The Level of Preoperative Plasma KRAS Mutations and CEA Predict Survival of Patients Undergoing Surgery for Colorectal Cancer Liver Metastases
Abstract
:1. Introduction
2. Results
2.1. The Presence and the Level of KRAS Mutations in Tissue of Primary Tumor and Liver Metastases
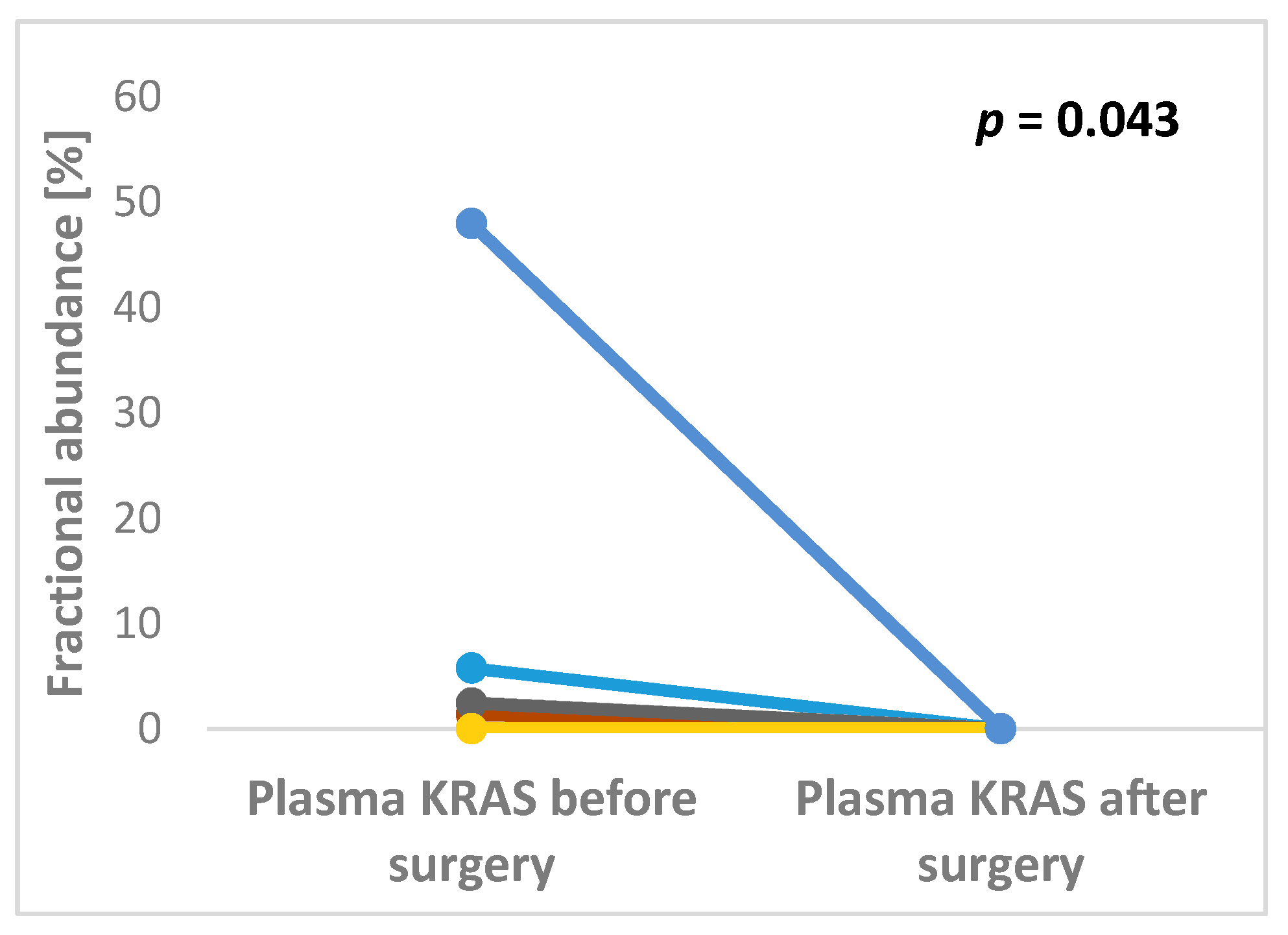
2.2. The Presence and the Level of KRAS Mutations in Plasma
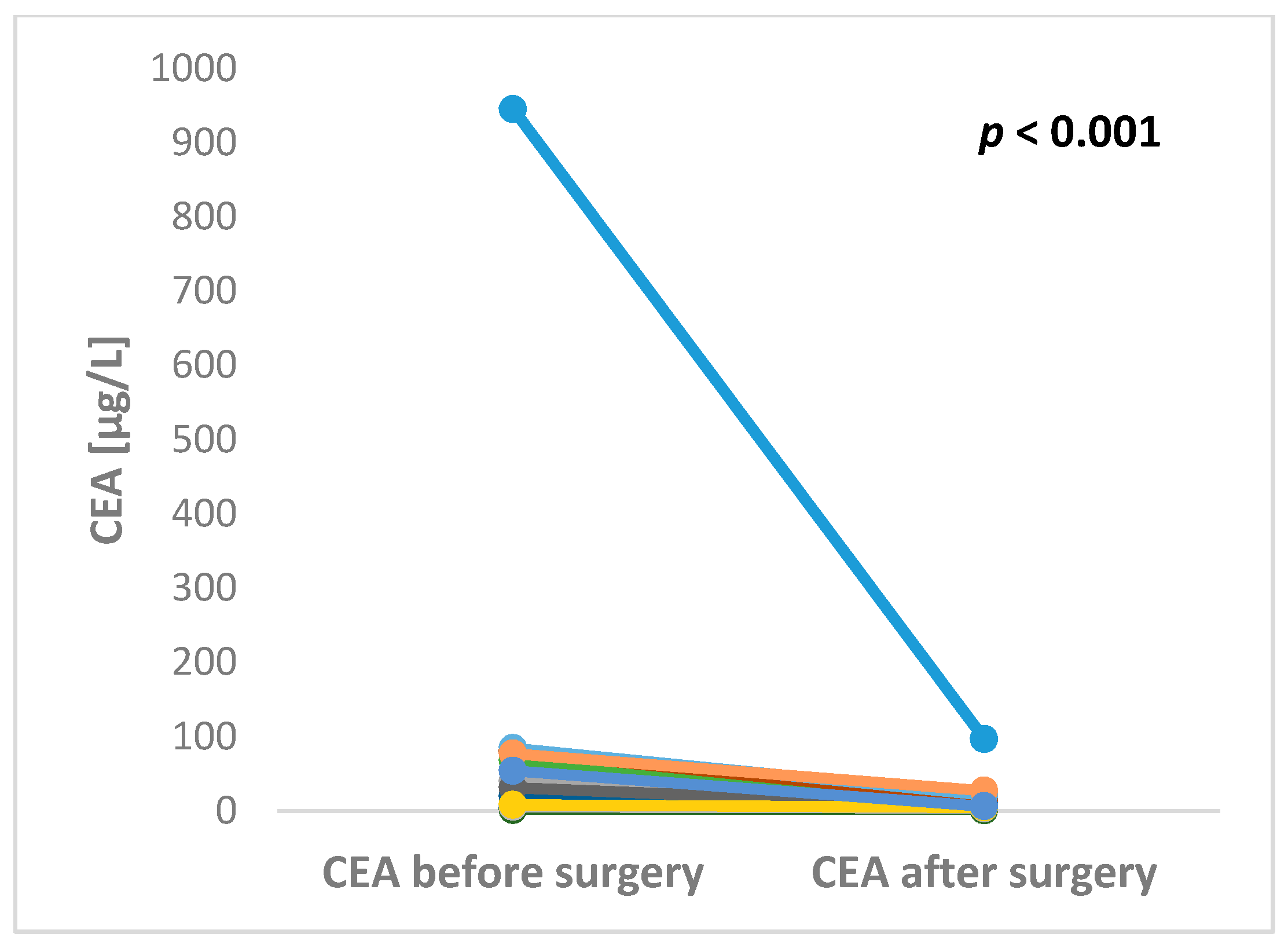
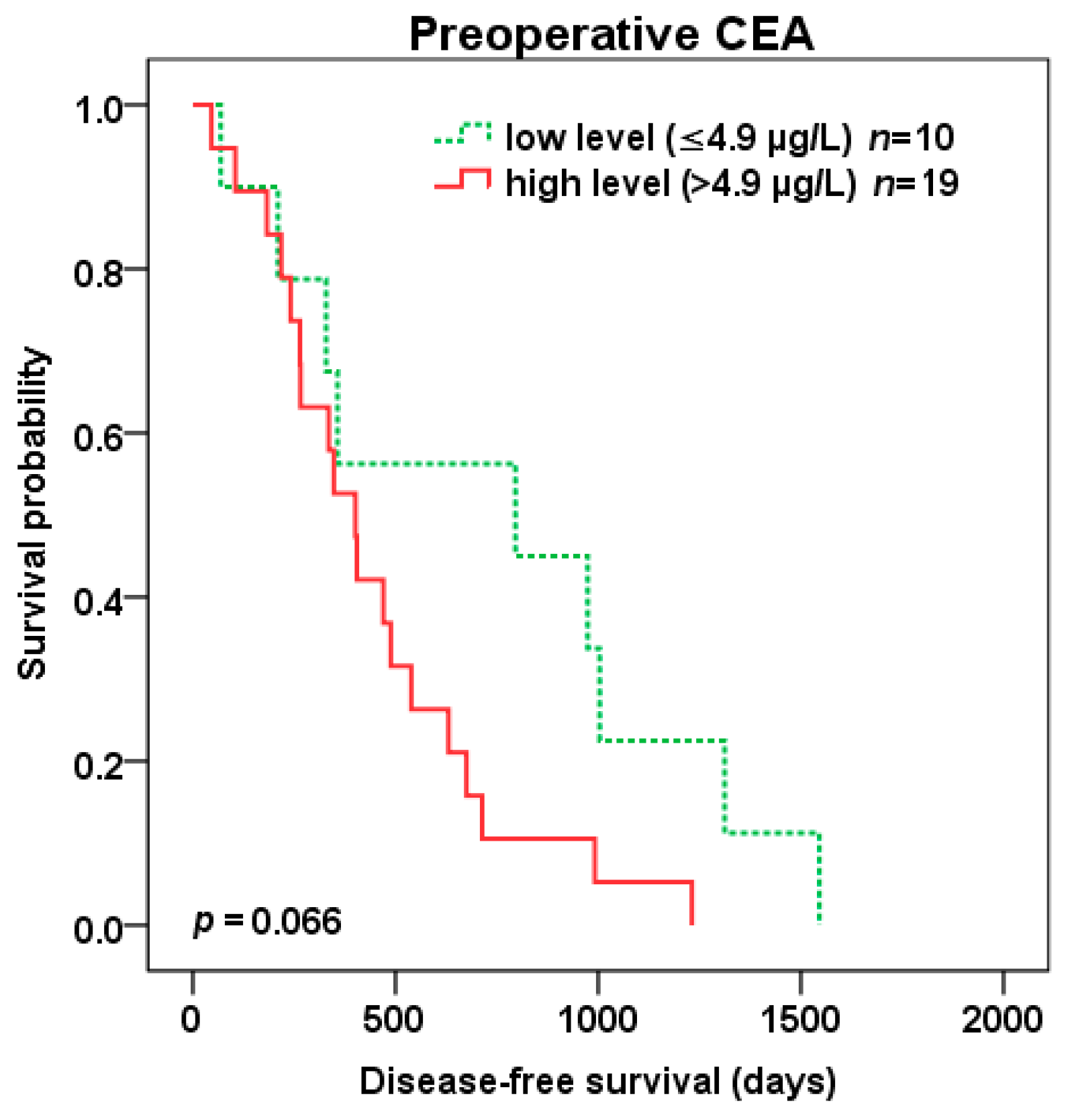
2.3. The Preoperative and Postoperative Serum Level of CEA in Patients Undergoing Liver Surgery
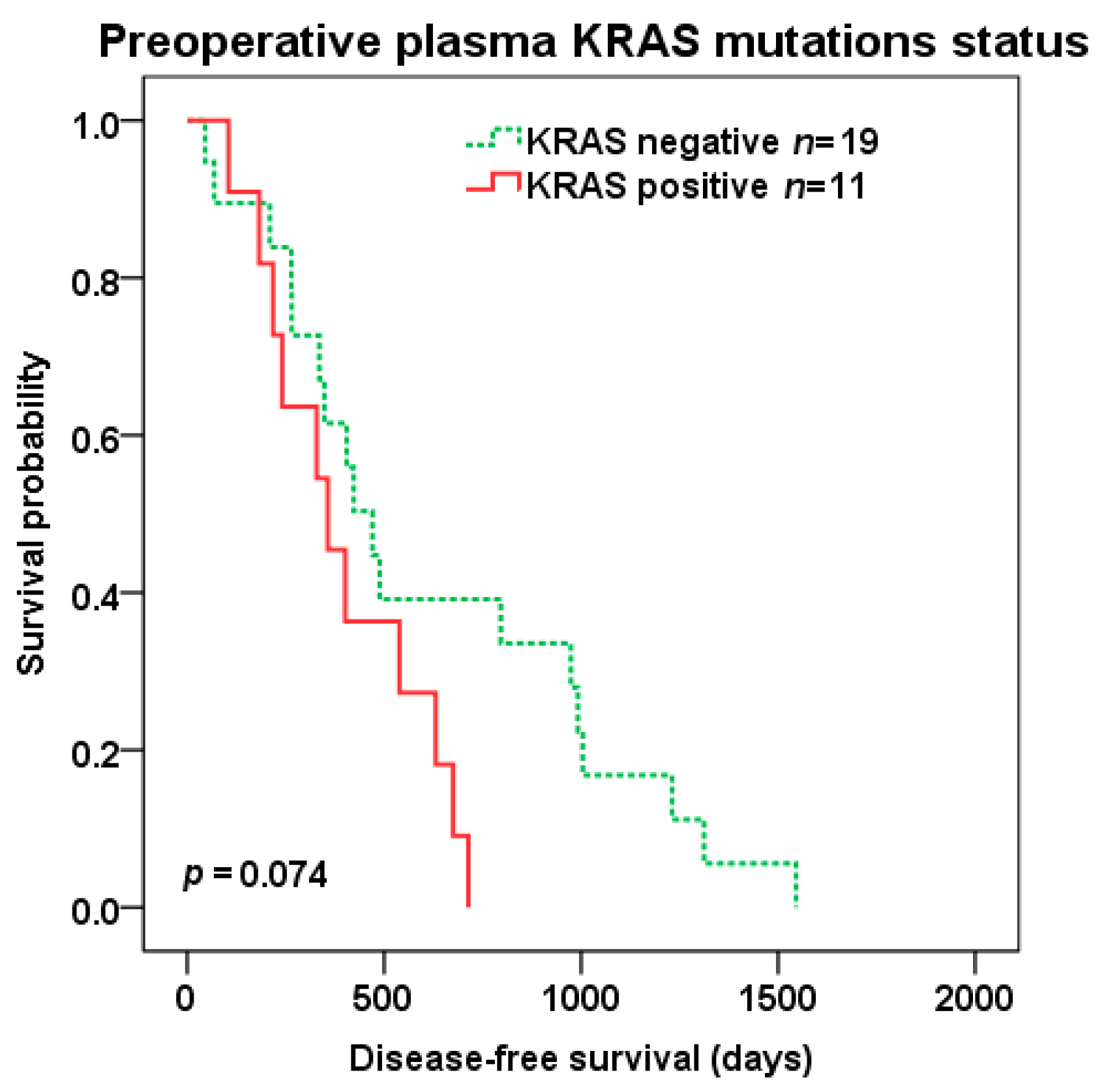
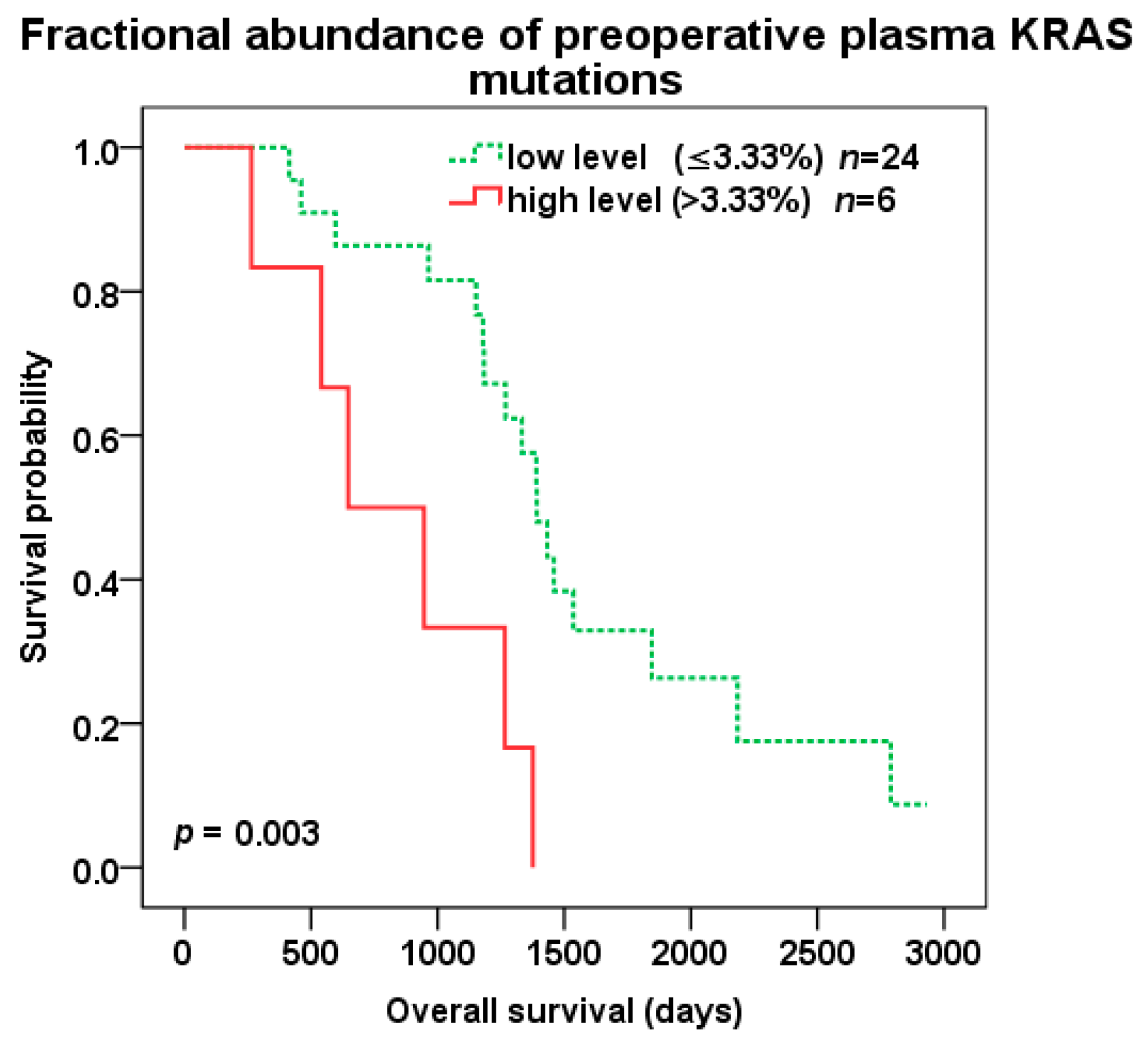
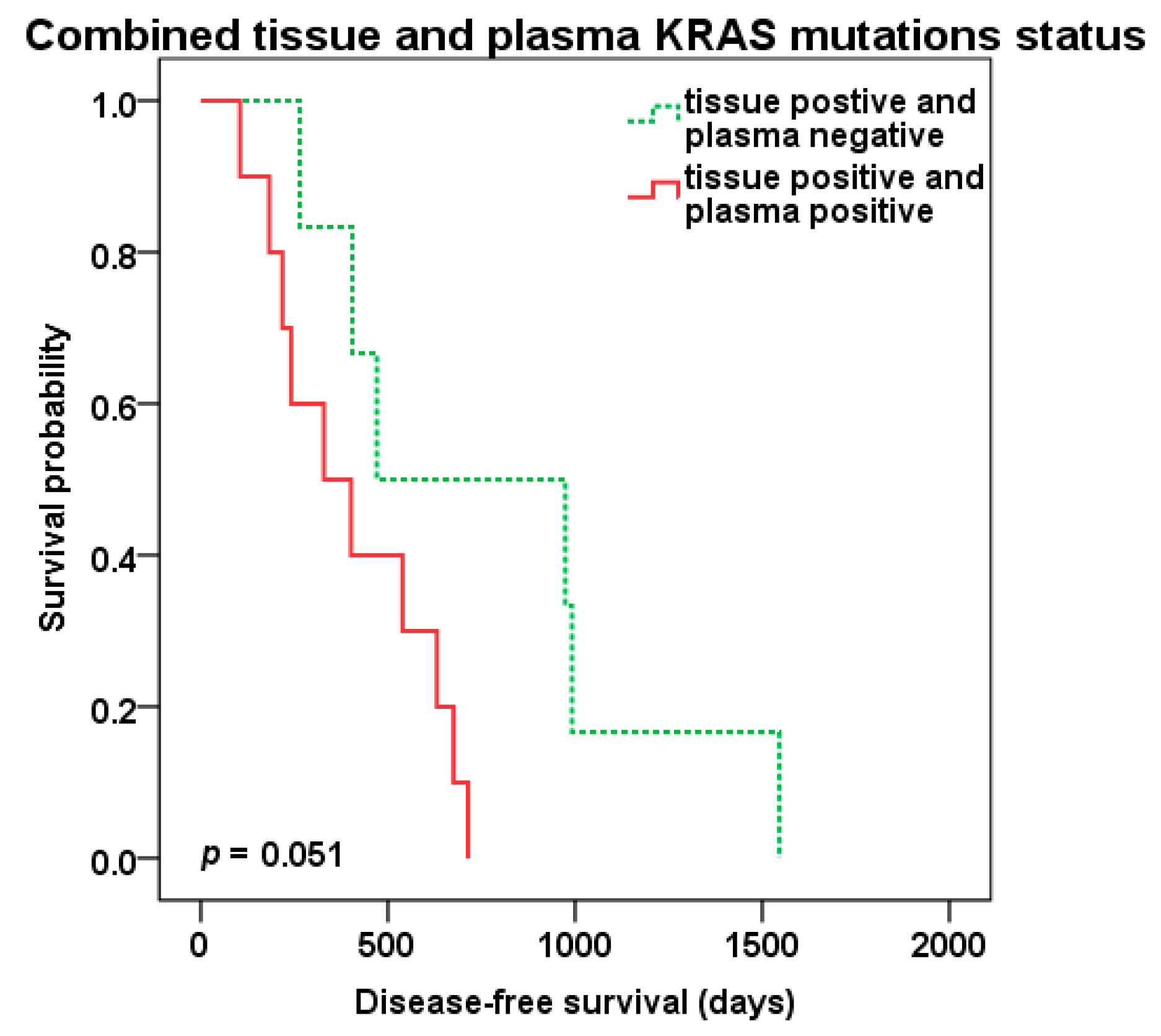
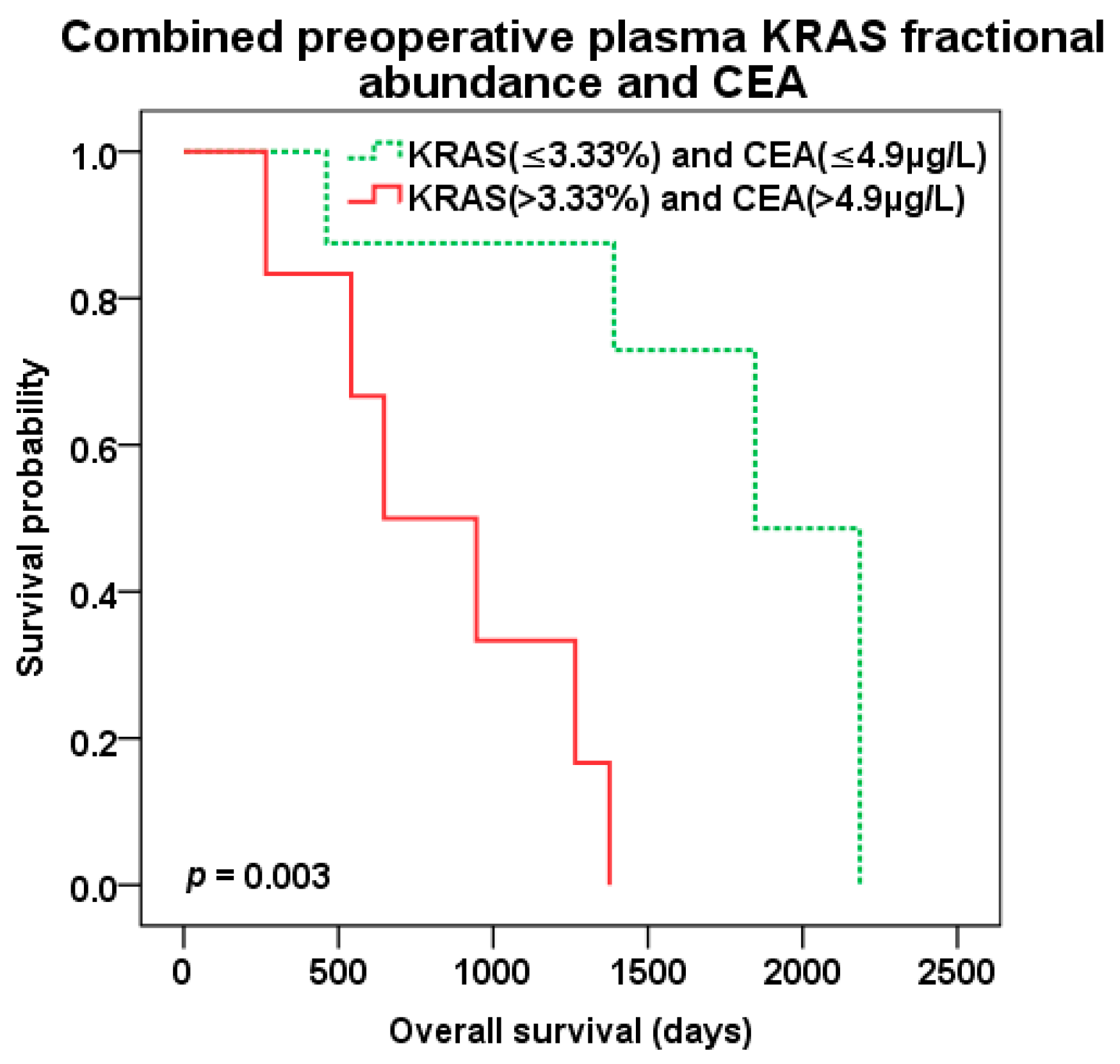
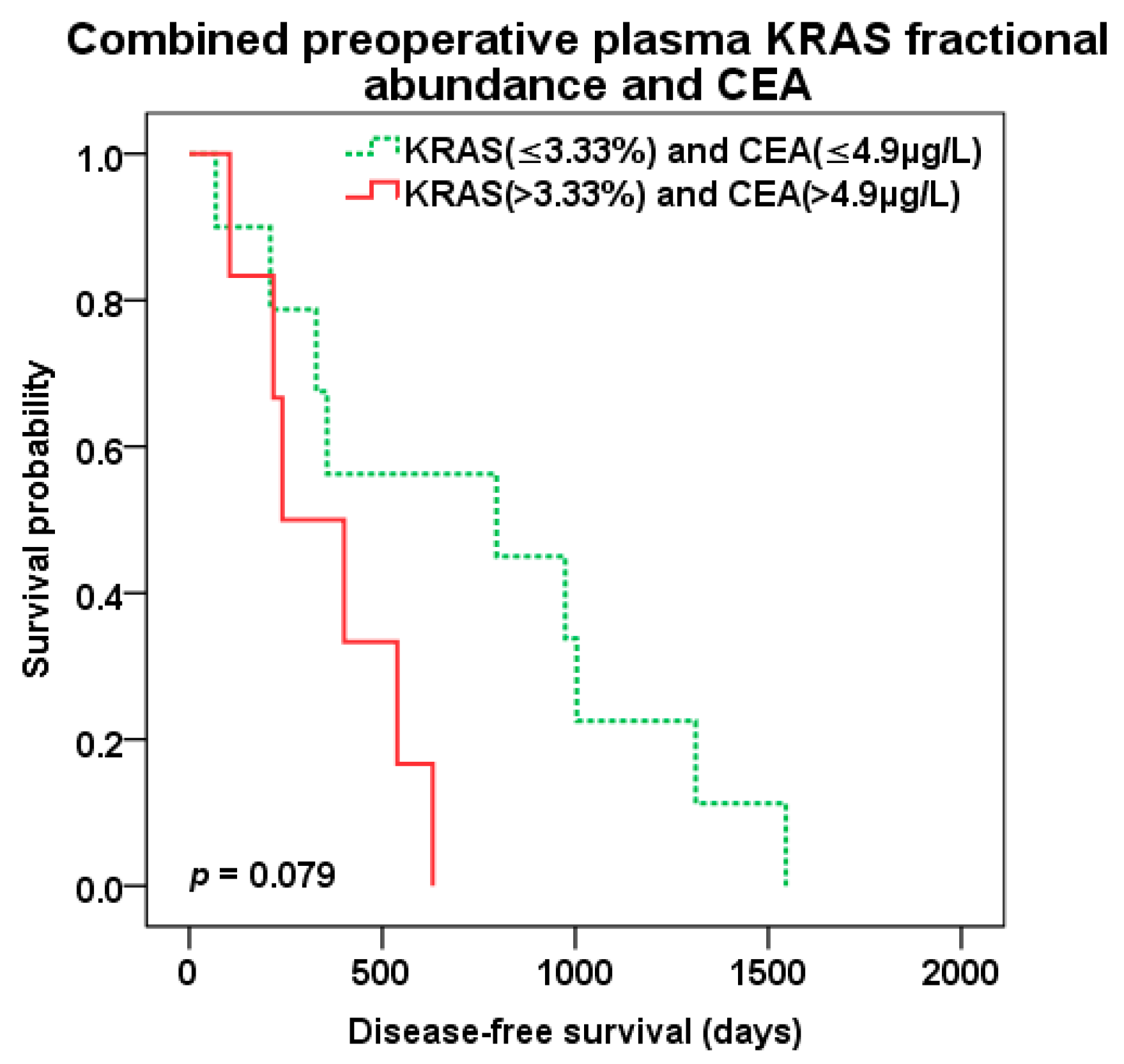
2.4. Survival Analysis
3. Discussion
4. Materials and Methods
4.1. Patients and Samples
4.2. Blood Samples
4.3. DNA Isolation
4.4. ddPCR Assay
4.5. Quantitative Measurement of CEA
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 95% CI | 95% Confidence interval |
| CEA | Carcinoembryonic antigen |
| CLM | Colorectal cancer liver metastases |
| CRC | Colorectal cancer |
| CTC | Circulating tumor cells |
| cfDNA | Circulating cell-free deoxyribonucleic acid |
| ctDNA | Circulating cell-free tumor deoxyribonucleic acid |
| ddPCR | Droplet digital polymerase chain reaction |
| DFS | Disease-free survival |
| DMFS | Distant metastasis free survival |
| DNA | Deoxyribonucleic acid |
| EGFR | Epidermal growth factor receptor |
| FFPE | Formalin-fixed, paraffin-embedded |
| FPR | False positive rate |
| HR | Hazard ratio |
| IUCC | International union against cancer |
| KRAS | Kirsten rat sarcoma viral oncogene homolog |
| mAb | Monoclonal antibody |
| miRNA | Micro-ribonucleic acid |
| NRAS | Neuroblastoma RAS viral oncogene homolog |
| OS | Overall survival |
| PCR | Polymerase chain reaction |
| pFA | Plasma KRAS fractional abundance |
| pKRAS- | Plasma KRAS negativity |
| pKRAS+ | Plasma KRAS positivity |
| RECIST | Response evaluation criteria in solid tumors |
| RFA | Radiofrequency ablation |
| tFA | Tissue KRAS fractional abundance |
| tKRAS+ | Tissue KRAS positivity |
