Cold Plasma Technology: A Sustainable Approach to Milk Preservation by Reducing Pathogens and Enhancing Oxidative Stability
Abstract
1. Introduction
2. Materials and Methods
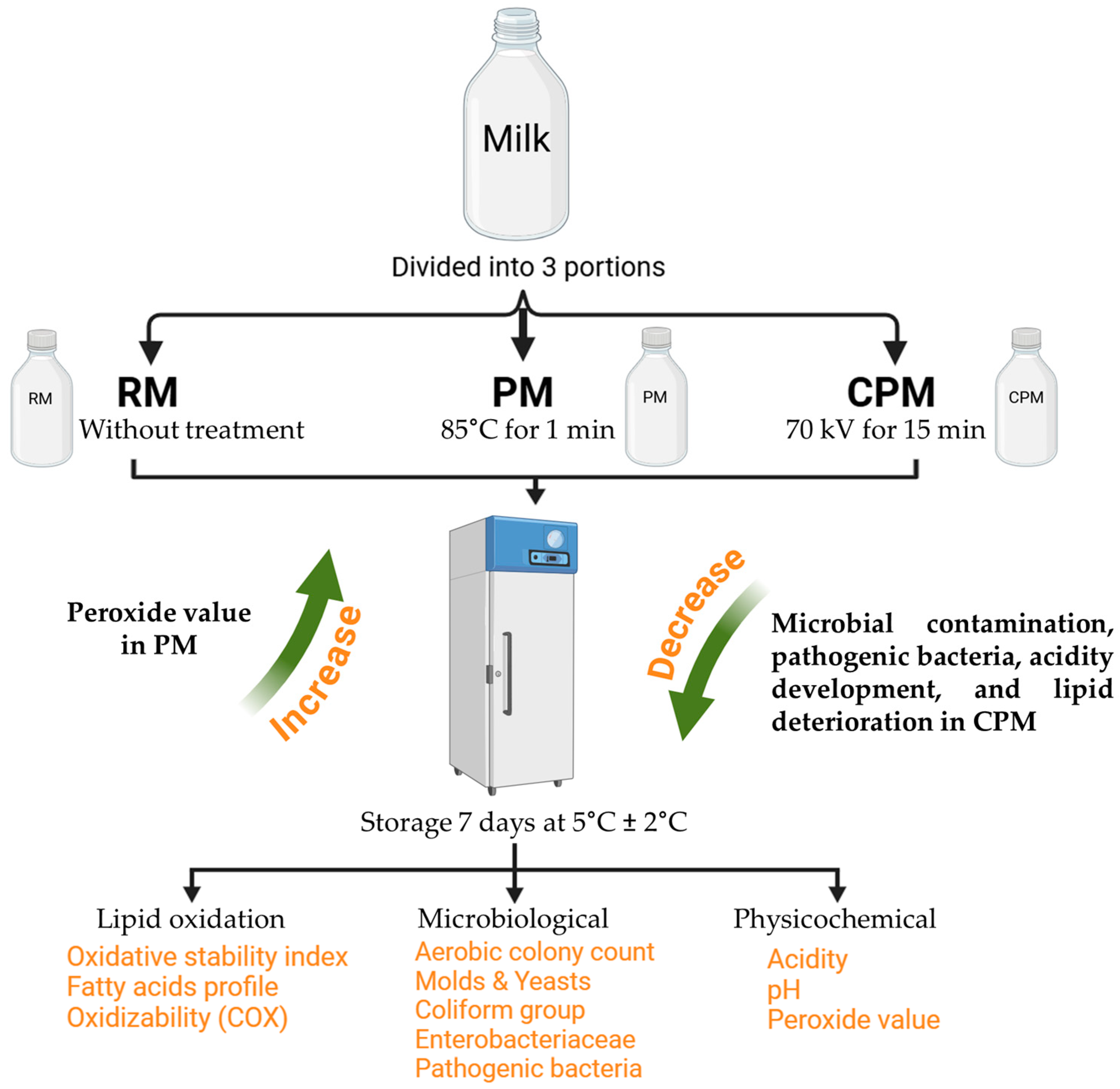
2.1. Sample Treatments
2.1.1. Pasteurization of Milk
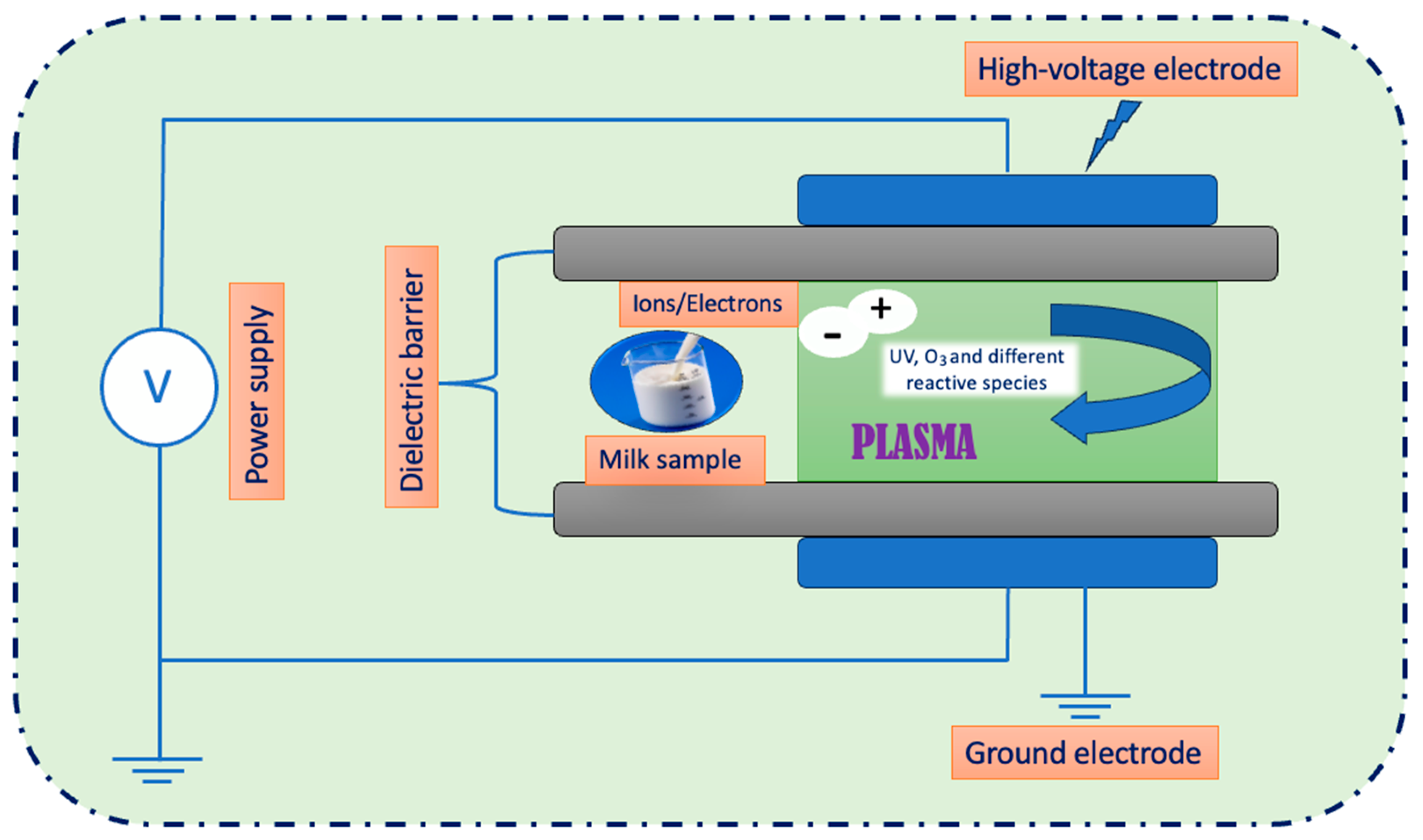
2.1.2. Treatments of Milk by Cold Plasma Dose
2.2. Methods
2.2.1. Chemical Analysis
2.2.2. The Microbiological Examinations for Milk Samples
2.2.3. Pathogenic Bacteria Examination for Milk Samples
2.2.4. Induction Period and Oxidative Stability Index (OSI)
2.2.5. Identification of Fatty Acids by Gas Chromatography (GC-FID)
2.2.6. Calculated Oxidizability (COX) Value
2.3. Statistical Analysis
3. Results and Discussion
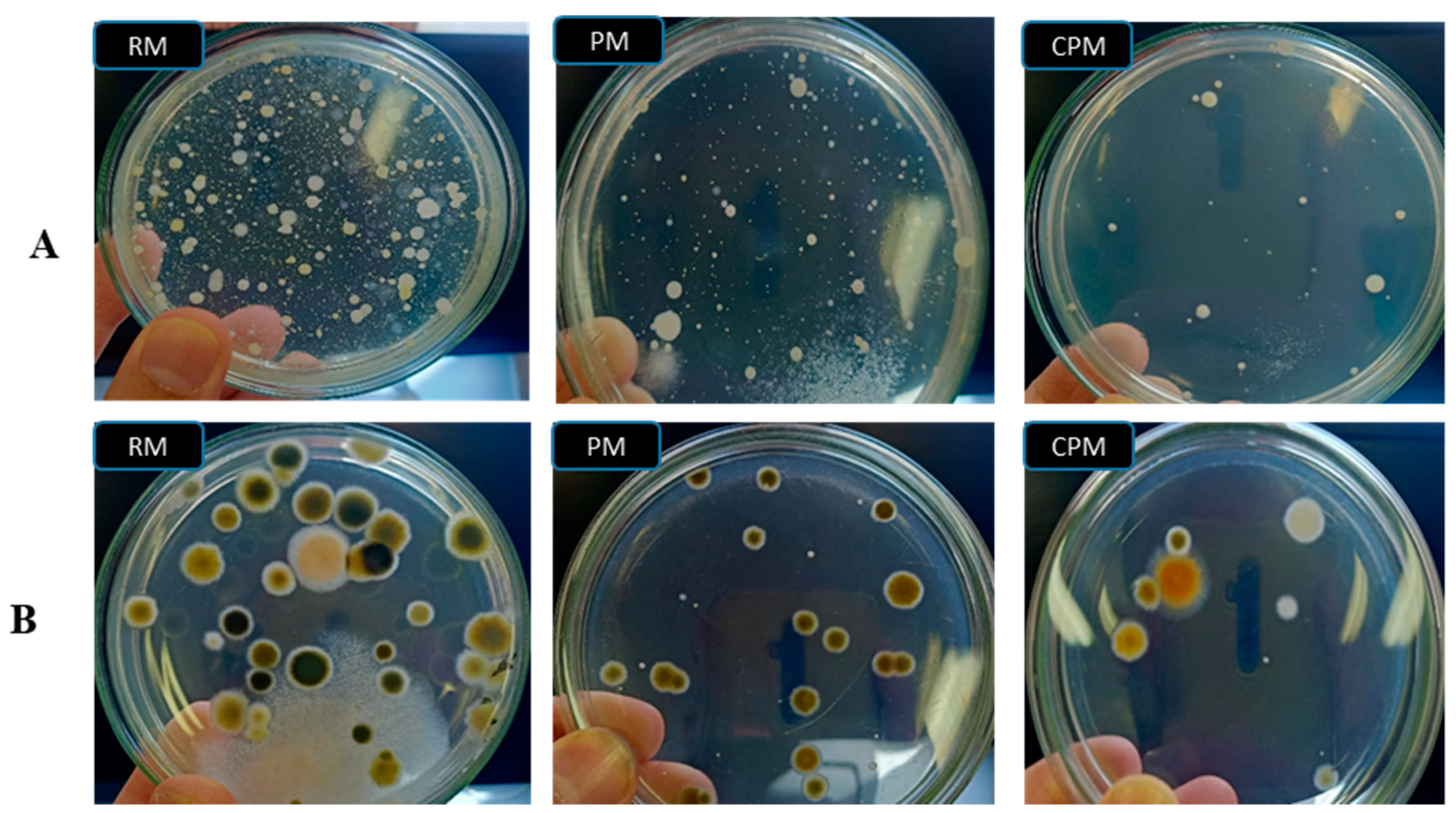
3.1. Microbiological Examination of Milk Sample
3.2. Changes in Quality Parameters for Milk Samples
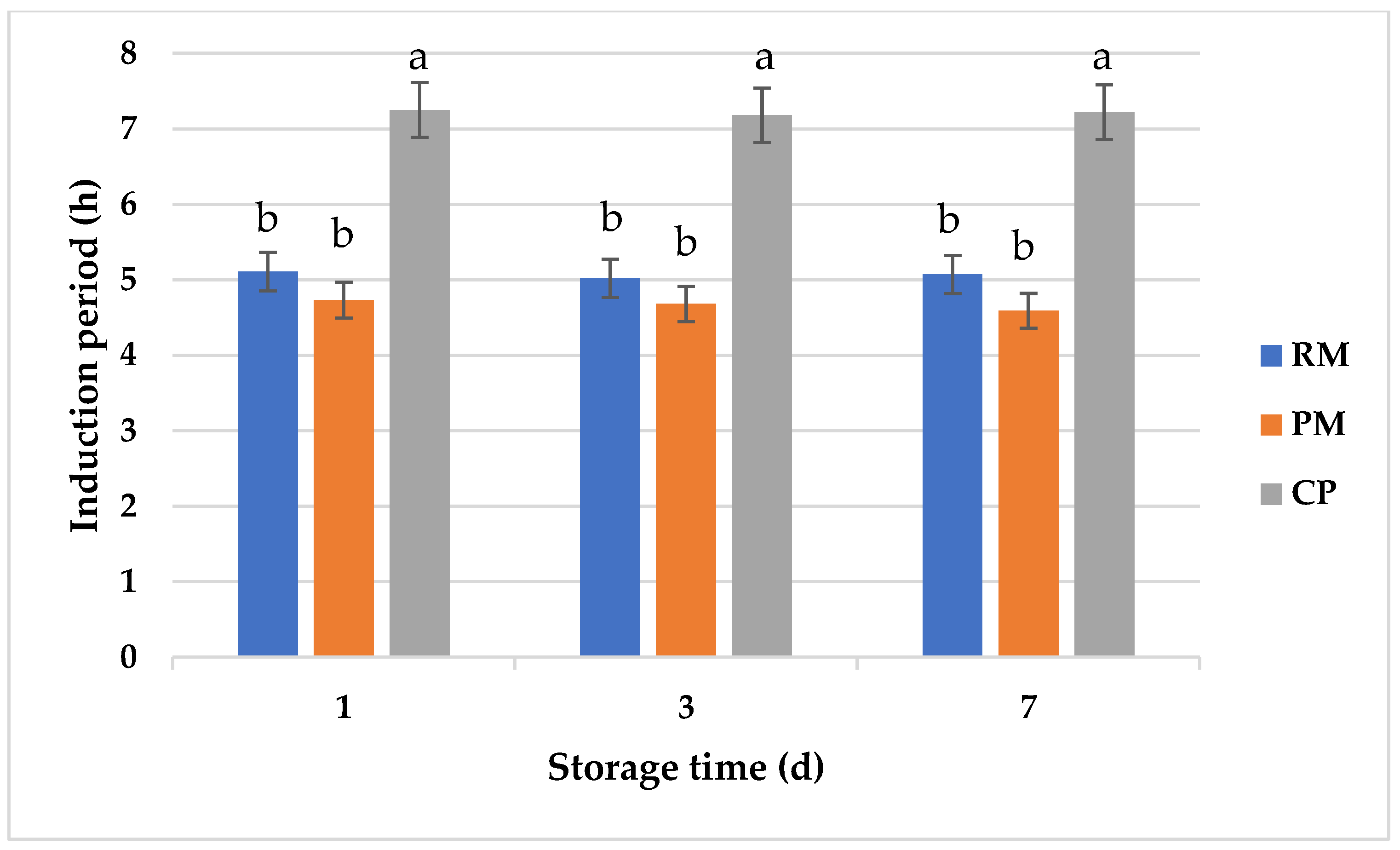
3.3. Oxidative Stability of Raw, Pasteurized, and Cold Plasma-Treated Milk Samples during Storage
3.4. Changes in Fatty Acids Profile
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Lei, Y.; Huang, S.; Dong, X.; Huang, J.; Huang, M. In-package cold plasma treatment of braised chicken: Voltage effect. Food Sci. Hum. Wellness 2022, 11, 845–853. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Nowacka, M.; Colussi, R.; Frasson, S.F.; Demirkesen, I.; Mert, B.; Singha, P.; Singh, S.K.; Falsafi, S.R. Impact of emerging non-thermal processing treatments on major food macromolecules: Starch, protein, and lipid. Trends Food Sci. Technol. 2023, 141, 104208. [Google Scholar] [CrossRef]
- Domonkos, M.; Tichá, P.; Trejbal, J.; Demo, P. Applications of Cold Atmospheric Pressure Plasma Technology in Medicine, Agriculture and Food Industry. Appl. Sci. 2021, 11, 4809. [Google Scholar] [CrossRef]
- Angela, D.M.; Gayle, B.M.; Tamer, A.; Wayne, H.; Mounir, L.; Susan, L.T. Cold Plasma Technology: Bactericidal Effects on Geobacillus Stearothermophilus and Bacillus Cereus Microorganisms. Am. Dent. Hyg. Assoc. 2009, 83, 55. [Google Scholar]
- Aguilar Uscanga, B.R.; Calderón Santoyo, M.; Ragazzo Sánchez, J.A.; Alemán Duarte, M.I.; Pérez Montaño, J.A.; Balcázar-López, E.; Solís Pacheco, J.R. Effect of the Application of Cold Plasma Energy on the Inactivation of Microorganisms, Proteins, and Lipids Deterioration in Adobera Cheese. J. Food Qual. 2022, 2022, 8230955. [Google Scholar] [CrossRef]
