258. [Google Scholar] [CrossRef] [PubMed]
- Grant, S. A Review of the Contaminants and Toxicity Associated with Particles in Stormwater Runoff. 2015. Available online: https://www.researchgate.net/publication/282219595_A_Review_of_the_Contaminants_and_Toxicity_Associated_with_particles_in_Stormwater_Runoff (accessed on 28 April 2024).
- Voutsa, D.; Hartmann, P.; Schaffner, C.; Giger, W. Benzotriazoles, alkylphenols and bisphenol A in municipal wastewaters and in the Glatt River, Switzerland. Environ. Sci. Pollut. Res. 2006, 13, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Guo, L.; Yin, D.; Ma, T.; Zhang, S.; Wang, C. Environmentally sustainable corrosion inhibitors used for electronics industry. Environ. Sustain. Corros. Inhib. 2022, 359–381. [Google Scholar] [CrossRef]
- Olds, H.T.; Corsi, S.R.; Rutter, T.D. Benzotriazole concentrations in airport runoff are reduced following changes in airport deicer formulations. Integr. Environ. Assess. Manag. 2022, 18, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Cantwell, M.G.; Sullivan, J.C.; Burgess, R.M. Benzotriazoles: History, Environmental Distribution, and Potential Ecological Effects. Compr. Anal. Chem. 2015, 67, 513–545. [Google Scholar]
- Global Industry Analysts, Inc. Benzotriazole: Global Strategic Business Report. 2022. Available online: https://www.researchandmarkets.com/reports/5301677/benzotriazole-global-strategic-business-report (accessed on 28 April 2024).
- Alotaibi, M.D.; McKinley, A.J.; Patterson, B.M.; Reeder, A.Y. Benzotriazoles in the Aquatic Environment: A Review of Their Occurrence, Toxicity, Degradation and Analysis. Water. Air. Soil Pollut. 2015, 226, 226. [Google Scholar] [CrossRef]
- Kotowska, U.; Sokołowska, J.S.; Piekutin, J. Simultaneous determination of low molecule benzotriazoles and benzotriazole UV stabilizers in wastewater by ultrasound—Assisted emulsification microextraction followed by GC–MS detection. Sci. Rep. 2021, 11, 10098. [Google Scholar] [CrossRef] [PubMed]
- Krautsieder, A.; Sharifi, N.; Madden, D.C.; Sonke, J.; Routh, A.F.; Clarke, S.M. Corrosion inhibitor distribution on abrasive-blasted steels. J. Colloid Interface Sci. 2023, 634, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Asimakopoulos, A.G.; Wang, L.; Thomaidis, N.S.; Kannan, K. Benzotriazoles and benzothiazoles in human urine from several countries: A perspective on occurrence, biotransformation, and human exposure. Environ. Int. 2013, 59, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Cottin, G.; Houde, M.; Giraudo, M. Transcriptomic, cellular and life-history responses of Daphnia magna chronically exposed to benzotriazoles: Endocrine-disrupting potential and molting effects. PLoS ONE 2017, 12, e0171763. [Google Scholar] [CrossRef] [PubMed]
- Pillard, D.; Dufresne, D.; Hernandez, M. Toxicity of benzotriazole and benzotriazole derivatives to three aquatic species. Water Res. 2001, 35, 557–560. [Google Scholar] [CrossRef]
- Corsi, S.; Geis, S.W.; Rice, C.; Sheesley, R. Characterization of Aircraft Deicer and Anti-Icer Components and Toxicity in Airport Snowbanks and Snowmelt Runoff. Environ. Sci. Technol. 2006, 40, 3195–3202. [Google Scholar] [CrossRef]
