Molecular Targets and Emerging Therapies for Advanced Gallbladder Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Current Treatment Strategies for Advanced Gallbladder Cancer
2.1. First-Line Treatment
2.2. Second-Line Treatment
3. Molecular Characterization of GBC: The Basis for Precision Oncology
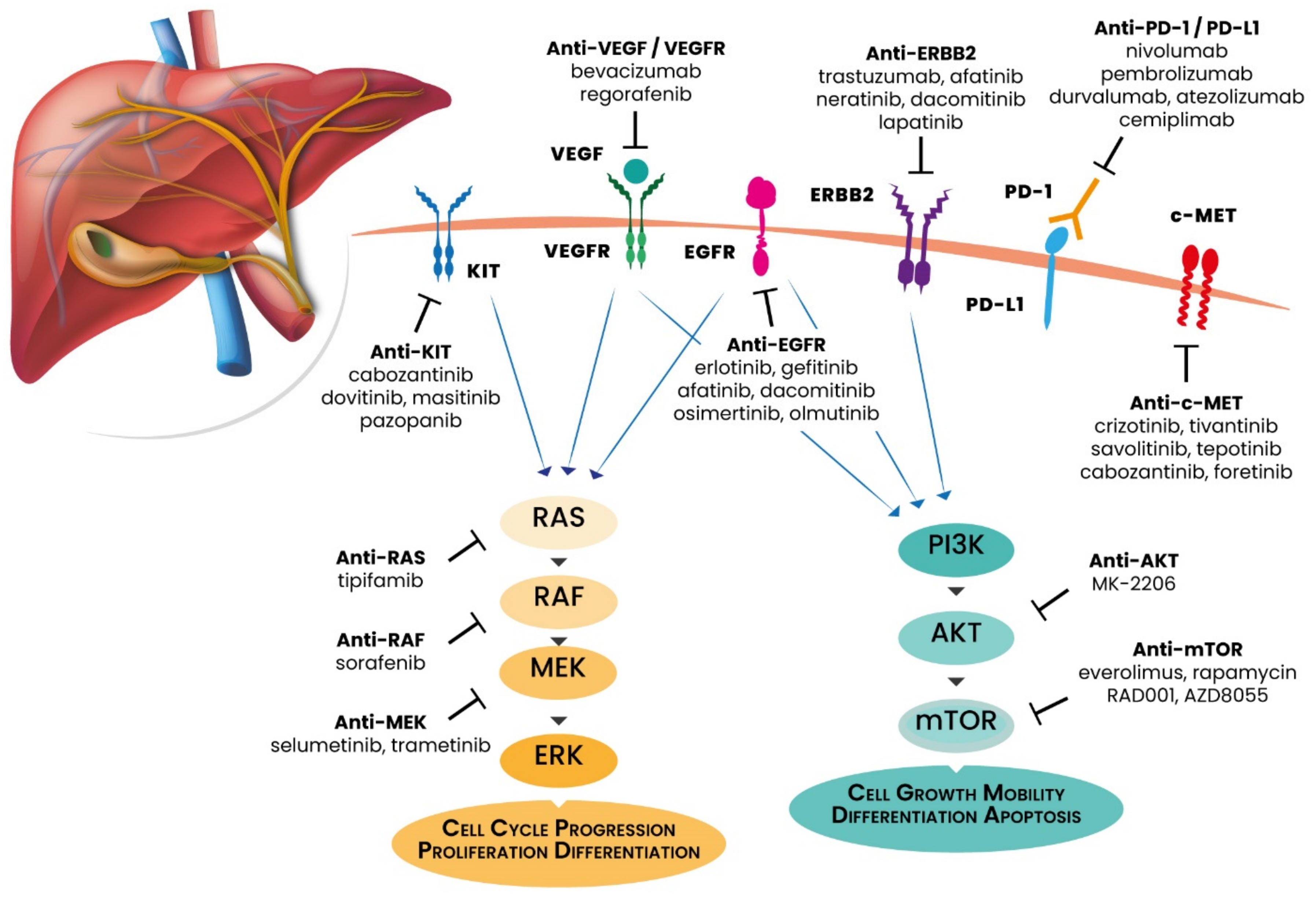
3.1. ERBB Receptors
3.2. PI3K/AKT/mTOR Pathway
3.3. MAPK Pathway
3.4. VEGF/VEGFR
3.5. DNA Damage Repair (DDR)
3.6. Programmed Death-1 (PD-1) and PD-Ligand 1 (PD-L1)
3.7. The Hedgehog Pathway
4. Advances in GBC Clinical Management Treatment
4.1. EGFR Inhibitors
4.2. HER2 Blockade
4.3. PI3K/AKT/mTOR Pathway Inhibitors
4.4. Mutated TP53 Inhibitors
4.5. VEGF/VEGFR Axis Inhibitors
4.6. Immunotherapy for GBC
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Sun, P.; Baria, K. The world-wide incidence of biliary tract cancer (BTC). J. Clin. Oncol. 2020, 38, 585. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Are, C.; Ahmad, H.; Ravipati, A.; Croo, D.; Clarey, D.; Smith, L.; Price, R.R.; Butte, J.M.; Gupta, S.; Chaturvedi, A.; et al. Global epidemiological trends and variations in the burden of gallbladder cancer. J. Surg. Oncol. 2017, 115, 580–590. [Google Scholar] [CrossRef]
- Hundal, R.; Shaffer, E.A. Gallbladder cancer: Epidemiology and outcome. Clin. Epidemiol. 2014, 6, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Eckel, F.; Schmid, R.M. Chemotherapy in advanced biliary tract carcinoma: A pooled analysis of clinical trials. Br. J. Cancer 2007, 96, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Hezel, A.F.; Deshpande, V.; Zhu, A.X. Genetics of biliary tract cancers and emerging targeted therapies. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 3531–3540. [Google Scholar] [CrossRef] [Green Version]
- Bridgewater, J.; Lopes, A.; Wasan, H.; Malka, D.; Jensen, L.; Okusaka, T.; Knox, J.; Wagner, D.; Cunningham, D.; Shannon, J.; et al. Prognostic factors for progression-free and overall survival in advanced biliary tract cancer. Ann. Oncol. 2016, 107, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Sohal, D.P.S.; Shrotriya, S.; Abazeed, M.; Cruise, M.; Khorana, A. Molecular characteristics of biliary tract cancer. Crit. Rev. Oncol. Hematol. 2016, 107, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [Green Version]
- Morizane, C.; Okusaka, T.; Mizusawa, J.; Katayama, H.; Ueno, M.; Ikeda, M.; Ozaka, M.; Okano, N.; Sugimori, K.; Fukutomi, A.; et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: The FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.; Kanai, M.; Kobayashi, S.; Eguchi, H.; Baba, H.; Seo, S.; Taketomi, A.; Takayama, T.; Yamaue, H.; Ishioka, C.; et al. Randomized phase III study of gemcitabine, cisplatin plus S-1 (GCS) versus gemcitabine, cisplatin (GC) for advanced biliary tract cancer (KHBO1401-MITSUBA). Ann. Oncol. 2018, 29, viii205. [Google Scholar] [CrossRef]
- Sharma, A.; Kalyan Mohanti, B.; Pal Chaudhary, S.; Sreenivas, V.; Kumar Sahoo, R.; Kumar Shukla, N.; Thulkar, S.; Pal, S.; Deo, S.V.; Pathy, S.; et al. Modified gemcitabine and oxaliplatin or gemcitabine + cisplatin in unresectable gallbladder cancer: Results of a phase III randomised controlled trial. Eur. J. Cancer 2019, 123, 162–170. [Google Scholar] [CrossRef]
- Kim, S.T.; Kang, J.H.; Lee, J.; Lee, H.W.; Oh, S.Y.; Jang, J.S.; Lee, M.A.; Sohn, B.S.; Yoon, S.Y.; Choi, H.J.; et al. Capecitabine plus oxaliplatin versus gemcitabine plus oxaliplatin as first-line therapy for advanced biliary tract cancers: A multicenter, open-label, randomized, phase III, noninferiority trial. Ann. Oncol. 2019, 30, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, S.H.; Chang, H.M.; Kim, J.S.; Choi, H.J.; Lee, M.A.; Chang, J.S.; Jeung, H.C.; Kang, J.H.; Lee, H.W.; et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2012, 13, 181–188. [Google Scholar] [CrossRef]
- Phelip, J.M.; Edeline, J.; Blanc, J.F.; Barbier, E.; Michel, P.; Bourgeois, V.; Neuzillet, C.; Malka, D.; Manfredi, S.; Desrame, J. Modified FOLFIRINOX versus CisGem first-line chemotherapy for locally advanced non resectable or metastatic biliary tract cancer (AMEBICA)-PRODIGE 38: Study protocol for a randomized controlled multicenter phase II/III study. Dig. Liver Dis. 2019, 51, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R. Articles Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer ( ABC-06 ): A phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021, 2045, 1–12. [Google Scholar] [CrossRef]