- Yepez, X.; Illera, A.E.; Baykara, H.; Keener, K. Recent Advances and Potential Applications of Atmospheric Pressure Cold Plasma Technology for Sustainable Food Processing. Foods 2022, 11, 1833. [Google Scholar] [CrossRef]
- Abbas, H.M.; Abd El-Gawad, M.A.; Kassem, J.M.; Salama, M. Application of fat replacers in dairy products: A review. Foods Raw Mater. 2023, 12, 319–333. [Google Scholar] [CrossRef]
- Segat, A.; Misra, N.N.; Cullen, P.J.; Innocente, N. Atmospheric pressure cold plasma (ACP) treatment of whey protein isolate model solution. Innov. Food Sci. Emerg. Technol. 2015, 29, 247–254. [Google Scholar] [CrossRef]
- Ribeiro, K.C.S.; Coutinho, N.M.; Silveira, M.R.; Rocha, R.S.; Arruda, H.S.; Pastore, G.M.; Neto, R.P.C.; Tavares, M.I.B.; Pimentel, T.C.; Silva, P.H.F.; et al. Impact of cold plasma on the techno-functional and sensory properties of whey dairy beverage added with xylooligosaccharide. Food Res. Int. 2021, 142, 110232. [Google Scholar] [CrossRef]
- Nikmaram, N.; Keener, K.M. The effects of cold plasma technology on physical, nutritional, and sensory properties of milk and milk products. LWT 2022, 154, 112729. [Google Scholar] [CrossRef]
- Akarca, G.; Atik, A.; Atik, İ.; Denizkara, A.J. The use of cold plasma technology in solving the mold problem in Kashar cheese. J. Food Sci. Technol. 2023, 60, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Nasiru, M.M.; Boateng, E.F.; Alnadari, F.; Umair, M.; Wang, Z.; Senan, A.M.; Yan, W.; Zhuang, H.; Zhang, J. Dielectric Barrier Discharge Cold Atmospheric Plasma Treatment of Egg White Protein: Insights into the Functional, Rheological, and Structural Properties. Food Bioprocess Technol. 2024, 17, 955–976. [Google Scholar] [CrossRef]
- Pérez-Andrés, J.M.; Álvarez, C.; Cullen, P.J.; Tiwari, B.K. Effect of cold plasma on the techno-functional properties of animal protein food ingredients. Innov. Food Sci. Emerg. Technol. 2019, 58, 102205. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Zhang, Y.; Lü, X.; Zhao, L.; Song, Y.; Zhang, L.; Jiang, H.; Zhang, J.; Ge, W. Processing sheep milk by cold plasma technology: Impacts on the microbial inactivation, physicochemical characteristics, and protein structure. LWT 2022, 153, 112573. [Google Scholar] [CrossRef]
- Abbas, H.; Kassem, J.M.; Salama, M.; Abd Elhamid, L.B. MILK BIOACTIVE LIPIDS AS POTENTIAL HEALTHY FRACTIONS: A REVIEW. Egypt. J. Chem. 2022, 65, 1389–1408. [Google Scholar] [CrossRef]