| TNM | Tumor, node, metastases |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engstrand, J.; Nilsson, H.; Strömberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases—A population-based study on incidence, management and survival. BMC Cancer 2018, 18, 78. [Google Scholar] [CrossRef] [PubMed]
- Golubnitschaja, O.; Polivka, J.; Yeghiazaryan, K.; Berliner, L. Liquid biopsy and multiparametric analysis in management of liver malignancies: New concepts of the patient stratification and prognostic approach. EPMA J. 2018, 9, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Kow, A.W.C. Hepatic metastasis from colorectal cancer. J. Gastrointest. Oncol. 2019, 10, 1274–1298. [Google Scholar] [CrossRef] [PubMed]
- Rosty, C.; Young, J.P.; Walsh, M.D.; Clendenning, M.; Walters, R.J.; Pearson, S.; Pavluk, E.; Nagler, B.; Pakenas, D.; Jass, J.R.; et al. Colorectal carcinomas with KRAS mutation are associated with distinctive morphological and molecular features. Mod. Pathol. 2013, 26, 825–834. [Google Scholar] [CrossRef] [Green Version]
- Normanno, N.; Tejpar, S.; Morgillo, F.; De Luca, A.; Van Cutsem, E.; Ciardiello, F. Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat. Rev. Clin. Oncol. 2009, 6, 519–527. [Google Scholar] [CrossRef]
- Malumbres, M.; Barbacid, M. RAS oncogenes: The first 30 years. Nat. Rev. Cancer 2003, 3, 459–465. [Google Scholar] [CrossRef]
- Vasioukhin, V.; Anker, P.; Maurice, P.; Lyautey, J.; Lederrey, C.; Stroun, M. Point mutations of the N-ras gene in the blood plasma DNA of patients with myelodysplastic syndrome or acute myelogenous leukaemia. Br. J. Haematol. 1994, 86, 774–779. [Google Scholar] [CrossRef]
- Sorenson, G.D. Detection of mutated KRAS2 sequences as tumor markers in plasma/serum of patients with gastrointestinal cancer. Clin. Cancer Res. 2000, 6, 2129–2137. [Google Scholar] [CrossRef]
- Sorenson, G.D.; Pribish, D.M.; Valone, F.H.; Memoli, V.A.; Bzik, D.J.; Yao, S.L. Soluble normal and mutated DNA sequences from single-copy genes in human blood. Cancer Epidemiol. Biomark. Prev. 1994, 3, 67–71. [Google Scholar]
