Vertical Features of Volatile Organic Compounds and Their Potential Photochemical Reactivities in Boundary Layer Revealed by In-Situ Observations and Satellite Retrieval
Abstract
:1. Introduction
2. Materials and Methods
2.1. Observation Sites, Instruments, and Measurements
2.1.1. Observation Sites
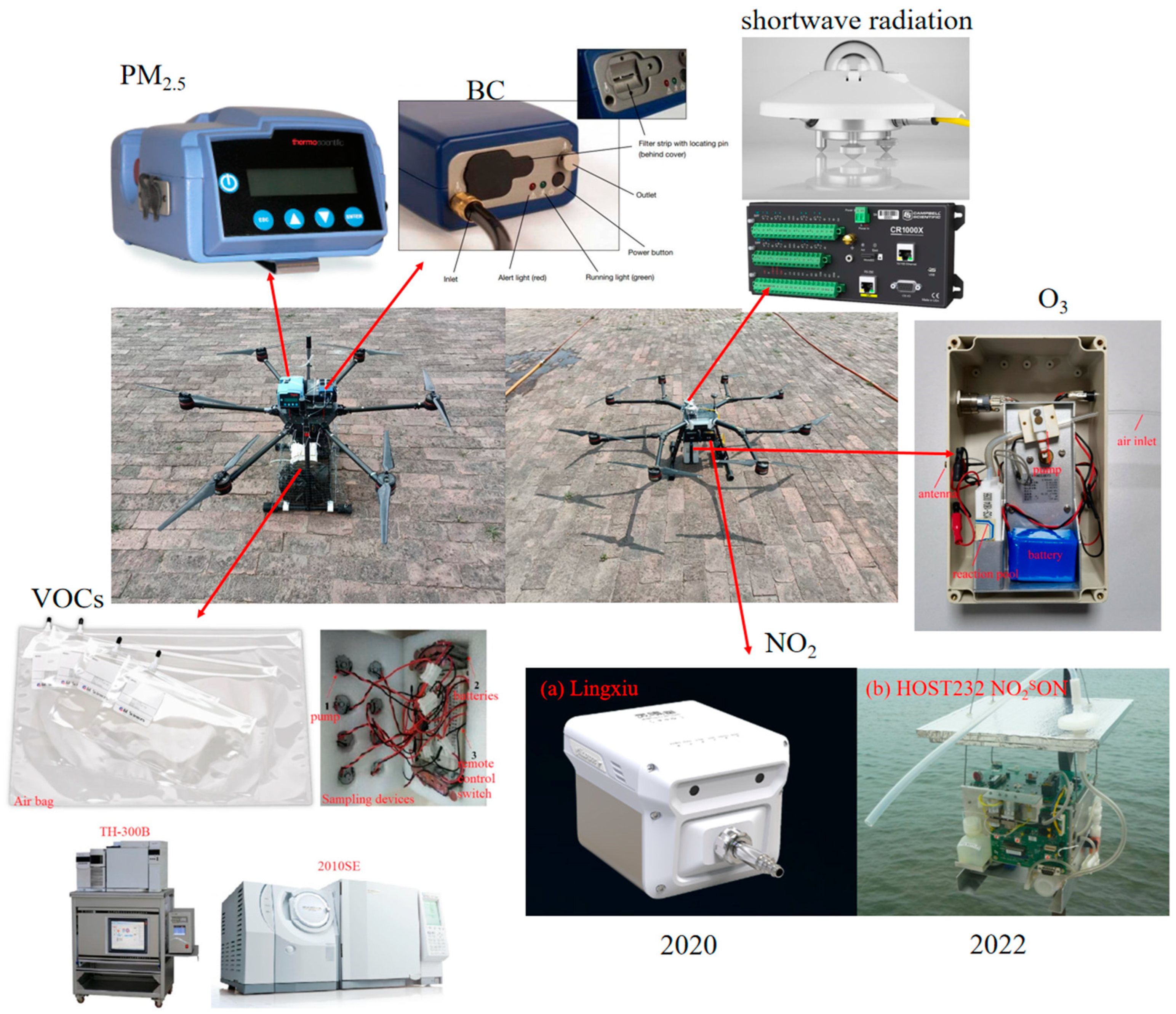
2.1.2. Observation Platforms, Instruments, and Sampling Methods
2.1.3. Quality Assurance and Quality Control
2.2. Observation Data and Methods
2.2.1. Observation Data Filtering
2.2.2. Calculation of BL NO2 Vertical Column Concentration
2.2.3. Calculation of the OH and NO3 Loss Rates
2.3. Satellite Data Sources
2.3.1. TROPOMI
2.3.2. TROPOMI Observations Used in This Study
3. Results
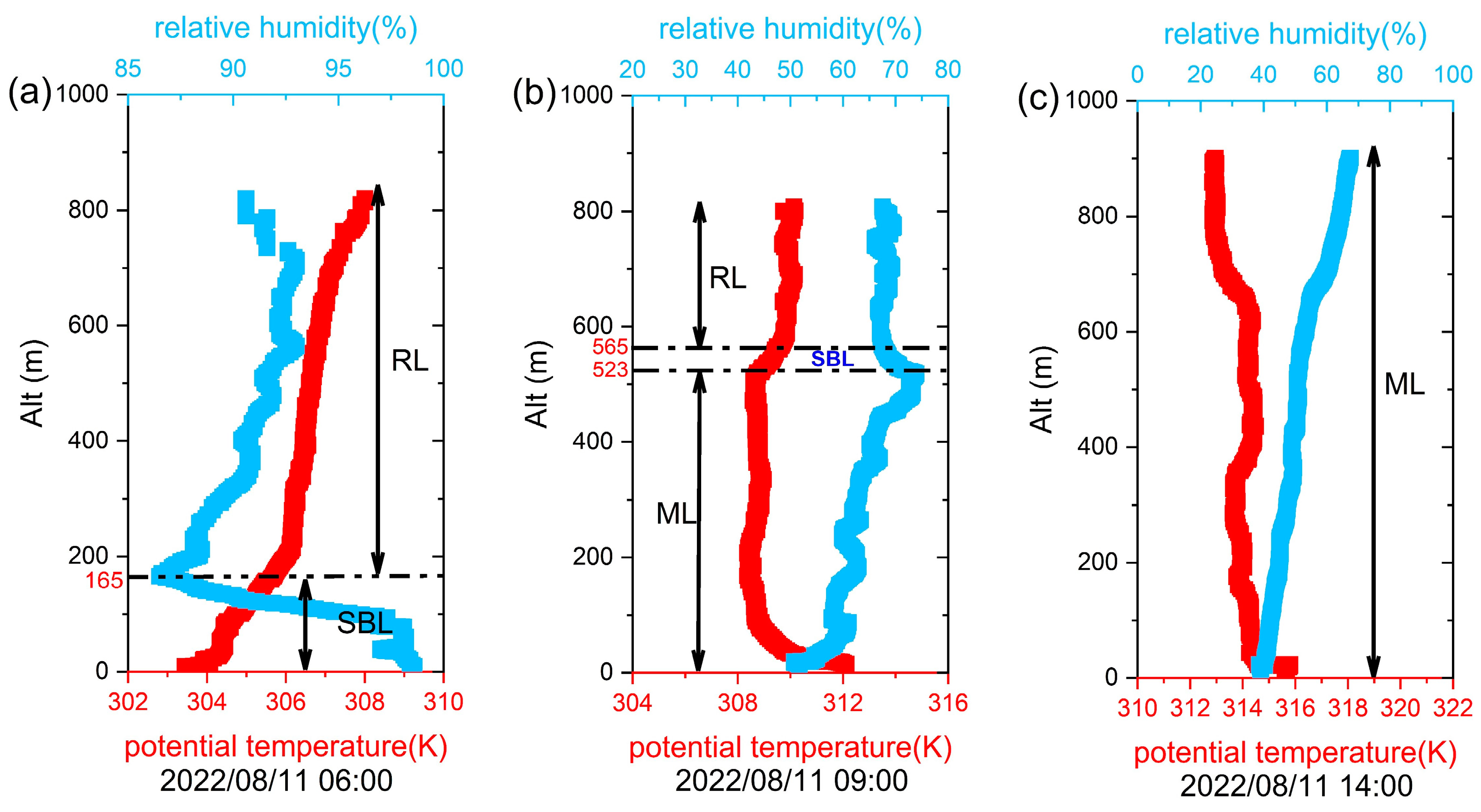
3.1. Thermal Layer Classification of BL and Characteristics of Vertical VOCs and NO2
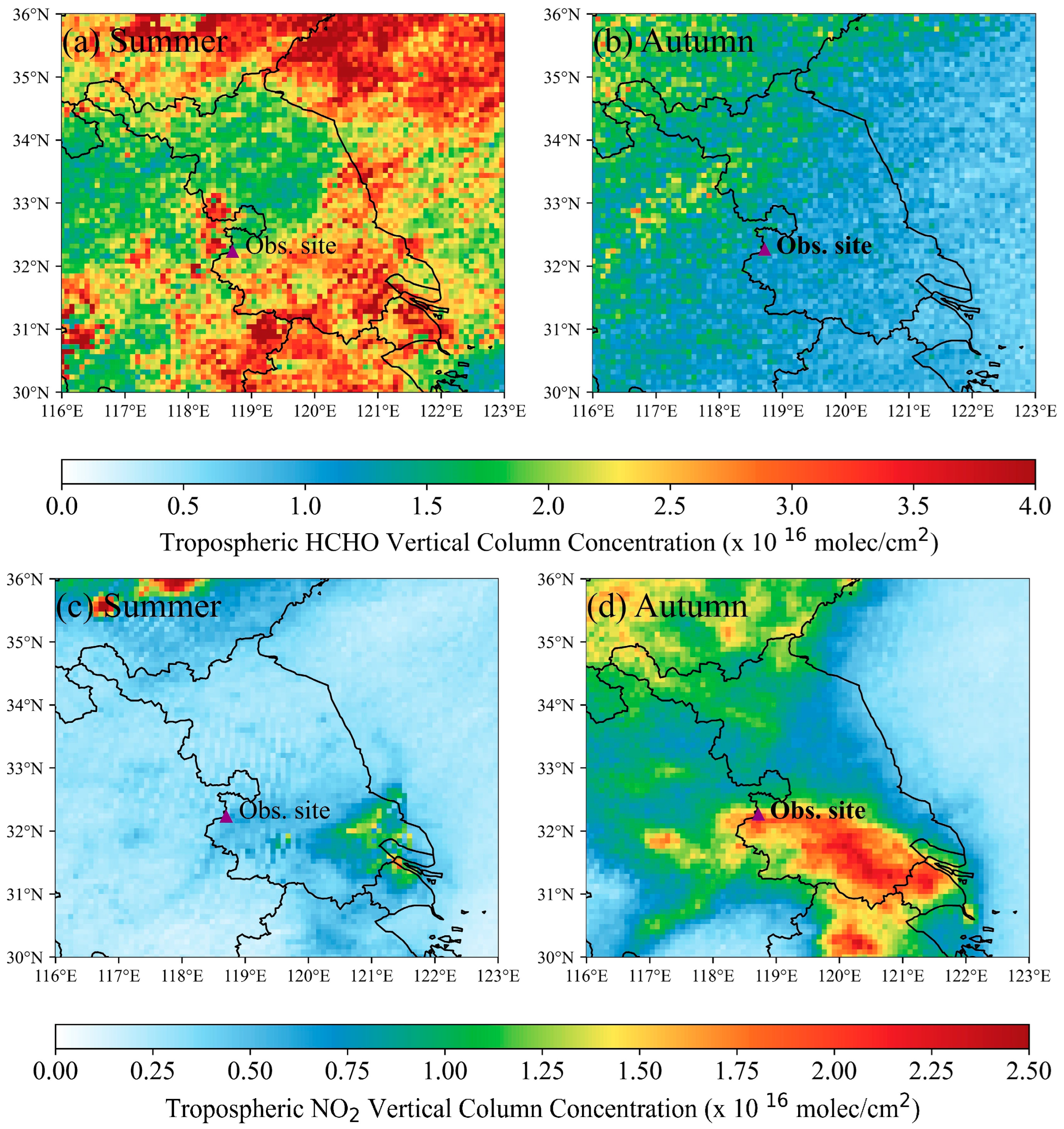
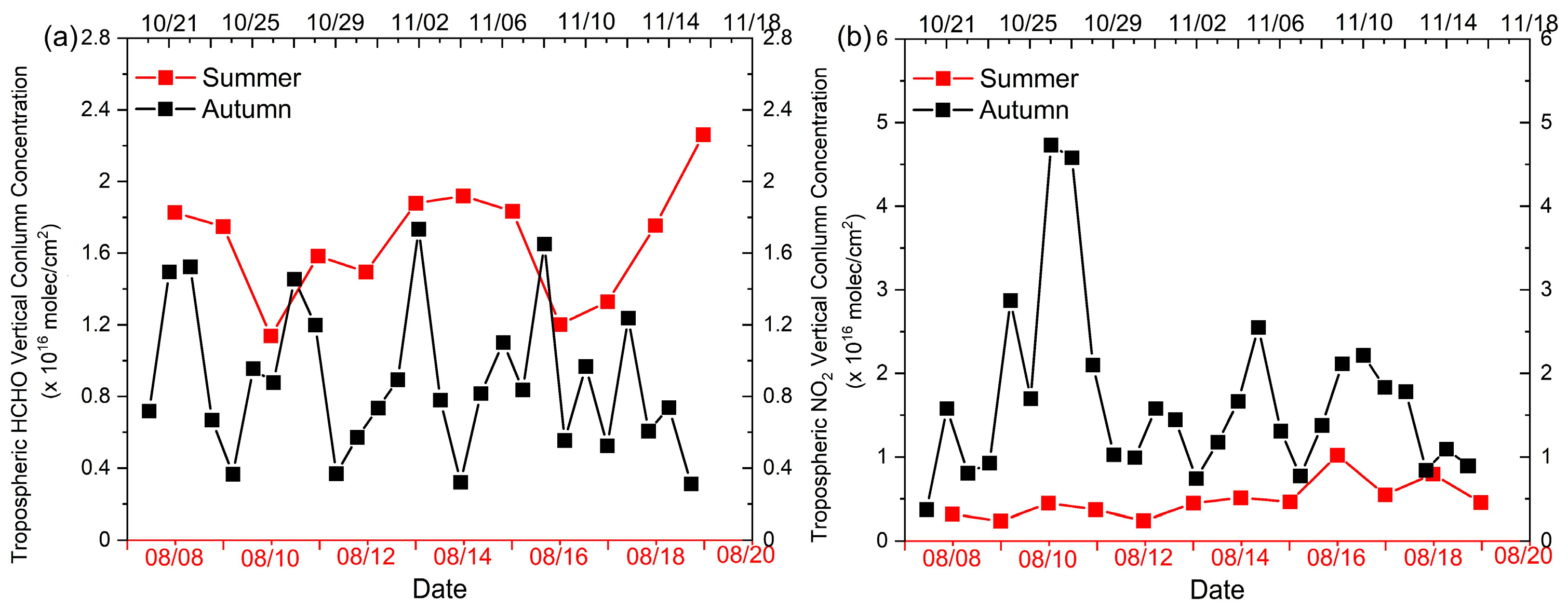
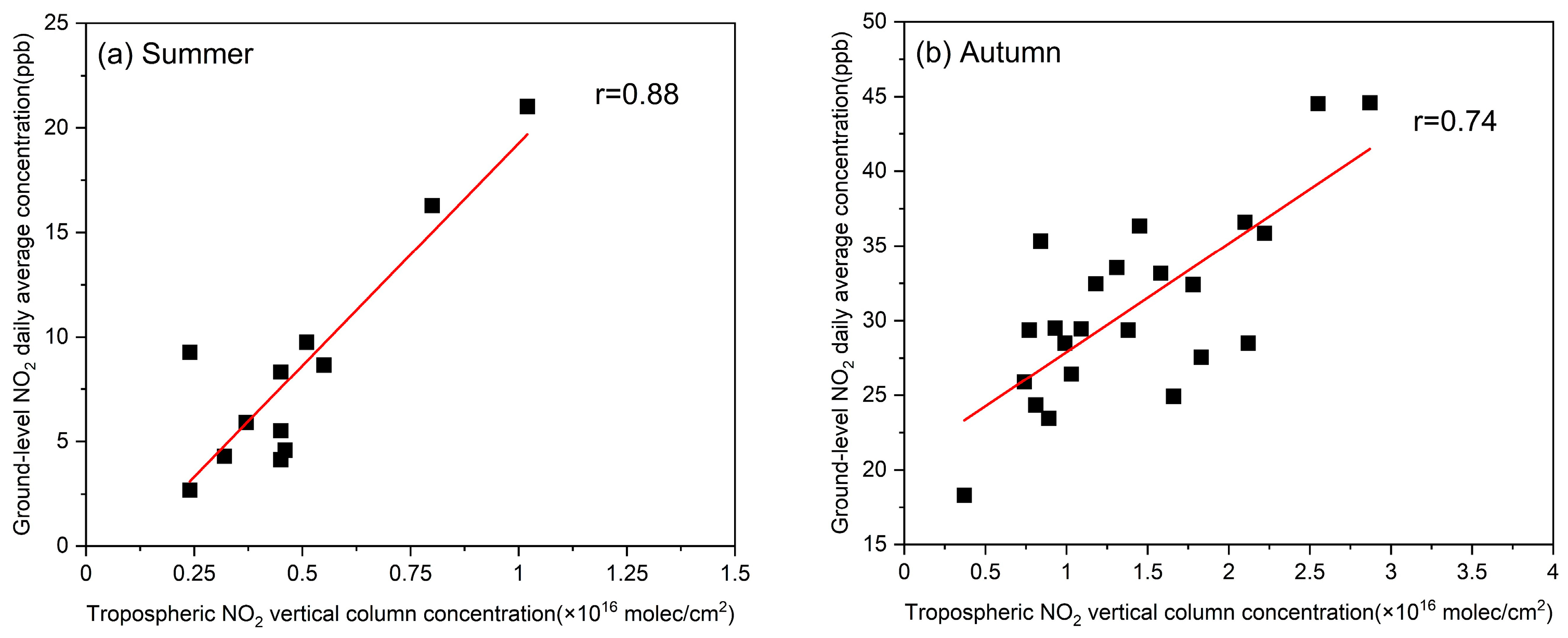
3.2. Satellite Data and Observations
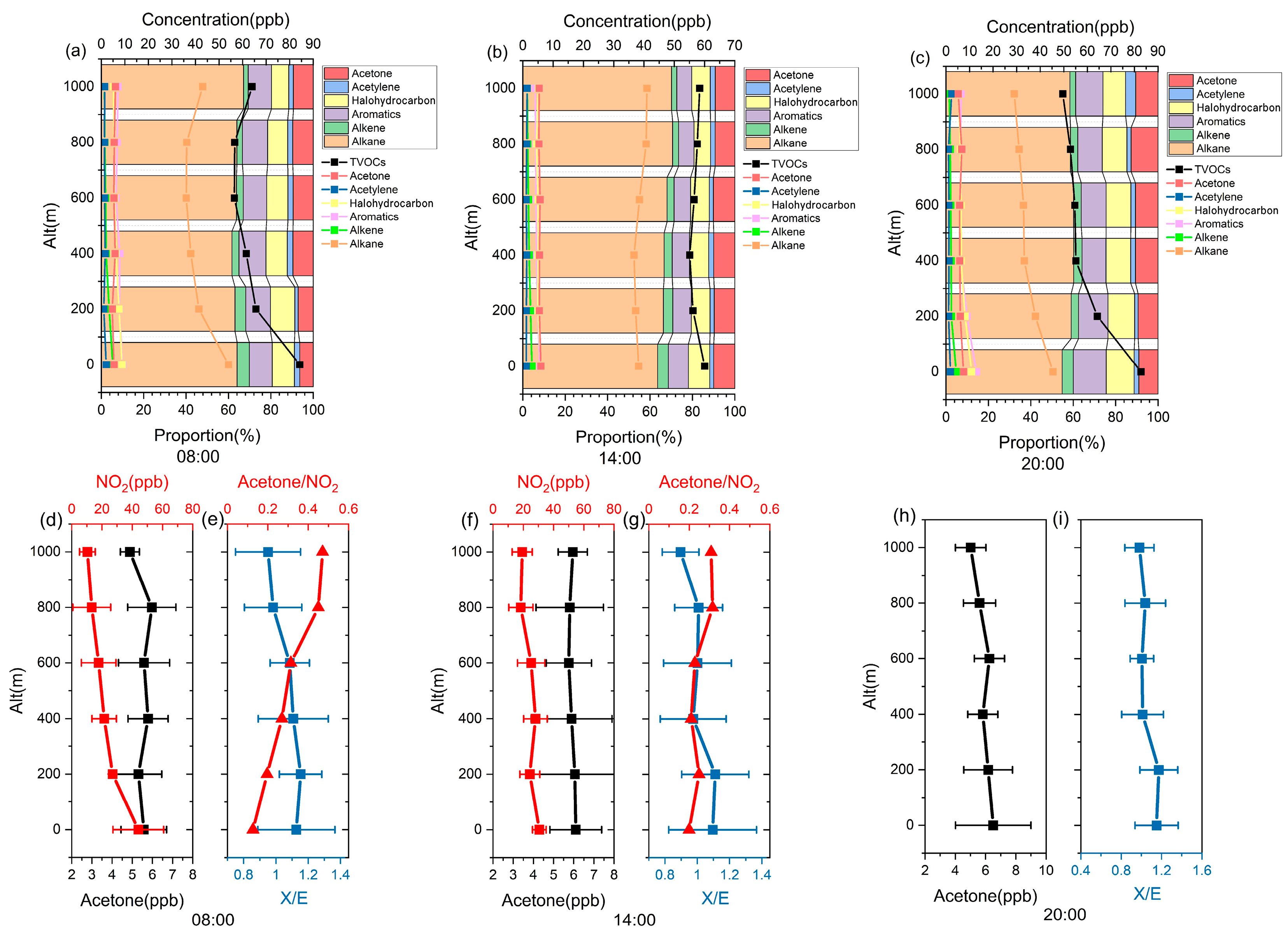
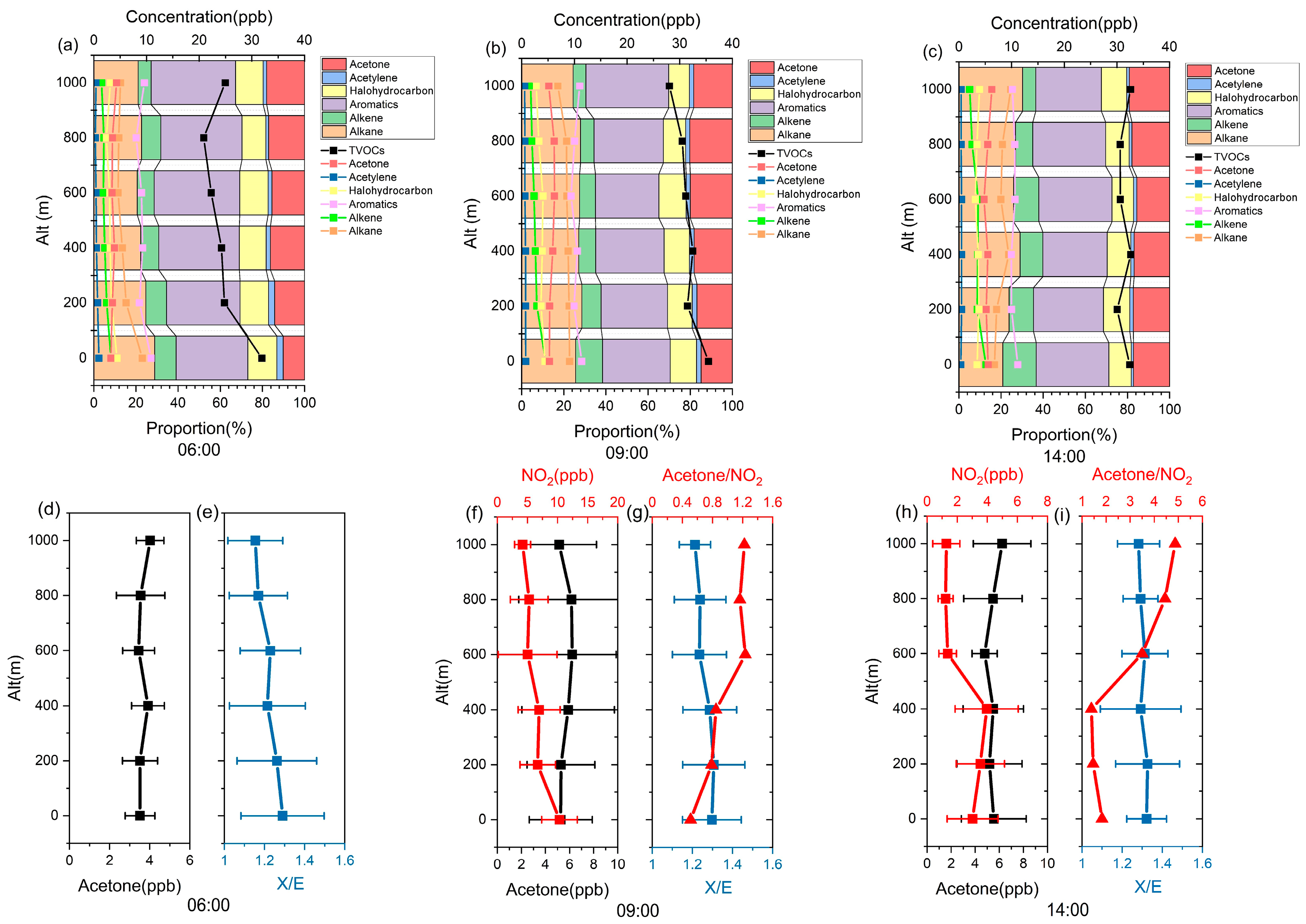
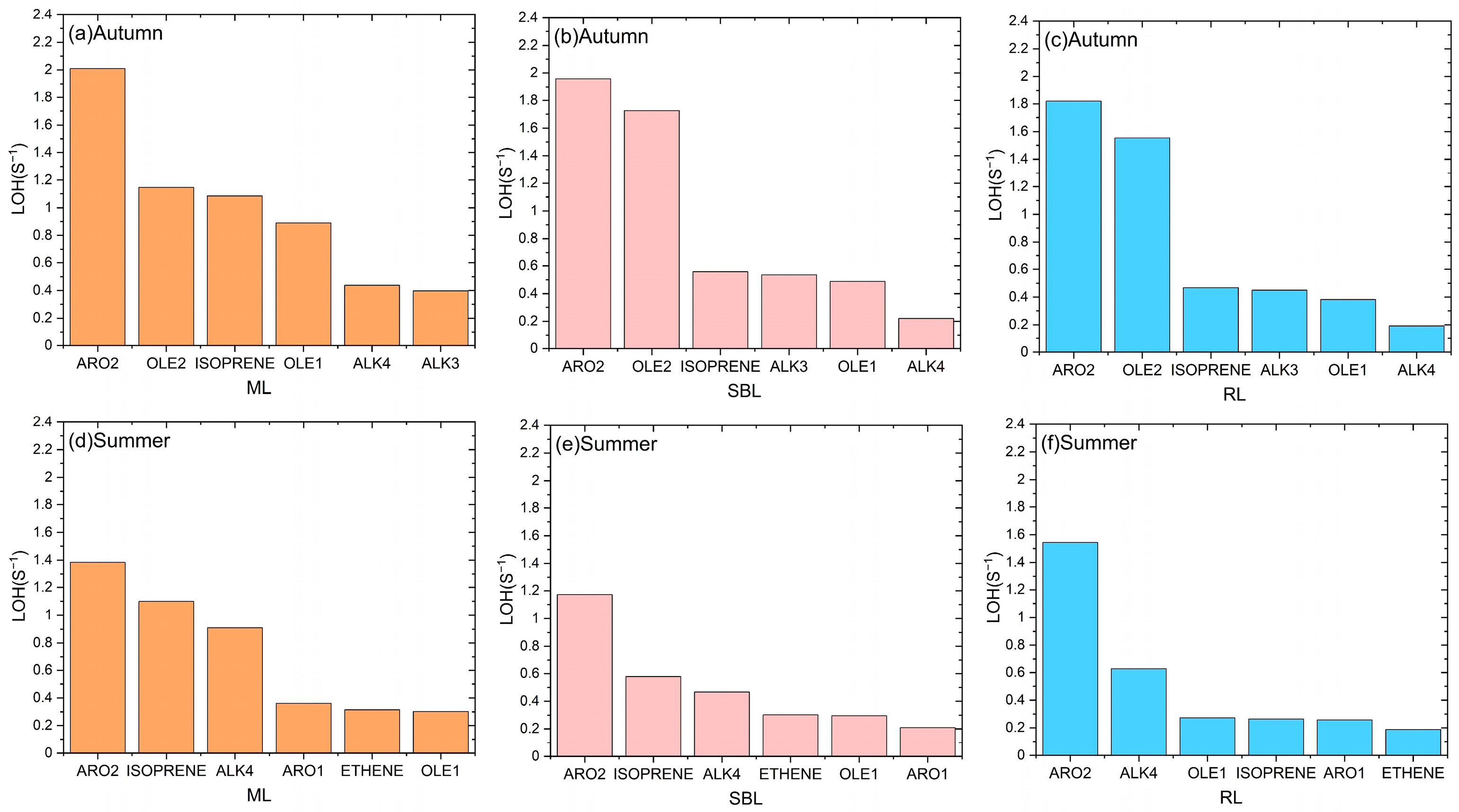
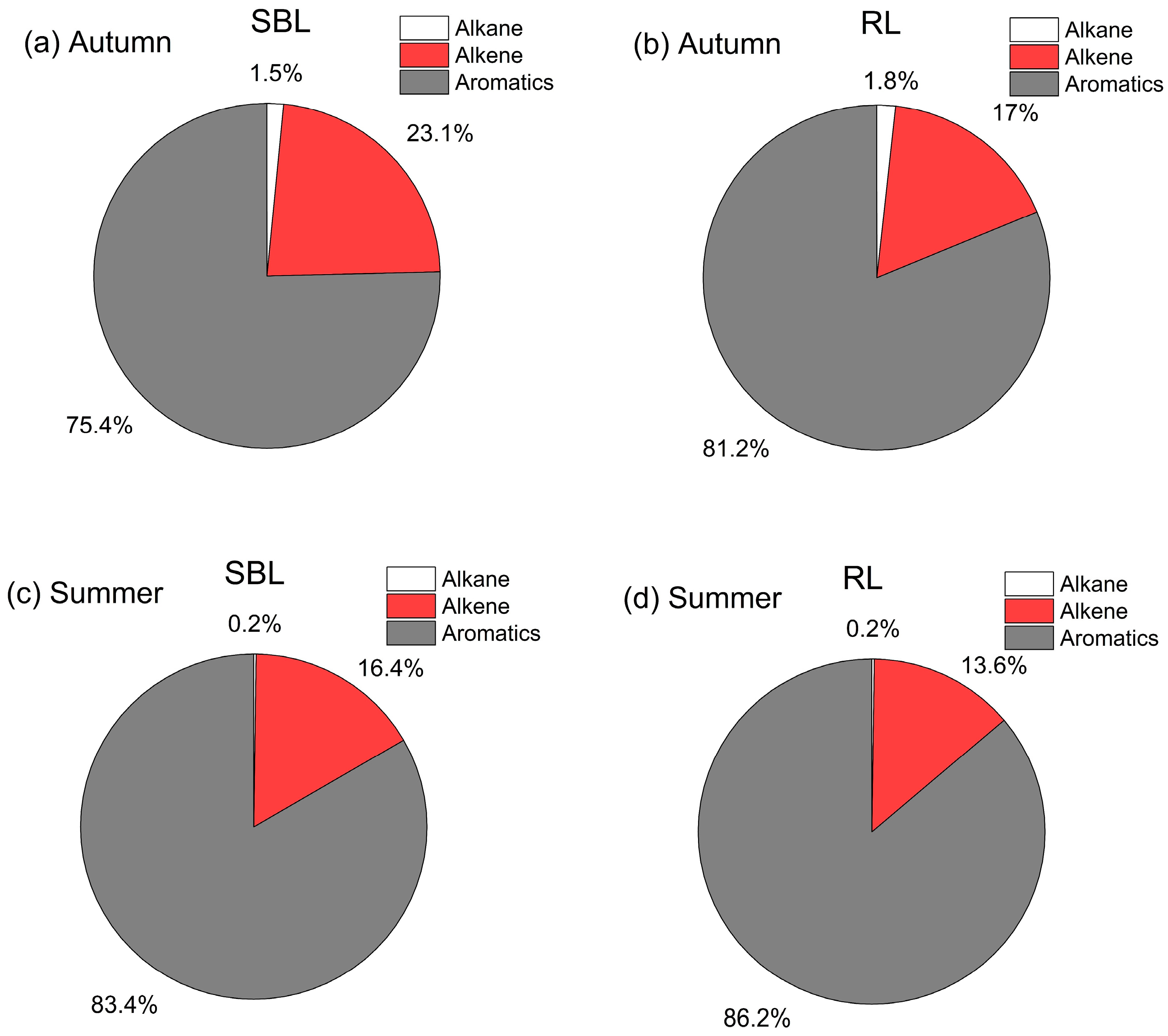
3.3. Differences in VOC Photochemical Reactivity in Different Layers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shao, M.; Yuan, B.; Wang, M.; Zheng, J.Y.; Liu, Y.; Lu, S.H. Sources and Atmospheric Chemical Reactions of Volatile Organic Compounds (VOCs); Science Press: Beijing, China, 2020; Chapter 1; pp. 3–4. ISBN 9787030658760. [Google Scholar]
- Liu, Y.X.; Geng, G.N.; Cheng, J.; Liu, Y.; Xiao, Q.Y.; Liu, L.K.; Shi, Q.R.; Tong, D.; He, K.B.; Zhang, Q. Drivers of Increasing Ozone during the Two Phases of Clean Air Actions in China. Environ. Sci. Technol. 2023, 57, 8954–8964. [Google Scholar] [CrossRef] [PubMed]
- Haagen-Smit, A.J. Chemistry, and physiology of Los Angeles smog. Ind. Eng. Chem. 1952, 44, 1342–1346. [Google Scholar] [CrossRef]
- Guo, H.; Lee, S.; Louie, P.; Ho, K. Characterization of hydrocarbons, halocarbons, and carbonyls in the atmosphere of Hong Kong. Chemosphere 2004, 10, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.Q.; Han, M.; Ran, L.; Han, S.Q.; Zhao, C.S. Characterization and ozone production potential analysis of non-methane organic compounds in the central urban area of Tianjin in summer. Environ. Sci. 2011, 31, 373–380. [Google Scholar]