- Silveira, M.R.; Coutinho, N.M.; Esmerino, E.A.; Moraes, J.; Fernandes, L.M.; Pimentel, T.C.; Freitas, M.Q.; Silva, M.C.; Raices, R.S.L.; Senaka Ranadheera, C.; et al. Guava-flavored whey beverage processed by cold plasma technology: Bioactive compounds, fatty acid profile and volatile compounds. Food Chem. 2019, 279, 120–127. [Google Scholar] [CrossRef]
- Abbas, H.; Abd Elhamid, L.B.; Kassem, J.M.; Salama, M. Vital lipids contents in Buffalo butter oil and its fractions prepared by dry fractionation. Egypt. J. Chem. 2021, 64, 949–956. [Google Scholar] [CrossRef]
- Coutinho, N.M.; Silveira, M.R.; Pimentel, T.C.; Freitas, M.Q.; Moraes, J.; Fernandes, L.M.; Silva, M.C.; Raices, R.S.L.; Ranadheera, C.S.; Borges, F.O.; et al. Chocolate milk drink processed by cold plasma technology: Physical characteristics, thermal behavior and microstructure. LWT 2019, 102, 324–329. [Google Scholar] [CrossRef]
- A.O.A.C. Association of Official Analytical Chemists. Official Methods of Analysis, 19th ed.; Benjamin Franklin Station: Washington, DC, USA, 2012. [Google Scholar]
- Sedik, A.A.; Salama, M.; Fathy, K.; Salama, A. Cold plasma approach fortifies the topical application of thymoquinone intended for wound healing via up-regulating the levels of TGF-ß, VEGF, and α-SMA in rats. Int. Immunopharmacol. 2023, 122, 110634. [Google Scholar] [CrossRef]
- Wai, H.H.; Shiekh, K.A.; Jafari, S.; Kijpatanasilp, I.; Assatarakul, K. Ultraviolet irradiation as alternative non-thermal cold pasteurization to improve quality and microbiological parameters of mango juice during cold storage. Int. J. Food Microbiol. 2024, 415, 110632. [Google Scholar] [CrossRef]
- Abdallah-Ruiz, A.; Wood, L.S.; Kim, T.; Schilling, W.; White, S.B.; Chen, B.-Y.; Durango-Villadiego, A.; Silva, J.L. Microbial Indicators and Possible Focal Points of Contamination during Production and Processing of Catfish. Foods 2022, 11, 2778. [Google Scholar] [CrossRef] [PubMed]
- Fouad, M.T.; Abu-El Khair, A.G.; El-Sayed, S.M.; Shazly, A.B.; El-Sayed, H.S. Bio-Labneh fortified with functional microcapsules filled with chickpea flour and probiotics. Biocatal. Agric. Biotechnol. 2022, 42, 102345. [Google Scholar] [CrossRef]
- Polo, A.; Cappello, C.; Carafa, I.; Da Ros, A.; Baccilieri, F.; Di Cagno, R.; Gobbetti, M. A novel functional herbal tea containing probiotic Bacillus coagulans GanedenBC30: An in vitro study using the Simulator of the Human Intestinal Microbial Ecosystem (SHIME). J. Funct. Foods 2022, 88, 104873. [Google Scholar] [CrossRef]