- Karapetis, C.S.; Khambata-Ford, S.; Jonker, D.J.; O’Callaghan, C.J.; Tu, D.; Tebbutt, N.C.; Simes, R.J.; Chalchal, H.; Shapiro, J.D.; Robitaille, S.; et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N. Engl. J. Med. 2008, 359, 1757–1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douillard, J.-Y.; Oliner, K.S.; Siena, S.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N. Engl. J. Med. 2013, 369, 1023–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peeters, M.; Oliner, K.S.; Price, T.J.; Cervantes, A.; Sobrero, A.F.; Ducreux, M.; Hotko, Y.; André, T.; Chan, E.; Lordick, F.; et al. Analysis of KRAS/NRAS Mutations in a Phase III Study of Panitumumab with FOLFIRI Compared with FOLFIRI Alone as Second-line Treatment for Metastatic Colorectal Cancer. Clin. Cancer Res. 2015, 21, 5469–5479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Cutsem, E.; Lenz, H.-J.; Köhne, C.-H.; Heinemann, V.; Tejpar, S.; Melezínek, I.; Beier, F.; Stroh, C.; Rougier, P.; van Krieken, J.H.; et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J. Clin. Oncol. 2015, 33, 692–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allegra, C.J.; Rumble, R.B.; Hamilton, S.R.; Mangu, P.B.; Roach, N.; Hantel, A.; Schilsky, R.L. Extended RAS Gene Mutation Testing in Metastatic Colorectal Carcinoma to Predict Response to Anti-Epidermal Growth Factor Receptor Monoclonal Antibody Therapy: American Society of Clinical Oncology Provisional Clinical Opinion Update 2015. J. Clin. Oncol. 2016, 34, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- Schmiegel, W.; Scott, R.J.; Dooley, S.; Lewis, W.; Meldrum, C.J.; Pockney, P.; Draganic, B.; Smith, S.; Hewitt, C.; Philimore, H.; et al. Blood-based detection of RAS mutations to guide anti-EGFR therapy in colorectal cancer patients: Concordance of results from circulating tumor DNA and tissue-based RAS testing. Mol. Oncol. 2017, 11, 208–219. [Google Scholar] [CrossRef] [Green Version]
- García-Foncillas, J.; Tabernero, J.; Élez, E.; Aranda, E.; Benavides, M.; Camps, C.; Jantus-Lewintre, E.; López, R.; Muinelo-Romay, L.; Montagut, C.; et al. Prospective multicenter real-world RAS mutation comparison between OncoBEAM-based liquid biopsy and tissue analysis in metastatic colorectal cancer. Br. J. Cancer 2018, 119, 1464–1470. [Google Scholar] [CrossRef]