- Fornaro, L.; Cereda, S.; Aprile, G.; Di Girolamo, S.; Santini, D.; Silvestris, N.; Lonardi, S.; Leone, F.; Milella, M.; Vivaldi, C.; et al. Multivariate prognostic factors analysis for second-line chemotherapy in advanced biliary tract cancer. Br. J. Cancer 2014, 110, 2165–2169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verlingue, L.; Malka, D.; Allorant, A.; Massard, C.; Ferté, C.; Lacroix, L.; Rouleau, E.; Auger, N.; Ngo, M.; Nicotra, C.; et al. Precision medicine for patients with advanced biliary tract cancers: An effective strategy within the prospective MOSCATO-01 trial. Eur. J. Cancer 2017, 87, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Morizane, C.; Ueno, M.; Ikeda, M.; Okusaka, T.; Ishii, H.; Furuse, J. New developments in systemic therapy for advanced biliary tract cancer. Jpn. J. Clin. Oncol. 2018, 48, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Javle, M.; Pang, F.; Zhao, W.; Abdel-Wahab, R.; Chen, X.; Meric-Bernstam, F.; Chen, H.; Borad, M.J.; Liu, Y.; et al. Somatic genetic aberrations in gallbladder cancer: Comparison between Chinese and US patients. Hepatobiliary Surg. Nutr. 2019, 8, 604–614. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Z.; Li, X.; Ye, J.; Wu, X.; Tan, Z.; Liu, C.; Shen, B.; Wang, X.A.; Wu, W.; et al. Whole-exome and targeted gene sequencing of gallbladder carcinoma identifies recurrent mutations in the ErbB pathway. Nat. Genet. 2014, 46, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Bekaii-Saab, T.; Jain, A.; Wang, Y.; Kelley, R.K.; Wang, K.; Kang, H.C.; Catenacci, D.; Ali, S.; Krishnan, S.; et al. Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer 2016, 122, 3838–3847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z. ErbB Receptors and Cancer. Methods Mol. Biol. 2017, 1652, 3–35. [Google Scholar] [CrossRef]
- Hsu, W.-H.; Yang, J.C.-H.; Mok, T.S.; Loong, H.H. Overview of current systemic management of EGFR-mutant NSCLC. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, i3–i9. [Google Scholar] [CrossRef]
- Li, Q.-H.; Wang, Y.-Z.; Tu, J.; Liu, C.-W.; Yuan, Y.-J.; Lin, R.; He, W.-L.; Cai, S.-R.; He, Y.-L.; Ye, J.-N. Anti-EGFR therapy in metastatic colorectal cancer: Mechanisms and potential regimens of drug resistance. Gastroenterol. Rep. 2020, 8, 179–191. [Google Scholar] [CrossRef]
- Pignochino, Y.; Sarotto, I.; Peraldo-Neia, C.; Penachioni, J.Y.; Cavalloni, G.; Migliardi, G.; Casorzo, L.; Chiorino, G.; Risio, M.; Bardelli, A.; et al. Targeting EGFR/HER2 pathways enhances the antiproliferative effect of gemcitabine in biliary tract and gallbladder carcinomas. BMC Cancer 2010, 10, 631. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, K.; Dobashi, Y.; Suzuki, S.; Fujii, H.; Takeda, Y.; Ooi, A. Amplification and overexpression of c-erbB-2, epidermal growth factor receptor, and c-met in biliary tract cancers. J. Pathol. 2005, 206, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Barreto, S.G.; Dutt, A.; Chaudhary, A. A genetic model for gallbladder carcinogenesis and its dissemination. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Schwaederle, M.; Arguello, D.; Millis, S.Z.; Gatalica, Z.; Kurzrock, R. HER2 expression status in diverse cancers: Review of results from 37,992 patients. Cancer Metastasis Rev. 2015, 34, 157–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roa, I.; de Toro, G.; Schalper, K.; de Aretxabala, X.; Churi, C.; Javle, M. Overexpression of the HER2/neu gene: A new therapeutic possibility for patients with advanced gallbladder cancer. Gastrointest. Cancer Res. 2014, 7, 42–48. [Google Scholar] [PubMed]
- Li, M.; Liu, F.; Zhang, F.; Zhou, W.; Jiang, X.; Yang, Y.; Qu, K.; Wang, Y.; Ma, Q.; Wang, T.; et al. Genomic ERBB2/ERBB3 mutations promote PD-L1-mediated immune escape in gallbladder cancer: A whole-exome sequencing analysis. Gut 2019, 68, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geyer, C.E.; Forster, J.; Lindquist, D. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. Adv. Breast Cancer 2008, 5, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shitara, K.; Bang, Y.-J.; Iwasa, S.; Sugimoto, N.; Ryu, M.-H.; Sakai, D.; Chung, H.-C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N. Engl. J. Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef]