- Zahran, H.A.; Tawfeuk, H.Z. Physicochemical properties of new peanut (Arachis hypogaea L.) varieties. OCL 2019, 26, 19. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Hammond, E.G. Analysis of oleate, linoleate and linolenate hydroperoxides in oxidized ester mixtures. Lipids 1980, 15, 379–385. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT User’s Guide Statistics (Released 9.2); SAS Institute Inc.: Cary, NC, USA, 2004. [Google Scholar]
- Abdelnasser Ahmed, S.; Hassanin Mahmoud Mostafa, A.; El-Sherbini, M.; Abdelkhalek, A. Assessment of Microbial Safety and Quality of Market Raw Milk and Pasteurized Milk Sold in Dakahlia Governorate, Egypt. J. Adv. Vet. Res. 2022, 12, 456–461. [Google Scholar]
- El-Diasty, E.M.; El-Kaseh, R. Microbiological monitoring of raw milk and yoghurt samples collected from El-Beida city. Arab J. Biotechnol. 2009, 12, 57–64. [Google Scholar]
- El Zubeir, I.E.; Ahmed, M.I. The hygienic quality of raw milk produced by some dairy farms in Khartoum State, Sudan. Res. J. Microbiol. 2007, 2, 988–991. [Google Scholar]
- Ledenbach, L.H.; Marshall, R.T. Microbiological Spoilage of Dairy Products. In Compendium of the Microbiological Spoilage of Foods and Beverages; Sperber, W.H., Doyle, M.P., Eds.; Springer: New York, NY, USA, 2009; pp. 41–67. [Google Scholar]
- Hasan, M.A.; Islam, M.A.; Mahmud, M.S.; Uddin, A.A.; Ahmed, S. Microbial analysis of raw and pasteurized milk from selected areas of Dinajpur, Bangladesh. Asian J. Med. Biol. Res. 2015, 1, 292–296. [Google Scholar] [CrossRef][Green Version]
- Baggio, A.; Marino, M.; Innocente, N.; Celotto, M.; Maifreni, M. Antimicrobial effect of oxidative technologies in food processing: An overview. Eur. Food Res. Technol. 2020, 246, 669–692. [Google Scholar] [CrossRef]
- Guo, L.; Zhao, Y.; Liu, D.; Liu, Z.; Chen, C.; Xu, R.; Tian, M.; Wang, X.; Chen, H.; Kong, M.G. Cold atmospheric-pressure plasma induces DNA–protein crosslinks through protein oxidation. Free Radic. Res. 2018, 52, 783–798. [Google Scholar] [CrossRef] [PubMed]
- Dezest, M.; Bulteau, A.-L.; Quinton, D.; Chavatte, L.; Le Bechec, M.; Cambus, J.P.; Arbault, S.; Nègre-Salvayre, A.; Clément, F.; Cousty, S. Oxidative modification and electrochemical inactivation of Escherichia coli upon cold atmospheric pressure plasma exposure. PLoS ONE 2017, 12, e0173618. [Google Scholar] [CrossRef] [PubMed]
- Govaert, M.; Smet, C.; Vergauwen, L.; Ećimović, B.; Walsh, J.L.; Baka, M.; Van Impe, J. Influence of plasma characteristics on the efficacy of Cold Atmospheric Plasma (CAP) for inactivation of Listeria monocytogenes and Salmonella Typhimurium biofilms. Innov. Food Sci. Emerg. Technol. 2019, 52, 376–386. [Google Scholar] [CrossRef]