- Sulej, A.M.; Polkowska, Z.; Astel, A.; Namieśnik, J. Analytical procedures for the determination of fuel combustion products, anti-corrosive compounds, and de-icing compounds in airport runoff water samples. Talanta 2013, 117, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Tangtian, H.; Bo, L.; Wenhua, L.; Paul, K.S.; Shin, R.S.S.W. Estrogenic potential of benzotriazole on marine medaka (Oryzias melastigma). Ecotoxicol. Environ. Saf. 2012, 80, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.Q.; Liu, Y.S.; Xiong, Q.; Cai, W.W.; Ying, G.G. Occurrence, toxicity and transformation of six typical benzotriazoles in the environment: A review. Sci. Total Environ. 2019, 661, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J. ScienceDirect Micro- and nanoplastics in the environment: Research and policymaking. Curr. Opin. Environ. Sci. Health 2018, 1, 12–16. [Google Scholar]
- Zhou, Y.; Qu, J.; Liu, W.; Liao, J.; Li, Y.; Zhao, H.; Li, J.; Jin, H.; Liu, H.; Fang, J.; et al. Early pregnancy exposure to benzotriazoles and benzothiazoles in relation to gestational diabetes mellitus: A prospective cohort study. Environ. Int. 2020, 135, 105360. [Google Scholar] [CrossRef] [PubMed]
- Cancilla, D.A.; Holtkamp, A.; Matassa, L.; Fang, X. Isolation and characterization of Microtox®-active components from aircraft de-icing/anti-icing fluids. Environ. Toxicol. Chem. 1997, 16, 430–434. [Google Scholar]
- Cancilla, D.A.; Martinez, J.; Van Aggelen, G.C. Detection of aircraft De-icing/Antiicing Fluid Additives in a Perched Water Monitoring Well at an International Airport. Environ. Sci. Technol. 1998, 32, 3834–3835. [Google Scholar] [CrossRef]
- Snow, N.H. Chapter 8—Applications. Sep. Sci. Technol. 2020, 12, 269–295. [Google Scholar]
- Sulej-Suchomska, A.M.; Polkowska, Ż.; Chmiel, T.; Dymerski, T.M.; Kokot, Z.J.; Namieśnik, J. Solid phase microextraction-comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry: A new tool for determining PAHs in airport runoff water samples. Anal. Methods 2016, 8, 4509–4520. [Google Scholar] [CrossRef]
- Ieda, T.; Ochiai, N.N.; Miyawaki, T.; Ohura, T.; Horii, Y. Environmental analysis of chlorinated and brominated polyciclic aromatic hydrocarbons by comprehensive two-dimentional gas chromatography coupled to high-resolution time-of-flight mass spectrometry. J. Chromatogr. A 2011, 1218, 3224–3232. [Google Scholar] [CrossRef]
- Frysinger, G.S.; Gaines, R.B.; Reddy, C.M. GC × GC—A new analytical tool for environmental forensics. Environ. Forensics 2002, 3, 27–34. [Google Scholar]
- Pani, O.; Górecki, T. Comprehensive two-dimensional gas chromatography (GC × GC) in environmental analysis and monitoring. Anal. Bioanal. Chem. 2006, 386, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Mladenov, N.; Dodder, N.G.; Steinberg, L.; Richardot, W.; Johnson, J.; Martincigh, B.S.; Buckley, C.; Lawrence, T.; Hoh, E. Persistence and removal of trace organic compounds in centralized and decentralized wastewater treatment systems. Chemosphere 2022, 286, 131621. [Google Scholar] [CrossRef] [PubMed]
- Stefanuto, P.H.; Smolinska, A.; Focant, J.F. Advanced chemometric and data handling tools for GC × GC-TOF-MS: Application of chemometrics and related advanced data handling in chemical separations. Trends Anal. Chem. 2021, 139, 116251. [Google Scholar] [CrossRef]
- Fischer, K.; Fries, E.; Körner, W.; Schmalz, C.; Zwiener, C. New developments in the trace analysis of organic water pollutants. Appl. Microbiol. Biotechnol. 2012, 94, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Vaye, O.; Ngumbu, R.S.; Xia, D. A review of the application of comprehensive two-dimensional gas chromatography MS-based techniques for the analysis of persistent organic pollutants and ultra-trace level of organic pollutants in environmental samples. Rev. Anal. Chem. 2022, 41, 63–73. [Google Scholar] [CrossRef]
