Maternal Obesity and Excessive Gestational Weight Gain Influence Endocannabinoid Levels in Human Milk Across Breastfeeding: Potential Implications for Offspring Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Study Design and Eligibility Criteria
2.2. Breast Milk Collection and Handling
2.3. Milk Protein, Triglyceride, and Hormone Quantification
2.4. Milk Endocannabinoid Quantification
2.5. Statistical Analysis
3. Results
3.1. Anthropometric and Demographic Profile of the Participants
3.2. Macronutrient and Hormone Profile in Human Milk
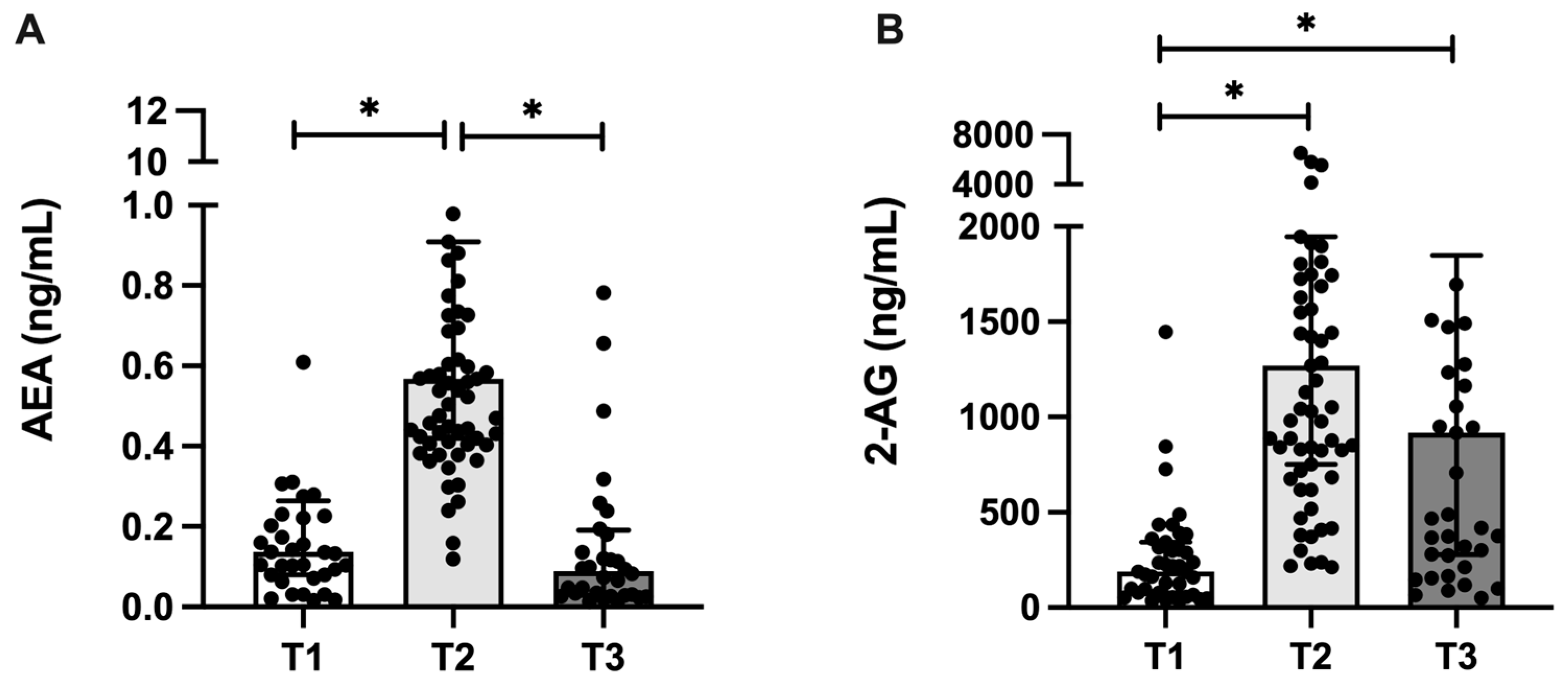
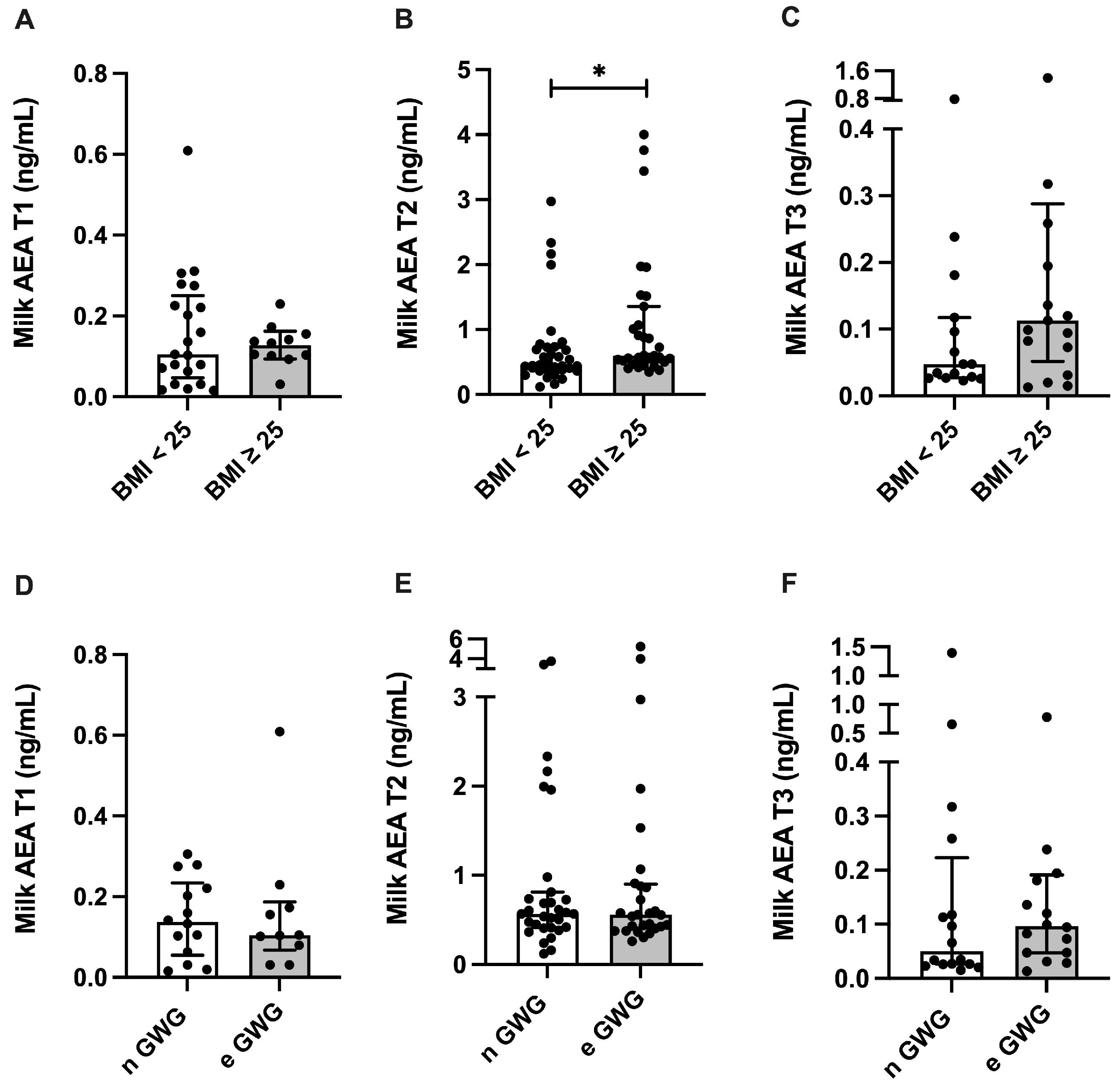
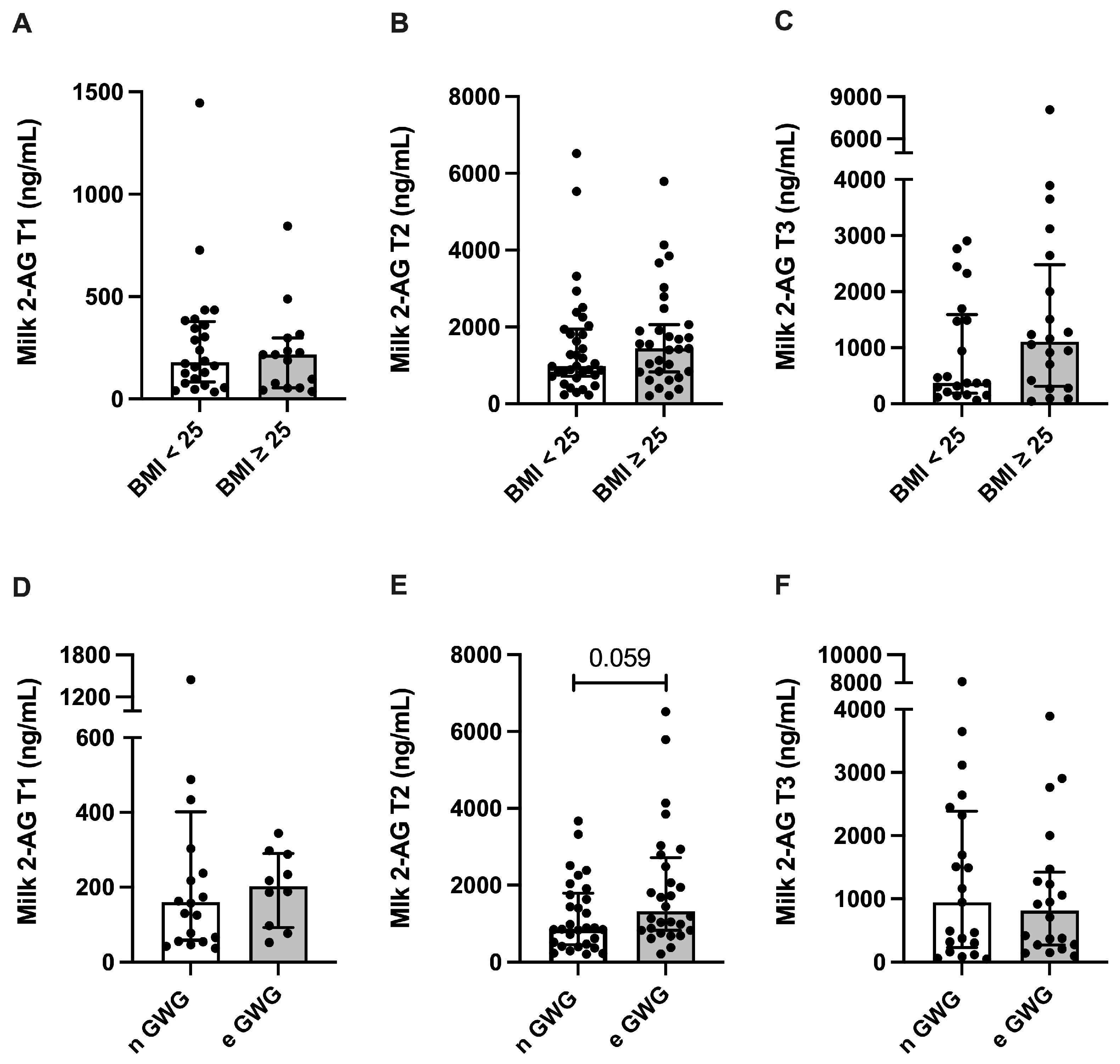
3.3. Endocannabinoid Profile in Human Milk
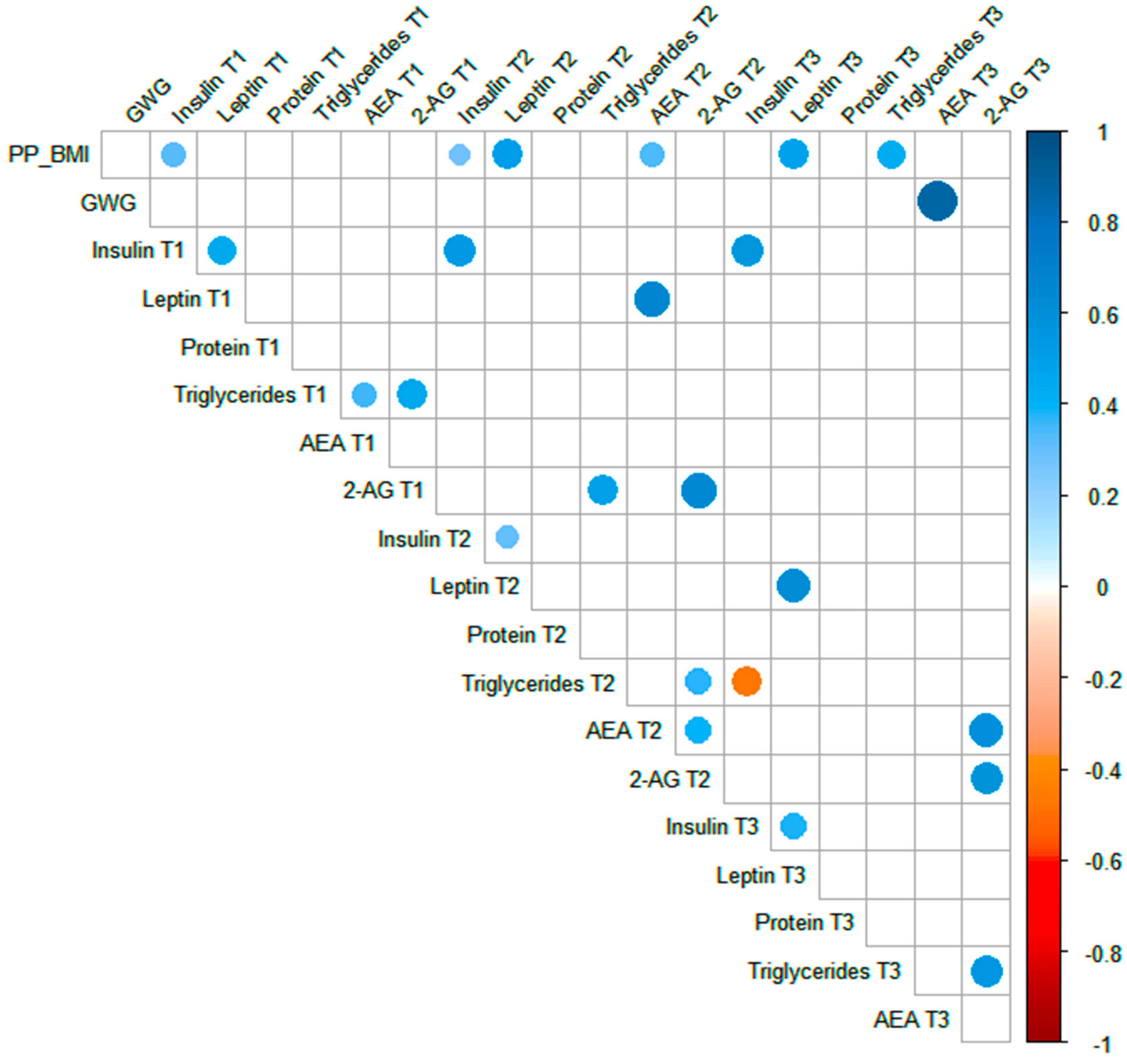
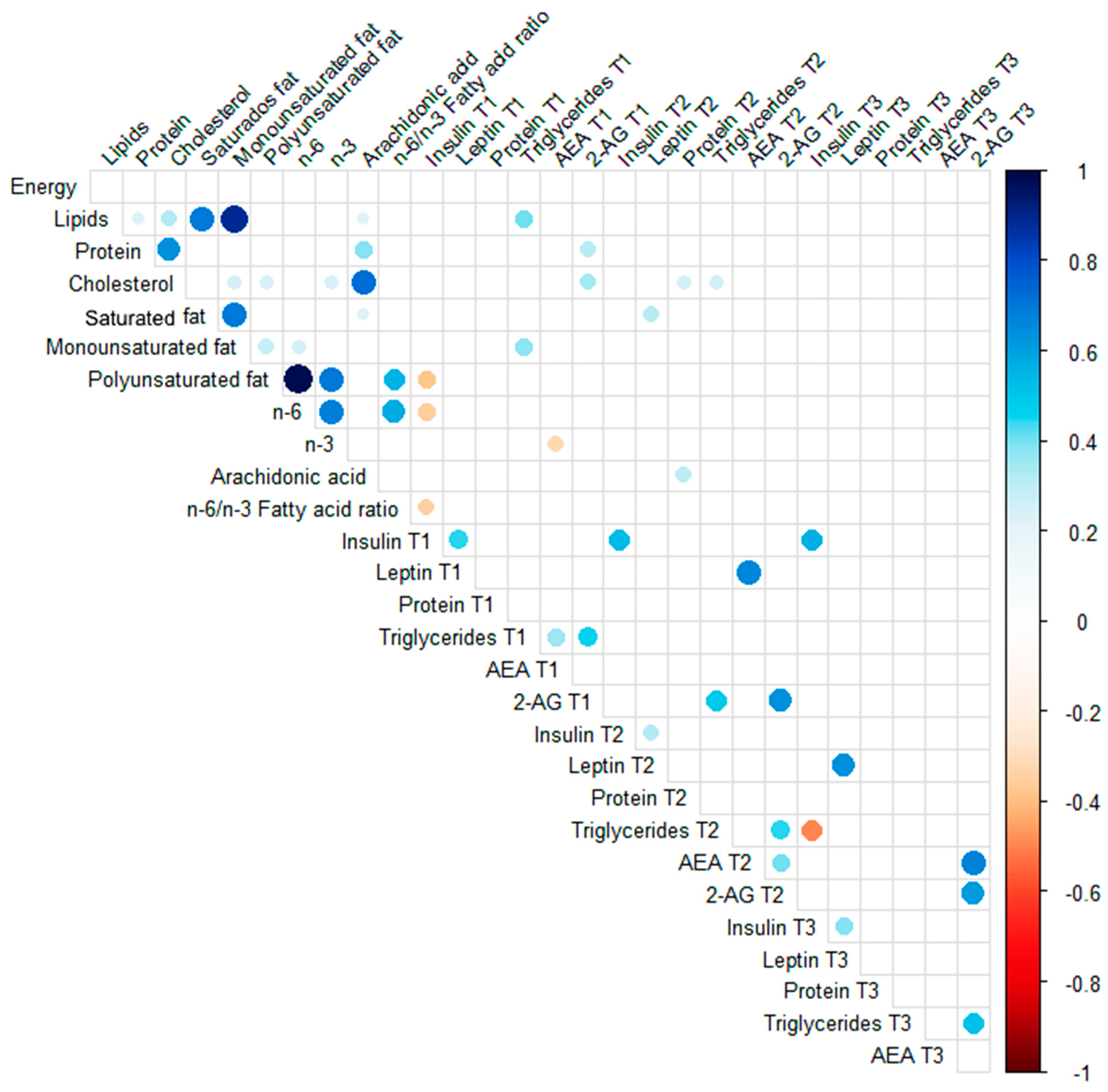
3.4. Spearman Correlations
3.5. Linear Regression Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, G.K.; DiBari, J.N. Marked Disparities in Pre-Pregnancy Obesity and Overweight Prevalence among US Women by Race/Ethnicity, Nativity/Immigrant Status, and Sociodemographic Characteristics, 2012–2014. J. Obes. 2019, 2019, 2419263. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, A.K.; Gregory, E.C.W. Prepregnancy Body Mass Index and Infant Outcomes by Race and Hispanic Origin: United States, 2020. Natl. Vital. Stat. Rep. 2021, 70, 1–8. [Google Scholar]
- Kac, G. Nutritional Status of Children and Mothers: Prevalence of Anthropometric Indicators of Brazilian Under-Five Children and Their Biological Mothers; Federal University of Rio de Janeiro: Rio de Janeiro, Brazil, 2022; Available online: https://enani.nutricao.ufrj.br/ (accessed on 11 March 2025).