- Ali, A.; Kim, Y.H.; Lee, J.Y.; Lee, S.; Uhm, H.S.; Cho, G.; Park, B.J.; Choi, E.H. Inactivation of Propionibacterium acnes and its biofilm by non-thermal plasma. Curr. Appl. Phys. 2014, 14, S142–S148. [Google Scholar] [CrossRef]
- Han, L.; Patil, S.; Boehm, D.; Milosavljević, V.; Cullen, P.J.; Bourke, P. Mechanisms of Inactivation by High-Voltage Atmospheric Cold Plasma Differ for Escherichia coli and Staphylococcus aureus. Appl. Environ. Microbiol. 2016, 82, 450–458. [Google Scholar] [CrossRef]
- Timmons, C.; Pai, K.; Jacob, J.; Zhang, G.; Ma, L.M. Inactivation of Salmonella enterica, Shiga toxin-producing Escherichia coli, and Listeria monocytogenes by a novel surface discharge cold plasma design. Food Control 2018, 84, 455–462. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, Y.; Sun, K.; Chen, Q.; Shen, F.; Zhang, J.; Yao, M.; Zhu, T.; Fang, J. Rapid Inactivation of Biological Species in the Air using Atmospheric Pressure Nonthermal Plasma. Environ. Sci. Technol. 2012, 46, 3360–3368. [Google Scholar] [CrossRef]
- Bermudez-Aguirre, D. Chapter 2—Advances in the inactivation of microorganisms and viruses in food and model systems using cold plasma. In Advances in Cold Plasma Applications for Food Safety and Preservation; Bermudez-Aguirre, D., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 49–91. [Google Scholar]
- Cullen, P.J.; Misra, N.N.; Han, L.; Bourke, P.; Keener, K.; Donnell, C.O.; Moiseev, T.; Mosnier, J.P.; Milosavljević, V. Inducing a Dielectric Barrier Discharge Plasma within a Package. IEEE Trans. Plasma Sci. 2014, 42, 2368–2369. [Google Scholar] [CrossRef]
- Deng, S.; Ruan, R.; Mok, C.K.; Huang, G.; Lin, X.; Chen, P. Inactivation of Escherichia coli on Almonds Using Nonthermal Plasma. J. Food Sci. 2007, 72, M62–M66. [Google Scholar] [CrossRef]
- Nwabor, O.F.; Onyeaka, H.; Miri, T.; Obileke, K.; Anumudu, C.; Hart, A. A Cold Plasma Technology for Ensuring the Microbiological Safety and Quality of Foods. Food Eng. Rev. 2022, 14, 535–554. [Google Scholar] [CrossRef]
- Gavahian, M.; Chu, Y.-H.; Mousavi Khaneghah, A.; Barba, F.J.; Misra, N.N. A critical analysis of the cold plasma induced lipid oxidation in foods. Trends Food Sci. Technol. 2018, 77, 32–41. [Google Scholar] [CrossRef]
- Korachi, M.; Ozen, F.; Aslan, N.; Vannini, L.; Guerzoni, M.E.; Gottardi, D.; Ekinci, F.Y. Biochemical changes to milk following treatment by a novel, cold atmospheric plasma system. Int. Dairy J. 2015, 42, 64–69. [Google Scholar] [CrossRef]