- Lecomte, T.; Berger, A.; Zinzindohoué, F.; Micard, S.; Landi, B.; Blons, H.; Beaune, P.; Cugnenc, P.-H.; Laurent-Puig, P. Detection of free-circulating tumor-associated DNA in plasma of colorectal cancer patients and its association with prognosis. Int. J. Cancer 2002, 100, 542–548. [Google Scholar] [CrossRef]
- Lindforss, U.; Zetterquist, H.; Papadogiannakis, N.; Olivecrona, H. Persistence of K-ras mutations in plasma after colorectal tumor resection. Anticancer Res. 2005, 25, 657–661. [Google Scholar]
- Trevisiol, C.; Di Fabio, F.; Nascimbeni, R.; Peloso, L.; Salbe, C.; Ferruzzi, E.; Salerni, B.; Gion, M. Prognostic value of circulating KRAS2 gene mutations in colorectal cancer with distant metastases. Int. J. Biol. Markers 2006, 21, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Spindler, K.-L.G.; Pallisgaard, N.; Vogelius, I.; Jakobsen, A. Quantitative cell-free DNA, KRAS, and BRAF mutations in plasma from patients with metastatic colorectal cancer during treatment with cetuximab and irinotecan. Clin. Cancer Res. 2012, 18, 1177–1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.; Liu, X.; Wang, Y.; Ge, F.; Zhao, C.; Fu, Y.; Lin, L.; Xu, J. Correlation analysis between abundance of K-ras mutation in plasma free DNA and its correlation with clinical outcome and prognosis in patients with metastatic colorectal cancer. Zhonghua Zhong Liu Za Zhi 2013, 35, 666–671. [Google Scholar] [PubMed]
- Spindler, K.G.; Appelt, A.L.; Pallisgaard, N.; Andersen, R.F.; Jakobsen, A. KRAS-mutated plasma DNA as predictor of outcome from irinotecan monotherapy in metastatic colorectal cancer. Br. J. Cancer 2013, 109, 3067–3072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.-M.; Liu, X.-J.; Ge, F.-J.; Lin, L.; Wang, Y.; Sharma, M.R.; Liu, Z.-Y.; Tommasi, S.; Paradiso, A. KRAS mutations in tumor tissue and plasma by different assays predict survival of patients with metastatic colorectal cancer. J. Exp. Clin. Cancer Res. 2014, 33, 104. [Google Scholar] [CrossRef]
- Siravegna, G.; Mussolin, B.; Buscarino, M.; Corti, G.; Cassingena, A.; Crisafulli, G.; Ponzetti, A.; Cremolini, C.; Amatu, A.; Lauricella, C.; et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat. Med. 2015, 21, 795–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spindler, K.L.G.; Pallisgaard, N.; Andersen, R.F.; Brandslund, I.; Jakobsen, A. Circulating free DNA as biomarker and source for mutation detection in metastatic colorectal cancer. PLoS ONE 2015, 10, e0108247. [Google Scholar] [CrossRef] [PubMed]