- Brenes-Martin, F.; Melero-Jimenez, V.; Lopez-Guerrero, M.A.; Calero-Ruiz, M.M.; Vazquez-Fonseca, L.; Abalos-Martinez, J.; Quintero-Prado, R.; Torrejon, R.; Visiedo, F.; Bugatto, F. First Trimester Evaluation of Maternal Visceral Fat and Its Relationship with Adverse Pregnancy Outcomes. Biology 2023, 12, 144. [Google Scholar] [CrossRef]
- Inzani, I.; Ozanne, S.E. Programming by maternal obesity: A pathway to poor cardiometabolic health in the offspring. Proc. Nutr. Soc. 2022, 81, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Poston, L.; Caleyachetty, R.; Cnattingius, S.; Corvalan, C.; Uauy, R.; Herring, S.; Gillman, M.W. Preconceptional and maternal obesity: Epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016, 4, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.L.L.; Freitas-Costa, N.; da Silva Rosa Freire, S.; Figueiredo, A.C.C.; Padilha, M.; Alves-Santos, N.H.; Kac, G. Association of pre-pregnancy maternal overweight/obesity and dietary intake during pregnancy with the concentrations of persistent organic pollutants in the human milk of women from Rio de Janeiro, Brazil. Environ. Sci. Pollut. Res. Int. 2023, 30, 44999–45014. [Google Scholar] [CrossRef]
- Batalha, M.A.; Ferreira, A.L.L.; Freitas-Costa, N.C.; Figueiredo, A.C.C.; Carrilho, T.R.B.; Shahab-Ferdows, S.; Hampel, D.; Allen, L.H.; Perez-Escamilla, R.; Kac, G. Factors associated with longitudinal changes in B-vitamin and choline concentrations of human milk. Am. J. Clin. Nutr. 2021, 114, 1560–1573. [Google Scholar] [CrossRef]
- Samuel, T.M.; Zhou, Q.; Giuffrida, F.; Munblit, D.; Verhasselt, V.; Thakkar, S.K. Nutritional and Non-nutritional Composition of Human Milk Is Modulated by Maternal, Infant, and Methodological Factors. Front. Nutr. 2020, 7, 576133. [Google Scholar] [CrossRef]
- Bautista, C.J.; Montano, S.; Ramirez, V.; Morales, A.; Nathanielsz, P.W.; Bobadilla, N.A.; Zambrano, E. Changes in milk composition in obese rats consuming a high-fat diet. Br. J. Nutr. 2016, 115, 538–546. [Google Scholar] [CrossRef]
- Castillo, P.; Kuda, O.; Kopecky, J.; Pomar, C.A.; Palou, A.; Palou, M.; Pico, C. Reverting to a Healthy Diet during Lactation Normalizes Maternal Milk Lipid Content of Diet-Induced Obese Rats and Prevents Early Alterations in the Plasma Lipidome of the Offspring. Mol. Nutr. Food Res. 2022, 66, e2200204. [Google Scholar] [CrossRef]
- Franco, J.G.; Fernandes, T.P.; Rocha, C.P.; Calvino, C.; Pazos-Moura, C.C.; Lisboa, P.C.; Moura, E.G.; Trevenzoli, I.H. Maternal high-fat diet induces obesity and adrenal and thyroid dysfunction in male rat offspring at weaning. J. Physiol. 2012, 590, 5503–5518. [Google Scholar] [CrossRef]
- Dias-Rocha, C.P.; Costa, J.C.B.; Oliveira, Y.S.; Fassarella, L.B.; Woyames, J.; Atella, G.C.; Santos, G.R.C.; Pereira, H.M.G.; Pazos-Moura, C.C.; Almeida, M.M.; et al. Maternal high-fat diet decreases milk endocannabinoids with sex-specific changes in the cannabinoid and dopamine signaling and food preference in rat offspring. Front. Endocrinol. 2023, 14, 1087999. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The developmental origins of adult disease. J. Am. Coll. Nutr. 2004, 23, 588S–595S. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, J.; Renault, K.M.; Garcia-Calzon, S.; Perfilyev, A.; Estampador, A.C.; Norgaard, K.; Lind, M.V.; Vaag, A.; Hjort, L.; Michaelsen, K.F.; et al. Lifestyle Intervention in Pregnant Women With Obesity Impacts Cord Blood DNA Methylation Which Associates With Body Composition in the Offspring. Diabetes 2021, 70, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Alba-Linares, J.J.; Perez, R.F.; Tejedor, J.R.; Bastante-Rodriguez, D.; Ponce, F.; Carbonell, N.G.; Zafra, R.G.; Fernandez, A.F.; Fraga, M.F.; Lurbe, E. Maternal obesity and gestational diabetes reprogram the methylome of offspring beyond birth by inducing epigenetic signatures in metabolic and developmental pathways. Cardiovasc. Diabetol. 2023, 22, 44. [Google Scholar] [CrossRef]
- Si, J.; Meir, A.Y.; Hong, X.; Wang, G.; Huang, W.; Pearson, C.; Adams, W.G.; Wang, X.; Liang, L. Maternal pre-pregnancy BMI, offspring epigenome-wide DNA methylation, and childhood obesity: Findings from the Boston Birth Cohort. BMC Med. 2023, 21, 317. [Google Scholar] [CrossRef]
- Akurati, S.; Hanlon, E.C. Beyond the Scale: Exploring the Endocannabinoid System’s Impact on Obesity. Curr. Diabetes Rep. 2024, 25, 6. [Google Scholar] [CrossRef]
- Veilleux, A.; Di Marzo, V.; Silvestri, C. The Expanded Endocannabinoid System/Endocannabinoidome as a Potential Target for Treating Diabetes Mellitus. Curr. Diabetes Rep. 2019, 19, 117. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Piscitelli, F.; Martella, A.; Mazzarella, E.; Allara, M.; Palmieri, V.; Parrella, C.; Capasso, R.; Di Marzo, V. Analysis of the “endocannabinoidome” in peripheral tissues of obese Zucker rats. Prostaglandins Leukot. Essent. Fatty Acids 2013, 89, 127–135. [Google Scholar] [CrossRef]
- Di Marzo, V.; Silvestri, C. Lifestyle and Metabolic Syndrome: Contribution of the Endocannabinoidome. Nutrients 2019, 11, 1956. [Google Scholar] [CrossRef]