| Gross Composition | Content (%) |
|---|---|
| Total solids | 17.33 ± 0.3 |
| Fat | 7.19 ± 0.5 |
| Protein | 3.84 ± 0.4 |
| Lactose | 5.50 ± 0.4 |
| Ash | 0.87 ± 0.1 |
| Microbiological Examination | Storage Period (Days) | log/CFU per mL | ||
|---|---|---|---|---|
| Raw Milk | Pasteurized Milk | Cold Plasma Milk | ||
| Total bacterial count | 1 | 8.4 ± 0.01 C,a | 6.1 ± 0.00 C,b | 4.2 ± 0.02 C,c |
| 3 | 8.8 ± 0.01 B,a | 6.2 ± 0.0 B,b | 4.6 ± 0.01 B,c | |
| 7 | 9.6 ± 0.02 A,a | 6.4 ± 0.02 A,b | 4.9 ± 0.0 A,c | |
| Mold and Yeast | 1 | 6.2 ± 0.03 A,a | 3.6 ± 0.01 A,c | 3.7 ± 0.02 A,b |
| 3 | 5.0 ± 0.0 B,a | 3.6 ± 0.0 A,b | 2.3 ± 0.01 B,c | |
| 7 | 4.6 ± 0.01 C,a | 1.3 ± 0.03 B,b | <1 ± 0.04 C,c | |
| Coliform bacterial Group | 1 | 4.5 ± 0.01 C,a | 1.3 ± 0.01 C,b | 1.2 ± 0.02 C,c |
| 3 | 4.8 ± 0.01 B,a | 1.6 ± 0.0 B,b | 1.4 ± 0.01 B,c | |
| 7 | 4.9 ± 0.05 A,a | 1.7 ± 0.0 A,b | 1.5 ± 0.01 A,c | |
| Enterobacteriaceae | 1 | 4.7 ± 0.01 C,a | <1 ± 0.01 C,b | <1 ± 0.2 B,b |
| 3 | 5.1 ± 0.05 A,a | 1.3 ± 0.02 A,b | 1.2 ± 0.02 A,c | |
| 7 | 4.9 ± 0.0 B,a | 1.0 ± 0.0 B,c | 1.1 ± 0.06 A,b | |
| Pathogenic Bacteria | Storage Period (Days) | log/CFU per mL | ||
|---|---|---|---|---|
| Raw Milk | Pasteurized Milk | Cold Plasma Milk | ||
| Listeria monocytogenes | 1 | 4.0 ± 0.03 C,a | 1.0 ± 0.03 C,b | <1 ± 0.05 C,c |
| 3 | 4.5 ± 0.01 B,a | 1.3 ± 0.02 B,b | 1.1 ± 0.03 B,c | |
| 7 | 4.7 ± 0.01 A,a | 1.7 ± 0.01 A,b | 1.5 ± 0.01 A,c | |
| Salmonella typhimurium | 1 | + | - | - |
| 3 | + | - | - | |
| 7 | + | - | - | |
| Bacillus cereus | 1 | 4.2 ± 0.02 C,a | <1 ± 0.2 B,b | <1 ± 0.04 B,b |
| 3 | 4.6 ± 0.01 B,a | 1.3 ± 0.02 A,b | 1.2 ± 0.02 A,c | |
| 7 | 4.8 ± 0.01 A,a | 1.3 ± 0.02 A,b | 1.2 ± 0.02 A,c | |
| Staphylococcus aureus | 1 | 4.5 ± 0.02 C,a | <1 ± 0.0 A,b | <1 ± 0.0 A,b |
| 3 | 4.7 ± 0.02 B,a | <1 ± 0.0 A,b | <1 ± 0.0 A,b | |
| 7 | 4.9 ± 0.01 A,a | <1 ± 0.0 A,b | <1 ± 0.0 A,b | |
| E. coli | 1 | 4.4 ± 0.01 C,a | <1 ± 0.0 A,b | <1 ± 0.0 A,b |
| 3 | 4.7 ± 0.01 B,a | <1 ± 0.0 A,b | <1 ± 0.0 A,b | |
| 7 | 4.8 ± 0.01 A,a | <1 ± 0.0 A,b | <1 ± 0.0 A,b | |
| Parameters | Storage Period (Days) | Raw Milk | Pasteurized Milk | Cold Plasma Milk |
|---|---|---|---|---|
| Acidity (%) | 1 | 0.31 ± 0.01 a,C | 0.31± 0.01 a,C | 0.27 ± 0.02 b,C |
| 3 | 0.75 ± 0.05 a,B | 0.60 ± 0.07 b,B | 0.47 ± 0.02 c,B | |
| 7 | 1.49 ± 0.07 a,A | 1.45 ± 0.03 a,A | 0.92 ± 0.05 b,A | |