- Garzón, J.P.; Huertas, J.I.; Magaña, M.; Huertas, M.E.; Cárdenas, B.; Watanabe, T.; Maeda, T.; Wakamatsu, S.; Blanco, S. Volatile organic compounds in the atmosphere of Mexico City. Atmos. Environ. 2015, 119, 415–429. [Google Scholar] [CrossRef]
- Zhang, Y.X.; An, J.L.; Wang, J.X.; Shi, Y.Z.; Liu, J.D.; Liang, J.S. Source apportionment and ozone contribution assessment of volatile organic compounds in an industrial area in Nanjing. Environ. Sci. 2018, 39, 502–510. [Google Scholar]
- Gee, I.L.; Sollars, C.J. Ambient air levels of volatile organic compounds in Latin American and Asian cities. Chemosphere 1998, 36, 2497–2506. [Google Scholar] [CrossRef]
- Lanzerstorfer, C.H.; Puxbaum, H. Volatile hydrocarbons in and around Vienna, Austria. Water Air Soil Pollut. 1990, 51, 345–355. [Google Scholar] [CrossRef]
- Nelson, P.F.; Quigley, S.M. Nonmethane hydrocarbons in the atmosphere of Sydney, Australia. Environ. Sci. Technol. 1982, 16, 650–655. [Google Scholar] [CrossRef]
- National Research Council (NRC). Rethinking the Ozone Problem in Urban and Regional Air Pollution; The National Academies Press: Washington, DC, USA, 1991; pp. 233–234. [Google Scholar]
- Tsujino, Y.; Kuwata, K. Sensitive flame ionization detector for the determination of traces of atmospheric hydrocarbons by capillary gas chromatography. J. Chromatogr. A 1993, 642, 383–388. [Google Scholar] [CrossRef]
- Duan, J.; Tan, J.; Yang, L.; Wu, S.; Hao, J. Concentration, sources, and ozone formation potential of volatile organic compounds (VOCs) during ozone episode in Beijing. Atmos. Res. 2008, 88, 25–35. [Google Scholar] [CrossRef]
- Mo, Z.; Shao, M.; Lu, S.; Niu, H.; Zhou, M.; Sun, J. Characterization of non-methane hydrocarbons and their sources in an industrialized coastal city, Yangtze River Delta, China. Sci. Total Environ. 2017, 593–594, 641. [Google Scholar] [CrossRef]
- Liu, B.; Liang, D.; Yang, J.; Dai, Q.; Bi, X.; Feng, Y.; Yuan, J.; Xiao, Z.; Zhang, Y.; Xu, H. Characterization and source apportionment of volatile organic compounds based on 1-year of observational data in Tianjin, China. Environ. Pollut. 2016, 218, 757–769. [Google Scholar] [CrossRef]