- Struk-Sokołowska, J.; Gwoździej-Mazur, J.; Jurczyk, Ł.; Jadwiszczak, P.; Kotowska, U.; Piekutin, J.; Canales, F.A.; Kaźmierczak, B. Environmental risk assessment of low molecule benzotriazoles in urban road rainwaters in Poland. Sci. Total Environ. 2022, 839, 156246. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.-L.; Chen, Y.; Yang, G.-P.; Chen, R. Simultaneous determination of benzothiazoles, benzotriazoles, and benzotriazole UV absorbers by solid-phase extraction-gas chromatography-mass spectrometry. Environ. Sci. Pollut. Res. 2023, 30, 45315–45330. [Google Scholar] [CrossRef] [PubMed]
- Carpinteiro, I.; Abuín, B.; Rodríguez, I.; Cela, R.; Ramil, M. Headspace solid-phase microextraction followed by gas chromatography tandem mass spectrometry for the sensitive determination of benzotriazole UV stabilizers in water samples. Anal. Bioanal. Chem. 2010, 397, 829–839. [Google Scholar] [CrossRef]
- Zhang, Z.-M.; Zhang, H.-H.; Zhang, J.; Wang, Q.-W.; Yang, G.-P. Occurrence, distribu_tion, and ecological risks of phthalate esters in the seawater and sediment of Changjiang River estuary and its adjacent area. Sci. Total Environ. 2018, 619–662, 93–102. [Google Scholar] [CrossRef]
- Perrodin, Y.; Boillot, C.; Angerville, R.; Donguy, G.; Emmanual, E. Ecological risk as_sessment of urban and industrial systems: A review. Sci. Total Environ. 2011, 409, 5162–5176. [Google Scholar] [CrossRef] [PubMed]
- EU. Technical Guidance Document (TGD) in Support of Com_mission Directive 93/67/EEC on Risk Assessment for New Notified Substances, Commis_sion Regulation (EC) No 1488/94 on Risk Assessment for Existing Substances and Directive 98/8/EC of the European. Publications Office of the European Union. 2003. Available online: https://op.europa.eu/en/publication-detail/-/publication/212940b8-3e55-43f8-8448-ba258d0374bb/language-en (accessed on 28 April 2024).
- Directive 2000/60/EC of the European Parliament and of the Council, Establishing a Framework for Community Action in the Field of Water Policy. 2000. Available online: https://www.eea.europa.eu/policy-documents/directive-2000-60-ec-of (accessed on 28 April 2024).
- Damalas, D.E.; Bletsou, A.A.; Agalou, A.; Beis, D.; Thomaidis, N.S. Assessment of the acute toxicity, uptake and biotransformation potential of benzotriazoles in zebrafish (Danio rerio) larvae combining HILIC- with RPLC-HRMS for high-throughput identifica_tion. Environ. Sci. Technol. 2018, 52, 6023–6031. [Google Scholar] [CrossRef]
- Seeland, A.; Oetken, M.; Kiss, A.; Fries, E.; Oehlmann, J. Acute and chronic toxicity of benzotriazoles to aquatic organisms. Environ. Sci. Pollut. Res. 2012, 19, 1781–1790. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.S.; Davis, L.C.; Erickson, L.E.; Callender, T.M. Sorption and partitioning parameters of benzotriazole compounds. Microchem. J. 2004, 77, 9–17. [Google Scholar] [CrossRef]
- Pawliszyn, J. Handbook of Sample Preparation; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Pawliszyn, J. 1—Solid-Phase Microextraction in Perspective. In Handbook of Solid Phase Microextraction; Pawliszyn, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1–12. [Google Scholar] [CrossRef]
- Spietelun, A.; Kloskowski, A.; Chrzanowski, W.; Namieśnik, J. Understanding solid-phase microextraction: Key factors influencing the extraction process and trends in improving the technique. Chem. Rev. 2013, 113, 1667–1685. [Google Scholar] [CrossRef] [PubMed]