- El Messaoudi, S.; Mouliere, F.; Du Manoir, S.; Bascoul-Mollevi, C.; Gillet, B.; Nouaille, M.; Fiess, C.; Crapez, E.; Bibeau, F.; Theillet, C.; et al. Circulating DNA as a Strong Multimarker Prognostic Tool for Metastatic Colorectal Cancer Patient Management Care. Clin. Cancer Res. 2016, 22, 3067–3077. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Shen, K.; Li, B.; Li, R.; Wang, Z.; Xie, Z. Clinical significance and diagnostic value of serum NSE, CEA, CA19-9, CA125 and CA242 levels in colorectal cancer. Oncol. Lett. 2020, 20, 742–750. [Google Scholar] [CrossRef]
- Kang, H.Y.; Choe, E.K.; Park, K.J.; Lee, Y. Factors Requiring Adjustment in the Interpretation of Serum Carcinoembryonic Antigen: A Cross-Sectional Study of 18,131 Healthy Nonsmokers. Gastroenterol. Res. Pract. 2017, 2017, 9858931. [Google Scholar] [CrossRef]
- Hall, C.; Clarke, L.; Pal, A.; Buchwald, P.; Eglinton, T.; Wakeman, C.; Frizelle, F. A Review of the Role of Carcinoembryonic Antigen in Clinical Practice. Ann. Coloproctol. 2019, 35, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Wahid, A.; Cydzik, M.; Fischer, N.W.; Prodeus, A.; Shively, J.E.; Martel, A.; Alminawi, S.; Ghorab, Z.; Berinstein, N.L.; Gariépy, J. Serum-derived carcinoembryonic antigen (CEA) activates fibroblasts to induce a local re-modeling of the extracellular matrix that favors the engraftment of CEA-expressing tumor cells. Int. J. Cancer 2018, 143, 1963–1977. [Google Scholar] [CrossRef] [PubMed]
- Calinescu, A.; Turcu, G.; Nedelcu, R.I.; Brinzea, A.; Hodorogea, A.; Antohe, M.; Diaconu, C.; Bleotu, C.; Pirici, D.; Jilaveanu, L.B.; et al. On the Dual Role of Carcinoembryonic Antigen-Related Cell Adhesion Molecule 1 (CEACAM1) in Human Malignancies. J Immunol. Res. 2018, 2018, 7169081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pesta, M.; Kucera, R.; Topolcan, O.; Karlikova, M.; Houfkova, K.; Polivka, J.; Macanova, T.; Machova, I.; Slouka, D.; Kulda, V. Plasma microRNA Levels Combined with CEA and CA19-9 in the Follow-Up of Colorectal Cancer Patients. Cancers 2019, 11, 864. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.