- Atkinson, R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- Sillman, S. The relation between ozone, NOx, and hydrocarbons in urban and polluted rural environments. Atmos. Environ. 1999, 33, 1821–1845. [Google Scholar] [CrossRef]
- Li, K.; Jacob, D.J.; Shen, L.; Lu, X.; De Smedt, I.; Liao, H. Increases in surface ozone pollution in China from 2013 to 2019: Anthropogenic and meteorological influences. Atmos. Chem. Phys. 2020, 20, 11423–11433. [Google Scholar] [CrossRef]
- Liu, Y.F.; Song, M.D.; Liu, X.G.; Zhang, Y.P.; Hui, L.R.; Kong, L.W.; Zhang, Y.Y.; Zhang, C.; Qu, Y.; An, J.L.; et al. Characterization and sources of volatile organic compounds (VOCs) and their related changes during ozone pollution days in 2016 in Beijing, China. Environ. Pollut. 2020, 257, 113599. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.S.; Wang, H.Y.; Zhang, L.; Guo, J.; Li, Z.G.; Wu, K.; Zhu, G.Y.; Hou, D.L.; Su, H.Y.; Sun, Z.B.; et al. Characteristics of volatile organic compounds (VOCs) based on multisite observations in Hebei province in the warm season in 2019. Atmos. Environ. 2021, 256, 118435. [Google Scholar] [CrossRef]
- Hui, L.R.; Liu, X.G.; Tan, Q.W.; Feng, M.; An, J.L.; Qu, Y.; Zhang, Y.H.; Deng, Y.J.; Zhai, R.X.; Wang, Z. VOC characteristics, chemical reactivity and sources in urban Wuhan, central China. Atmos. Environ. 2020, 224, 117340. [Google Scholar] [CrossRef]
- Lin, X.; Zhu, B.; An, J.L.; Yang, H. Potential contribution of secondary organic aerosols and ozone of VOCs in the Northern Suburb of Nanjing. China Environ. Sci. 2015, 35, 976–986. [Google Scholar]
- Calfapietra, C.; Fares, S.; Manes, F.; Morani, A.; Sgrigna, G.; Loreto, F. Role of biogenic volatile organic compounds (BVOC) emitted by urban trees on ozone concentration in cities: A review. Environ. Pollut. 2013, 183, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, L.; An, J.; Huang, L.; Yan, R.; Huang, C.; Wang, H.; Wang, Q.; Wang, M.; Zhang, W. Estimation of biogenic VOC emissions and its impact on ozone formation over the Yangtze River Delta region, China. Atmos. Environ. 2018, 186, 113–128. [Google Scholar] [CrossRef]
- Zhang, H.N.; Zhang, Y.L.; Huang, Z.H.; Acton, W.J.F.; Wang, Z.Y.; Nemitz, E.; Langford, B.; Mullinger, N.; Davison, B.; Shi, Z.B.; et al. Vertical profiles of biogenic volatile organic compounds as observed online at a tower in Beijing. J. Environ. Sci. 2020, 95, 33–42. [Google Scholar] [CrossRef]