- Merkle, S.; Kleeberg, K.K.; Fritsche, J. Recent Developments and Applications of Solid Phase Microextraction (SPME) in Food and Environmental Analysis—A Review. Chromatography 2015, 2, 293–381. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Małgorzata, R.; Namieśnik, J. Solid Phase Microextraction: State of the Art, Opportunities and Applications; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2017. [Google Scholar]
- Han, L.; Jiang, S.; Wang, Q. Application of a single-drop microextraction for the analysis of organophosphorus pesticides in juice. J. Chromatogr. A 2006, 1114, 269–273. [Google Scholar]
- Barros, E.P.; Moreira, N.; Pereira, G.E.; Leite SG, F.; Rezende, C.M.; de Pinho, P.G. Development and validation of automatic HS-SPME with a gas chromatography-ion trap/mass spectrometry method for analysis of volatiles in wines. Talanta 2012, 101, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Pawliszyn, J. Headspace solid phas microextraction. Anal. Chem. 1993, 65, 1843. [Google Scholar] [CrossRef]
- Costas, M.; Pena-Pereira, F.J. Method Development with Miniaturized Sample Preparation Techniques 6 Method Development with Miniaturized Sample Preparation Techniques; De Gruyter Open Ltd.: Warsaw, Poland; Berlin, Germany, 2015. [Google Scholar]
- Sulej-Suchomska, A.M.; Polkowska, Z.; Kokot, Z.J.; de la Guardia, M.; Namieśnik, J. Determination of antifreeze substances in the airport runoff waters by solid-phase microextraction and gas chromatography-mass spectrometry method. Microchem. J. 2016, 126, 466–473. [Google Scholar] [CrossRef]
- Marriott, P.; Shellie, R. Principles and applications of comprehensivetwo-dimensional gas chromatography. Trends Anal. Chem. 2002, 21, 9–10. [Google Scholar] [CrossRef]
- Skoczyńska, E.; Korytar, P.; Boer, J. Maximilizing Chromatographic Information from Environmental Extracts by GC × GC-TOF-MS. Environ. Sci. Technol. 2008, 42, 6611–6618. [Google Scholar] [CrossRef]
- Breedveld, G.D.; Roseth, R.; Sparrevik, M.; Hartnik, T.; Hem, L.J. Persistence of the de-icing additive benzotriazole at an abandoned airport. Water Air Soil Pollut. 2003, 3, 91–101. [Google Scholar] [CrossRef]
- Kale, U.; Jankovics, I.; Nagy, A.; Rohács, D. Towards sustainability in air traffic management. Sustainability 2021, 13, 5451. [Google Scholar] [CrossRef]
- Xiong, C.; Beckmann, V.; Tan, R. Effects of infrastructure on land use and land cover change (LUCC): The case of Hangzhou international airport, China. Sustainability 2018, 10, 2013. [Google Scholar] [CrossRef]
- EPA. Part 122—EPA Administered Permit Programs: The National Pollutant Discharge Elimination System. 33 U.S.C. 1251. Available online: https://www3.epa.gov/npdes/pubs/cafo_part122.pdf (accessed on 28 April 2024).
- ICAO. Water Management at Airports. 2019. Available online: https://www.icao.int/environmental-protection/Documents/Water%20management%20at%20airports.pdf (accessed on 28 April 2024).
- Jaiyeola, A.T. The management and treatment of airport rainwater in a water-scarce environment. Int. J. Environ. Sci. Technol. 2017, 14, 421–434. [Google Scholar] [CrossRef]
- Brtnický, M.; Pecina, V.; Baltazár, T.; Vašinová Galiová, M.; Baláková, L.; Bęś, A.; Radziemska, M. Environmental Impact Assessment of Potentially Toxic Elements in Soils Near the Runway at the International Airport in Central Europe. Sustainability 2020, 12, 7224. [Google Scholar] [CrossRef]
- Surya, B.; Hamsina, H.; Ridwan, R.; Baharuddin, B.; Menne, F.; Fitriyah, A.T.; Rasyidi, E.S. The Complexity of Space Utilization and Environmental Pollution Control in the Main Corridor of Makassar City, South Sulawesi, Indonesia. Sustainability 2020, 12, 9244. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).