- Corti, F.; Nichetti, F.; Raimondi, A.; Niger, M.; Prinzi, N.; Torchio, M.; Tamborini, E.; Perrone, F.; Pruneri, G.; Di Bartolomeo, M.; et al. Targeting the PI3K/AKT/mTOR pathway in biliary tract cancers: A review of current evidences and future perspectives. Cancer Treat. Rev. 2019, 72, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Turkes, F.; Carmichael, J.; Cunningham, D.; Starling, N. Contemporary Tailored Oncology Treatment of Biliary Tract Cancers. Gastroenterol. Res. Pract. 2019, 2019, 7698786. [Google Scholar] [CrossRef] [PubMed]
- Zuo, M.; Rashid, A.; Churi, C.; Vauthey, J.-N.; Chang, P.; Li, Y.; Hung, M.-C.; Li, D.; Javle, M. Novel therapeutic strategy targeting the Hedgehog signalling and mTOR pathways in biliary tract cancer. Br. J. Cancer 2015, 112, 1042–1051. [Google Scholar] [CrossRef] [Green Version]
- Leal, P.; Garćia, P.; Sandoval, A.; Letelier, P.; Brebi, P.; Ili, C.; Álvarez, H.; Tapia, O.; Roa, J.C. Immunohistochemical expression of phospho-mTOR is associated with poor prognosis in patients with gallbladder adenocarcinoma. Arch. Pathol. Lab. Med. 2013, 137, 552–557. [Google Scholar] [CrossRef]
- Lunardi, A.; Webster, K.A.; Papa, A.; Padmani, B.; Clohessy, J.G.; Bronson, R.T.; Pandolfi, P.P. Role of aberrant PI3K pathway activation in gallbladder tumorigenesis. Oncotarget 2014, 5, 894–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, Y.J.; Weng, H.; Ye, Y.Y.; Hu, Y.P.; Bao, R.F.; Cao, Y.; Wang, X.A.; Zhang, F.; Xiang, S.S.; Li, H.F.; et al. SPOCK1 as a potential cancer prognostic marker promotes the proliferation and metastasis of gallbladder cancer cells by activating the PI3K/AKT pathway. Mol. Cancer 2015, 14. [Google Scholar] [CrossRef] [Green Version]
- Robert, C.; Karaszewska, B.; Schachter, J.; Rutkowski, P.; Mackiewicz, A.; Stroiakovski, D.; Lichinitser, M.; Dummer, R.; Grange, F.; Mortier, L.; et al. Improved Overall Survival in Melanoma with Combined Dabrafenib and Trametinib. N. Engl. J. Med. 2015, 372, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Van Cutsem, E.; Desai, J.; Yoshino, T.; Wasan, H.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E–Mutated Colorectal Cancer. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef] [Green Version]
- Valle, J.W.; Lamarca, A.; Goyal, L.; Barriuso, J.; Zhu, A.X. New horizons for precision medicine in biliary tract cancers. Cancer Discov. 2017, 7, 943–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokoyama, M.; Ohnishi, H.; Ohtsuka, K.; Matsushima, S.; Ohkura, Y.; Furuse, J.; Watanabe, T.; Mori, T.; Sugiyama, M. KRAS Mutation as a Potential Prognostic Biomarker of Biliary Tract Cancers. Jpn. Clin. Med. 2016, 7, 33–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deshpande, V.; Nduaguba, A.; Zimmerman, S.M.; Kehoe, S.M.; Macconaill, L.E.; Lauwers, G.Y.; Ferrone, C.; Bardeesy, N.; Zhu, A.X.; Hezel, A.F. Mutational profiling reveals PIK3CA mutations in gallbladder carcinoma. BMC Cancer 2011, 11, 60. [Google Scholar] [CrossRef] [Green Version]
- Goeppert, B.; Frauenschuh, L.; Renner, M.; Roessler, S.; Stenzinger, A.; Klauschen, F.; Warth, A.; Vogel, M.N.; Mehrabi, A.; Hafezi, M.; et al. BRAF V600E-specific immunohistochemistry reveals low mutation rates in biliary tract cancer and restriction to intrahepatic cholangiocarcinoma. Mod. Pathol. 2014, 27, 1028–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.C.; Tsai, C.C.; Chan, C.C. Mutation analysis and copy number changes of KRAS and BRAF genes in Taiwanese cases of biliary tract cholangiocarcinoma. J. Formos. Med. Assoc. 2017, 116, 464–468. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Bang, S.S.; Jee, S.; Park, S.; Shin, S.-J.; Jang, K. Prevalence and Clinicopathological Significance of MET Overexpression and Gene Amplification in Patients with Gallbladder Carcinoma. Cancer Res. Treat. 2020, 52, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Moon, W.S.; Park, H.S.; Lee, H.; Pai, R.; Tarnawski, A.S.; Kim, K.R.; Jang, K.Y. Co-Expression of Cox-2, C-Met and β-catenin in Cells Forming Invasive front of Gallbladder Cancer. Cancer Res. Treat. 2005, 37, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simone, V.; Brunetti, O.; Lupo, L.; Testini, M.; Maiorano, E.; Simone, M.; Longo, V.; Rolfo, C.; Peeters, M.; Scarpa, A.; et al. Targeting angiogenesis in biliary tract cancers: An open option. Int. J. Mol. Sci. 2017, 18, 418. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Liu, M.; Cai, X.; Xie, L.; She, F.; Chen, Y. Serum vascular endothelial growth factor-C levels predict lymph node metastasis and prognosis of patients with gallbladder cancer. Oncol. Lett. 2018, 16, 6065–6070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.N.; Cao, W.G.; Wang, X.; Wang, Q.; Gu, B.X.; Yang, Q.C.; Hu, J.B.; Liu, H.; Zheng, S. Prognostic impact of vascular endothelial growth factor-A expression in resected gallbladder carcinoma. Tumour Biol. 2011, 32, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Hu, Y.; Li, Y.; Shao, R.; Liu, F.; Liu, Y. Overview of current targeted therapy in gallbladder cancer. Signal Transduct. Target. Ther. 2020, 5, 230. [Google Scholar] [CrossRef]