- Mo, Z.W.; Huang, S.; Yuan, B.; Pei, C.L.; Song, Q.C.; Qi, J.P.; Wang, M.; Wang, B.L.; Wang, C.; Li, M.; et al. Deriving emission fluxes of volatile organic compounds from tower observation in the Pearl River Delta, China. Sci. Total Environ. 2020, 741, 139763. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Tang, G.Q.; Wang, Y.H.; Mai, R.; Yao, D.; Kang, Y.Y.; Wang, Q.L.; Wang, Y.S. Vertical Evolution of Boundary Layer Volatile Organic Compounds in Summer over the North China Plain and the Differences with Winter. Adv. Atmos. Sci. 2021, 38, 1165–1176. [Google Scholar] [CrossRef]
- Wu, S.; Tang, G.Q.; Wang, Y.H.; Yang, Y.; Yao, D.; Zhao, W.; Gao, W.K.; Sun, J.; Wang, Y.S. Vertically decreased VOC concentration and reactivity in the planetary boundary layer in winter over the North China Plain. Atmos. Res. 2020, 240, 104930. [Google Scholar] [CrossRef]
- Liu, Y.H.; Wang, H.L.; Jing, S.G.; Zhou, M.; Lou, S.R.; Qu, K.; Qiu, W.Y.; Wang, Q.; Li, S.L.; Gao, Y.Q.; et al. Vertical profiles of volatile organic compounds in suburban Shanghai. Adv. Atmos. Sci. 2021, 38, 1177–1187. [Google Scholar] [CrossRef]
- Vo, T.D.H.; Lin, C.; Weng, C.E.; Yuan, C.S.; Lee, C.W.; Hung, C.H.; Bui, X.T.; Lo, K.C.; Lin, J.X. Vertical stratification of volatile organic compounds and their photochemical product formation potential in an industrial urban area. J. Environ. Manag. 2018, 217, 327–336. [Google Scholar] [CrossRef]
- Xue, L.K.; Wang, T.; Simpson, I.J.; Ding, A.J.; Gao, J.; Blake, D.R.; Wang, X.Z.; Wang, W.X.; Lei, H.C.; Jin, D.Z. Vertical distributions of non-methane hydrocarbons and halocarbons in the lower troposphere over northeast China. Atmos. Environ. 2011, 45, 6501–6509. [Google Scholar] [CrossRef]
- Benish, S.E.; He, H.; Ren, X.R.; Roberts, S.J.; Salawitch, R.J.; Li, Z.Q.; Wang, F.; Wang, Y.Y.; Zhang, F.; Shao, M.; et al. Measurement report: Aircraft observations of ozone, nitrogen oxides, and volatile organic compounds over Hebei Province, China. Atmos. Chem. Phys. 2020, 20, 14523–14545. [Google Scholar] [CrossRef]
- Sangiorgi, G.; Ferrero, L.; Perrone, M.; Bolzacchini, E.; Duane, M.; Larsen, B. Vertical distribution of hydrocarbons in the low troposphere below and above the mixing height: Tethered balloon measurements in Milan, Italy. Environ. Pollut. 2011, 159, 3545–3552. [Google Scholar] [CrossRef] [PubMed]