- Engeli, S.; Bohnke, J.; Feldpausch, M.; Gorzelniak, K.; Janke, J.; Batkai, S.; Pacher, P.; Harvey-White, J.; Luft, F.C.; Sharma, A.M.; et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes 2005, 54, 2838–2843. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.J.; Genelhu, V.; Pimentel, M.M.; Celoria, B.M.; Mangia, R.F.; Aveta, T.; Silvestri, C.; Di Marzo, V.; Francischetti, E.A. Circulating Endocannabinoids and the Polymorphism 385C>A in Fatty Acid Amide Hydrolase (FAAH) Gene May Identify the Obesity Phenotype Related to Cardiometabolic Risk: A Study Conducted in a Brazilian Population of Complex Interethnic Admixture. PLoS ONE 2015, 10, e0142728. [Google Scholar] [CrossRef]
- Mattelaer, N.; Van der Schueren, B.; Van Oudenhove, L.; Weltens, N.; Vangoitsenhoven, R. The circulating and central endocannabinoid system in obesity and weight loss. Int. J. Obes. 2024, 48, 1363–1382. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.M.; Dias-Rocha, C.P.; Calvino, C.; Trevenzoli, I.H. Lipid endocannabinoids in energy metabolism, stress and developmental programming. Mol. Cell Endocrinol. 2022, 542, 111522. [Google Scholar] [CrossRef] [PubMed]
- Uthayakumaran, K.; Sunil, M.; Ratcliffe, E.M. Evaluating the Role of the Endocannabinoid System in Axon Guidance: A Literature Review. Cannabis Cannabinoid Res. 2024, 9, 12–20. [Google Scholar] [CrossRef]
- Song, C.G.; Kang, X.; Yang, F.; Du, W.Q.; Zhang, J.J.; Liu, L.; Kang, J.J.; Jia, N.; Yue, H.; Fan, L.Y.; et al. Endocannabinoid system in the neurodevelopment of GABAergic interneurons: Implications for neurological and psychiatric disorders. Rev. Neurosci. 2021, 32, 803–831. [Google Scholar] [CrossRef]
- Paraiso-Luna, J.; Aguareles, J.; Martin, R.; Ayo-Martin, A.C.; Simon-Sanchez, S.; Garcia-Rincon, D.; Costas-Insua, C.; Garcia-Taboada, E.; de Salas-Quiroga, A.; Diaz-Alonso, J.; et al. Endocannabinoid signalling in stem cells and cerebral organoids drives differentiation to deep layer projection neurons via CB(1) receptors. Development 2020, 147, dev192161. [Google Scholar] [CrossRef]
- Boquien, C.Y. Human Milk: An Ideal Food for Nutrition of Preterm Newborn. Front. Pediatr. 2018, 6, 295. [Google Scholar] [CrossRef]
- Verduci, E.; Gianni, M.L.; Vizzari, G.; Vizzuso, S.; Cerasani, J.; Mosca, F.; Zuccotti, G.V. The Triad Mother-Breast Milk-Infant as Predictor of Future Health: A Narrative Review. Nutrients 2021, 13, 486. [Google Scholar] [CrossRef]
- Gouveia-Figueira, S.; Nording, M.L. Development and validation of a sensitive UPLC-ESI-MS/MS method for the simultaneous quantification of 15 endocannabinoids and related compounds in milk and other biofluids. Anal. Chem. 2014, 86, 1186–1195. [Google Scholar] [CrossRef]
- Gaitan, A.V.; Wood, J.T.; Zhang, F.; Makriyannis, A.; Lammi-Keefe, C.J. Endocannabinoid Metabolome Characterization of Transitional and Mature Human Milk. Nutrients 2018, 10, 1294. [Google Scholar] [CrossRef]
- Gaitan, A.V.; Wood, J.T.; Solomons, N.W.; Donohue, J.A.; Ji, L.; Liu, Y.; Nikas, S.P.; Zhang, F.; Allen, L.H.; Makriyannis, A.; et al. Endocannabinoid Metabolome Characterization of Milk from Guatemalan Women Living in the Western Highlands. Curr. Dev. Nutr. 2019, 3, nzz018. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gouveia-Figueira, S.; Domellof, M.; Zivkovic, A.M.; Nording, M.L. Oxylipins, endocannabinoids, and related compounds in human milk: Levels and effects of storage conditions. Prostaglandins Other Lipid Mediat. 2016, 122, 28–36. [Google Scholar] [CrossRef]
- Fradet, A.; Castonguay-Paradis, S.; Dugas, C.; Perron, J.; St-Arnaud, G.; Marc, I.; Doyen, A.; Flamand, N.; Dahhani, F.; Di Marzo, V.; et al. The human milk endocannabinoidome and neonatal growth in gestational diabetes. Front. Endocrinol. 2024, 15, 1415630. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Carlson, M.E.; Kuchel, G.A.; Newman, J.W.; Watkins, B.A. Dietary DHA reduces downstream endocannabinoid and inflammatory gene expression and epididymal fat mass while improving aspects of glucose use in muscle in C57BL/6J mice. Int. J. Obes. 2016, 40, 129–137. [Google Scholar] [CrossRef]
- Fisk, H.L.; Childs, C.E.; Miles, E.A.; Ayres, R.; Noakes, P.S.; Paras-Chavez, C.; Kuda, O.; Kopecky, J.; Antoun, E.; Lillycrop, K.A.; et al. Dysregulation of endocannabinoid concentrations in human subcutaneous adipose tissue in obesity and modulation by omega-3 polyunsaturated fatty acids. Clin. Sci. 2021, 135, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Freitas, H.R.; Isaac, A.R.; Malcher-Lopes, R.; Diaz, B.L.; Trevenzoli, I.H.; De Melo Reis, R.A. Polyunsaturated fatty acids and endocannabinoids in health and disease. Nutr. Neurosci. 2017, 21, 695–714. [Google Scholar] [CrossRef]
- Ferreira, A.L.; Alves, R.; Figueiredo, A.; Alves-Santos, N.; Freitas-Costa, N.; Batalha, M.; Yonemitsu, C.; Manivong, N.; Furst, A.; Bode, L.; et al. Human Milk Oligosaccharide Profile Variation Throughout Postpartum in Healthy Women in a Brazilian Cohort. Nutrients 2020, 12, 790. [Google Scholar] [CrossRef]
- Weight Gain During Pregnancy: Reexamining the Guidelines; Rasmussen, K.M., Yaktine, A.L., Eds.; National Academies Press: Washington, DC, USA, 2009. [Google Scholar] [CrossRef]