| pH | 1 | 5.90 ± 0.2 a,A | 6.00 ± 0.2 ab,A | 6.10 ± 0.2 b,A |
| 3 | 5.97 ± 0.3 a,A | 5.85 ± 0.4 a,A | 5.55 ± 0.3 a,B | |
| 7 | 4.80 ± 0.3 a,B | 4.80 ± 0.2 a,B | 5.10 ± 0.3 b,B | |
| Peroxide value (meq/kg) | 1 | 0.42 ± 0.01 a,C | 1.52 ± 0.07 b,C | 0.47 ± 0.05 a,C |
| 3 | 0.51 ± 0.03 a,B | 1.86 ± 0.08 b,B | 0.52 ± 0.06 a,B | |
| 7 | 0.78 ± 0.05 a,A | 1.97 ± 0.07 b,A | 0.79 ± 0.06 a,A |
| Fatty Acids | RT (Min) | Concentration % | |||||
|---|---|---|---|---|---|---|---|
| Zero-Time | 7 days Storage | ||||||
| RMF | PMF | CPMF | RMF | PMF | CPMF | ||
| Butyric acid (C4:0) | 1.81 | 3.21 ± 0.15 | 1.91 ± 0.14 | 1.89 ± 0.23 | 3.23 ± 0.16 | 4.76 ± 0.37 | 3.15 ± 0.29 |
| Caproic acid (C6:0) | 2.38 | 3.03 ± 0.22 | 2.88 ± 0.26 | 1.67 ± 0.21 | 2.68 ± 0.29 | 2.53 ± 0.54 | 2.35 ± 0.36 |
| Caprylic acid (C8:0) | 3.63 | 2.41 ± 0.10 | 1.82 ± 0.09 | 1.35 ± 0.19 | 1.56 ± 0.55 | 1.43 ± 0.31 | 1.28 ± 0.18 |
| Capric acid (C10:0) | 5.35 | 2.97 ± 0.11 | 3.89 ± 0.25 | 3.21 ± 0.12 | 3.55 ± 0.43 | 2.35 ± 0.26 | 2.31 ± 0.11 |
| Caproleic acid (C10:1) | 5.87 | 0.53 ± 0.17 | 0.44 ± 0.06 | 0.23 ± 0.05 | 0.41 ± 0.09 | 0.38 ± 0.09 | 0.18 ± 0.05 |
| Lauric acid (C12:0) | 7.26 | 4.16 ± 0.15 | 4.13 ± 0.22 | 3.41 ± 0.65 | 3.98 ± 0.53 | 2.98 ± 0.14 | 3.17 ± 0.45 |
| Tridecanoic acid (C13:0) | 8.43 | 0.34 ± 0.05 | 0.52 ± 0.03 | 0.18 ± 0.02 | 0.52 ± 0.10 | 0.78 ± 0.11 | 0.85 ± 0.09 |
| Tridecenoic acid (C13:1) | 8.75 | 0.69 ± 0.04 | 0.61 ± 0.06 | ND | 0.47 ± 0.04 | ND | 0.22 ± 0.10 |
| Myristic acid (C14:0) | 9.11 | 9.65 ± 0.56 | 10.63 ± 0.62 | 9.59 ± 0.94 | 11.72 ± 0.72 | 9.56 ± 0.87 | 10.28 ± 0.83 |
| Myristoleic acid (14.1) | 9.39 | 1.49 ± 0.18 | 0.97 ± 0.16 | 0.76 ± 0.11 | 1.16 ± 0.49 | 1.24 ± 0.06 | 1.39 ± 0.18 |
| Myristolinoleic acid (14.2) | 9.75 | 1.18 ± 0.09 | 0.94 ± 0.11 | 0.66 ± 0.08 | 0.89 ± 0.23 | 1.06 ± 0.13 | 1.52 ± 0.25 |
| Pentadecanoic acid (C15:0) | 10.01 | 1.78 ± 0.05 | 1.35 ± 0.08 | 1.12 ± 0.11 | 0.75 ± 0.12 | 2.14 ± 0.47 | 2.13 ± 0.10 |
| Cis-10-Pentadecenoic (C15:1) | 10.56 | 0.56 ± 0.01 | 0.42 ± 0.02 | 0.53 ± 0.07 | 0.62 ± 0.08 | 0.93 ± 0.15 | 1.58 ± 0.11 |
| Palmitic acid (C16:0) | 11.04 | 31.27 ± 1.34 | 28.24 ± 1.04 | 34.21 ± 2.05 | 31.84 ± 1.35 | 27.73 ± 1.19 | 22.97 ± 1.12 |
| Palmitoleic acid (C16:1 n9) | 11.17 | 1.87 ± 0.22 | 1.77 ± 0.13 | 1.24 ± 0.63 | 1.98 ± 0.22 | 2.46 ± 0.33 | 3.40 ± 0.35 |
| Palmitoleic acid (C16:1 n7) | 11.21 | 0.46 ± 0.15 | 0.26 ± 0.05 | 0.77 ± 0.12 | 0.87 ± 0.16 | 1.02 ± 0.25 | 2.01 ± 0.08 |