- Wohrnschimmel, H.; Marquez, C.; Mugica, V.; Stahel, W.A.; Staehelin, J.; Cardenas, B.; Blanco, S. Vertical profiles, and receptor modeling of volatile organic compounds over Southeastern Mexico City. Atmos. Environ. 2006, 40, 5125–5136. [Google Scholar] [CrossRef]
- Koßmann, M.; Vogel, H.; Vogel, B.; Vogtlin, R.; Corsmeier, U.; Fiedler, F.; Klemm, O.; Schlager, H. The composition and vertical distribution of volatile organic compounds in southwestern Germany, eastern France, and northern Switzerland during the TRACT campaign in September 1992. Phys. Chem. Earth 1996, 21, 429–433. [Google Scholar] [CrossRef]
- Zhang, K.; Xiu, G.L.; Zhou, L.; Bian, Q.G.; Duan, Y.S.; Fei, D.N.; Wang, D.F.; Fu, Q.Y. Vertical distribution of volatile organic compounds within the lower troposphere in late spring of Shanghai. Atmos. Environ. 2018, 186, 150–157. [Google Scholar] [CrossRef]
- Sillman, S.; Logan, J.A.; Wofsy, S.C. The sensitivity of ozone to nitrogen oxides and hydrocarbons in regional ozone episodes. J. Geophys. Res. Atmos. 1990, 95, 1837–1851. [Google Scholar] [CrossRef]
- Fuglestvedt, J.S.; Berntsen, T.K.; Isaksen, I.S.A.; Mao, H.T.; Liang, X.Z.; Wang, W.C. Climatic forcing of nitrogen oxides through changes in tropospheric ozone and methane; global 3D model studies. Atmos. Environ. 1999, 33, 961–977. [Google Scholar] [CrossRef]
- Huang, X.F.; Zhang, B.; Xia, S.Y.; Han, Y.; Wang, C.; Yu, G.H.; Feng, N. Sources of oxygenated volatile organic compounds (OVOCs) in urban atmospheres in North and South China. Environ. Pollut. 2020, 261, 114152. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Goldstein, A.H.; Keywood, M.D.; Gao, S.; Varutbangkul, V.; Bahreini, R.; Ng, N.L.; Flagan, R.C.; Seinfeld, J.H. Gas-phase products and secondary aerosol yields from the ozonolysis of ten different terpenes. J. Geophys. Res. Atmos. 2006, 111, D7. [Google Scholar] [CrossRef]
- Fan, M.Y.; Zhang, Y.L.; Lin, Y.C.; Li, L.; Ye, F.; Hu, J.; Cao, F. Source apportionments of atmospheric volatile organic compounds in Nanjing, China during high ozone pollution season. Chemosphere 2021, 263, 128025. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zhao, Y.; Zhang, J.; Zhang, L. Variability and sources of ambient volatile organic compounds based on online measurements in a suburban region of Nanjing, Eastern China. Aerosol Air Qual. Res. 2020, 20, 606–619. [Google Scholar] [CrossRef]