- Xu, D.; Li, J.; Jiang, F.; Cai, K.; Ren, G. The Effect and Mechanism of Vascular Endothelial Growth Factor (VEGF) on Tumor Angiogenesis in Gallbladder Carcinoma. Iran. J. Public Health 2019, 48, 713–721. [Google Scholar]
- Mishra, K.; Behari, A.; Kapoor, V.K.; Khan, M.S.; Prakash, S.; Agrawal, S. Vascular endothelial growth factor single-nucleotide polymorphism in gall bladder cancer. J. Gastroenterol. Hepatol. 2013, 28, 1678–1685. [Google Scholar] [CrossRef]
- Banimohamad-Shotorbani, B.; Kahroba, H.; Sadeghzadeh, H.; Wilson, D.M., III; Maadi, H.; Samadi, N.; Hejazi, M.S.; Farajpour, H.; Onari, B.N.; Sadeghi, M.R. DNA damage repair response in mesenchymal stromal cells: From cellular senescence and aging to apoptosis and differentiation ability. Ageing Res. Rev. 2020, 62, 101125. [Google Scholar] [CrossRef]
- Abdel-Wahab, R.; Yap, T.A.; Madison, R.; Pant, S.; Cooke, M.; Wang, K.; Zhao, H.; Bekaii-Saab, T.; Karatas, E.; Kwong, L.N.; et al. Genomic profiling reveals high frequency of DNA repair genetic aberrations in gallbladder cancer. Sci. Rep. 2020, 10, 22087. [Google Scholar] [CrossRef] [PubMed]
- Spizzo, G.; Puccini, A.; Xiu, J.; Goldberg, R.M.; Grothey, A.; Shields, A.F.; Arora, S.P.; Khushman, M.; Salem, M.E.; Battaglin, F.; et al. Molecular profile of BRCA-mutated biliary tract cancers. ESMO Open 2020, 5, e000682. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, J.; Li, R.; Han, Z.; Li, L.; Li, G.; Yang, B.; Yin, Q.; Wang, Y.; Ke, Y.; et al. Effectiveness of Olaparib Treatment in a Patient with Gallbladder Cancer with an ATM-Inactivating Mutation. Oncologist 2020, 25, 375–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Gao, L.; Qiu, M.; Cao, D. Olaparib Treatment in a Patient with Advanced Gallbladder Cancer Harboring BRCA1 Mutation. Onco. Targets. Ther. 2021, 14, 2815–2819. [Google Scholar] [CrossRef]
- Xie, Y.; Jiang, Y.; Yang, X.-B.; Wang, A.-Q.; Zheng, Y.-C.; Wan, X.-S.; Sang, X.-T.; Wang, K.; Zhang, D.-D.; Xu, J.-J.; et al. Response of BRCA1-mutated gallbladder cancer to olaparib: A case report. World J. Gastroenterol. 2016, 22, 10254–10259. [Google Scholar] [CrossRef]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H.J. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020, 27, S87–S97. [Google Scholar] [CrossRef]
- Lin, J.; Long, J.; Wan, X.; Chen, J.; Bai, Y.; Wang, A.; Yang, X.; Wu, Y.; Robson, S.C.; Sang, X.; et al. Classification of gallbladder cancer by assessment of CD8+ TIL and PD-L1 expression. BMC Cancer 2018, 18, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mody, K.; Starr, J.; Saul, M.; Poorman, K.; Weinberg, B.A.; Salem, M.E.; VanderWalde, A.; Shields, A.F. Patterns and genomic correlates of PD-L1 expression in patients with biliary tract cancers. J. Gastrointest. Oncol. 2019, 10, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Nam, A.-R.; Bang, J.-H.; Park, J.-E.; Kim, T.-Y.; Lee, K.-H.; Han, S.-W.; Im, S.-A.; Kim, T.-Y.; Bang, Y.-J.; et al. Soluble programmed death-ligand 1 (sPDL1) and neutrophil-to-lymphocyte ratio (NLR) predicts survival in advanced biliary tract cancer patients treated with palliative chemotherapy. Oncotarget 2016, 7, 76604–76612. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Kim, K.; Kim, M.; Kim, Y.M.; Suh, J.H.; Cha, H.J.; Choi, H.J. Programmed death-ligand 1 expression and its correlation with clinicopathological parameters in gallbladder cancer. J. Pathol. Transl. Med. 2020, 54, 154–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neyaz, A.; Husain, N.; Kumari, S.; Gupta, S.; Shukla, S.; Arshad, S.; Anand, N.; Chaturvedi, A. Clinical relevance of PD-L1 expression in gallbladder cancer: A potential target for therapy. Histopathology 2018, 73, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, M.; Tian, H.; de Sauvage, F.J. The hedgehog signaling pathway in cancer. Clin. Cancer Res. 2006, 12, 5924–5928. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wu, T.; Lu, J.; Cao, Y.; Song, N.; Yang, T.; Dong, R.; Yang, Y.; Zang, L.; Du, X.; et al. Immunohistochemical evidence of the prognostic value of hedgehog pathway components in primary gallbladder carcinoma. Surg. Today 2012, 42, 770–775. [Google Scholar] [CrossRef]