- Barbieri, P.; Crivellenti, L.C.; Nishimura, R.Y.; Sartorelli, D.S. Validation of a food frequency questionnaire to assess food group intake by pregnant women. J. Hum. Nutr. Diet. 2015, 28 (Suppl. 1), 38–44. [Google Scholar] [CrossRef]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S; discussion 1229S–1231S. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Mi, L.; Li, C.; Zhang, Y.; Bi, R.; Pang, J.; Li, Y. Comparative Proteomic Analysis of Proteins in Breast Milk during Different Lactation Periods. Nutrients 2022, 14, 3648. [Google Scholar] [CrossRef]
- Grapov, D.; Lemay, D.G.; Weber, D.; Phinney, B.S.; Azulay Chertok, I.R.; Gho, D.S.; German, J.B.; Smilowitz, J.T. The human colostrum whey proteome is altered in gestational diabetes mellitus. J. Proteome Res. 2015, 14, 512–520. [Google Scholar] [CrossRef]
- Obermann-Borst, S.A.; Eilers, P.H.; Tobi, E.W.; de Jong, F.H.; Slagboom, P.E.; Heijmans, B.T.; Steegers-Theunissen, R.P. Duration of breastfeeding and gender are associated with methylation of the LEPTIN gene in very young children. Pediatr. Res. 2013, 74, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, L.; Perng, W.; Harman, E.; Das, A.; Pennathur, S.; Gregg, B. Impact of maternal overweight and obesity on milk composition and infant growth. Matern. Child. Nutr. 2020, 16, e12979. [Google Scholar] [CrossRef]
- Bzikowska-Jura, A.; Czerwonogrodzka-Senczyna, A.; Oledzka, G.; Szostak-Wegierek, D.; Weker, H.; Wesolowska, A. Maternal Nutrition and Body Composition During Breastfeeding: Association with Human Milk Composition. Nutrients 2018, 10, 1379. [Google Scholar] [CrossRef] [PubMed]
- Bobinski, R.; Bobinska, J. Fatty acids of human milk—A review. Int. J. Vitam. Nutr. Res. 2022, 92, 280–291. [Google Scholar] [CrossRef]
- Bouret, S.G. Developmental programming of hypothalamic melanocortin circuits. Exp. Mol. Med. 2022, 54, 403–413. [Google Scholar] [CrossRef]
- Lemas, D.J.; Young, B.E.; Baker, P.R., II; Tomczik, A.C.; Soderborg, T.K.; Hernandez, T.L.; de la Houssaye, B.A.; Robertson, C.E.; Rudolph, M.C.; Ir, D.; et al. Alterations in human milk leptin and insulin are associated with early changes in the infant intestinal microbiome. Am. J. Clin. Nutr. 2016, 103, 1291–1300. [Google Scholar] [CrossRef]
- Sinkiewicz-Darol, E.; Adamczyk, I.; Lubiech, K.; Pilarska, G.; Twaruzek, M. Leptin in Human Milk-One of the Key Regulators of Nutritional Programming. Molecules 2022, 27, 3581. [Google Scholar] [CrossRef]
- Logan, C.A.; Koenig, W.; Rothenbacher, D.; Genuneit, J. Determinants of leptin in human breast milk: Results of the Ulm SPATZ Health Study. Int. J. Obes. 2019, 43, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Logan, C.A.; Siziba, L.P.; Koenig, W.; Carr, P.; Brenner, H.; Rothenbacher, D.; Genuneit, J. Leptin in Human Milk and Child Body Mass Index: Results of the Ulm Birth Cohort Studies. Nutrients 2019, 11, 1883. [Google Scholar] [CrossRef]
- Andreas, N.J.; Hyde, M.J.; Herbert, B.R.; Jeffries, S.; Santhakumaran, S.; Mandalia, S.; Holmes, E.; Modi, N. Impact of maternal BMI and sampling strategy on the concentration of leptin, insulin, ghrelin and resistin in breast milk across a single feed: A longitudinal cohort study. BMJ Open 2016, 6, e010778. [Google Scholar] [CrossRef]
- Sims, C.R.; Lipsmeyer, M.E.; Turner, D.E.; Andres, A. Human milk composition differs by maternal BMI in the first 9 months postpartum. Am. J. Clin. Nutr. 2020, 112, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Sadr Dadres, G.; Whitaker, K.M.; Haapala, J.L.; Foster, L.; Smith, K.D.; Teague, A.M.; Jacobs, D.R., Jr.; Kharbanda, E.O.; McGovern, P.M.; Schoenfuss, T.C.; et al. Relationship of Maternal Weight Status Before, During, and After Pregnancy with Breast Milk Hormone Concentrations. Obesity 2019, 27, 621–628. [Google Scholar] [CrossRef]
- Pico, C.; Palou, M.; Pomar, C.A.; Rodriguez, A.M.; Palou, A. Leptin as a key regulator of the adipose organ. Rev. Endocr. Metab. Disord. 2022, 23, 13–30. [Google Scholar] [CrossRef]
- Little, T.J.; Cvijanovic, N.; DiPatrizio, N.V.; Argueta, D.A.; Rayner, C.K.; Feinle-Bisset, C.; Young, R.L. Plasma endocannabinoid levels in lean, overweight, and obese humans: Relationships to intestinal permeability markers, inflammation, and incretin secretion. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E489–E495. [Google Scholar] [CrossRef]
- Azar, S.; Sherf-Dagan, S.; Nemirovski, A.; Webb, M.; Raziel, A.; Keidar, A.; Goitein, D.; Sakran, N.; Shibolet, O.; Tam, J.; et al. Circulating Endocannabinoids Are Reduced Following Bariatric Surgery and Associated with Improved Metabolic Homeostasis in Humans. Obes. Surg. 2019, 29, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Flamand, N.; Pelicaen, R.; Maiter, D.; Thissen, J.P.; Loumaye, A.; Hermans, M.P.; Everard, A.; Delzenne, N.M.; Di Marzo, V.; et al. Linking the Endocannabinoidome with Specific Metabolic Parameters in an Overweight and Insulin-Resistant Population: From Multivariate Exploratory Analysis to Univariate Analysis and Construction of Predictive Models. Cells 2021, 10, 71. [Google Scholar] [CrossRef]