- Zhao, Q.; Bi, J.; Liu, Q.; Ling, Z.; Shen, G.; Chen, F.; Ma, Z. Sources of volatile organic compounds and policy implications for regional ozone pollution control in an urban location of Nanjing, East China. Atmos. Chem. Phys. 2020, 20, 3905–3919. [Google Scholar] [CrossRef]
- Tan, P.; Lian, J.; Li, X. Stability of volatile organic compounds in three sampling devices. Anal. Chem. 2006, 34, 187–191. [Google Scholar]
- Hui, L.; Liu, X.; Tan, Q.; Feng, M.; An, J.; Qu, Y.; Zhang, Y.; Cheng, N. VOC characteristics, sources, and contributions to SOA formation during haze events in Wuhan, Central China. Sci. Total Environ. 2019, 650, 2624–2639. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wu, F.; Hu, B.; Tang, G.; Wang, Y. VOC characteristics, emissions, and contributions to SOA formation during hazy episodes. Atmos. Environ. 2016, 141, 56–570. [Google Scholar] [CrossRef]
- Wang, M.; Zeng, L.; Lu, S.; Shao, M.; Liu, X.; Yu, X.; Chen, W.; Yuan, B.; Zhang, Q.; Hu, M.; et al. Development, and validation of a cryogen-free automatic gas chromatograph system (GC-MS/FID) for online measurements of volatile organic compounds. Anal. Methods 2014, 6, 9424–9434. [Google Scholar] [CrossRef]
- Li, C.; Liu, M.; Hu, Y.; Zhou, R.; Huang, N.; Wu, W.; Liu, C. Spatial distribution characteristics of gaseous pollutants and particulate matter inside a city in the heating season of Northeast China. Sustain. Cities Soc. 2020, 61, 102302. [Google Scholar] [CrossRef]
- Senarathna, M.; Priyankara, S.; Jayaratne, R.; Weerasooriya, R.; Morawska, L.; Bowatte, G. Measuring TrafficRelated Air Pollution Using Smart Sensors in Sri Lanka: Before and During a New Traffic Plan. Geogr. Environ. Sustain. 2022, 15, 27–36. [Google Scholar] [CrossRef]
- Sluis, W.W.; Allaart, M.A.; Piters, A.J.; Gast, L.F.L. The development of a nitrogen dioxide sonde. Atmos. Meas. Technol. 2010, 3, 1753–1762. [Google Scholar] [CrossRef]
- HJ 1010-2018; Technical Requirements, and Detection Methods for Continuous Monitoring System of Volatile Organic Compounds in Environmental Air by Gas Chromatography. China Environmental Publishing Group: Beijing, China, 2019.