- Matsushita, S.; Onishi, H.; Nakano, K.; Nagamatsu, I.; Imaizumi, A.; Hattori, M.; Oda, Y.; Tanaka, M.; Katano, M. Hedgehog signaling pathway is a potential therapeutic target for gallbladder cancer. Cancer Sci. 2014, 105, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Malka, D.; Cervera, P.; Foulon, S.; Trarbach, T.; de la Fouchardière, C.; Boucher, E.; Fartoux, L.; Faivre, S.; Blanc, J.F.; Viret, F.; et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): A randomised, open-label, non-comparative phase 2 trial. Lancet Oncol. 2014, 15, 819–828. [Google Scholar] [CrossRef]
- Chen, J.S.; Hsu, C.; Chiang, N.J.; Tsai, C.S.; Tsou, H.H.; Huang, S.F.; Bai, L.Y.; Chang, I.C.; Shiah, H.S.; Ho, C.L.; et al. A KRAS mutation status-stratified randomized phase II trial of gemcitabine and oxaliplatin alone or in combination with cetuximab in advanced biliary tract cancer. Ann. Oncol. 2015, 26, 943–949. [Google Scholar] [CrossRef]
- Leone, F.; Marino, D.; Cereda, S.; Filippi, R.; Belli, C.; Spadi, R.; Nasti, G.; Montano, M.; Amatu, A.; Aprile, G.; et al. Panitumumab in combination with gemcitabine and oxaliplatin does not prolong survival in wild-type KRAS advanced biliary tract cancer: A randomized phase 2 trial (Vecti-BIL study). Cancer 2016, 122, 574–581. [Google Scholar] [CrossRef]
- Jensen, L.H.; Fernebro, E.; Ploen, J.; Eberhard, J.; Lindebjerg, J.; Jakobsen, A.K.M. Randomized phase II crossover trial exploring the clinical benefit from targeting EGFR or VEGF with combination chemotherapy in patients with non-resectable biliary tract cancer. J. Clin. Oncol. 2015, 33, 4071. [Google Scholar] [CrossRef]
- Gruenberger, B.; Schueller, J.; Heubrandtner, U.; Wrba, F.; Tamandl, D.; Kaczirek, K.; Roka, R.; Freimann-Pircher, S.; Gruenberger, T. Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: A phase 2 study. Lancet Oncol. 2010, 11, 1142–1148. [Google Scholar] [CrossRef]
- Jensen, L.H.; Lindebjerg, J.; Ploen, J.; Hansen, T.F.; Jakobsen, A. Phase II marker-driven trial of panitumumab and chemotherapy in KRAS wild-type biliary tract cancer. Ann. Oncol. 2012, 23, 2341–2346. [Google Scholar] [CrossRef]
- Hezel, A.F.; Noel, M.S.; Allen, J.N.; Abrams, T.A.; Yurgelun, M.; Faris, J.E.; Goyal, L.; Clark, J.W.; Blaszkowsky, L.S.; Murphy, J.E.; et al. Phase II study of gemcitabine, oxaliplatin in combination with panitumumab in KRAS wild-type unresectable or metastatic biliary tract and gallbladder cancer. Br. J. Cancer 2014, 111, 430–436. [Google Scholar] [CrossRef]
- Cai, W.; Yuan, Y.; Ge, W.; Fan, Y.; Liu, X.; Wu, D.; Hu, H. EGFR target therapy combined with GEMOX for advanced biliary tract cancers: A meta-analysis based on RCTs. J. Cancer 2018, 9, 1476–1485. [Google Scholar] [CrossRef]
- Rizzo, A.; Frega, G.; Ricci, A.D.; Palloni, A.; Abbati, F.; de Lorenzo, S.; Deserti, M.; Tavolari, S.; Brandi, G. Anti-EGFR monoclonal antibodies in advanced biliary tract cancer: A systematic review and meta-analysis. In Vivo 2020, 34, 479–488. [Google Scholar] [CrossRef] [Green Version]
- Philip, P.A.; Mahoney, M.R.; Allmer, C.; Thomas, J.; Pitot, H.C.; Kim, G.; Donehower, R.C.; Fitch, T.; Picus, J.; Erlichman, C. Phase II study of erlotinib in patients with advanced biliary cancer. J. Clin. Oncol. 2006, 24, 3069–3074. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, R.K.; Belani, C.P.; Singh, D.A.; Tanaka, M.; Lenz, H.J.; Yen, Y.; Kindler, H.L.; Iqbal, S.; Longmate, J.; MacK, P.C.; et al. A phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemother. Pharmacol. 2009, 64, 777–783. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Rankin, C.; Siegel, A.B.; Iqbal, S.; Gong, I.Y.; Micetich, K.C.; Kayaleh, O.R.; Lenz, H.J.; Blanke, C.D. S0941: A phase 2 SWOG study of sorafenib and erlotinib in patients with advanced gallbladder carcinoma or cholangiocarcinoma. Br. J. Cancer 2014, 110, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.M.; Hainsworth, J.D.; Swanton, C.; Burris, H.A.; Kurzrock, R.; Sweeney, C.; Meric-Bernstam, F.; Spigel, D.R.; Bose, R.; Guo, S.; et al. Pertuzumab + trastuzumab for HER2-positive metastatic biliary cancer: Preliminary data from MyPathway. J. Clin. Oncol. 2017, 35, 402. [Google Scholar] [CrossRef]