- Datta, P.; Melkus, M.W.; Rewers-Felkins, K.; Patel, D.; Bateman, T.; Baker, T.; Hale, T.W. Human Milk Endocannabinoid Levels as a Function of Obesity and Diurnal Rhythm. Nutrients 2021, 13, 2297. [Google Scholar] [CrossRef]
- Hanlon, E.C.; Leproult, R.; Stuhr, K.L.; Doncheck, E.M.; Hillard, C.J.; Van Cauter, E. Circadian Misalignment of the 24-hour Profile of Endocannabinoid 2-Arachidonoylglycerol (2-AG) in Obese Adults. J. Clin. Endocrinol. Metab. 2020, 105, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Muldoon, P.P.; Chen, J.; Harenza, J.L.; Abdullah, R.A.; Sim-Selley, L.J.; Cravatt, B.F.; Miles, M.F.; Chen, X.; Lichtman, A.H.; Damaj, M.I. Inhibition of monoacylglycerol lipase reduces nicotine withdrawal. Br. J. Pharmacol. 2015, 172, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Smaga, I.; Jastrzebska, J.; Zaniewska, M.; Bystrowska, B.; Gawlinski, D.; Faron-Gorecka, A.; Broniowska, Z.; Miszkiel, J.; Filip, M. Changes in the Brain Endocannabinoid System in Rat Models of Depression. Neurotox. Res. 2017, 31, 421–435. [Google Scholar] [CrossRef]
- Gaitan, A.V.; Wood, J.T.; Liu, Y.; Ji, L.; Nikas, S.P.; Makriyannis, A.; Lammi-Keefe, C.J. Maternal Dietary Fatty Acids and Their Relationship to Derived Endocannabinoids in Human Milk. J. Hum. Lact. 2021, 37, 813–820. [Google Scholar] [CrossRef]
- Bruun, S.; Gouveia-Figueira, S.; Domellof, M.; Husby, S.; Neergaard Jacobsen, L.; Michaelsen, K.F.; Fowler, C.J.; Zachariassen, G. Satiety Factors Oleoylethanolamide, Stearoylethanolamide, and Palmitoylethanolamide in Mother’s Milk Are Strongly Associated with Infant Weight at Four Months of Age-Data from the Odense Child Cohort. Nutrients 2018, 10, 1747. [Google Scholar] [CrossRef] [PubMed]
- Budday, S.; Steinmann, P.; Kuhl, E. Physical biology of human brain development. Front. Cell Neurosci. 2015, 9, 257. [Google Scholar] [CrossRef]
- Fride, E.; Braun, H.; Matan, H.; Steinberg, S.; Reggio, P.H.; Seltzman, H.H. Inhibition of milk ingestion and growth after administration of a neutral cannabinoid CB1 receptor antagonist on the first postnatal day in the mouse. Pediatr. Res. 2007, 62, 533–536. [Google Scholar] [CrossRef]

| Variables | n = 92 |
|---|---|
| Median [IQR] | |
| Maternal age (years) | 26.0 [22.2–31.4] |
| Education (schooling years) | 12.0 [9.00–12.0] |
| Pre-pregnancy BMI (kg/m2) | 24.4 [21.1–28.8] |
| Total GWG (kg) | 11.8 [9.00–14.3] |
| Dietary intake | |
| Total energy intake (kcal) | 2740 [1950–3800] |
| Proteins (g) 1 | 113 [100–128] |
| Carbohydrates (g) 1 | 432 [389–461] |
| Lipids (g) 1 | 84.6 [75.1–93.3] |
| Saturated fatty acid (g) 1 | 34.1 [29.6–39.0] |
| Monounsaturated fatty acid (g) 1 | 28.5 [24.9–31.1] |
| Polyunsaturated fatty acid (g) 1 | 18.2 [15.6–22.0] |
| Omega-6 polyunsaturated fatty acid (g) 1 | 16.2 [14.1–19.7] |
| Omega-3 polyunsaturated fatty acid (g) 1 | 1.49 [1.28–1.72] |
| Arachidonic acid (g) 1 | 0.0936 [0.0702–0.134] |
| n-6/n-3 ratio | 10.6 [9.60–13.0] |
| Gestational age at delivery | 39.4 [38.9–40.9] |
| Delivery mode 2 | n (%) |
| Vaginal | 50 (58.8) |
| Caesarean | 35 (41.2) |
| Parity 3 | |
| Multiparous | 42 (46.2) |
| Primiparous | 49 (53.8) |
| Skin color 4 | |
| White | 11 (12.2) |
| Brown/Mixed color | 56 (62.2) |
| Black | 21 (23.3) |
| Yellow/Asian | 2 (2.2) |
| Pregestational BMI (kg/m2) 5 | |
| Underweight (<18.5) | 2 (2.3) |
| Normal weight (≥18.5 and <25.0) | 44 (50.6) |
| Overweight (≥25.0 and <30.0) | 26 (29.9) |
| Obese (≥30.0) | 14 (16.1) |
| Variables | Median [IQR] (n) | ||
|---|---|---|---|
| T1 | T2 | T3 | |
| Total protein (mg/mL) | 16.3 [15.0–18.1] * (40) | 13.5 [12.5–15.5] (65) | 15.4 [14.6–17.5] (39) |
| Total triglycerides (mg/mL) | 842.4 [773–966] (41) | 964.3 [848–1074] # (64) | 779.4 [674–903] (41) |
| Insulin (ng/mL) | 0.90 [0.55–1.21] (41) | 0.84 [0.70–1.21] (67) | 1.04 [0.87–1.70] (37) |
| Leptin (ng/mL) | 0.45 [0.34–0.72] (31) | 0.65 [0.45–0.75] (67) | 0.62 [0.44–0.81] (41) |
| Variables | Median [IQR] (n) | |
|---|---|---|
| BMI < 25 | BMI ≥ 25 | |
| Total protein (mg/mL) T1 | 16.0 [14.3–18.1] (24) | 17.3 [14.5–19.8] (16) |
| Total protein (mg/mL) T2 | 12.6 [11.7–15.3] (35) | 15.2 [13.0–17.1] (30) |
| Total protein (mg/mL) T3 | 15.1 [13.8–19.1] (20) | 15.5 [13.6–17.3] (19) |
| Total triglycerides (mg/mL) T1 | 912 [786–1113] (25) | 823 [704–1036] (16) |
| Total triglycerides (mg/mL) T2 | 1036 [803–1126] (35) | 855 [788–1071] (29) |
| Total triglycerides (mg/mL) T3 | 693 [605–851] (21) | 830 [767–1008] * (20) |
| Insulin (ng/mL) T1 | 0.58 [0.44–1.17] (25) | 1.14 [0.85–1.49] (16) |
| Insulin (ng/mL) T2 | 0.75 [0.61–1.21] (35) | 1.08 [0.71–1.56] (32) |
| Insulin (ng/mL) T3 | 1.04 [0.79–2.02] (21) | 1.23 [0.88–1.75] (20) |
| Leptin (ng/mL) T1 | 0.45 [0.16–0.77] (22) | 0.46 [0.35–0.89] (16) |
| Leptin (ng/mL) T2 | 0.45 [0.32–0.65] (35) | 0.85 [0.63–1.15] * (32) |
| Leptin (ng/mL) T3 | 0.44 [0.25–0.63] (21) | 0.95 [0.62–1.27] * (20) |
| n GWG | e GWG | |
| Total protein (mg/mL) T1 | 16.4 [14.8–18.4] (28) | 15.6 [14.5–19.8] (12) |
| Total protein (mg/mL) T2 | 12.6 [11.6–15.0] (36) | 15.9 [13.3–18.1] (28) |
| Total protein (ng/mL) T3 | 15.9 [13.7–19.3] (21) | 15.1 [14.4–17.3] (20) |
| Total triglycerides (ng/mL) T1 | 832 [742–1036] (29) | 844 [704–1139] (12) |