-L.; Gong, Y.-Z.; Kuang, J.-A.; Gao, F.; Tang, S.-Y.; Gan, J.-L. The prognostic value of the postoperative serum CEA levels/preoperative serum CEA levels ratio in colorectal cancer patients with high preoperative serum CEA levels. Cancer Manag. Res. 2019, 11, 7499–7511. [Google Scholar] [CrossRef] [Green Version]
- Tan, E.; Gouvas, N.; Nicholls, R.J.; Ziprin, P.; Xynos, E.; Tekkis, P.P. Diagnostic precision of carcinoembryonic antigen in the detection of recurrence of colorectal cancer. Surg. Oncol. 2009, 18, 15–24. [Google Scholar] [CrossRef]
- Park, I.J.; Choi, G.-S.; Lim, K.H.; Kang, B.M.; Jun, S.H. Serum carcinoembryonic antigen monitoring after curative resection for colorectal cancer: Clinical significance of the preoperative level. Ann. Surg. Oncol. 2009, 16, 3087–3093. [Google Scholar] [CrossRef]
- Nicholson, B.D.; Shinkins, B.; Pathiraja, I.; Roberts, N.W.; James, T.J.; Mallett, S.; Perera, R.; Primrose, J.N.; Mant, D. Blood CEA levels for detecting recurrent colorectal cancer. Cochrane. Database. Syst. Rev. 2015, CD011134. [Google Scholar] [CrossRef] [Green Version]
- Knijn, N.; Mekenkamp, L.J.M.; Klomp, M.; Vink-Börger, M.E.; Tol, J.; Teerenstra, S.; Meijer, J.W.R.; Tebar, M.; Riemersma, S.; van Krieken, J.H.J.M.; et al. KRAS mutation analysis: A comparison between primary tumours and matched liver metastases in 305 colorectal cancer patients. Br. J. Cancer 2011, 104, 1020–1026. [Google Scholar] [CrossRef] [Green Version]
- Mardinian, K.; Okamura, R.; Kato, S.; Kurzrock, R. Temporal and spatial effects and survival outcomes associated with concordance between tissue and blood KRAS alterations in the pan-cancer setting. Int. J. Cancer 2020, 146, 566–576. [Google Scholar] [CrossRef]
- Cassinotti, E.; Boni, L.; Segato, S.; Rausei, S.; Marzorati, A.; Rovera, F.; Dionigi, G.; David, G.; Mangano, A.; Sambucci, D.; et al. Free circulating DNA as a biomarker of colorectal cancer. Int. J. Surg. 2013, 11 (Suppl. 1), S54–S57. [Google Scholar] [CrossRef] [Green Version]