- Wang, W.; Hu, Z.; Huang, Y.; Zheng, H.; Sun, Q.; Yang, Q.; Zhang, Y.; Zhang, L.; Wang, W. Pretreatment with gemcitabine/5-fluorouracil enhances the cytotoxicity of trastuzumab to HER2-negative human gallbladder cancer cells In Vitro and In Vivo. BioMed Res. Int. 2019, 2019, 9205851. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.; Cleary, J.; Shapiro, G.; Braña, I.; Moreno, V.; Quinn, D.; Borad, M.; Loi, S.; Spanggaard, I.; Stemmer, S.; et al. Treating HER2-mutant advanced biliary tract cancer with neratinib: Benefits of HER2-directed targeted therapy in the phase 2 SUMMIT ‘basket’ trial. Ann. Oncol. 2019, 30, iv127. [Google Scholar] [CrossRef]
- Tan, A.C.; Oh, D.-Y.; Chao, Y.; Hsieh, C.-Y.; Chang, W.-L.; Isanto, F.; Chen, Y.-C.; McHale, M.; Lindmark, B.; Ng, M.C.H. Efficacy and safety of varlitinib, a reversible pan-HER tyrosine kinase inhibitor, in combination with platinum-based regimens in biliary tract cancers: A pooled analysis from three phase I studies. J. Clin. Oncol. 2019, 37, 331. [Google Scholar] [CrossRef]
- Jeong, H.; Jeong, J.H.; Kim, K.P.; Lee, S.S.; Oh, D.W.; Park, D.H.; Song, T.J.; Park, Y.; Hong, S.M.; Ryoo, B.Y.; et al. Feasibility of HER2-targeted therapy in advanced biliary tract cancer: A prospective pilot study of trastuzumab biosimilar in combination with gemcitabine plus cisplatin. Cancers 2021, 13, 161. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Hanna, D.L.; El-Khoueiry, A.B.; Kang, Y.-K.; Oh, D.-Y.; Chaves, J.M.; Rha, S.Y.; Hamilton, E.P.; Pant, S.; Javle, M.M.; et al. Zanidatamab (ZW25) in HER2-positive biliary tract cancers (BTCs): Results from a phase I study. J. Clin. Oncol. 2021, 39, 299. [Google Scholar] [CrossRef]
- Subbiah, V.; Lassen, U.; Élez, E.; Italiano, A.; Curigliano, G.; Javle, M.; de Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef]
- Ikeda, M.; Ioka, T.; Fukutomi, A.; Morizane, C.; Kasuga, A.; Takahashi, H.; Todaka, A.; Okusaka, T.; Creasy, C.L.; Gorman, S.; et al. Efficacy and safety of trametinib in Japanese patients with advanced biliary tract cancers refractory to gemcitabine. Cancer Sci. 2018, 109, 215–224. [Google Scholar] [CrossRef]
- Kim, R.D.; McDonough, S.; El-Khoueiry, A.B.; Bekaii-Saab, T.S.; Stein, S.M.; Sahai, V.; Keogh, G.P.; Kim, E.J.; Baron, A.D.; Siegel, A.B.; et al. Randomised phase II trial (SWOG S1310) of single agent MEK inhibitor trametinib Versus 5-fluorouracil or capecitabine in refractory advanced biliary cancer. Eur. J. Cancer 2020, 130, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Bekaii-Saab, T.; Phelps, M.A.; Li, X.; Saji, M.; Goff, L.; Kauh, J.S.W.; O’Neil, B.H.; Balsom, S.; Balint, C.; Liersemann, R.; et al. Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 2357–2363. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.W.; Lee, K.-H.; Kim, J.-W.; Suh, K.J.; Nam, A.-R.; Bang, J.-H.; Bang, Y.-J.; Oh, D.-Y. Enhanced antitumor effect of binimetinib in combination with capecitabine for biliary tract cancer patients with mutations in the RAS/RAF/MEK/ERK pathway: Phase Ib study. Br. J. Cancer 2019, 121, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Costello, B.A.; Borad, M.J.; Qi, Y.; Kim, G.P.; Northfelt, D.W.; Erlichman, C.; Alberts, S.R. Phase I trial of everolimus, gemcitabine and cisplatin in patients with solid tumors. Invest. New Drugs 2014, 32, 710–716. [Google Scholar] [CrossRef]
- Ahn, D.H.; Li, J.; Wei, L.; Doyle, A.; Marshall, J.L.; Schaaf, L.J.; Phelps, M.A.; Villalona-Calero, M.A.; Bekaii-Saab, T. Results of an abbreviated phase-II study with the Akt Inhibitor MK-2206 in Patients with Advanced Biliary Cancer. Sci. Rep. 2015, 5, 12122. [Google Scholar] [CrossRef] [PubMed]
- Yeung, Y.H.; Chionh, F.J.M.; Price, T.J.; Scott, A.M.; Tran, H.; Fang, G.; Skrinos, E.; Murone, C.; Mariadason, J.M.; Tebbutt, N.C. Phase II study of everolimus monotherapy as first-line treatment in advanced biliary tract cancer: RADichol. J. Clin. Oncol. 2014, 32, 4101. [Google Scholar] [CrossRef]
- Buzzoni, R.; Pusceddu, S.; Bajetta, E.; De Braud, F.; Platania, M.; Iannacone, C.; Cantore, M.; Mambrini, A.; Bertolini, A.; Alabiso, O.; et al. Activity and safety of RAD001 (everolimus) in patients affected by biliary tract cancer progressing after prior chemotherapy: A phase II ITMO study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 1597–1603. [Google Scholar] [CrossRef] [PubMed]