| Total triglycerides (mg/mL) T2 | 1014 [803–1084] (36) | 951 [796–1084] (28) |
| Total triglycerides (mg/mL) T3 | 779 [605–1008] (21) | 798 [666–967] (19) |
| Insulin (ng/mL) T1 | 0.73 [0.44–1.49] (21) | 1.01 [0.58–1.22] (20) |
| Insulin (ng/mL) T2 | 0.96 [0.61–1.51] (31) | 0.78 [0.68–1.29] (36) |
| Insulin (ng/mL) T3 | 1.44 [0.81–3.12] (15) | 1.02 [0.87–1.70] (26) |
| Leptin (ng/mL) T1 | 0.50 [0.24–0.67] (18) | 0.40 [0.34–0.93] (20) |
| Leptin (ng/mL) T2 | 0.44 [0.32–0.75] (31) | 0.72 [0.59–1.03] * (36) |
| Leptin (ng/mL) T3 | 0.62 [0.24–1.16] (15) | 0.62 [0.38–0.97] (26) |
| Independent Variables | Unadjusted models | ||
|---|---|---|---|
| AEA | |||
| Categorical Variable (yes/no) | β | CI (95%) | p |
| BMI ≥ 25 kg/m2 | 0.64 | –0.97; 2.26 | 0.432 |
| Gestational Weight Gain 1 | 2.37 | –1.65; 6.38 | 0.239 |
| 2-AG | |||
| Categorical Variable (yes/no) | β | CI (95%) | p |
| BMI ≥ 25 kg/m2 | 174.60 | –398.5; 747.6 | 0.546 |
| Gestational Weight Gain1 | 1366.6 | 362.4; 2370.7 | 0.009 |
| Independent Variables | Adjusted models | ||
| AEA | |||
| Categorical Variable (yes/no) | β | CI (95%) | p |
| BMI ≥ 25 kg/m2 | 1.81 | –0.18; 3.80 | 0.073 |
| Gestational Weight Gain 1 | 3.51 | –1.22; 8.23 | 0.140 |
| 2-AG | |||
| Categorical Variable (yes/no) | β | CI (95%) | p |
| BMI ≥ 25 kg/m2 | 413.93 | –251.9; 1079.8 | 0.219 |
| Gestational Weight Gain 1 | 1629.51 | 466.7; 2792.3 | 0.008 |
| Independent Variables | Unadjusted Models | ||
|---|---|---|---|
| AEA | |||
| Dietary intake | β | CI (95%) | p |
| Total lipids (g) | −0.01 | −0.05; 0.04 | 0.807 |
| Cholesterol (mg) | −0.00 | −0.00; 0.00 | 0.608 |
| Saturated fatty acid (g) | −0.03 | −0.11; 0.05 | 0.429 |
| Monounsaturated fatty acid (g) | −0.01 | −0.15; 0.14 | 0.909 |
| Polyunsaturated fatty acid (g) | −0.02 | −0.14; 0.10 | 0.756 |
| Fatty acid n-6 (g) | −0.02 | −0.15; 0.11 | 0.785 |
| Fatty acid n-3 (g) | −0.33 | −2.31; 1.66 | 0.745 |
| Arachidonic acid (g) | 5.24 | −5.54; 16.03 | 0.336 |
| Ratio n-6/n-3 | −0.01 | −0.25; 0.24 | 0.952 |
| 2-AG | |||
| Dietary intake | β | CI (95%) | p |
| Total lipids (g) | −5.33 | −21.09; 10.43 | 0.503 |
| Cholesterol (mg) | −0.19 | −1.50; 1.11 | 0.770 |
| Saturated fatty acid (g) | −14.81 | −42.38; 12.77 | 0.382 |
| Monounsaturated fatty acid (g) | −22.68 | −74.03; 28.66 | 0.378 |
| Polyunsaturated fatty acid (g) | 8.00 | −35.59; 51.61 | 0.716 |
| Fatty acid n-6 (g) | 13.52 | −32.12; 59.17 | 0.557 |
| Fatty acid n-3 (g) | 114.6 | −598.50; 827.77 | 0.745 |
| Arachidonic acid (g) | 1492.3 | −2393.96; 5378.56 | 0.447 |
| Ratio n-6/n-3 | 60.38 | −26.32–147.08 | 0.170 |
| AEA | |||
| Dietary intake | β | CI (95%) | p |
| Total lipids (g) | 0.01 | −0.05; 7.41 | 0.815 |
| Cholesterol (mg) | 0.00 | −0.00; 0.00 | 0.716 |
| Saturated fatty acid (g) | −0.03 | −0.11; 0.05 | 0.416 |
| Monounsaturated fatty acid (g) | −0.01 | −0.16; 0.13 | 0.850 |
| Polyunsaturated fatty acid (g) | −0.01 | −0.14; 0.12 | 0.886 |
| Fatty acid n-6 (g) | −0.01 | −0.15; 0.13 | 0.861 |
| Fatty acid n-3 (g) | 0.01 | −2.02; 2.18 | 0.944 |
| Arachidonic acid (g) | 4.37 | −7.00; 15.75 | 0.446 |
| Ratio n-6/n-3 | −0.01 | −0.29; 0.24 | 0.847 |
| 2-AG | |||
| Dietary intake | β | CI (95%) | p |
| Total lipids (g) | −2.98 | −18.13; 12.17 | 0.696 |
| Cholesterol (mg) | −0.38 | −1.65; 0.89 | 0.557 |
| Saturated fatty acid (g) | −8.73 | −36.13; 18.67 | 0.528 |
| Monounsaturated fatty acid (g) | −22.63 | −71.68; 26.41 | 0.361 |
| Polyunsaturated fatty acid (g) | −12.63 | −56.26; 30.98 | 0.566 |
| Fatty acid n-6 (g) | −10.59 | −56.76; 35.57 | 6.649 |
| Fatty acid n-3 (g) | −50.32 | −756.26; 655.63 | 0.887 |
| Arachidonic acid (g) | 2039.36 | −1766.42; 5845.14 | 0.290 |
| Ratio n-6/n-3 | 22.26 | −66.14; 110.66 | 0.617 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pontes, T.F.; Reis, G.; Santos, G.R.C.; Pereira, H.M.G.; Kac, G.; Ferreira, A.L.L.; Trevenzoli, I.H. Maternal Obesity and Excessive Gestational Weight Gain Influence Endocannabinoid Levels in Human Milk Across Breastfeeding: Potential Implications for Offspring Development. Nutrients 2025, 17, 1344. https://doi.org/10.3390/nu17081344
Pontes TF, Reis G, Santos GRC, Pereira HMG, Kac G, Ferreira ALL, Trevenzoli IH. Maternal Obesity and Excessive Gestational Weight Gain Influence Endocannabinoid Levels in Human Milk Across Breastfeeding: Potential Implications for Offspring Development. Nutrients. 2025; 17(8):1344. https://doi.org/10.3390/nu17081344
Chicago/Turabian StylePontes, Tatiana F., Gabriel Reis, Gustavo R. C. Santos, Henrique M. G. Pereira, Gilberto Kac, Ana L. L. Ferreira, and Isis H. Trevenzoli. 2025. "Maternal Obesity and Excessive Gestational Weight Gain Influence Endocannabinoid Levels in Human Milk Across Breastfeeding: Potential Implications for Offspring Development" Nutrients 17, no. 8: 1344. https://doi.org/10.3390/nu17081344
APA StylePontes, T. F., Reis, G., Santos, G. R. C., Pereira, H. M. G., Kac, G., Ferreira, A. L. L., & Trevenzoli, I. H. (2025). Maternal Obesity and Excessive Gestational Weight Gain Influence Endocannabinoid Levels in Human Milk Across Breastfeeding: Potential Implications for Offspring Development. Nutrients, 17(8), 1344. https://doi.org/10.3390/nu17081344