- Frattini, M.; Gallino, G.; Signoroni, S.; Balestra, D.; Battaglia, L.; Sozzi, G.; Leo, E.; Pilotti, S.; Pierotti, M.A. Quantitative analysis of plasma DNA in colorectal cancer patients: A novel prognostic tool. Ann. N. Y. Acad. Sci. 2006, 1075, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Vymetalkova, V.; Cervena, K.; Bartu, L.; Vodicka, P. Circulating Cell-Free DNA and Colorectal Cancer: A Systematic Review. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [Green Version]
- Reinert, T.; Schøler, L.V.; Thomsen, R.; Tobiasen, H.; Vang, S.; Nordentoft, I.; Lamy, P.; Kannerup, A.-S.; Mortensen, F.V.; Stribolt, K.; et al. Analysis of circulating tumour DNA to monitor disease burden following colorectal cancer surgery. Gut 2016, 65, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Carpinetti, P.; Donnard, E.; Bettoni, F.; Asprino, P.; Koyama, F.; Rozanski, A.; Sabbaga, J.; Habr-Gama, A.; Parmigiani, R.B.; Galante, P.A.F.; et al. The use of personalized biomarkers and liquid biopsies to monitor treatment response and disease recurrence in locally advanced rectal cancer after neoadjuvant chemoradiation. Oncotarget 2015, 6, 38360–38371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobin, L.H.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) TNM Classification of Malignant Tumours, 7th ed.; Wiley-Blackwell: Chichester/West Sussex, UK; Hoboken, NJ, USA, 2011; ISBN 978-1-4443-3241-4. [Google Scholar]
- Schwartz, L.H.; Litière, S.; de Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Tissue Origin | CRC | ||
|---|---|---|---|
| KRAS Status | KRAS Negative | KRAS Positive | |
| CLM | KRAS negative | 28 | 3 |
| KRAS positive | 1 | 29 | |
| Tissue vs. Plasma Origin | CLM Tissue | ||
|---|---|---|---|
| KRAS Status | KRAS Negative | KRAS Positive | |
| Plasma | KRAS negative | 12 | 6 |
| KRAS positive | 1 | 10 |
| The Multivariate Survival Analysis | DFS | OS | ||
|---|---|---|---|---|
| Status of Biomarkers and Clinical Characteristics | HR (95% Confidence Interval) | Significance | HR (95% Confidence Interval) | Significance |
| Plasma KRAS fractional abundance [cut-off 3.33%] | ||||
| Low level | 1.000 | 1.000 | ||
| High level | 2.542 (0.823–7.853) | p = 0.105 | 11.732 (2.729–50.432) | p= 0.001 |
| CEA [cut-off 4.9 µg/L] | ||||
| Low level | 1.000 | 1.000 | ||
| High level | 3.264 (1.119–9.521) | p= 0.03 | 5.409 (1.272–22.998) | p= 0.022 |
| Extend of liver resection | ||||