- Makower, D.; Rozenblit, A.; Kaufman, H.; Edelman, M.; Lane, M.E.; Zwiebel, J.; Haynes, H.; Wadler, S. Phase II clinical trial of intralesional administration of the oncolytic adenovirus ONYX-015 in patients with hepatobiliary tumors with correlative p53 studies. Clin. Cancer Res. 2003, 9, 693–702. [Google Scholar]
- Zhu, A.X.; Blaszkowsky, L.S.; Ryan, D.P.; Clark, J.W.; Muzikansky, A.; Horgan, K.; Sheehan, S.; Hale, K.E.; Enzinger, P.C.; Bhargava, P.; et al. Phase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Lubner, S.J.; Mahoney, M.R.; Kolesar, J.L.; Loconte, N.K.; Kim, G.P.; Pitot, H.C.; Philip, P.A.; Picus, J.; Yong, W.-P.; Horvath, L.; et al. Report of a multicenter phase II trial testing a combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer: A phase II Consortium study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 3491–3497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyer, R.V.; Groman, A.; Ma, W.W.; Malhotra, U.; Iancu, D.; Grande, C.; Bekaii-Saab, T.S. Gemcitabine (G), capecitabine (C) and bevacizumab (BV) in patients with advanced biliary cancers (ABC): Final results of a multicenter phase II study. J. Clin. Oncol. 2015, 33, 4078. [Google Scholar] [CrossRef]
- Sun, W.; Patel, A.; Normolle, D.; Patel, K.; Ohr, J.; Lee, J.J.; Bahary, N.; Chu, E.; Streeter, N.; Drummond, S. A phase 2 trial of regorafenib as a single agent in patients with chemotherapy-refractory, advanced, and metastatic biliary tract adenocarcinoma. Cancer 2019, 125, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Ikeda, M.; Sasaki, T.; Nagashima, F.; Mizuno, N.; Shimizu, S.; Ikezawa, H.; Hayata, N.; Nakajima, R.; Morizane, C. Phase 2 study of lenvatinib monotherapy as second-line treatment in unresectable biliary tract cancer: Primary analysis results. BMC Cancer 2020, 20, 1105. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.H.; Thongprasert, S.; Lee, J.; Doval, D.C.; Park, S.H.; Park, J.O.; Park, Y.S.; Kang, W.K.; Lim, H.Y. A phase II study of sunitinib as a second-line treatment in advanced biliary tract carcinoma: A multicentre, multinational study. Eur. J. Cancer 2012, 48, 196–201. [Google Scholar] [CrossRef]
- Santoro, A.; Gebbia, V.; Pressiani, T.; Testa, A.; Personeni, N.; Arrivas Bajardi, E.; Foa, P.; Buonadonna, A.; Bencardino, K.; Barone, C.; et al. A randomized, multicenter, phase II study of vandetanib monotherapy versus vandetanib in combination with gemcitabine versus gemcitabine plus placebo in subjects with advanced biliary tract cancer: The VanGogh study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Arkenau, H.-T.; Martin-Liberal, J.; Calvo, E.; Penel, N.; Krebs, M.G.; Herbst, R.S.; Walgren, R.A.; Widau, R.C.; Mi, G.; Jin, J.; et al. Ramucirumab Plus Pembrolizumab in Patients with Previously Treated Advanced or Metastatic Biliary Tract Cancer: Nonrandomized, Open-Label, Phase I Trial (JVDF). Oncologist 2018, 23, 1407. [Google Scholar] [CrossRef] [Green Version]
- Golan, T.; Raitses-Gurevich, M.; Kelley, R.K.; Bocobo, A.G.; Borgida, A.; Shroff, R.T.; Holter, S.; Gallinger, S.; Ahn, D.H.; Aderka, D.; et al. Overall Survival and Clinical Characteristics of BRCA-Associated Cholangiocarcinoma: A Multicenter Retrospective Study. Oncologist 2017, 22, 804–810. [Google Scholar] [CrossRef] [Green Version]
- Ricci, A.D.; Rizzo, A.; Bonucci, C.; Tober, N.; Palloni, A.; Mollica, V.; Maggio, I.; Deserti, M.; Tavolari, S.; Brandi, G. PARP Inhibitors in Biliary Tract Cancer: A New Kid on the Block? Medicines 2020, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.D.; Chung, V.; Alese, O.B.; El-Rayes, B.F.; Li, D.; Al-Toubah, T.E.; Schell, M.J.; Zhou, J.-M.; Mahipal, A.; Kim, B.H.; et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients With Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020, 6, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Ueno, M.; Morizane, C.; Kobayashi, S.; Ohno, I.; Kondo, S.; Okano, N.; Kimura, K.; Asada, S.; Namba, Y.; et al. A multicenter, open-label, phase I study of nivolumab alone or in combination with gemcitabine plus cisplatin in patients with unresectable or recurrent biliary tract cancer. J. Clin. Oncol. 2019, 37, 306. [Google Scholar] [CrossRef]