| Heptadecanoic acid (C17:0) | 12.28 | 0.62 ± 0.11 | 0.22 ± 0.02 | 0.62 ± 0.08 | 1.09 ± 0.15 | 1.34 ± 0.22 | 1.65 ± 0.04 |
| Cis-10-Heptadecanoic acid (C17:1) | 12.35 | ND | ND | 0.32 ± 0.01 | 1.16 ± 0.05 | ND | 0.31 ± 0.02 |
| Stearic acid (C18:0) | 13.54 | 6.59 ± 0.37 | 8.27 ± 0.37 | 8.94 ± 0.87 | 7.64 ± 0.85 | 9.98 ± 1.16 | 9.51 ± 0.85 |
| Oleic acid (C18:1n9c) | 13.58 | 24.11 ± 0.95 | 26.65 ± 0.99 | 24.15 ± 1.22 | 19.63 ± 0.86 | 22.34 ± 1.12 | 22.68 ± 1.03 |
| Linoleic acid (C18:2n6c) | 14.18 | 2.33 ± 0.73 | 2.74 ± 0.35 | 4.27 ± 0.48 | 2.11 ± 0.07 | 2.33 ± 0.15 | 2.49 ± 0.63 |
| γ- Linolenic acid (C18:3n6, CLA) | 14.99 | 0.32 ± 0.03 | 0.82 ± 0.06 | 0.37 ± 0.03 | 0.65 ± 0.04 | 1.07 ± 0.11 | 2.83 ± 0.22 |
| α- Linolenic acid (C18:3n3) | 15.45 | 0.43 ± 0.01 | 0.36 ± 0.02 | 0.51 ± 0.09 | 1.12 ± 0.10 | 1.15 ± 0.07 | 1.45 ± 0.16 |
| Arachididc acid (C20:0) | 16.62 | ND | 0.16 ± 0.03 | ND | 0.37 ± 0.05 | 0.44 ± 0.20 | 0.29 ± 0.03 |
| ∑ SFA | --- | 66.03 ± 1.94 | 64.02 ± 1.15 | 66.19 ± 1.02 | 68.93 ± 1.78 | 66.02 ± 1.34 | 59.94 ± 1.34 |
| ∑ UFA | --- | 33.97 ± 1.55 | 35.98 ± 2.17 | 33.81 ± 1.82 | 31.07 ± 1.11 | 33.98 ± 1.35 | 40.06 ± 1.15 |
| ∑ MUFA | --- | 29.71 ± 1.09 | 31.12 ± 1.83 | 28.00 ± 0.86 | 26.3 ± 0.88 | 28.37 ± 1.27 | 31.77 ± 1.52 |
| ∑ PUFA | --- | 4.26 ± 0.65 | 4.86 ± 0.78 | 5.81 ± 0.66 | 4.77 ± 0.35 | 5.61 ± 0.66 | 8.29 ± 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, H.M.; Altamim, E.A.; Salama, M.; Fouad, M.T.; Zahran, H.A. Cold Plasma Technology: A Sustainable Approach to Milk Preservation by Reducing Pathogens and Enhancing Oxidative Stability. Sustainability 2024, 16, 8754. https://doi.org/10.3390/su16208754
Abbas HM, Altamim EA, Salama M, Fouad MT, Zahran HA. Cold Plasma Technology: A Sustainable Approach to Milk Preservation by Reducing Pathogens and Enhancing Oxidative Stability. Sustainability. 2024; 16(20):8754. https://doi.org/10.3390/su16208754
Chicago/Turabian StyleAbbas, Hayam M., Ebtehal A. Altamim, Mohamed Salama, Mohamed T. Fouad, and Hamdy A. Zahran. 2024. "Cold Plasma Technology: A Sustainable Approach to Milk Preservation by Reducing Pathogens and Enhancing Oxidative Stability" Sustainability 16, no. 20: 8754. https://doi.org/10.3390/su16208754
APA StyleAbbas, H. M., Altamim, E. A., Salama, M., Fouad, M. T., & Zahran, H. A. (2024). Cold Plasma Technology: A Sustainable Approach to Milk Preservation by Reducing Pathogens and Enhancing Oxidative Stability. Sustainability, 16(20), 8754. https://doi.org/10.3390/su16208754