- Lu, Y.; Zhu, B.; Huang, Y.; Shi, S.S.; Wang, H.L.; An, J.L.; Yu, X.N. Vertical distributions of black carbon aerosols over rural areas of the Yangtze River Delta in winter. Sci. Total Environ. 2019, 661, 1–9. [Google Scholar] [CrossRef]
- Atkinson, R.; Arey, J. Atmospheric degradation of volatile organic compounds. Chem. Rev. 2003, 103, 4605–4638. [Google Scholar] [CrossRef]
- Veefkind, J.P.; Aben, I.; McMullan, K.; Forster, H.; Vries, J.D.; Otter, G.; Claas, J.; Eskes, H.J.; Haan, J.F.D.; Kleipool, Q.; et al. TROPOMI on the ESA Sentinel-5 Precursor: A GMES mission for global observations of the atmospheric composition for climate, air quality and ozone layer applications. Remote Sens. Environ. 2012, 120, 70–83. [Google Scholar] [CrossRef]
- Ljubiša, B.; Remco, B.; Werner, D.; Jonathan, K.-A.; Quintus, K.; Jonatan, L.; Erwin, L.; Antje, L.; Nico, R.; Joost, S.; et al. Algorithm Theoretical Basis Document for the TROPOMI L01b Data Processor, Issue 10.0.0; Koninklijk Nederlands Meteorologisch Instituut (KNMI): De Bilt, The Netherlands, 2022. [Google Scholar]
- Van, G.J.; Boersma, K.F.; Eskes, H.; Sneep, M.; Linden, M.T.; Zara, M.; Veefkind, J.P. S5P TROPOMI NO2 slant column retrieval: Method, stability, uncertainties, and comparisons with OMI. Atmos. Meas. Technol. 2020, 13, 1315–1335. [Google Scholar]
- Su, W.; Liu, C.; Chan, K.L.; Hu, Q.H.; Liu, H.R.; Ji, X.G.; Zhu, Y.Z.; Liu, T.; Zhang, C.X.; Chen, Y.J.; et al. An improved TROPOMI tropospheric HCHO retrieval over China. Atmos. Meas. Technol. 2020, 13, 6271–6292. [Google Scholar] [CrossRef]
- Eskes, H.J.; Eichmann, K.-U. S5P MPC Product Readme Nitrogen Dioxide, Report S5P-MPC-KNMI-PRF-NO2, Version 1.4; ESA: Paris, France, 2019. [Google Scholar]
- Eskes, H.J.; van Geffen, J.H.G.M.; Boersma, K.F.; Eichmann, K.-U.; Apituley, A.; Pedergnana, M.; Sneep, M.; Veefkind, J.P.; Loyola, D. S5P/TROPOMI Level-2 Product User Manual Nitrogen Dioxide, Report S5P-KNMI-L2-0021-MA, Version 3.0.0; ESA: Paris, France, 2019. [Google Scholar]
- Lambert, J.-C.; Keppens, A.; Hubert, D.; Langerock, B.; Eichmann, K.-U.; Kleipool, Q.; Sneep, M.; Verhoelst, T.; Wagner, T.; Weber, M.; et al. Quarterly Validation Report of the Copernicus Sentinel-5 Precursor Operational Data Products, 05: April 2018–November 2019; Koninklijk Nederlands Meteorologisch Instituut (KNMI): De Bilt, The Netherlands, 2019; p. 151. [Google Scholar]
- Stull, R.B. An Introduction to Boundary Layer Meteorology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1988. [Google Scholar]
- Wei, W.; Chen, S.S.; Wang, Y.; Cheng, L.; Wang, X.Q.; Cheng, S.Y. The impacts of VOCs on PM2.5 increasing via their chemical losses’ estimates: A case study in a typical industrial city of China. Atmos. Environ. 2022, 273, 118978. [Google Scholar] [CrossRef]
- Singh, H.B.; O’hara, D.; Herlth, D.; Sachse, W.; Blake, D.R.; Bradshaw, J.D.; Kanakidou, M.; Crutzen, P.J. Acetone in the atmosphere: Distribution, sources, and sinks. J. Geophys. Res. Atmos. 1994, 99, 1805–1819. [Google Scholar] [CrossRef]
- Zhao, S.; Hu, B.; Liu, H.; Du, C.; Xia, X.; Wang, Y. The influence of aerosols on the NO2 photolysis rate in a suburban site in North China. Sci. Total Environ. 2021, 767, 144788. [Google Scholar] [CrossRef] [PubMed]
- Lefer, B.L.; Hall, S.R.; Cinquini, L.; Shetter, R.E.; Barrick, J.D.; Crawford, J.H. Comparison of airborne NO2 photolysis frequency measurements during PEM-Tropics, B. J. Geophys. Res. Atmospheres. 2001, 106, 32645–32656. [Google Scholar] [CrossRef]
- Meng, Z.Y.; Ding, G.A.; Xu, X.B.; Xu, X.D.; Yu, H.Q.; Wang, S.F. Vertical distributions of SO2 and NO2 in the lower atmosphere in Beijing urban areas, China. Sci. Total Environ. 2008, 390, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.R.; Crawford, J.H.; Fried, A.; Walega, J.; Weinheimer, A.; Wisthaler, A.; Müller, M.; Mikoviny, T.; Chen, G.; Shook, M.; et al. new insights into the column CH2O/NO2 ratio as an indicator of near-surface ozone sensitivity. J. Geophys. Res. Atmos. 2017, 122, 8885–8907. [Google Scholar] [CrossRef]
- Jin, X.; Fiore, A.M.; Murray, L.T.; Valin, L.C.; Lamsal, L.N.; Duncan, B.; Folkert Boersma, K.; De Smedt, I.; Abad, G.G.; Chance, K.; et al. Evaluating a space-based indicator of surface ozone-NOx-VOC sensitivity over midlatitude source regions and application to decadal trends. J. Geophys. Res. Atmos. 2017, 122, 10439–10461. [Google Scholar] [CrossRef]
- Chan, K.L.; Wang, Z.; Ding, A.; Heue, K.P.; Shen, Y.; Wang, J.; Zhang, F.; Shi, Y.; Hao, N.; Wenig, M. MAX-DOAS measurements of tropospheric NO2 and HCHO in Nanjing and a comparison to ozone monitoring instrument observations. Atmos. Chem. Phys. 2019, 19, 10051–10071. [Google Scholar] [CrossRef]
- Ren, B.; Xie, P.; Xu, J.; Li, A.; Qin, M.; Hu, R.; Zhang, T.; Fan, G.; Tian, X.; Zhu, W.; et al. Vertical characteristics of NO2 and HCHO, and the ozone formation regimes in Hefei, China. Sci. Total Environ. 2022, 823, 153425. [Google Scholar] [CrossRef] [PubMed]
- Souri, A.H.; Johnson, M.S.; Wolfe, G.M.; Crawford, J.H.; Fried, A.; Wisthaler, A.; Brune, W.H.; Blake, D.R.; Weinheimer, A.J.; Verhoelst, T.; et al. Characterization of errors in satellite-based HCHO/NO2 tropospheric column ratios with respect to chemistry, column-to-PBL translation, spatial representation, and retrieval uncertainties. Atmos. Chem. Phys. 2023, 23, 1963–1986. [Google Scholar] [CrossRef]
- Wang, Y.; Dörner, S.; Donner, S.; Böhnke, S.; De Smedt, I.; Dickerson, R.R.; Dong, Z.; He, H.; Li, Z.; Li, Z.; et al. Vertical profiles of NO2, SO2, HONO, HCHO, CHOCHO and aerosols derived from MAX-DOAS measurements at a rural site in the central western North China Plain and their relation to emission sources and effects of regional transport. Atmos. Chem. Phys. 2019, 19, 5417–5449. [Google Scholar] [CrossRef]