- Villanueva, L.; Lwin, Z.; Chung, H.C.; Gomez-Roca, C.; Longo, F.; Yanez, E.; Senellart, H.; Doherty, M.; García-Corbacho, J.; Hendifar, A.E.; et al. Lenvatinib plus pembrolizumab for patients with previously treated biliary tract cancers in the multicohort phase II LEAP-005 study. J. Clin. Oncol. 2021, 39, 321. [Google Scholar] [CrossRef]
- Hack, S.P.; Verret, W.; Mulla, S.; Liu, B.; Wang, Y.; Macarulla, T.; Ren, Z.; El-Khoueiry, A.B.; Zhu, A.X. IMbrave 151: A randomized phase II trial of atezolizumab combined with bevacizumab and chemotherapy in patients with advanced biliary tract cancer. Ther. Adv. Med. Oncol. 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Chen, Z.; Liu, Y.; Xiong, J.; Ren, Z.; Meng, Z.; Gu, S.; Wang, L.; Zou, J. A phase II study of anti–PD-1 antibody camrelizumab plus FOLFOX4 or GEMOX systemic chemotherapy as first-line therapy for advanced hepatocellular carcinoma or biliary tract cancer. J. Clin. Oncol. 2019, 37, 4074. [Google Scholar] [CrossRef]
- Ioka, T.; Ueno, M.; Oh, D.-Y.; Fujiwara, Y.; Chen, J.-S.; Doki, Y.; Mizuno, N.; Park, K.; Asagi, A.; Hayama, M.; et al. Evaluation of safety and tolerability of durvalumab (D) with or without tremelimumab (T) in patients (pts) with biliary tract cancer (BTC). J. Clin. Oncol. 2019, 37, 387. [Google Scholar] [CrossRef]
| Study Code | Cohort | Drug Investigated | Phase | Setting (line) | Drug Target |
|---|---|---|---|---|---|
| NCT03260712 | BTC and GBC | Pembrolizumab | II | Palliative (I) | PD-1 |
| NCT03111732 | BTC and GBC | Pembrolizumab | II | Palliative (II>) | PD-1 |
| NCT02834013 | Solid tumors, GBC | nivolumab and ipilimumab | II | Palliative (II>) | PD1 + CTLA-4 |
| NCT03473574 | CCA and GBC | durvalumab and tremelimumab | II R | Palliative (I) | PDL1 + CTLA-4 |
| NCT03201458 | CCA and GBC | atezolizumab, cobimetinib | II R | Palliative (II>) | PD-L1 + MEK |
| NCT01308840 | BTC and GBC | Panitumumab | II | Palliative (I) | EGFR |
| NCT01267344 | BTC and GBC | Cetuximab | II R | Palliative (I) | EGFR |
| NCT00478140 | BTC and GBC | Trastuzumab | II | Palliative (II>) | HER2 |
| NCT00361231 | BTC and GBC | Bevacizumab | II | Palliative (I>) | VEGFR |
| NCT00356889 | BTC and GBC | Bevacizumab, erlotinib | II | Palliative (I) | VEGF + EGFR |
| NCT02520141 | BTC and GBC | Ramucirumab | II | Palliative (I>) | VEGFR |
| NCT02115542 | BTC and GBC | Regorafenib | II | Palliative (III>) | RAF |
| NCT00919061 | BTC and GBC | Sorafenib | II | Palliative (I) | RAF |
| NCT00238212 | BTC and GBC | Sorafenib | II | Palliative (II>) | RAF |
| NCT00832637 | BTC and GBC | Erlotinib | II | Palliative (I) | EGFR |
| NCT00033462 | BTC, GBC and LC | Erlotinib | II | Palliative (II>) | EGFR |
| NCT01093222 | BTC and GBC | Erlotinib, sorafenib | II | Palliative (I) | EGFR + RAF |
| NCT00350753 | BTC and GBC | Erlotinib, bevacizumab | II | Palliative (II>) | EGFR + VEGF |
| NCT04183712 | GBC | Afatinib | II R | Adjuvant | HER/HER EGFR |
| NCT02992340 | BTC and GBC | Varlitinib, cisplatin, gemcitabine | I–II | Palliative (I) | HER2/EGFR |
| NCT03093870 | BRC and GBC | Varlitinib, capecitabine | II–III | Palliative (II) | HER2/EGFR |
| NCT02151084 | BTC and GBC | Selumetinib | II R | Palliative (I) | MEK |
| NCT01242605 | BTC and GBC | Selumetinib | I | Palliative (I) | MEK |
| NCT02042443 | BTC and GBC | Trametinib | II R | Palliative (II>) | MEK |
| NCT03027284 | CCA and GBC | Merestinib | I | Palliative (I) | C-met |
| NCT02631590 | BTC and GBC | Copanlisib | II | Palliative (I) | PI3K |
| NCT01425879 | eCCA and GBC | MK2206 | II | Palliative (II>) | Akt |
| NCT00949949 | BTC and GBC | Everolimus | I | Palliative (I) | mTor |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canale, M.; Monti, M.; Rapposelli, I.G.; Ulivi, P.; Sullo, F.G.; Bartolini, G.; Tiberi, E.; Frassineti, G.L. Molecular Targets and Emerging Therapies for Advanced Gallbladder Cancer. Cancers 2021, 13, 5671. https://doi.org/10.3390/cancers13225671
Canale M, Monti M, Rapposelli IG, Ulivi P, Sullo FG, Bartolini G, Tiberi E, Frassineti GL. Molecular Targets and Emerging Therapies for Advanced Gallbladder Cancer. Cancers. 2021; 13(22):5671. https://doi.org/10.3390/cancers13225671
Chicago/Turabian StyleCanale, Matteo, Manlio Monti, Ilario Giovanni Rapposelli, Paola Ulivi, Francesco Giulio Sullo, Giulia Bartolini, Elisa Tiberi, and Giovanni Luca Frassineti. 2021. "Molecular Targets and Emerging Therapies for Advanced Gallbladder Cancer" Cancers 13, no. 22: 5671. https://doi.org/10.3390/cancers13225671