| R0 | 1.000 | 1.000 | ||
| R1 | 3.309 (1.09–10.045) | p= 0.035 | 6.054 (1.499–24.451) | p= 0.011 |
| RFA 1 | 2.895 (0.891–9.406) | p = 0.077 | 7.368 (1.479–36.7) | p= 0.015 |
| Biological treatment | ||||
| bevacizumab or cetuximab | 1.000 | 1.000 | ||
| n.a. | 1.546 (0.633–3.774) | p = 0.339 | 0.828 (0.326–2.104) | p = 0.692 |
| Patient Characteristics | Number of Patients (%) |
|---|---|
| All patients in the study | 71 (100%) |
| Gender | |
| Female | 29 (41%) |
| Male | 42 (59%) |
| Mean age at liver surgery (min-max) | 62.7 (29–77) years |
| Extent of liver surgery | |
| R0 resection | 38 (54%) |
| R1 resection | 21 (30%) |
| RFA 1 | 11 (16%) |
| Adjuvant chemotherapy | |
| Yes | 55 (77%) |
| No | 16 (23%) |
| Adjuvant biological therapy (bevacizumab or cetuximab) | |
| Yes | 15 (21%) |
| No | 56 (79%) |
| Tumor grade (available in 44 pts.) | |
| Grade 1 | 15 (21%) |
| Grade 2 | 23 (32%) |
| Grade 3 | 6 (9%) |
| Number of liver metastases | |
| 1 | 35 (49%) |
| ≥2 | 36 (51%) |
| Mean size of metastases (min-max) | 38.5 (6–130) mm |
| Extrahepatic disease | |
| Yes | 4 (6%) |
| No | 67 (94%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polivka, J.; Windrichova, J.; Pesta, M.; Houfkova, K.; Rezackova, H.; Macanova, T.; Vycital, O.; Kucera, R.; Slouka, D.; Topolcan, O. The Level of Preoperative Plasma KRAS Mutations and CEA Predict Survival of Patients Undergoing Surgery for Colorectal Cancer Liver Metastases. Cancers 2020, 12, 2434. https://doi.org/10.3390/cancers12092434
Polivka J, Windrichova J, Pesta M, Houfkova K, Rezackova H, Macanova T, Vycital O, Kucera R, Slouka D, Topolcan O. The Level of Preoperative Plasma KRAS Mutations and CEA Predict Survival of Patients Undergoing Surgery for Colorectal Cancer Liver Metastases. Cancers. 2020; 12(9):2434. https://doi.org/10.3390/cancers12092434
Chicago/Turabian StylePolivka, Jiri, Jindra Windrichova, Martin Pesta, Katerina Houfkova, Hana Rezackova, Tereza Macanova, Ondrej Vycital, Radek Kucera, David Slouka, and Ondrej Topolcan. 2020. "The Level of Preoperative Plasma KRAS Mutations and CEA Predict Survival of Patients Undergoing Surgery for Colorectal Cancer Liver Metastases" Cancers 12, no. 9: 2434. https://doi.org/10.3390/cancers12092434