- Kovacs, T.A.; Brune, W.H. Total OH loss rate measurement. J. Atmos. Chem. 2001, 39, 105–122. [Google Scholar] [CrossRef]
- Geyer, A.; Platt, U. Temperature dependence of the NO3 loss frequency: A new indicator for the contribution of NO3 to the oxidation of monoterpenes and NOx removal in the atmosphere. J. Geophys. Res. Atmos. 2002, 107, ACL-8. [Google Scholar] [CrossRef]
- Carter, W.P.L. Development of the SAPRC-07 chemical mechanism. Atmos. Environ. 2010, 44, 5324–5335. [Google Scholar] [CrossRef]
- Bonsang, B.; Martin, D.; Lambert, G.; Kanakidou, M.; Le Roulley, J.C.; Sennequier, G. Vertical distribution of nonmethane hydrocarbons in the remote marine boundary layer. J. Geophys. Res. 1991, 96, 7313–7324. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y.; Wu, F.; Tang, G.; Wang, L.; Wang, Y.; Yang, Y. Vertical characteristics of VOCs in the lower troposphere over the North China Plain during pollution periods. Environ. Pollut. 2018, 236, 907–915. [Google Scholar] [CrossRef]



| Averaged | Standard Deviation | Maximum | Minimum | |||||
|---|---|---|---|---|---|---|---|---|
| Autumn | Summer | Autumn | Summer | Autumn | Summer | Autumn | Summer | |
| Temperature (°C) | 15.2 | 33.5 | 3.8 | 4.0 | 24.0 | 41.2 | 4.0 | 26.0 |
| Wind speed (m/s) | 2.2 | 2.8 | 1.3 | 1.7 | 7.0 | 8.0 | 0.3 | 0.2 |
| NO2 (ppb) | 30.9 | 8.4 | 13.7 | 3.9 | 45.3 | 18.7 | 4.9 | 1.3 |
| TVOCs (ppb) | 61.0 | 33.9 | 20.5 | 14.6 | 132.1 | 77.7 | 33.4 | 17.0 |
| Autumn (2020) | ||||||
| Alkane | Alkene | Aromatics | Acetylene | Acetone | TVOCs | |
| ML | 40.3 ± 18.0 (64.9%) | 3.1 ± 2.4 (5.0%) | 5.8 ± 2.7 (9.3%) | 1.3 ± 0.8 (2.1%) | 5.5 ± 1.1 (8.8%) | 62.1 ± 21.8 |
| SBL | 37.9 ± 14.9 (60.4%) | 2.5 ± 1.7 (4.0%) | 8.1 ± 4.8 (12.9%) | 1.6 ± 1.0 (2.6%) | 5.9 ± 1.8 (9.4%) | 62.7 ± 20.0 |
| RL | 38.3 ± 16.8 (64.0%) | 1.9 ± 1.4 (3.2%) | 6.2 ± 2.4 (10.4%) | 1.3 ± 0.8 (2.2%) | 6.0 ± 1.9 (10.0%) | 59.9 ± 19.4 |
| Summer (2022) | ||||||
| ML | 8.3 ± 5.7 (26.1%) | 3.5 ± 3.1 (11.0%) | 10.5 ± 5.9 (33.0%) | 0.5 ± 0.3 (1.6%) | 5.4 ± 2.5 (17.1%) | 31.9 ± 13.8 |
| SBL | 7.8 ± 3.0 (27.5%) | 2.9 ± 1.5 (10.1%) | 9.9 ± 5.4 (34.7%) | 0.6 ± 0.3 (2.0%) | 3.4 ± 1.1 (11.8%) | 28.6 ± 8.6 |
| RL | 6.9 ± 5.0 (23.9%) | 2.3 ± 1.6 (8.0%) | 10.9 ± 7.3 (37.9%) | 0.5 ± 0.3 (1.6%) | 5.0 ± 3.3 (17.4%) | 28.8 ± 14.1 |
| Autumn (2020) | ||||||||||||
| Dates | 1020 | 1022 | 1023 | 1024 | 1028 | 1029 | 1030 | 1031 | 1102 | 1103 | 1104 | 1105 |
| Tropospheric NO2 vertical column (×1016 molec/cm2) | 0.37 | 0.81 | 0.93 | 2.87 | 2.10 | 1.03 | 0.99 | 1.58 | 0.74 | 1.18 | 1.66 | 2.55 |
| BL NO2 vertical column (×1016 molec/cm2) | 0.21 | 0.34 | 0.37 | 1.64 | 1.44 | 0.55 | 0.56 | 0.89 | 0.37 | 0.52 | 1.06 | 1.49 |
| BL/Tropospheric (%) | 56.76 | 41.98 | 39.78 | 57.14 | 68.57 | 53.40 | 56.57 | 56.33 | 50.00 | 44.07 | 63.86 | 58.43 |
| dates | 1106 | 1108 | 1109 | 1110 | 1111 | 1112 | 1114 | 1115 | Averaged | |||
| Tropospheric NO2 vertical column (×1016 molec/cm2) | 1.31 | 1.38 | 2.12 | 2.22 | 1.83 | 1.78 | 1.09 | 0.89 | ||||
| BL NO2 vertical column (×1016 molec/cm2) | 0.93 | 0.85 | 1.02 | 1.48 | 1.32 | 0.89 | 0.72 | 0.39 | ||||
| BL/Tropospheric (%) | 70.99 | 61.59 | 48.11 | 66.67 | 72.13 | 50.00 | 66.06 | 43.82 | 55.87 | |||
| Summer (2022) | ||||||||||||
| dates | 0808 | 0810 | 0811 | 0812 | 0813 | 0814 | 0815 | 0816 | 0817 | 0818 | 0819 | Averaged |
| Tropospheric NO2 vertical column (×1016 molec/cm2) | 0.32 | 0.45 | 0.37 | 0.24 | 0.45 | 0.51 | 0.46 | 1.02 | 0.55 | 0.80 | 0.45 | |
| BL NO2 vertical column (×1016 molec/cm2) | 0.21 | 0.28 | 0.19 | 0.09 | 0.12 | 0.34 | 0.24 | 0.57 | 0.27 | 0.43 | 0.16 | |
| BL/Tropospheric (%) | 65.63 | 62.22 | 51.35 | 37.50 | 26.67 | 66.67 | 52.17 | 55.88 | 49.09 | 53.75 | 35.56 | 50.59 |
| Autumn (2020) | ||||
| Alkane (s−1) | Alkene (s−1) | Aromatics (s−1) | LOH (s−1) | |
| ML | 2.8 ± 0.6 | 2.6 ± 1.3 | 1.5 ± 0.7 | 6.9 ± 1.3 |
| SBL | 2.6 ± 0.4 | 1.3 ± 0.3 | 2.3 ± 0.4 | 6.3 ± 0.9 |
| RL | 2.3 ± 0.4 | 1.1 ± 0.2 | 2.0 ± 0.5 | 5.5 ± 0.8 |
| Summer (2022) | ||||
| ML | 1.3 ± 0.3 | 1.2 ± 0.4 | 2.5 ± 0.7 | 5.0 ± 1.1 |
| SBL | 1.1 ± 0.2 | 0.9 ± 0.2 | 2.2 ± 0.5 | 4.3 ± 0.6 |
| RL | 1.0 ± 0.1 | 0.7 ± 0.3 | 2.2 ± 0.3 | 4.0 ± 0.7 |
| Autumn (2020) | ||||
| Alkane (s−1) | Alkene (s−1) | Aromatics (s−1) | LNO3 (s−1) | |
| SBL | 3.1 × 10−4 ± 0.6 × 10−4 | 4.6 × 10−3 ± 1.1 × 10−3 | 1.5 × 10−2 ± 0.5 × 10−2 | 2.0 × 10−2 ± 0.5 × 10−2 |
| RL | 2.9 × 10−4 ± 0.9 × 10−4 | 2.8 × 10−3 ± 0.8 × 10−3 | 1.3 × 10−2 ± 0.4 × 10−2 | 1.6 × 10−2 ± 0.3 × 10−2 |
| Summer (2022) | ||||
| SBL | 4.1 × 10−5 ± 0.7 × 10−5 | 3.0 × 10−3 ± 0.4 × 10−3 | 1.5 × 10−2 ± 0.3 × 10−2 | 1.9 × 10−2 ± 0.5 × 10−2 |
| RL | 3.9 × 10−5 ± 0.3 × 10−5 | 2.2 × 10−3 ± 0.3 × 10−3 | 1.4 × 10−2 ± 0.4 × 10−2 | 1.7 × 10−2 ± 0.4 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Zhu, B.; Shi, S.; Jiang, Z.; Hou, X.; An, J.; Xia, L. Vertical Features of Volatile Organic Compounds and Their Potential Photochemical Reactivities in Boundary Layer Revealed by In-Situ Observations and Satellite Retrieval. Remote Sens. 2024, 16, 1403. https://doi.org/10.3390/rs16081403
Yang S, Zhu B, Shi S, Jiang Z, Hou X, An J, Xia L. Vertical Features of Volatile Organic Compounds and Their Potential Photochemical Reactivities in Boundary Layer Revealed by In-Situ Observations and Satellite Retrieval. Remote Sensing. 2024; 16(8):1403. https://doi.org/10.3390/rs16081403
Chicago/Turabian StyleYang, Siqi, Bin Zhu, Shuangshuang Shi, Zhuyi Jiang, Xuewei Hou, Junlin An, and Li Xia. 2024. "Vertical Features of Volatile Organic Compounds and Their Potential Photochemical Reactivities in Boundary Layer Revealed by In-Situ Observations and Satellite Retrieval" Remote Sensing 16, no. 8: 1403. https://doi.org/10.3390/rs16081403






